Abstract
Rationale and Objective
Computed tomography (CT) of the chest can be used to assess pectoralis muscle area (PMA) and subcutaneous adipose tissue area (SAT). Adipose tissue content is associated with inflammatory mediators in chronic obstructive pulmonary disease (COPD) subjects. Based on gender differences in body composition, we aimed to assess the hypothesis that in subjects with COPD the relationships between PMA, SAT, and blood biomarkers of inflammation differ by gender.
Materials and Methods
We compared chest CT measures of PMA and SAT on a single slice at aortic arch and supraesternal notch levels from 73 subjects (28 women) with COPD between genders. The relationships of PMA and SAT to biomarkers were assessed using within-gender regression models.
Results
Women had a lower PMA and higher SAT than men (difference range for PMA, 13.3–22.8 cm2; for SAT, 11.8–12.4 cm2; P<0.05 for all comparisons) at both anatomic levels. These differences in PMA and SAT remained significant after adjustment for age and body mass index. Within-gender regression models adjusted for age showed that SAT was directly associated with C-reactive protein (for aortic arch level, P=0.04) and fibrinogen (for both anatomic locations, P=0.003) only in women, whereas PMA was not associated with any biomarkers in either gender.
Conclusion
It appears that in subjects with COPD there are gender-based differences in the relationships between subcutaneous adipose tissue and inflammatory biomarkers.
Introduction
Chronic obstructive pulmonary disease (COPD) affects approximately 28.9 million people in the United States.1 It is increasingly recognized that altered body composition is common in COPD and represents a clinically relevant process in patients suffering from this condition.2–4 For example, a low body mass index (BMI) is associated with increasing mortality.4 Furthermore, dissecting the components of body composition in COPD subjects gives additional understanding of extra-pulmonary features of the disease. Prior investigation has demonstrated that the prevalence of low fat free mass prevalence was higher than that of low BMI.5. Together these findings suggest that characterizing distinct body components beyond BMI is of clinical importance.
There are several methods available for the determination of body composition including skin fold thickness, bioimpedance, and dual energy X-ray absorbance (DXA).6 However, measures of skin fold thickness have been found to underestimate fat free mass6 and both bioimpedance and DXA are not widely available. Prior investigations7,8 have suggested that computed tomographic (CT) measures may provide additional insight into the body composition of smokers. Marquis et al found that mid thigh muscular cross sectional area was a stronger predictor of mortality in COPD than BMI.7 We also observed that mid thigh muscle loss is even present in smokers with mild COPD.9 We have demonstrated that CT measures of pectoralis muscle area (PMA) on a single axial slice may be a more clinically relevant measure of COPD-related outcomes than BMI in the prediction of including spirometric measures of lung function, symptoms and exercise capacity.10
Subcutaneous adipose tissue (SAT) can also be assessed on axial CT images of the chest and can provide additional understanding of body composition in COPD. Chest CT measures of muscle area and fat area, however, may vary according to the anatomical location of the measurement and to gender differences in body composition such as the presence of breast tissue in women in the anterior chest wall. Additionally, adipose tissue depots might be a source of inflammatory mediators and contribute to low-grade inflammatory state in COPD.11 Gender differences in adipose tissue metabolism and inflammatory biomarker levels have been documented.12,13 For example, smoking women have lower level of C reactive protein.13 Thus, exploring gender differences in the relationships between distinct body composition components and inflammatory mediators can further the understanding of extra-pulmonary manifestations of COPD. In this study we aimed to (1) assess the reproducibility of CT measurements of SAT and PMA; (2) examine the association between these body composition metrics and blood inflammatory mediators; and (3) compare PMA SAT, and their association with inflammatory mediators in men vs. women. We hypothesize that in COPD subjects CT measures of PMA and SAT are correlated with inflammatory mediators and that these correlations differ between genders. To test this hypothesis, we measured PMA and SAT on CT scans of subjects from The Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) Study14 at two specific anatomic levels of the chest and correlated these findings with inflammatory mediators measured from peripheral blood.
