Abstract
Diabetic retinopathy is the lead among causes of blindness in North America. Glucose-induced endothelial injury is the most important cause of diabetic retinopathy and other vascular complications. Extracellular signal-regulated kinase 5 (ERK5), also known as big mitogen-activated protein kinase 1 (BMK1), is a member of mitogen-activated protein kinases (MAPK) family. Physiologically, it is critical for cardiovascular development and maintenance of the endothelial cell integrity. Extracellular signal-regulated kinase 5 is protective for endothelial cells under stimulation and stress. Decreased activation of ERK5 results in increased endothelial cell death. Extracellular signal-regulated kinase 5 signaling may be subject to alteration by hyperglycemia, while signaling pathway including ERK5 may be subject to alteration during pathogenesis of diabetic complications. In this review, the role of ERK5 in diabetic macro- and microvascular complications with a focus on diabetic retinopathy are summarized and discussed.
Key Words: Endothelial Cells, ERK5, Diabetic Retinopathy
INTRODUCTION
Chronic complications are the leading cause of mortality and morbidity in all types of diabetes (1, 2). Vascular endothelium is a primary organ affected in chronic diabetic complications wherein it acts both the target organ and potential mediator (1, 3). Chronic complications typically develop after 10 to 20 years of diabetes, and include both macroangiopathy and microangiopathy. Macroangiopathy is an accelerated form of atherosclerosis, a pathological process initiated by injury of endothelial cells seen in diabetes. This increases the risk of myocardial infarction, stroke, intermittent claudication and the ischemic gangrene (4).
Diabetes also causes microvascular complications such as diabetic retinopathy (DR) and nephropathy (5). Diabetic retinopathy is a severe complication of diabetes, manifesting primarily as vascular changes (structural and functional) in the retina. Diabetic retinopathy may result in vision loss, and it is the most common cause of blindness in North America in the age group 25–74 years (6). It has two phases, non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) (7, 8). In NPDR phase, the vessels in the retina are weakened and leaky, forming microaneurysms and retinal hemorrhages, which leads to decreased vision. Proliferative diabetic retinopathy is an advanced stage in which new, but fragile, therefore delicate blood vessels develop on the surface of the retina or on the optic disk. Consequently, they rupture easily what makes the cause to tractional retinal detachment and blindness (9). Several growth and vasoactive factors are implicated in the development of PDR (10). Vascular endothelial growth factor (VEGF) plays a significant role in mediating intraocular neovascularization in patients with DR (11). Inhibition of ocular VEGF by intravitreal injection of anti-VEGF drug has emerged as a promising treatment for PDR (12, 13).
Diabetic nephropathy is a progressive kidney disease caused by microangiopathy in the renal glomeruli. It is characterized by nephrotic syndrome and diffuse glomerulosclerosis (14) and is a common cause of dialysis in Western countries.
HYPERGLYCEMIA IS DIRECTLY RELATED TO ENDOTHELIAL DYSFUNCTION IN DIABETES
Diabetes-associated conditions such as hypertension, dyslipidemia and insulin resistance are correlated to impaired endothelial function (1, 2, 4). However, hyperglycemia is most commonly causally associated with endothelial dysfunction in chronic diabetic complications such as DR (1, 15). Evidences demonstrate impaired endothelial vasodilator function during either acute or chronic hyperglycemia both in human (16-18) and in animal diabetes (19, 20). In addition, hyperglycemia is known to increase endothelial permeability to macromolecules, delay cell replication, increase the secretion of sclerotic matrix proteins, increase adhesive properties for leukocytes and decrease the secretion of the pro-fibrinolytic agents, such as tissue plasminogen activator (tPA) (1). Both the Diabetes Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes Study (UKPDS) have demonstrated correlations between poor glycemic control and increased incidences of microvascular complications in patients with diabetes (21, 22). Other clinical trials have also shown that macrovascular complications such as coronary (23) and peripheral artery disease (24) are related to glycemic levels.
STRUCTURE OF ERK5
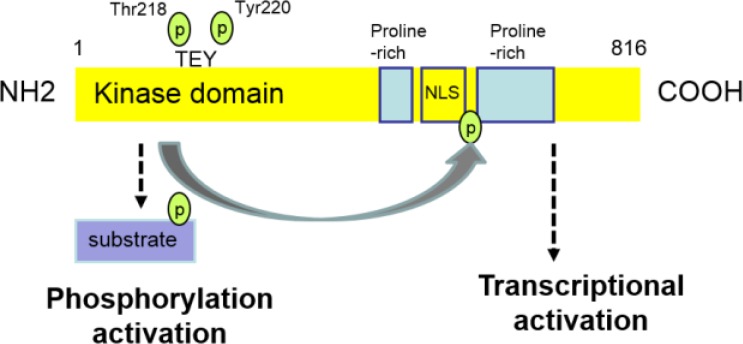
Human ERK5 is 816 amino acids protein of with a predicted molecular mass of 98 kDa. Extracellular signal-regulated kinase 5 is encoded by MAPK7 gene, present in the majority of mammals (sharing 80-98% homology). It is more than twice the size of the other MAPKs due to its unique C-terminal. The N-terminal of MAPK’s catalytic domain share more than 50% homology with ERK1/2, which contains the Thr–Glu–Tyr (TEY) dual phosphorylation pattern in the activation loop (Fig. 1) (29). The C-terminal of ERK5 contains a nuclear localization signal (NLS) crucial for the nuclear localization of ERK5 upon stimulation; and two proline-rich regions that may serve as binding sites for Src homology 3 (SH3) domain containing proteins (29,32,33) (Fig. 1).
Figure 1.
Structure and activation of ERK5.
KINASE ACTIVATION OF ERK5
Mitogen- activated protein kinases signaling cascade consists of three sequentially activated kinases: MEKK, MEK, and MAPK. These kinase module relay signals from extracellular agonists to cellular targets. The signaling modules in the ERK5 pathway are composed of MEKK2/MEKK3, MEK5 and ERK5 (Fig. 2) (28, 29, 38, 39). MEKK2/MEKK3 phosphorylate MEK5 on Ser311 and Thr315, resulting in an increase in MEK5 activities (38). Extracellular-signal-regulated kinase 5 is activated by dual phosphorylation at Thr218/Tyr220 by an upstream kinase MEK5 (28, 29, 40). MEK5 preferentially phosphorylates ERK5 on Thr218, which might induce a conformational change and subsequent phosphorylation of Tyr220 (41). Active ERK5 can undergo auto-phosphorylation on the C-terminal at a number of residues including Thr28, Ser421, Ser433, Ser496, Ser731, and Thr733, leading to an enhancement of ERK5 transcriptional activity as described below. Activated ERK5 also phosphorylates MEK5 at residues 129, 137, 142 and 149 which are located in the region that is thought to interact with ERK5 (41). PKCζ, an atypical protein kinase C, has been reported to interact with MEK5 in EGF-induced activation of ERK5 (42, 43). Interestingly, a recent study demonstrated that PKCζ is directly associated with ERK5. PKCζ mediates inhibitory phosphorylation of ERK5 by binding and phosphorylating serine 486, thus suppressing ERK5 function in TNFα-mediated inflammatory process (44).
