Abstract
Background:
Traditionally Trichodesma indicum has been used for its therapeutic effect in folk medicine that include anti-inflammatory, analgesic and anticancer properties. In this work, we validate the anticancer potential of the plant.
Aims:
To screen the shoot extracts T. indicum for their antimitotic and antiproliferative activities.
Materials and Methods:
The dried aerial parts of T. indicum were successively extracted with petroleum ether, successive chloroform extract (SCH), successive ethanol extract (SEE) and water. The plant extracts were subjected to study of in vitro antioxidant activity using 2,2’- diphenyl-1-picrylhydrazyl, 2,2’- azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical inhibition systems. The extracts were also tested for their in vitro antimitotic activity in Allium cepa root and antiproliferative activity using the yeast model and five human cell lines (MCF-7, HOP-62, MOLT-4, HCT-15 and PRO).
Result and Conclusion:
The mitotic index for SCH and SEE was found to be 12.01 ± 1.34 and 12.99 ± 0.25 mg/mL, respectively. The IC50 value in the antiproliferative assay was found to be 30.14s–35.36 mg/mL for SCH and SEE respectively. Both SCH and SEE extracts showed significant antimitotic and antiproliferative activity when compared to the standard methothreaxate, vincreastine and adriamycin. Among the extracts, SEE showed strong inhibition against MCF-7 and MOLT-4 cell lines at concentration <30 μg/mL. Phytochemical analysis of extracts indicated the presence of β-sitosterol, gallic acid and catechin. Based on these results, it is concluded that T. indicum may be a good candidate for the treatment of a variety of cancer. Thus, its traditional use is validated.
KEY WORDS: Allium cepa root inhibition, anticancer, antimitotic, antiproliferative, catechin, gallic acid, high-performance liquid chromatography, sulforhodamine-B assay, β-sitosterol
INTRODUCTION
Cancer is a disease of abnormal cell growth in which normal cells are converted to masses that divide in an uncontrolled manner. The occurrence of cancer is due to the effect of chemicals, viruses, free radicals and some environmental and routine life factors. Between 1990, there was a 22% increase in cancer incidence and mortality, with over 10 million new cases and over 6 million deaths worldwide till 2000 (excluding nonmelanoma skin cancers). Deaths due to cancer are projected to continuously increase, and it has been estimated that there will be 11.5 million deaths due to cancer in the year 2030.[1] Traditional treatments for cancer like chemotherapy, radiotherapy and surgery provide only partial and transient relief. Also the above treatments and synthetic anticancer drugs are costly and beyond the reach of the general public. Hence, alternative herbal remedies that are commonly available and comparatively economical are to be explored.[2] Plant drugs have been used to treat cancer for a long time in both traditional and modern societies since the discovery of anticancer potentials of podophyllotoxin and Taxus, and Cathranthus. These drugs and their derivatives are now well established as a potential anticancer drug.[3]
The plant Trichodesma indicum is an important member of Boraginaceae family. Phytochemically it contains monocrotolin, suspinine alkaloids, hexcosane, amylin and lupeol triterpenoids. Traditionally the root paste of T. indicum is used to treat swelling of the joints and as an emollient.[4] The leaves of this plant are used to treat cancer.[5] A literature review revealed that in spite of the anticancer potentials of this plant in traditional medicine, the plant is yet to be validated for its anticancer property. Hence, in the present study, we evaluated the antimitotic, and antiproliferative activities of T. indicum with the aim of establishing a new anticancer drug for the global market.
MATERIALS AND METHODS
Herbal material collection, extraction and standardization
The aerial parts of T. indicum were collected during August to September, 2009 from hilly area of Medghat (21°26′45″N 77°11′50″E), Amravati District, Maharashtra and they were authenticated by Prof. Dr. Bhowagaokar (Taxonomist), Botany Department, VIHS Amravati, Maharashtra. The herbarium (plant specimen) of the plant was submitted at VIHS, Amravati for future reference (accession number VMT 36). The air dried powder material (10 kg) was extracted successively with petroleum ether, chloroform, ethanol by hot continuous method (soxhlation). The aqueous extract has been prepared by maceration technique. The solvent was evaporated to dryness under pressure using rotary flash evaporator to obtain solid extracts. The total alkaloid, phenolic and flavonoid contents of the extracts were determined using standard method.[6]
In vitro antioxidant activity
2,2’-diphenyl-1-picrylhydrazyl
The 2,2’-diphenyl-1-picrylhydrazyl (DPPH) + quenching capacity of extracts was measured as per the Kalaskar and Surana.[7] The percentage inhibition was calculated with respect to control. Ascorbic acid was used as a standard compound in DPPH assay. The antiradical activity was expressed as IC50 (μg/mL).
2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical cation scavenging assay
Study of 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical cation scavenging activity was performed using the method reported by Kalaskar and Surana.[7] The absorbance was read at 734 nm after 5 min of reaction. The percentage inhibition was calculated from the control.
Antimitotic activity
The antimitotic assay was performed as described by Sehgal et al.[8] In this method, Allium cepa roots were used for evaluation of antimitotic potential. A. cepa bulbs purchased from the local market were sprouted in water for 48 h at room temperature. The bulbs with uniform developed roots were selected for the study. The roots were dipped in the plant extract (10 mg/mL) for 3 h. Water was used as a solvent for dilution and as a blank. Methothrexate used as a standard. After 3 h, the root tips were fixed in the fixing solution of acetic acid and alcohol (1:3). Squash preparation was made by staining with acetocarmine stain. The mitotic index was calculated by following formula:
Mitotic index = Number of dividing cells/total number of cells observed × −100
Antiproliferative activity using the yeast model
Antiproliferative study was evaluated by yeast Saccharocymes cerevisiae model according to the method of Simon.[9] The yeast culture was obtained from National Chemical Laboratory, Pune.