Materials and Methods
Population
The ECLIPSE Study is a non-interventional, multicenter, longitudinal study designed to identify factors that predict COPD progression as well as disease subtypes and biomarkers that may be useful as surrogate end-points. ECLIPSE enrolled smokers (≥10 pack-years) aged 40–75 years with Chronic Obstructive Lung Disease (GOLD) stage II–IV COPD (n=2162).14 All subjects provided informed consent to participate in the study. For this study we used a convenience sample of 73 ECLIPSE subjects with COPD (28 females) who had fat free mass (FFM) data collected. Body composition was assessed using bioelectrical impedance analysis as detailed elsewhere.3 Briefly, FFM was computed using sex-specific equations,15 and fat mass was calculated by subtracting FFM from weight. In order to take into account differences in body surface, fat mass index (FMI) was computed by dividing fat mass by squared height and expressed as kg/m2.
Imaging assessment
All subjects underwent a low dose volumetric CT scan of the chest at baseline and at years 1 and 3 of follow-up with the following protocol: 120 kV peak, 40 mA and 1.00 or 1.25-mm slice thickness at full inspiration. In this study we used the baseline CT data to perform body composition measures. The radiation was estimated at 5 mSv for each subject for the entire ECLIPSE protocol.14
Pectoralis muscle area (PMA) and subcutaneous adipose tissue area (SAT) measurements
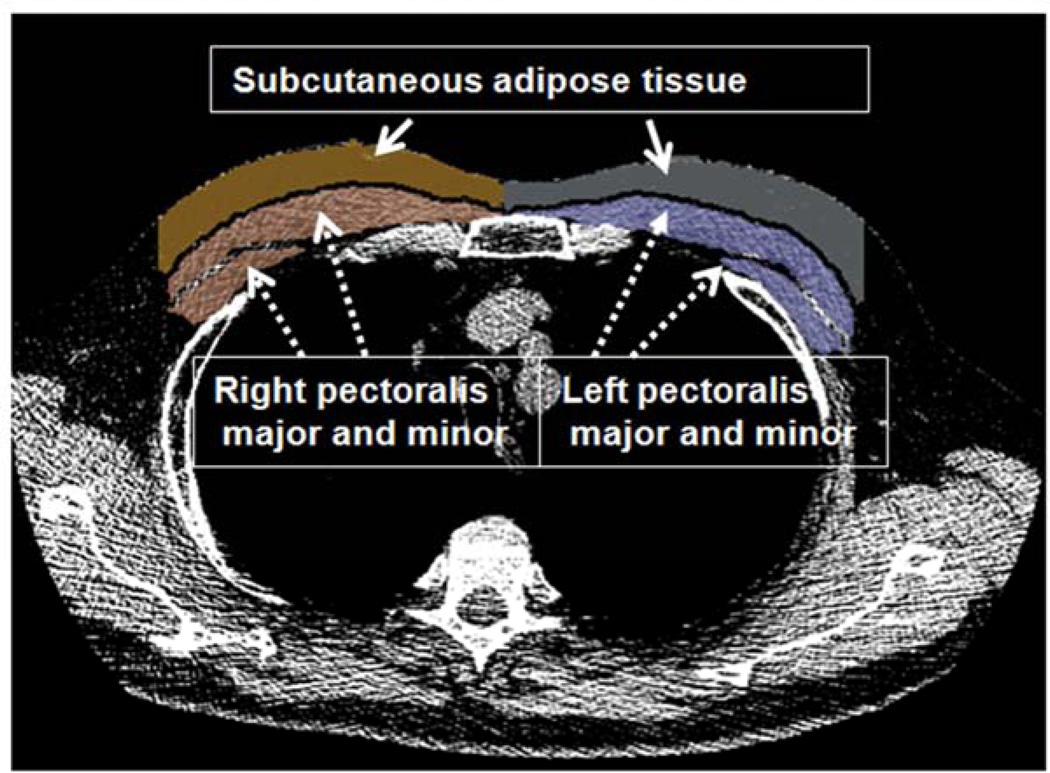
Measures of PMA and SAT were performed using custom software by a pulmonologist who was blinded to subjects’ data. PMA and SAT were measured on a single-axial slice of the CT scans as follows: The reader visually identified the superior aspect of the aortic arch and then scrolled towards the apex of the lungs to identify the first axial image above the arch and the first image above the supraesternal notch of the sternum. These slices were selected because they were easy to identify and could be replicated across a large cohort of subjects. The left and right pectoralis major and minor muscles were then identified on the anterior chest and their edges manually segmented using a pre-defined attenuation range of −50 and 90 Hounsfield Units. The SAT was defined as the region of interest between the pectoralis major muscles and skin surface on those same axial slices and their edges were manually determined using a range of −200 and 0 Hounsfield Units. We used this limited subcutaneous fat region because the entire circumference of the chest was not available in many CT scans (Figure 1). Both PMA and SAT are reported as the aggregate area in cm2 of the right and left pectoralis major and minor and the right and left fat areas, respectively, assessed in those axial planes. In order to evaluate the intra- and inter-reader reproducibility of PMA and SAT at these two locations, a second reader measured a random sample of 20 CT scans two times.