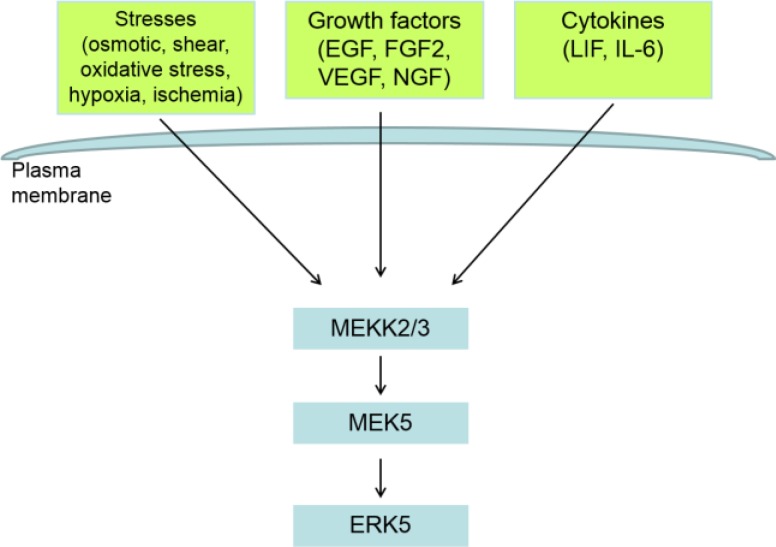
Figure 2.
Activators of ERK5 Pathway.
The signaling modules in the ERK5 pathway are composed of MEKK2/MEKK3, MEK5, and ERK5. ERK5 is activated by a variety of stimuli. It can be activated by serum and a range of growth factors including EGF, FGF2, VEGF, and nerve growth factor (NGF). It can also be activated by cytokines such as leukemia inhibitory factor (LIF) and IL-6. Additionally, range of stress stimuli such as osmotic (58), fluid shear(30), or oxidative stresses; hypoxia (59) or ischemia (60) may activate ERK5.
G-proteins are involved in the activation of ERK5 by growth factors (61). In addition, studies have shown that PKCζ mediates ERK5 activation by G protein-coupled receptors (GPCR) (42, 44, 62). It has been also reported that G protein acts as an adaptor protein in PKCζ-mediated ERK5 activation by GPCR (62).
TRANSCRIPTIONAL ACTIVATION OF ERK5
The C-terminal region of ERK5 contains a transcriptional activation domain, which is required for maximal transcriptional activity of target molecules (32, 45, 46). Activated ERK5 phosphorylates itself at the C-terminal at a number of residues (41) and auto-phosphorylation of C-terminal region of ERK5 leads to enhanced transcriptional activity (45, 47). Once stimulated, phosphorylation of ERK5 results in the activation of the kinase activity. Extracellular-signal-regulated kinase 5 phosphorylates both downstream target molecules and their C-terminal region (Fig. 1). Thus, auto-phosphorylation of the C-terminal leads to a further increase in the transcription activity of target molecules (47). In addition, Morimoto et al. showed that the activated kinase activity of ERK5 is required for the C-terminal mediated transcriptional activation of downstream targets. Mutation of phosphorylatable Thr and Ser residues to unphosphorylatable Ala significantly reduces the transcriptional activation effect of ERK5 (47). Interestingly, C-terminal also regulates the kinase activation of N-terminal. Deletion of C-terminal results in a dramatic increase in kinase activation of N-terminal (32).
REGULATORS OF ERK5 SIGNALING
Similar to other MAPKs, ERK5 is activated by a variety of stimuli (Fig. 2). Studies have revealed that it is activated by serum (48), a range of growth factors including epidermal growth factor (EGF) (49), fibroblast growth factor-2 (FGF-2) (50), VEGF (31), and by cytokines such as LIF (51) and interleukin 6 (IL-6) (52). Additionally, NGF, use the ERK5 pathway to mediate its effects on neuronal cells, ECs as well as other cell types (53-56). We found that recombinant NGF stimulated ERK5 activation in the basal and high glucose conditions in ECs (57).
SUBSTRATES OF ERK5 SIGNALING
A number of molecules have been identified as substrates of the ERK5 pathway. The transcription factors of the myocyte enhancer factor 2 (MEF2) family are best-characterized substrates of ERK5 (48, 63, 64). MEF2 is a four-membered family of transcription factors including MEF2A, MEF2B, MEF2C, and MEF2D. ERK5 phosphorylates and activates MEF2A, MEF2C and MEF2D, but not MEF2B (48, 63). The C-terminal tail of ERK5 contains an MEF2-interacting region and a transcriptional activation domain essential for co-activation of MEF2 (45). Activation of the MEF2 by the ERK5 is indispensable for EC survival and proliferation (48, 65). In addition, Krueppel-like factor 2 (KLF2) is identified as an ERK5 responsive gene and ERK5 drives KLF2 transcription by activating MEF2 (66). Krueppel-like factor 2 plays an important role in regulating inflammation, angiogenesis and maintaining the vascular quiescence (66-70). Studies in our lab suggest that MEF2 and KLF2 may be mediators of ERK5 signaling in the regulation of vasoactive factors involved in chronic diabetic complications (36, 37, 57). It has been shown that KLF2 lentivirus transfection inhibits transforming growth factor beta 1 (TGFβ1) signaling (71). We found a significant inhibition of TGFβ1 signaling after CAMEK5 transfection, and an increase of TGFβ1 mRNA after siERK5 transfection, suggesting that TGFβ1 signaling mediates the effect of ERK5 in high glucose conditions (57).
Ets-domain transcription factor (Sap1a) as well as serum- and glucocorticoid-inducible kinase (SGK) have also been identified as the downstream targets of ERK5 and play an important role in cell proliferation induced by growth factors (31, 55). Moreover, the ERK5 signaling pathway stimulates the transcriptional activity of c-Fos and Fra-1 (fos-related antigen 1) and members of the AP-1 (activator protein 1) family (46). Other downstream substrates of ERK5 include Cx43 (connexin 43 - a gap junction protein) (72), BAD (Bcl2 associated death promoted - a pro-apoptotic member of Bcl-2 family) (73), C-Myc proto-oncogene (74) and CREB (cAMP response element binding protein) (54).
ERK5 IN ENDOTHELIAL CELLS
Extracellular-signal-regulated kinase 5 is highly expressed in the ECs and is essential for maintaining endothelial function and blood vessel integrity (31). Extracellular-signal-regulated kinase 5-deletion is lethal as seen in ERK5-/- mice who die around embryonic day 10 due to cardiovascular defects (59, 75, 76). Similar phenotypic abnormalities are observed in the MEKK3−/−, MEK5−/− and MEF2−/− embryos, suggesting that the MEKK3/MEK5/ERK5/MEF2 cascade is critical to the cardiovascular development (77-79). Additional studies employing targeted deletion of ERK5 gene in mice have shown that ERK5 is essential in EC physiology, but not in the cardiac development (80). Endothelial cells specific ERK5 ablation generates the same heart defects as those observed in global ERK5 knockout mutants, whereas cardiomyocyte specific ERK5 deletion mice are normal (80). These results indicate that ERK5 is critical for endothelial cell function and that the abnormal heart development in the mice lacking ERK5 is a consequence of endothelial cell dysfunction (80). Additionally, ERK5 is required to maintain vascular integrity in adult mice. Adult mice display hemorrhages in multiple organs and die within 2–4 weeks after deletion of ERK5 (80). In addition to these in vivo studies, ERK5 has been shown to be essential for endothelial cells survival in vitro (73, 80). Deletion of ERK5 induces profound endothelial cell apoptosis. Introduction of exogenous ERK5 can prevent endothelial cells from cell death (80). Similarly, activation of ERK5 by constitutively active MEK5 (CAMEK5) significantly improved cell viability and decreased apoptosis induced by growth factor deprivation (73). In addition, CAMEK5 inhibited growth factor deprivation-induced apoptosis, whereas dominant negative ERK5 (DNERK5) stimulated apoptosis in endothelial cells (73). ERK5 pathway also mediates the shear stress-induced antiapoptotic effect in endothelial cells (30, 73). Inhibition of ERK5 activity by overexpression of dominant negative ERK5 reduces endothelial-protective effect of shear stress (73). Analysis of antiapoptotic mechanisms of ERK5 showed that MEF2C, a direct substrate of ERK5 mediates endothelial cell survival signal (80).