Figure 1.
Axial slice at aortic arch level showing muscles and fat segmentation. The segmented pectoralis muscle major and minor (dotted arrows) and the subcutaneous adipose tissue (solid arrows) are shown. The pectoralis muscle area (PMA) and subcutaneous adipose tissue area (SAT) are computed as aggregate measures and reported in cm2.
Lung function assessment and biomarkers collection
At baseline and at each subsequent visit, patients underwent spirometric evaluation (Viasys MasterScope) before and 15 minutes after inhaling 400 µg of the bronchodilator salbutamol. Forced expiratory volume in 1 second (FEV1) from spirometry is reported as percent predicted values.16 The following biomarkers in blood were collected at baseline and stored at −80°C until they were analyzed: C-reactive protein (CRP), interleukin-6 (IL-6), and fibrinogen.16 Biomarkers are reported as mean (interquantile range) as in a prior ECLIPSE report.16
Statistical analysis
The intra- and inter-reader agreement of PMA and SAT was assessed with the concordance correlation coefficient (CCC)17 and a Bland-Altman analysis.18 We used these tests because they provide complementary information on assessing agreement of CT measures between readers. Differences in subjects’ characteristics at baseline and PMA and SAT between genders were assessed using a Wilcoxon rank-sum test since some of the variables did not follow normality. Pairwise correlations of PMA and SAT to anthropometric measures and biomarkers by gender were performed using a Spearman correlation. Liner regression models for PMA, SAT, and biomarkers were also performed. These 3 dependent variables were log transformed to normalize them. The models were adjusted for age and BMI. A P<0.05 was considered significant. All the analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC).
Results
Clinical and biomarkers data
Clinical and biomarkers data by gender is shown in Table 1. Female subjects had significantly lower stature, body mass index (BMI), and fat free mass than males. In contrast, females had higher FMI than males. Females also had marginally significant (p=0.065) lower levels of IL-6. No differences in age, dyspnea score, FEV1 % predicted, CRP, and fibrinogen were found.
Table 1.
Baseline characteristics of the 73 selected subjects by gender
| Variable | Female (n=28) |
Male (n=45) |
P |
|---|---|---|---|
| Age, y | 64.5 (59.0 – 67.0) | 62.0 (59.0 – 67.0) | 0.97 |
| Pack years of smoking | 43.5 (29.5 – 53.0) | 44.0 (30.0 – 68.0) | 0.66 |
| BMI, kg/m2 | 24.3 (22.3 – 26.4) | 28.6 (23.9 – 32.6) | 0.0045 |
| Fat free mass, kg | 37.3 (34.1 – 41.8) | 59.4 (54.4 – 67.4) | <0.0001 |
| Fat mass index, kg/m2 | 15.2 (14.1 – 16.3) | 10.6 (10.1 – 12.4) | <0.0001 |
| FEV1 % predicted | 45.6 (34 – 53.5) | 43.5 (31.2 – 54.9) | 0.84 |
| Biomarkers | |||
| C-reactive protein, µg/ml | 4.2 (1.3 – 4.8) | 5.1 (1.4 – 6.6) | 0.75 |
| Fibrinogen, mg/dl | 458.5 (425.0 – 507.0) | 449 (386.0 – 485.0) | 0.226 |
| Interleukin-6, pg/ml | 2.6 (0.5 – 2.3) | 6.6 (0.9 – 3.2) | 0.065 |
Continuous variables are presented as median (interquantile range) except for biomarkers (mean [interquantile rage]). Missing data, BMI 7; Fat mass index 7; C-reactive protein 3; Interleukin-6 7
Intra- and inter-reader assessment of PMA and SAT
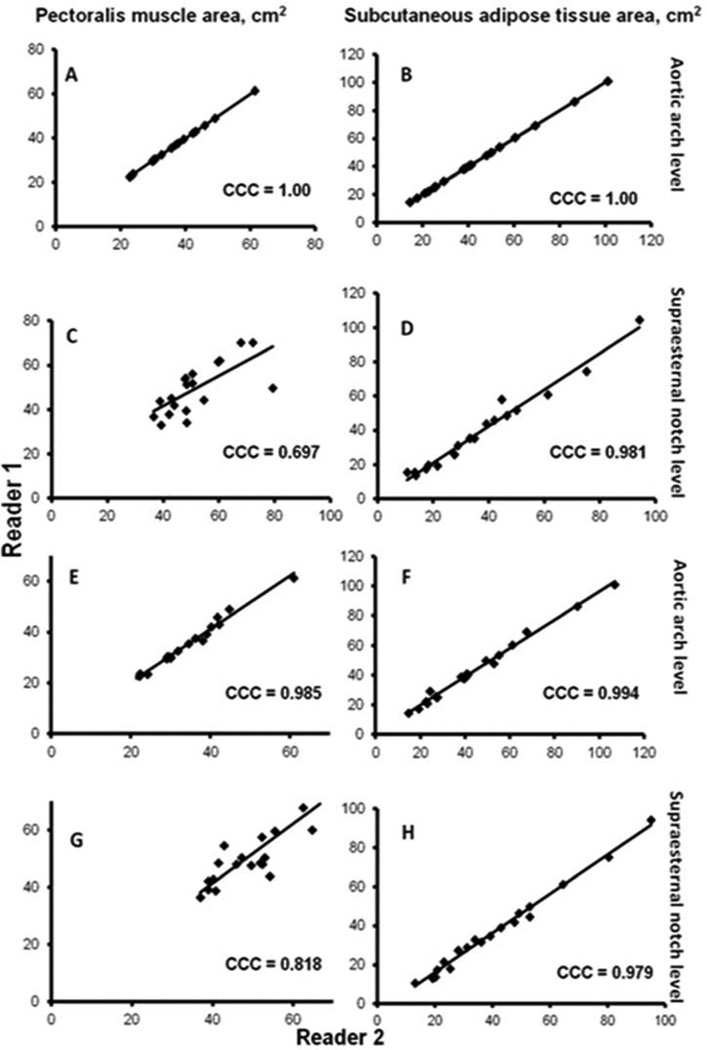
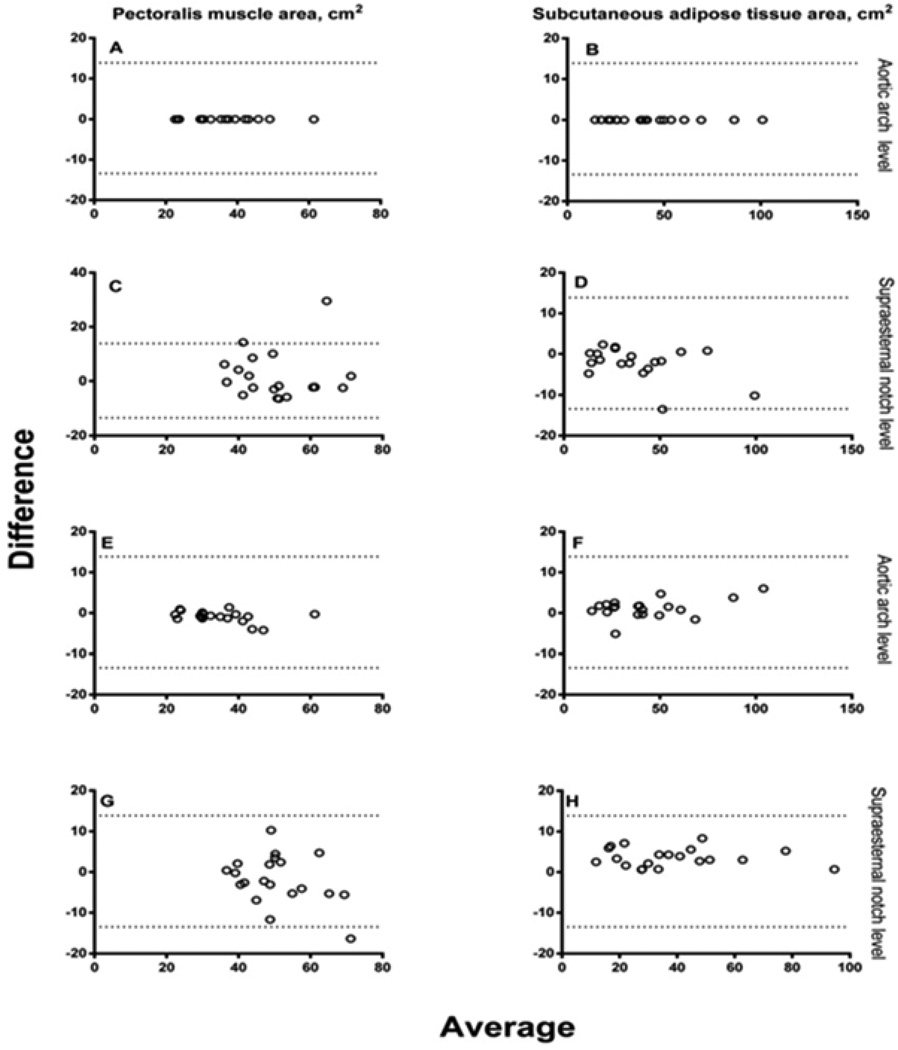
The intra-reader CCC of both PMA and SAT at aortic arch level was 1.00 and corresponding values at supraesternal notch level were 0.697 and 0.981. The inter-reader CCC of PMA and SAT at aortic arch level were 0.985 and 0.994, respectively, and 0.818 and 0.979 at supraesternal notch level, respectively (Figure 2A). The Bland-Altman plots did not show a systematic bias across the range of PMA and SAT values between readers (Figure 2B). The correlation between measures of PMA at aortic arch and supraesternal notch levels was 0.81 and the corresponding value for SAT was 0.95.