ERK5 IN DIABETIC RETINOPATHY
Our study has demonstrated the existence of the initial ERK5 activation in ECs because of glucose administration, followed by decreased activation upon prolonged glucose exposure. Decreased ERK5 signaling may contribute to increased vasoactive factors and extracellular matrix accumulation (36, 37, 57). In keeping with our data, a previous study showed glucose-induced initial ERK5 activation in pulmonary artery ECs (81).
Endothelin-1 (ET-1) is a potent vasoconstriction factor whose role has been implicated in the pathogenesis of DR (82-84). Blockade ET increases retinal blood flow and prevents DR (82, 83). Decreased ERK5 activation and increased ET-1 expression were observed in ECs treated with high glucose (36). We also observed similar changes in retinal tissues of diabetic rats (36). Activation of ERK5 by CAMEK5 upregulated KLF2 and suppressed both basal and glucose-induced ET-1 expression in ECs. In contrast, ERK5 siRNA transfection resulted in decreased ERK5, KLF2 and increased ET-1 expression (36).
Vascular endothelial growth factor is a major contributor of retinal neovascularization in DR (85, 86). Elevated VEGF mRNA and protein expression have been confirmed in the patient with DR (87-89). Extracellular-signal-regulated kinase 5 has been shown to take part in the regulation of VEGF. Vascular endothelial growth factor expression is upregulated in ERK5 knockout mice (59, 66, 90, 91). Further in vitro studies showed that ERK5 repressed VEGF expression by negatively regulating hypoxia inducible factor-1α (HIF1α) in bovine lung microvascular ECs (92). Hypoxia inducible factor-1α is a strong mediator of angiogenesis in hypoxia by regulating VEGF (93, 94). High glucose induces a state of pseudo-hypoxia in diabetic complications (95, 96). It is, therefore, possible that decreased ERK5 signaling may promote glucose-induced VEGF production and angiogenesis via HIF1α. A recent study has further shown that constitutive activation of ERK5 signaling strongly inhibited EC migration, whereas ERK5 siRNA transfection increases migration (97). Similarly, our experiments showed that ERK5 siRNA enhances tube formation and VEGF expression in the ECs. Constitutively activation of ERK5 by CAMEK5 reduced both basal and glucose-induced VEGF expression (37). In addition, we observed decreased ERK5 signaling and increased VEGF expression in the retina of diabetic rats (37).
Fibronectin (FN) is an important component of the extracellular matrix, which plays a significant role in EC adhesion, migration, growth and proliferation (98, 99). FN overproduction is a characteristic feature of DR. Studies in our lab, and others have shown that the synthesis of FN is upregulated in diabetes and ECs treated with glucose (100-103). We have found a significant decrease of FN mRNA and protein following CAMEK5 transduction in basal and high glucose conditions (57). In contrast, ERK5 siRNA transfection and DNMEK5 transduction lead to an increase of FN synthesis. Moreover, our study has demonstrated that TGFβ1 signaling mediates the effect of ERK5 on FN. Furthermore, we have observed that FN expression in retinal tissues of diabetic rats is increased while ERK5 activation is decreased (57). These data suggested that decreased ERK5 signaling is important in glucose-induced FN overproduction and DR. A diagrammatic representation of such mechanisms is outlined in Fig. 3.
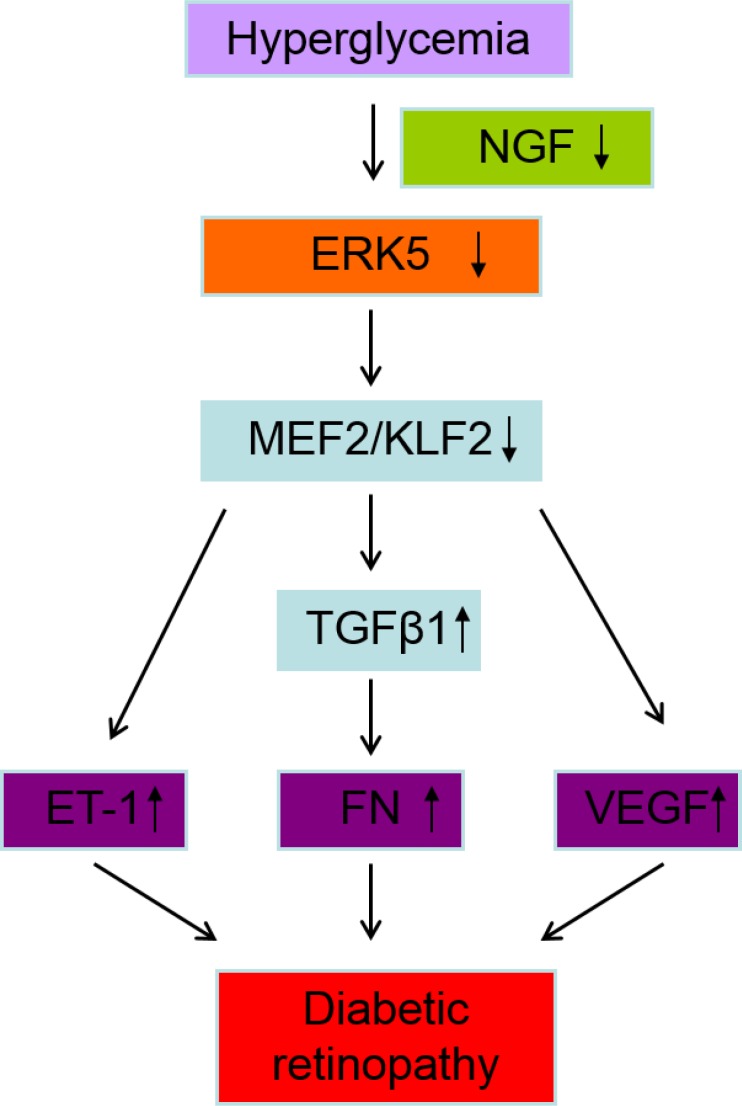
Figure 3.
A diagrammatic representation of the main conclusions of this study, outlining possible role of ERK5 in DR.
Hyperglycemia decreased activation of ERK5, which lead to upregulation of ET-1, VEGF, FN expression, and function, subsequently possibly contributing to DR. NGF mediated hyperglycemia-induced ERK5 alteration. ERK5 exerted its effect on endothelial cells via MEF2/KLF2 and TGFβ1.