Figure 2.
A Regression line plots and the concordance correlations coefficients (CCC) values of the reproducibility of pectoralis muscle area (PMA) and subcutaneous adipose tissue area (SAT) measurements between two readers. The intra-reader reproducibility of PMA at aortic arch (A) and supraesternal notch levels (C) and of SAT (B, D) are shown. Corresponding plots and CCC values for inter-reader reproducibility of PMA at aortic arch (E) and supraesternal notch levels (G) and of SAT (F, H) are also shown.
B Bland-Altman plots of the reproducibility of pectoralis muscle area (PMA) and subcutaneous adipose tissue area (SAT) measurements between two readers. Dotted lines represent the 95% confidence intervals. Plots for intra-reader reproducibility of PMA at aortic arch (A) and supraesternal notch levels (C) and of SAT (B, D) are shown. Corresponding plots for inter-reader reproducibility of PMA at aortic arch (E) and supraesternal notch levels (G) and of SAT (F, H) are also shown.
Comparison of PMA and SAT by gender
Regardless of the anatomic level of measurement, female subjects had lower PMA than males (mean of 13.3 cm2 and 22.8 cm2 lower in women at aortic arch and supraesternal notch levels, respectively; for both P<0.0001). An opposing significant difference in SAT was observed in both locations (mean of 12.4 cm2 and 11.8 cm2 higher in women at aortic arch and supraesternal notch levels, respectively) (Table 2). In regression models adjusted for age and BMI, the differences in PMA and SAT between genders persisted (for all comparisons, P<0.0001).
Table 2.
Pectoralis muscles area (PMA) and subcutaneous adipose tissue area (SAT) in COPD subjects by gender
| CT Measurement | Female Median (IQR) |
Male Median (IQR) |
P |
|---|---|---|---|
| PMA at aortic arch, cm2 | 24.2 (19.5 – 27.2) | 37.0 (31.1 – 40.7) | <0.0001 |
| PMA at supraesternal notch, cm2 | 39.8 (33.7 – 42.9) | 63.0 (52.3 – 70.8) | <0.0001 |
| SAT at aortic arch, cm2 | 49.0 (35.2 – 68.7) | 38.3 (24.9 – 49.9) | 0.03 |
| SAT at supraesternal notch, cm2 | 47.1 (28.2 – 64.3) | 32.3 (23.0 – 51.3) | 0.04 |
Missing PMA and SAT data at supraesternal notch level for 1 subject due to image truncation; CT= computed tomography
Relationships between PMA, SAT, anthropometric variables, and biomarkers
There was a significant correlation between both PMA and SAT measured at both levels with BMI. These relationships were weaker in women. Similarly, the PMA and SAT at both levels correlated positively with FFM in both genders with the correlations coefficients being lower in women. SAT but not PMA correlated directly with FMI, CRP, and fibrinogen only in women at both anatomic levels (Tables 3A and 3B). The associations between SAT and fibrinogen remained significant in within-gender adjusted models for age (for both anatomic levels, P=0.003), while the association between SAT and CRP was significant at aortic arch level only (P=0.04) and near significance at supraesternal notch level (P=0.11). In contrast, BMI and FFM were not significantly associated with any above biomarkers within either gender (P>0.05 for CRP, fibrinogen, and IL-6). FMI was correlated with CRP only in women (r= 0.51; P=0.009).
Table 3.
| A Spearman correlation coefficients (r) between pectoralis muscles area (PMA) and subcutaneous adipose tissue area (SAT) and anthropometric and biomarker data in women | ||||
|---|---|---|---|---|
| PMA | SAT | |||
| Variable | Aortic arch level | Supraesternal notch level | Aortic arch level | Supraesternal notch level |
| Age | −0.33 | −0.21 | −0.07 | −0.01 |
| BMI, m/kg2 | 0.44* | 0.60** | 0.62** | 0.61** |
| Fat free mass, kg | 0.41* | 0.60** | 0.40* | 0.43* |
| Fat mass index, m/kg2 | 0.25 | 0.30 | 0.59** | 0.49* |
| Biomarkers | ||||
| C-reactive protein, µg/ml | 0.20 | 0.12 | 0.40* | 0.36* |
| Fibrinogen, mg/dl | 0.03 | 0.29 | 0.54** | 0.51** |
| Interleukin-6, pg/ml | 0.25 | 0.18 | 0.28 | 0.32 |
| B Spearman correlation coefficients (r) between pectoralis muscles area (PMA) and subcutaneous adipose tissue area (SAT) and anthropometric and biomarker data in men | ||||
|---|---|---|---|---|
| PMA | SAT | |||
| Variable | Aortic arch level | Supraesternal notch level | Aortic arch level | Supraesternal notch level |
| Age | −0.12 | −0.22 | −0.15 | −0.17 |
| BMI, m/kg2 | 0.58*** | 0.69*** | 0.89*** | 0.87*** |
| Fat free mass, kg | 0.44** | 0.64*** | 0.77*** | 0.75*** |
| Fat mass index, m/kg2 | 0.04 | −0.08 | −0.04 | −0.02 |
| Biomarkers | ||||
| C-reactive protein, µg/ml | 0.003 | 0.05 | 0.14 | 0.10 |
| Fibrinogen, mg/dl | −0.07 | −0.09 | 0.12 | 0.17 |
| Interleukin-6, pg/ml | −0.2 | −0.15 | −0.21 | −0.20 |
P<0.05;
P<0.01;
P<0.0001.
P>0.05 when the correlation coefficient has no star
P<0.01;
P<0.0001;
P>0.05 when the correlation coefficient has no star
Discussion
In this study we have demonstrated that chest CT scans can be used to assess body composition in subjects with COPD enrolled in the ECLIPSE study. We found that CT measurements of PMA at the aortic arch level and SAT at both aortic arch and supraesternal notch locations were highly reproducible. PMA was lower in female compared to male subjects, while the opposite was observed in SAT. Additionally, SAT was directly related to CRP and fibrinogen levels only in women whereas PMA was not related with any biomarkers within either gender.
In this study, we used a simple approach to assess pectoralis muscle and subcutaneous fat content by measuring their cross-sectional areas on a single-slice on existing chest CT scans. We found that the intra- and inter-reader agreement of PMA was high at aortic level and that of SAT was high at both anatomic locations, respectively. The PMA agreement at aortic arch level was greater than that reported in COPDGene subjects,19 which may be due to readers’ differences in experience with the task between the two studies. One explanation for the lower intra- and inter-reader agreement in PMA at the supraesternal level is that structures such as vessels surrounding the pectoralis muscle look similar in density on non-contrast CT, making the identification of this portion of this muscle harder than at the aortic arch level. However, we think that with proper training this CT technique is an easy-to-do, relatively fast assessment of the body composition making it usable in large-scale clinical and epidemiologic investigation.