ERK5 IN OTHER DIABETIC VASCULAR COMPLICATIONS
Macroangiopathy in diabetes is mainly due to an accelerated form of atherosclerosis (4). Steady and laminar blood flow is known to be atheroprotective and has been shown to be a strong activator of ERK5 (30). Also, ERK5 activation has been demonstrated to be atheroprotective. Increased plaque formation is observed in inducible EC-specific ERK5 knockout mice (104). In addition, inhibition of ERK5 activity by dominant negative ERK5 reduces the endothelial cell-protective effect of shear stress (73), indicating that the ERK5 mediates the shear stress-induced antiapoptotic effect in endothelial cells. This may be mediated by phosphorylation of BAD (73). Sohn et al. revealed that KLF2 mediates endothelial-protective effect of ERK5 (66). KLF2 is a critical transcriptional regulator for the vasoprotective effect of shear stress (67,105). In addition, laminar flow-induced ERK5 activation has been shown to confer an atheroprotective effect by inducing peroxisome proliferator-activated receptor gamma (PPARγ) (106) and inhibiting tumor necrosis factor α (TNFα) mediated adhesion molecule expression in endothelial cells (107).
However, SUMOylation inhibits a protective effect of ERK5 in diabetes (108), as small ubiquitin-like modifier (SUMO) covalently attaches to certain residues of specific target proteins and negatively regulates transcription factors (109,110). Increased ERK5 SUMOylation in diabetes inhibits shear stress-mediated ERK5’s transcription activity. Subsequently decreased KLF2 and endothelial nitric oxide synthase (eNOS) expression lead to endothelial dysfunction and accelerated atherosclerosis in diabetes (108). Extracellular-signal-regulated kinase 5 activity is also suppressed by p90 ribosomal S6 kinase (p90RSK) which is found to be increased in diabetic mouse vessels. p90 ribosomal S6 kinase -mediated reduction of ERK5 activity increased adhesion molecule1 and reduced eNOS expression, which contribute to atherosclerosis in diabetes (104).
Some studies have been performed to investigate further the role of ERK5 on diabetic nephropathy. A recent study on renal epithelial cells showed that the overexpression of ERK5 provided protection against renal ischemia-reperfusion injury (111). However, studies in mesangial cells have contradictory results. It has been reported that ERK5 activation stimulates mesangial cell proliferation and extracellular matrix accumulation (112,113). Similarly, ERK5 increases mesangial cell viability and collagen matrix accumulation in glomerulonephritis (114). The differences between mesangial cells and renal epithelial cells indicate that ERK5 signaling may regulate extracellular matrix production in a cell type-specific manner.
CONCLUSION
Chronic vascular complications are leading causes of morbidity and mortality in diabetes. Extracellular-signal-regulated kinase 5signaling plays a significant role in maintaining vascular integrity. A number of studies demonstrated that ERK5 is protective against endothelial injury in high glucose concentrations, and it exerts its effects by acting on multiple factors that are involved in regulating endothelial function. Hence, ERK5 may be a potential target for prevention and treatment of DR and other chronic diabetic complications.
DISCLOSURE
None Declared.
References
- 1.Laight DW, Carrier MJ, Anggard Endothelial cell dysfunction and the pathogenesis of diabetic macroangiopathy. Diabetes Metab Res Rev. 1999 Jul-Aug;15(4):274–82. doi: 10.1002/(sici)1520-7560(199907/08)15:4<274::aid-dmrr46>3.0.co;2-g. PMID: 10495476. [DOI] [PubMed] [Google Scholar]
- 2.Panus C, Mota M, Vladu D, Vanghelie L, Raducanu CL. The endothelial dysfunction in diabetes mellitus. Rom J Intern Med. 2003;41(1):27–33. PMID: 15529582. [PubMed] [Google Scholar]
- 3.Cosentino F, Luscher TF. Endothelial dysfunction in diabetes mellitus. J Cardiovasc Pharmacol. 1998;32(Suppl 3):S54–61. PMID: 9883749. [PubMed] [Google Scholar]
- 4.Guerci B, Bohme P, Kearney-Schwartz A, Zannad F, Drouin P. Endothelial dysfunction and type 2 diabetes Part 2: altered endothelial function and the effects of treatments in type 2 diabetes mellitus. Diabetes Metab. 2001 Sep;27(4 Pt 1):436–47. PMID: 11547217. [PubMed] [Google Scholar]
- 5.Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care. 1995 Feb;18(2):258–68. doi: 10.2337/diacare.18.2.258. PMID: 7729308. [DOI] [PubMed] [Google Scholar]
- 6.Aiello LP, Gardner TW, King GL, et al. Diabetic retinopathy. Diabetes Care. 1998 Jan;21(1):143–56. doi: 10.2337/diacare.21.1.143. PMID: 9538986. [DOI] [PubMed] [Google Scholar]
- 7.Hudson C. The clinical features and classification of diabetic retinopathy. Ophthalmic Physiol Opt. 1996 Sep;16(Suppl 2):S43–8. PMID: 9398920. [PubMed] [Google Scholar]
- 8.Khan ZA, Chakrabarti S. Cellular signaling and potential new treatment targets in diabetic retinopathy. Exp Diabetes Res. 2007;2007 doi: 10.1155/2007/31867. 1155/2007/31867. PMID: 18288248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007 Aug;298(8):902–16. doi: 10.1001/jama.298.8.902. PMID: 17712074. [DOI] [PubMed] [Google Scholar]
- 10.Khan ZA, Chakrabarti S. Growth factors in proliferative diabetic retinopathy. Exp Diabesity Res. 2003 Oct-Dec;4(4):287–301. doi: 10.1155/EDR.2003.287. PMID: 14668050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994 Dec ;331(22):1480–7. doi: 10.1056/NEJM199412013312203. PMID: 7526212. [DOI] [PubMed] [Google Scholar]
- 12.Abdallah W, Fawzi AA. Anti-VEGF therapy in proliferative diabetic retinopathy. Int Ophthalmol Clin. 2009 Spring;49(2):95–107. doi: 10.1097/IIO.0b013e31819fd84a. doi: 10.1097/IIO.0b013e31819fd84a. PMID: 19349790. [DOI] [PubMed] [Google Scholar]
- 13.Jardeleza MS, Miller JW. Review of anti-VEGF therapy in proliferative diabetic retinopathy. Semin Ophthalmol. 2009 Mar-Apr;24(2):87–92. doi: 10.1080/08820530902800330. doi: 10.1080/08820530902800330. PMID: 19373692. [DOI] [PubMed] [Google Scholar]
- 14.Tervaert TW, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010 Apr;21(4):556–63. doi: 10.1681/ASN.2010010010. doi: 10.1681/ASN.2010010010. Epub 2010 Feb 18. PMID: 20167701. [DOI] [PubMed] [Google Scholar]
- 15.Arosio E, Minuz P, Prior M. [Endothelial function and the microcirculation in diabetes mellitus] Ann Ital Med Int. 1999 Apr-Jun;14(2):106–13. PMID: 10399372. [PubMed] [Google Scholar]