CRP is a protein synthesized in hepatocytes, lymphocytes, and alveolar macrophages, and levels of CRP increase in response to acute and chronic infections.20 Fibrinogen is also an acute phase reactant produced in the liver.20,21 Previous studies have shown that both systemic inflammatory mediators are elevated12,20,22 and associated with relevant clinical outcomes23,24 and mortality25,26 in subjects with COPD. Moreover, adipose tissue depots including subcutaneous fat are associated with inflammatory mediators. For example, in COPD patients with high (vs. low) CRP had higher adipose tissue macrophages infiltration11 and in the Framingham study, a positive association between SAT content measured at abdominal level and fibrinogen was observed.27 Consistent with these findings, we observed that in women, greater SAT was linked to increased CRP and fibrinogen levels. While the reasons for these gender differences in the relationships of SAT and these biomarkers are unclear, it may involve differences in sex hormones regulation of these mediators, ageing, and the interaction between these processes and COPD. Although the sample size of our study is small, our findings may suggest that SAT has a sex-specific exacerbating effect on mediators of inflammation.
In this study, we also observed that PMA was lower in women than men, which is consistent with our data demonstrating lower fat free mass in female subjects and with others’ observations in peripheral skeletal muscles in patients with COPD.28 The magnitude of the observed difference in PMA at aortic level between genders was similar to that of COPDGene Study.19 In contrast, SAT was higher in women than men at both anatomic locations suggesting that this measurement is not influenced by the breast in women. Together these findings are in agreement with the notion that the clinical assessment of body composition in COPD should take into account more factors than a simple measure of BMI.29
Several limitations are worth of noting. We used a small, convenient sample of subjects from a large study, so caution should be exercised to generalize our findings. While CT measures of body composition were reproducible, they are likely dependent based on body position. For example, as one raises their hands above their head (the standard position for CT scanning) the pectoralis muscles become elongated and the PMA may be artificially decreased, and this factor might be important in subjects with severe COPD who may be limited to do this maneuver. Since the standard procedure for CT scanning in this study was with the hands above the head, we believe that this would not result in a specific gender bias. We used a CT cross-sectional area of muscle and adipose tissues on single slices as opposed to the gold standard measure of body composition (i.e. DXA). However, FFM measured using bioelectrical impedance has been repeatedly shown to correlate with COPD-related traits demonstrating it is utility in the research setting,3,4,19 and we observed that FFM measured with bioelectrical impedance was directly associated with PMA in both genders. Therefore, we believe that our findings indicate that CT measures of muscle and adipose tissue are relevant to this disease even though they do use the current reference standard.
It appears that there are gender differences in the relationships between CT measures of subcutaneous fat area and mediators of inflammation. While these findings are preliminary, we believe that CT measures of muscle and fat are a valuable tool to assess body composition in COPD and warrant further investigation in larger cohorts and longitudinal studies.
Acknowledgments
Funding
Dr. Diaz is supported by NIH grant 1K01HL118714-01 and the Brigham and Women’s Hospital Minority Faculty Career Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ford ES, Mannino DM, Wheaton AG, et al. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination surveys from 1988–1994 to 2007–2010. Chest. 2013;143:1395–1406. doi: 10.1378/chest.12-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sava F, Laviolette L, Bernard S, et al. The impact of obesity on walking and cycling performance and response to pulmonary rehabilitation in COPD. BMC Pulm Med. 2010;10:55. doi: 10.1186/1471-2466-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutten EP, Calverley PM, Casaburi R, et al. Changes in Body Composition in Patients with Chronic Obstructive Pulmonary Disease: Do They Influence Patient-Related Outcomes? Ann Nutr Metab. 2013;63:239–247. doi: 10.1159/000353211. [DOI] [PubMed] [Google Scholar]
- 4.Schols AM, Broekhuizen R, Weling-Scheepers CA, et al. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53–59. doi: 10.1093/ajcn.82.1.53. [DOI] [PubMed] [Google Scholar]
- 5.Kim SB, Kang YA, Jung JY, et al. Body mass index and fat free mass index in obstructive lung disease in Korea. Int J Tuberc Lung Dis. 2014;18:102–108. doi: 10.5588/ijtld.13.0212. [DOI] [PubMed] [Google Scholar]
- 6.Hronek M, Kovarik M, Aimova P, et al. Skinfold anthropometry--the accurate method for fat free mass measurement in COPD. COPD. 2013;10:597–603. doi: 10.3109/15412555.2013.781151. [DOI] [PubMed] [Google Scholar]
- 7.Marquis K, Debigare R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 8.Romme EA, Murchison JT, Edwards LD, et al. CT-measured bone attenuation in patients with chronic obstructive pulmonary disease: relation to clinical features and outcomes. J Bone Miner Res. 2013;28:1369–1377. doi: 10.1002/jbmr.1873. [DOI] [PubMed] [Google Scholar]
- 9.Diaz AA, Morales A, Diaz JC, et al. CT and physiologic determinants of dyspnea and exercise capacity during the six-minute walk test in mild COPD. Respir Med. 2013;107:570–579. doi: 10.1016/j.rmed.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 10.McDonald ML, Diaz AA, Ross JC, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc. 2014;11:326–334. doi: 10.1513/AnnalsATS.201307-229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Borst B, Gosker HR, Wesseling G, et al. Low-grade adipose tissue inflammation in patients with mild-to-moderate chronic obstructive pulmonary disease. Am J Clin Nutr. 2011;94:1504–1512. doi: 10.3945/ajcn.111.023911. [DOI] [PubMed] [Google Scholar]
- 12.Breyer MK, Rutten EP, Vernooy JH, et al. Gender differences in the adipose secretome system in chronic obstructive pulmonary disease (COPD): a pivotal role of leptin. Respir Med. 2011;105:1046–1053. doi: 10.1016/j.rmed.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Ahonen TM, Kautiainen HJ, Keinanen-Kiukaanniemi SM, et al. Gender difference among smoking, adiponectin, and high-sensitivity C-reactive protein. Am J Prev Med. 2008;35:598–601. doi: 10.1016/j.amepre.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) Eur Respir J. 2008;31:869–873. doi: 10.1183/09031936.00111707. [DOI] [PubMed] [Google Scholar]
- 15.Steiner MC, Barton RL, Singh SJ, et al. Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J. 2002;19:626–631. doi: 10.1183/09031936.02.00279602. [DOI] [PubMed] [Google Scholar]
- 16.Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 17.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 19.McDonald M-L, Diaz AA, Ross JC, et al. Quantitative CT measures of pectoralis muscle area and disease severity in COPD: a cross-sectional study. Annals of the ATS. 2014 doi: 10.1513/AnnalsATS.201307-229OC. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and markers of inflammation: data from the Third National Health and Nutrition Examination. Am J Med. 2003;114:758–762. doi: 10.1016/s0002-9343(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 21.Duvoix A, Dickens J, Haq I, et al. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax. 2013;68:670–676. doi: 10.1136/thoraxjnl-2012-201871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nillawar AN, Joshi KB, Patil SB, et al. Evaluation of HS-CRP and Lipid Profile in COPD. J Clin Diagn Res. 2013;7:801–803. doi: 10.7860/JCDR/2013/5187.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrari R, Tanni SE, Caram LM, et al. Three-year follow-up of Interleukin 6 and C-reactive protein in chronic obstructive pulmonary disease. Respir Res. 2013;14:24. doi: 10.1186/1465-9921-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309:2353–2361. doi: 10.1001/jama.2013.5732. [DOI] [PubMed] [Google Scholar]
- 25.Man SF, Connett JE, Anthonisen NR, et al. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61:849–853. doi: 10.1136/thx.2006.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahl M, Vestbo J, Lange P, et al. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–255. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 27.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 28.Seymour JM, Ward K, Sidhu PS, et al. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax. 2009;64:418–423. doi: 10.1136/thx.2008.103986. [DOI] [PubMed] [Google Scholar]
- 29.Maltais F. Body composition in COPD: looking beyond BMI [Editorial] Int J Tuberc Lung Dis. 2014;18:3–4. doi: 10.5588/ijtld.13.0868. [DOI] [PubMed] [Google Scholar]