- 16.Akbari CM, Saouaf R, Barnhill DF, Newman PA, LoGerfo FW, Veves A. Endothelium-dependent vasodilatation is impaired in both microcirculation and macrocirculation during acute hyperglycemia. J Vasc Surg. 1998 Oct;28(4):687–94. doi: 10.1016/s0741-5214(98)70095-3. PMID: 9786265. [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Park KW, Kim YS, et al. Effects of acute hyperglycemia on endothelium-dependent vasodilation in patients with diabetes mellitus or impaired glucose metabolism. Endothelium. 2003;10(2):65–70. doi: 10.1080/10623320303362. 18. PMID: 12791513. [DOI] [PubMed] [Google Scholar]
- 18.Williams SB, Goldfine AB, Timimi FK, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998 May ;97(17):1695–701. doi: 10.1161/01.cir.97.17.1695. PMID: 9591763. [DOI] [PubMed] [Google Scholar]
- 19.Pieper GM, Meier DA, Hager SR. Endothelial dysfunction in a model of hyperglycemia and hyperinsulinemia. Am J Physiol. 1995 Sep;269(3 Pt 2):H845–50. doi: 10.1152/ajpheart.1995.269.3.H845. PMID: 7573526. [DOI] [PubMed] [Google Scholar]
- 20.Tesfamariam B, Cohen RA. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol. 1992 Aug;263(2 Pt 2):H321–6. doi: 10.1152/ajpheart.1992.263.2.H321. PMID: 151012. [DOI] [PubMed] [Google Scholar]
- 21.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993 Sep;329(14):977–86. doi: 10.1056/NEJM199309303291401. PMID: 8366922. [DOI] [PubMed] [Google Scholar]
- 22.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998 Sep ;352(9131):837–53. PMID: 9742976. [PubMed] [Google Scholar]
- 23.Kuusisto J, Mykkanen L, Pyorala K, Laakso M. NIDDM and its metabolic control predict coronary heart disease in elderly subjects. Diabetes. 1994 Aug;43(8):960–7. doi: 10.2337/diab.43.8.960. PMID: 8039603. [DOI] [PubMed] [Google Scholar]
- 24.Beks PJ, Mackaay AJ, de Neeling JN, de Vries H, Bouter LM, Heine RJ. Peripheral arterial disease in relation to glycaemic level in an elderly Caucasian population: the Hoorn study. Diabetologia. 1995 Jan;38(1):86–96. doi: 10.1007/BF02369357. PMID: 7744233. [DOI] [PubMed] [Google Scholar]
- 25.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001 Mar ;410(6824):37–40. doi: 10.1038/35065000. PMID: 11242034. [DOI] [PubMed] [Google Scholar]
- 26.Pearson G, Robinson F, Beers GT, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001 Apr;22(2):153–83. doi: 10.1210/edrv.22.2.0428. PMID: 11294822. [DOI] [PubMed] [Google Scholar]
- 27.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999 Jan;79(1):143–80. doi: 10.1152/physrev.1999.79.1.143. PMID: 9922370. [DOI] [PubMed] [Google Scholar]
- 28.Lee JD, Ulevitch RJ, Han J. Primary structure of BMK1: a new mammalian map kinase. Biochem Biophys Res Commun. 1995 Aug ;213(2):715–24. doi: 10.1006/bbrc.1995.2189. PMID: 7646528. [DOI] [PubMed] [Google Scholar]
- 29.Zhou G, Bao ZQ, Dixon JE. Components of a new human protein kinase signal transduction pathway. J Biol Chem. 1995 May 26;270(21):12665–9. doi: 10.1074/jbc.270.21.12665. PMID: 7759517. [DOI] [PubMed] [Google Scholar]
- 30.Yan C, Takahashi M, Okuda M, Lee JD, Berk BC. Fluid shear stress stimulates big mitogen-activated protein kinase 1 (BMK1) activity in endothelial cells Dependence on tyrosine kinases and intracellular calcium. J Biol Chem. 1999 Jan ;274(1):143–50. doi: 10.1074/jbc.274.1.143. PMID: 9867822. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi M, Tapping RI, Chao TH, et al. BMK1 mediates growth factor-induced cell proliferation through direct cellular activation of serum and glucocorticoid-inducible kinase. J Biol Chem. 2001 Mar ;276(12):8631–4. doi: 10.1074/jbc.C000838200. Epub 2001 Jan 31. PMID: 11254654. [DOI] [PubMed] [Google Scholar]
- 32.Buschbeck M, Ullrich A. The unique C-terminal tail of the mitogen-activated protein kinase ERK5 regulates its activation and nuclear shuttling. J Biol Chem. 2005 Jan 28;280(4):2659–67. doi: 10.1074/jbc.M412599200. Epub 2004 Nov 17. PMID: 15548525. [DOI] [PubMed] [Google Scholar]
- 33.Yan C, Luo H, Lee JD, Abe J, Berk BC. Molecular cloning of mouse ERK5/BMK1 splice variants and characterization of ERK5 functional domains. J Biol Chem. 2001 Apr 6;276(14):10870–8. doi: 10.1074/jbc.M009286200. Epub 2001 Jan 3. PMID: 11139578. [DOI] [PubMed] [Google Scholar]
- 34.Nishimoto S, Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006 Aug;7(8):782–6. doi: 10.1038/sj.embor.7400755. PMID: 16880823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts OL, Holmes K, Muller J, Cross DA, Cross MJ. ERK5 and the regulation of endothelial cell function. Biochem Soc Trans. 2009 Dec;37(Pt 6):1254–9. doi: 10.1042/BST0371254. doi: 10.1042/BST0371254. PMID: 19909257. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Feng B, Chen S, Zuo Y, Chakrabarti S. Glucose-induced endothelin-1 expression is regulated by ERK5 in the endothelial cells and retina of diabetic rats. Can J Physiol Pharmacol. 2010 Jun;88(6):607–15. doi: 10.1139/Y10-033. doi: 10.1139/Y10-033. PMID: 20628425. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Zuo Y, Chakrabarti R, Feng B, Chen S, Chakrabarti S. ERK5 Contributes to VEGF Alteration in Diabetic Retinopathy. J Ophthalmol. 2010;2010 doi: 10.1155/2010/465824. doi: 10.1155/2010/465824. Epub 2010 Jun 30. PMID: 20671964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao TH, Hayashi M, Tapping RI, Kato Y, Lee JD. MEKK3 directly regulates MEK5 activity as part of the big mitogen-activated protein kinase 1 (BMK1) signaling pathway. J Ophthalmol. 2010;2010 doi: 10.1074/jbc.274.51.36035. doi: 10.1155/2010/465824. Epub 2010 Jun 30. PMID: 20671964. [DOI] [PubMed] [Google Scholar]
- 39.Sun W, Kesavan K, Schaefer BC, et al. MEKK2 associates with the adapter protein Lad/RIBP and regulates the MEK5-BMK1/ERK5 pathway. J Biol Chem. 2001 Feb ;276(7):5093–100. doi: 10.1074/jbc.M003719200. Epub 2000 Nov 9. PMID: 11073940. [DOI] [PubMed] [Google Scholar]
- 40.English JM, Vanderbilt CA, Xu S, Marcus S, Cobb MH. Isolation of MEK5 and differential expression of alternatively spliced forms. J Biol Chem. 1995 Dec ;270(48):28897–902. doi: 10.1074/jbc.270.48.28897. PMID: 7499418. [DOI] [PubMed] [Google Scholar]
- 41.Mody N, Campbell DG, Morrice N, Peggie M, Cohen P. An analysis of the phosphorylation and activation of extracellular-signal-regulated protein kinase 5 (ERK5) by mitogen-activated protein kinase kinase 5 (MKK5) in vitro. Biochem J. 2003 Jun 1;372(Pt 2):567–75. doi: 10.1042/BJ20030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diaz-Meco MT, Moscat J. MEK5, a new target of the atypical protein kinase C isoforms in mitogenic signaling. Mol Cell Biol. 2001 Feb;21(4):1218–27. doi: 10.1128/MCB.21.4.1218-1227.2001. PMID: 11158308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sumimoto H, Kamakura S, Ito T. Structure and function of the PB1 domain, a protein interaction module conserved in animals, fungi, amoebas, and plants. Sci STKE. 2007 Aug;2007(401) doi: 10.1126/stke.4012007re6. PMID: 17726178. [DOI] [PubMed] [Google Scholar]
- 44.Nigro P, Abe J, Woo CH, et al. PKCzeta decreases eNOS protein stability via inhibitory phosphorylation of ERK5. Blood. 2010 Sep;116(11):1971–9. doi: 10.1182/blood-2010-02-269134. doi: 10.1182/blood-2010-02-269134. Epub 2010 Jun 10. PMID: 20538799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasler HG, Victoria J, Duramad O, Winoto A. ERK5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Mol Cell Biol. 2000 Nov;20(22):8382–9. doi: 10.1128/mcb.20.22.8382-8389.2000. PMID: 11046135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terasawa K, Okazaki K, Nishida E. Regulation of c-Fos and Fra-1 by the MEK5-ERK5 pathway. Genes Cells. 2003 Mar;8(3):263–73. doi: 10.1046/j.1365-2443.2003.00631.x. PMID: 12622723. [DOI] [PubMed] [Google Scholar]
- 47.Morimoto H, Kondoh K, Nishimoto S, Terasawa K, Nishida E. Activation of a C-terminal transcriptional activation domain of ERK5 by autophosphorylation. J Biol Chem. 2007 Dec ;282(49):35449–56. doi: 10.1074/jbc.M704079200. Epub 2007 Oct 10. PMID: 17928297. [DOI] [PubMed] [Google Scholar]
- 48.Kato Y, Kravchenko VV, Tapping RI, Han J, Ulevitch RJ, Lee JD. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997 Dec ;16(23):7054–66. doi: 10.1093/emboj/16.23.7054. PMID: 9384584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998 Oct ;395(6703):713–6. doi: 10.1038/27234. PMID: 9790194. [DOI] [PubMed] [Google Scholar]
- 50.Kesavan K, Lobel-Rice K, Sun W, et al. MEKK2 regulates the coordinate activation of ERK5 and JNK in response to FGF-2 in fibroblasts. J Cell Physiol. 2004 Apr;199(1):140–8. doi: 10.1002/jcp.10457. PMID: 14978743. [DOI] [PubMed] [Google Scholar]
- 51.Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001 Jun 1;20(11):2757–67. doi: 10.1093/emboj/20.11.2757. PMID: 11387209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carvajal-Vergara X, Tabera S, Montero JC, et al. Multifunctional role of Erk5 in multiple myeloma. Blood. 2005 Jun 1;105(11):4492–9. doi: 10.1182/blood-2004-08-2985. Epub 2005 Feb 3. PMID: 15692064. [DOI] [PubMed] [Google Scholar]
- 53.Cavanaugh JE. Role of extracellular signal regulated kinase 5 in neuronal survival. Eur J Biochem. 2004 Jun;271(11):2056–9. doi: 10.1111/j.1432-1033.2004.04131.x. PMID: 15153094. [DOI] [PubMed] [Google Scholar]
- 54.Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci. 2001 Oct;4(10):981–8. doi: 10.1038/nn720. 11544482. [DOI] [PubMed] [Google Scholar]
- 55.Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases Identification and characterization of a signaling pathway to the nucleus. J Biol Chem. 1999 Sep;274(37):26563–71. doi: 10.1074/jbc.274.37.26563. PMID: 10473620. [DOI] [PubMed] [Google Scholar]
- 56.Obara Y, Yamauchi A, Takehara S, et al. ERK5 activity is required for nerve growth factor-induced neurite outgrowth and stabilization of tyrosine hydroxylase in PC12 cells. J Biol Chem. 2009 Aug;284(35):23564–73. doi: 10.1074/jbc.M109.027821. doi: 10.1074/jbc.M109.027821. Epub 2009 Jul 6. PMID: 19581298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Y, Feng B, Chen S, Chakrabarti S. ERK5 Regulates glucose-induced increased fibronectin production in the endothelial cells and in the retina in diabetes. nvest Ophthalmol Vis Sci. 2012 Dec ;53(13):8405–13. doi: 10.1167/iovs.12-10553. doi: 10.1167/iovs.12-10553. PMID: 23188731. [DOI] [PubMed] [Google Scholar]
- 58.Abe J, Kusuhara M, Ulevitch RJ, Berk BC, Lee JD. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J Biol Chem. 1996 Jul ;271(28):16586–90. doi: 10.1074/jbc.271.28.16586. PMID: 8663194. [DOI] [PubMed] [Google Scholar]
- 59.Sohn SJ, Sarvis BK, Cado D, Winoto A. ERK5 MAPK regulates embryonic angiogenesis and acts as a hypoxia-sensitive repressor of vascular endothelial growth factor expression. J Biol Chem. 2002 Nov ;277(45):43344–51. doi: 10.1074/jbc.M207573200. Epub 2002 Sep 6. PMID: 12221099. [DOI] [PubMed] [Google Scholar]
- 60.Takeishi Y, Abe J, Lee JD, Kawakatsu H, Walsh RA, Berk BC. Differential regulation of p90 ribosomal S6 kinase and big mitogen-activated protein kinase 1 by ischemia/reperfusion and oxidative stress in perfused guinea pig hearts. Circ Res. 1999 Dec ;85(12):1164–72. doi: 10.1161/01.res.85.12.1164. PMID: 10590243. [DOI] [PubMed] [Google Scholar]
- 61.Obara Y, Nakahata N. The signaling pathway leading to extracellular signal-regulated kinase 5 (ERK5) activation via G-proteins and ERK5-dependent neurotrophic effects. Mol Pharmacol. 2010 Jan;77(1):10–6. doi: 10.1124/mol.109.060236. doi: 10.1124/mol.109.060236. Epub 2009 Oct 26. PMID: 19858097. [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Hoz C, Sanchez-Fernandez G, Diaz-Meco MT, Moscat J, Mayor F, Ribas C. G alpha(q) acts as an adaptor protein in protein kinase C zeta (PKCzeta)-mediated ERK5 activation by G protein-coupled receptors (GPCR) J Biol Chem. 2010 Apr;285(18):13480–9. doi: 10.1074/jbc.M109.098699. doi: 10.1074/jbc.M109.098699. Epub 2010 Mar 3. PMID: 20200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kato Y, Zhao M, Morikawa A, et al. Big mitogen-activated kinase regulates multiple members of the MEF2 protein family. J Biol Chem. 2000 Jun ;275(24):18534–40. doi: 10.1074/jbc.M001573200. PMID: 10849446. [DOI] [PubMed] [Google Scholar]
- 64.Yang CC, Ornatsky OI, McDermott JC, Cruz TF, Prody CA. Interaction of myocyte enhancer factor 2 (MEF2) with a mitogen-activated protein kinase, ERK5/BMK1. Nucleic Acids Res. 1998 Oct 15;26(20):4771–7. doi: 10.1093/nar/26.20.4771. PMID: 9753748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olson EN. Undermining the endothelium by ablation of MAPK-MEF2 signaling. J Clin Invest. 2004 Apr;113(8):1110–2. doi: 10.1172/JCI21497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sohn SJ, Li D, Lee LK, Winoto A. Transcriptional regulation of tissue-specific genes by the ERK5 mitogen-activated protein kinase. Mol Cell Biol. 2005 Oct;25(19):8553–66. doi: 10.1128/MCB.25.19.8553-8566.2005. PMID: 16166637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boon RA, Horrevoets AJ. Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie. 2009 Jan;29(1):39–40. PMID: 19151844. [PubMed] [Google Scholar]
- 68.Dekker RJ, Boon RA, Rondaij MG, et al. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006 Jun ;107(11):4354–63. doi: 10.1182/blood-2005-08-3465. Epub 2006 Feb 2. PMID: 16455954. [DOI] [PubMed] [Google Scholar]
- 69.Senbanerjee S, Lin Z, Atkins GB, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004 May 17;199(10):1305–15. doi: 10.1084/jem.20031132. Epub 2004 May 10. PMID: 15136591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki T, Aizawa K, Matsumura T, Nagai R. Vascular implications of the Kruppel-like family of transcription factors. Arterioscler Thromb Vasc Biol. 2005 Jun;25(6):1135–41. doi: 10.1161/01.ATV.0000165656.65359.23. Epub 2005 Apr 7. PMID: 15817882. [DOI] [PubMed] [Google Scholar]
- 71.Boon RA, Fledderus JO, Volger OL, et al. KLF2 suppresses TGF-beta signaling in endothelium through induction of Smad7 and inhibition of AP-1. Arterioscler Thromb Vasc Biol. 2007 Mar;27(3):532–9. doi: 10.1161/01.ATV.0000256466.65450.ce. Epub 2006 Dec 28. [DOI] [PubMed] [Google Scholar]
- 72.Cameron SJ, Malik S, Akaike M, et al. Regulation of epidermal growth factor-induced connexin 43 gap junction communication by big mitogen-activated protein kinase1/ERK5 but not ERK1/2 kinase activation. J Biol Chem. 2003 May;278(20):18682–8. doi: 10.1074/jbc.M213283200. Epub 2003 Mar 12. PMID: 12637502. [DOI] [PubMed] [Google Scholar]
- 73.Pi X, Yan C, Berk BC. Big mitogen-activated protein kinase (BMK1)/ERK5 protects endothelial cells from apoptosis. Circ Res. 2004 Feb ;94(3):362–9. doi: 10.1161/01.RES.0000112406.27800.6F. Epub 2003 Dec 11. PMID: 14670836. [DOI] [PubMed] [Google Scholar]
- 74.English JM, Pearson G, Baer R, Cobb MH. Identification of substrates and regulators of the mitogen-activated protein kinase ERK5 using chimeric protein kinases. J Biol Chem. 1998 Feb ;273(7):3854–60. doi: 10.1074/jbc.273.7.3854. PMID: 9461566. [DOI] [PubMed] [Google Scholar]
- 75.Regan CP, Li W, Boucher DM, Spatz S, Su MS, Kuida K. Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects. Proc Natl Acad Sci U S A. 2002 Jul ;99(14):9248–53. doi: 10.1073/pnas.142293999. Epub 2002 Jul 1. PMID: 12093914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan L, Carr J, Ashby PR, Murry-Tait V, Thompson C, Arthur JS. Knockout of ERK5 causes multiple defects in placental and embryonic development. BMC Dev Biol. 2003 Dec;3 doi: 10.1186/1471-213X-3-11. PMID: 14675480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997 May;276(5317):1404–7. doi: 10.1126/science.276.5317.1404. PMID: 9162005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang X, Merritt AJ, Seyfried J, et al. Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol Cell Biol. 2005 Jan;25(1):336–45. doi: 10.1128/MCB.25.1.336-345.2005. PMID: 15601854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang J, Boerm M, McCarty M, et al. Mekk3 is essential for early embryonic cardiovascular development. Nat Genet. 2000 Mar;24(3):309–13. doi: 10.1038/73550. PMID: 10700190. [DOI] [PubMed] [Google Scholar]
- 80.Hayashi M, Kim SW, Imanaka-Yoshida K, et al. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. J Clin Invest. 2004 Apr;113(8):1138–48. doi: 10.1172/JCI19890. PMID: 15085193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu W, Schoenkerman A, Lowe WL Jr. Activation of members of the mitogen-activated protein kinase family by glucose in endothelial cells. Am J Physiol Endocrinol Metab. 2000 Oct;279(4):E782–90. doi: 10.1152/ajpendo.2000.279.4.E782. PMID: 11001759. [DOI] [PubMed] [Google Scholar]
- 82.Takagi C, Bursell SE, Lin YW, et al. Regulation of retinal hemodynamics in diabetic rats by increased expression and action of endothelin-1. Invest Ophthalmol Vis Sci. 1996 Nov;37(12):2504–18. PMID: 8933767. [PubMed] [Google Scholar]
- 83.Shaw SG, Boden JP, Biecker E, Reichen J, Rothen B. Endothelin antagonism prevents diabetic retinopathy in NOD mice: a potential role of the angiogenic factor adrenomedullin. Exp Biol Med (Maywood) 2006 Jun;231(6):1101–5. PMID: 16741057. [PubMed] [Google Scholar]
- 84.Khan ZA, Chakrabarti S. Endothelins in chronic diabetic complications. Can J Physiol Pharmacol. 2003 Jun;81(6):622–34. doi: 10.1139/y03-053. PMID: 12839273. [DOI] [PubMed] [Google Scholar]
- 85.Pe'er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest. 1995 Jun;72(6):638–45. PMID: 7540233. [PubMed] [Google Scholar]
- 86.Ray D, Mishra M, Ralph S, Read I, Davies R, Brenchley P. Association of the VEGF gene with proliferative diabetic retinopathy but not proteinuria in diabetes. Diabetes. 2004 Mar;53(3):861–4. doi: 10.2337/diabetes.53.3.861. PMID: 14988276. [DOI] [PubMed] [Google Scholar]
- 87.Boulton M, Foreman D, Williams G, McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol. 1998 May;82(5):561–8. doi: 10.1136/bjo.82.5.561. PMID: 9713066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lutty GA, McLeod DS, Merges C, Diggs A, Plouet J. Localization of vascular endothelial growth factor in human retina and choroid. Arch Ophthalmol. 1996 Aug;114(8):971–7. doi: 10.1001/archopht.1996.01100140179011. PMID: 8694733. [DOI] [PubMed] [Google Scholar]
- 89.Malecaze F, Clamens S, Simorre-Pinatel V, et al. Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol. 1994 Nov;112(11):1476–82. doi: 10.1001/archopht.1994.01090230090028. PMID: 7980139. [DOI] [PubMed] [Google Scholar]
- 90.Chen S, Apostolova MD, Cherian MG, Chakrabarti S. Interaction of endothelin-1 with vasoactive factors in mediating glucose-induced increased permeability in endothelial cells. Lab Invest. 2000 Aug;80(8):1311–21. doi: 10.1038/labinvest.3780139. PMID: 10950122. [DOI] [PubMed] [Google Scholar]
- 91.Gao R, Zhu BH, Tang SB, Wang JF, Ren J. Scutellarein inhibits hypoxia- and moderately-high glucose-induced proliferation and VEGF expression in human retinal endothelial cells. Acta Pharmacol Sin. 2008 Jun;29(6):707–12. doi: 10.1111/j.1745-7254.2008.00797.x. doi: 10.1111/j.1745-7254.2008.00797.x. PMID: 18501117. [DOI] [PubMed] [Google Scholar]
- 92.Pi X, Garin G, Xie L, et al. BMK1/ERK5 is a novel regulator of angiogenesis by destabilizing hypoxia inducible factor 1alpha. Circ Res. 2005 Jun;96(11):1145–51. doi: 10.1161/01.RES.0000168802.43528.e1. Epub 2005 May 5. PMID: 15879308. [DOI] [PubMed] [Google Scholar]
- 93.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–78. doi: 10.1146/annurev.cellbio.15.1.551. PMID: 10611972. [DOI] [PubMed] [Google Scholar]
- 94.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–5. doi: 10.1038/359843a0. PMID: 1279431. [DOI] [PubMed] [Google Scholar]
- 95.Van den Enden MK, Nyengaard JR, Ostrow E, Burgan JH, Williamson JR. Elevated glucose levels increase retinal glycolysis and sorbitol pathway metabolism Implications for diabetic retinopathy. Invest Ophthalmol Vis Sci. 1995 Jul;36(8):1675–85. PMID: 7601647. [PubMed] [Google Scholar]
- 96.Williamson JR, Chang K, Frangos M, et al. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993 Jun;42(6):801–13. doi: 10.2337/diab.42.6.801. PMID: 8495803. [DOI] [PubMed] [Google Scholar]
- 97.Spiering D, Schmolke M, Ohnesorge N, et al. MEK5/ERK5 signaling modulates endothelial cell migration and focal contact turnover. J Biol Chem. 2009 Sep;284(37):24972–80. doi: 10.1074/jbc.M109.042911. doi: 10.1074/jbc.M109.042911. Epub 2009 Jul 15. PMID: 19605361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Madri JA, Pratt BM, Yannariello-Brown J. Matrix-driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am J Pathol. 1988 Jul;132(1):18–27. PMID: 3394798. [PMC free article] [PubMed] [Google Scholar]
- 99.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002 Oct 15;115(Pt 20):3861–3. doi: 10.1242/jcs.00059. PMID: 12244123. [DOI] [PubMed] [Google Scholar]
- 100.Chen S, Mukherjee S, Chakraborty C, Chakrabarti S. High glucose-induced, endothelin-dependent fibronectin synthesis is mediated via NF-kappa B and AP-1. Am J Physiol Cell Physiol. 2003 Feb;284(2):C263–72. doi: 10.1152/ajpcell.00192.2002. Epub 2002 Sep 18. PMID: 12388107. [DOI] [PubMed] [Google Scholar]
- 101.Chen S, Khan ZA, Cukiernik M, Chakrabarti S. Differential activation of NF-kappa B and AP-1 in increased fibronectin synthesis in target organs of diabetic complications. Am J Physiol Endocrinol Metab. 2003 Jun;284(6):E1089–97. doi: 10.1152/ajpendo.00540.2002. Epub 2003 Feb 11. PMID: 12582013. [DOI] [PubMed] [Google Scholar]
- 102.Kaur H, Chen S, Xin X, Chiu J, Khan ZA, Chakrabarti S. Diabetes-induced extracellular matrix protein expression is mediated by transcription coactivator p300. Diabetes. 2006 Nov;55(11):3104–11. doi: 10.2337/db06-0519. PMID: 17065349. [DOI] [PubMed] [Google Scholar]
- 103.Roy S, Cagliero E, Lorenzi M. Fibronectin overexpression in retinal microvessels of patients with diabetes. Invest Ophthalmol Vis Sci. 1996 Feb;37(2):258–66. PMID: 8603829. [PubMed] [Google Scholar]
- 104.Le NT, Heo KS, Takei Y, et al. A crucial role for p90RSK-mediated reduction of ERK5 transcriptional activity in endothelial dysfunction and atherosclerosis. Circulation. 2013 Jan;127(4):486–99. doi: 10.1161/CIRCULATIONAHA.112.116988. doi: 10.1161/CIRCULATIONAHA.112.116988. Epub 2012 Dec 14. PMID: 23243209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parmar KM, Larman HB, Dai G, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006 Jan;116(1):49–58. doi: 10.1172/JCI24787. Epub 2005 Dec 8. PMID: 16341264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Akaike M, Che W, Marmarosh NL, et al. The hinge-helix 1 region of peroxisome proliferator-activated receptor gamma1 (PPARgamma1) mediates interaction with extracellular signal-regulated kinase 5 and PPARgamma1 transcriptional activation: involvement in flow-induced PPARgamma activation in endothelial cells. Mol Cell Biol. 2004 Oct;24(19):8691–704. doi: 10.1128/MCB.24.19.8691-8704.2004. PMID: 15367687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li L, Tatake RJ, Natarajan K, et al. Fluid shear stress inhibits TNF-mediated JNK activation via MEK5-BMK1 in endothelial cells. Biochem Biophys Res Commun. 2008 May ;370(1):159–63. doi: 10.1016/j.bbrc.2008.03.051. doi: 10.1016/j.bbrc.2008.03.051. Epub 2008 Mar 19. PMID: 18358237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Woo CH, Shishido T, McClain C, et al. Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ Res. 2008 Mar ;102(5):538–45. doi: 10.1161/CIRCRESAHA.107.156877. doi: 10.1161/CIRCRESAHA.107.156877. Epub 2008 Jan 24. PMID: 18218985. [DOI] [PubMed] [Google Scholar]
- 109.Verger A, Perdomo J, Crossley M. Modification with SUMO A role in transcriptional regulation. EMBO Rep. 2003 Feb;4(2):137–42. doi: 10.1038/sj.embor.embor738. PMID: 12612601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang X, Tournier C. Regulation of cellular functions by the ERK5 signalling pathway. Cell Signal. 2006 Jun;18(6):753–60. doi: 10.1016/j.cellsig.2005.11.003. Epub 2006 Jan 6. PMID: 16376520. [DOI] [PubMed] [Google Scholar]
- 111.Kawakami T, Park SW, Kaku R, Yang J. Extracellular-regulated-kinase 5-mediated renal protection against ischemia-reperfusion injury. Biochem Biophys Res Commun. 2012 Feb;418(4):603–8. doi: 10.1016/j.bbrc.2012.01.043. doi: 10.1016/j.bbrc.2012.01.043. Epub 2012 Jan 24. PMID: 22293190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dorado F, Velasco S, Esparis-Ogando A, et al. The mitogen-activated protein kinase Erk5 mediates human mesangial cell activation. Nephrol Dial Transplant. 2008 Nov;23(11):3403–11. doi: 10.1093/ndt/gfn333. doi: 10.1093/ndt/gfn333. Epub 2008 Jun 21. PMID: 18567890. [DOI] [PubMed] [Google Scholar]
- 113.Suzaki Y, Yoshizumi M, Kagami S, et al. BMK1 is activated in glomeruli of diabetic rats and in mesangial cells by high glucose conditions. Kidney Int. 2004 May;65(5):1749–60. doi: 10.1111/j.1523-1755.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- 114.Urushihara M, Takamatsu M, Shimizu M, et al. ERK5 activation enhances mesangial cell viability and collagen matrix accumulation in rat progressive glomerulonephritis. Am J Physiol Renal Physiol. 2010 Jan;298(1):F167–76. doi: 10.1152/ajprenal.00124.2009. doi: 10.1152/ajprenal.00124.2009. Epub 2009 Oct 21. PMID: 19846573. [DOI] [PubMed] [Google Scholar]