Preparation of yeast inoculums
The yeast cultured in potato dextrose broth by incubating at 37°C for 24 h, was referred as seeded broth. The seeded broth was diluted with sterilized distilled water, in order to get 25.4 × 104 cells (average).
Cell viability count
The yeast inoculums suspension culture (potato dextrose) was gently swirled to distribute the cells evenly. 0.5 mL of culture was transferred to Eppendroff tubes containing extracts and standard methothreaxate aseptically. One tube was kept as control. All tubes were incubated at 37°C. To the above cell suspension, 0.1% methylene blue dye was added and after few minutes, they were observed under low power microscope. The viable cells that failed to stain, and were transparent and oval shaped, while dead cells stained blue. The average number of cell was calculated using nephlometer and hemocytometer.
Antiproliferative activity on cell lines
The sulforhodamine-B, assay method[10] was used to describe the antiproliferative effect of extracts on five cell lines: MCF-7, HCT-15, MOLT-4, HOP-62 and PRO. This study was performed at the Advance Centre for Treatment Research and Education in Cancer, Mumbai. In the study cell concentration 4 × 103 cells/mL was used, and vincreastine sulfate and adriamycin was used as a positive control.
Phytochemical analysis
High-performance liquid chromatography (HPLC) analysis was performed on successive chloroform extract (SCH) and successive ethanol extract (SEE) using β-sitosterol, gallic acid and catechin (Sigma-Aldrich Chemie, Steinheim, Germany) as a standard marker. The separation of the components was performed on Phenomenix C18 column (250 mm × 4.6 mm I.D., 5 μm particle size). The β-sitosterol was identified using a solution of acetonitrile with water (in the ratio 90:10) as mobile phase at a flow rate 0.9 mL/min and detection was performed at 220 nm. The separation of gallic acid was achieved using methanol and water (in the ratio 70:30 v/v) as mobile phase, flow rate was adjusted to 0.7 mL/min and detection was performed at 280 nm. While, for catechin mobile phase acetonitrile and water (80:20 v/v) was used at flow rate 0.30 mL/min and detection was carried out at 280 nm.
RESULTS
In vitro antioxidant activity
The standardized extracts of the T. indicum were composed of a significant amount of total phenolics and flavonoids. The SEE showed highest polyphenolic content compared to the other extracts [Table 1].
Table 1.
Extractive yields in extracts
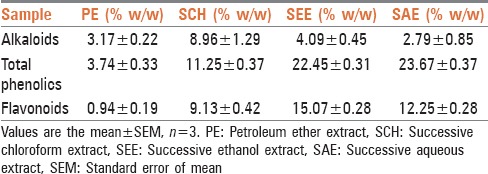
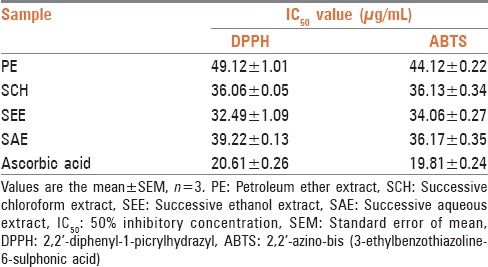
The extracts exhibited antioxidant activity in the DPPH and ABTS assays. The IC50 value obtained for DPPH, ABTS scavenging of SCH was 36.06 ± 0.05 and 36.13 ± 0.34 (% w/w). While in the SEE 32.49 ± 1.09 and 34.06 ± 0.27 (% w/w), respectively, which were found to be the least among all extracts and comparable to the reference standard ascorbic acid [Table 2].
Table 2.
Antioxidant effect (IC50) of extracts
Antimitotic activity
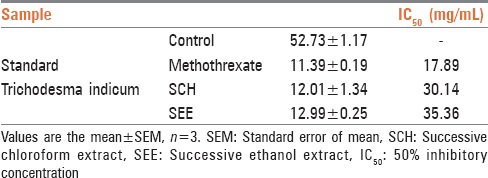
Antimitotic agents are the compounds that arrest cells multiplication in mitosis. In the antimitotic assay both the extracts SCH and SEE showed good inhibition of meristematic cell during different stages of the cell cycle [Figure 1]. The % mitotic index was 12.01 ± 1.34 for SCH and 12.99 ± 0.25 for SEE, which was close to standard, methothreaxate 11.39 ± 0.19.
Figure 1.
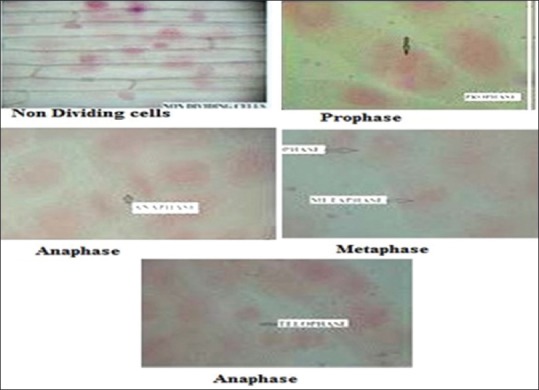
Stages of meristematic cell division of successive chloroform extract
Antiproliferative assay using the yeast model
To assess the possible antiproliferative effect of extracts of T. indicum as a first step toward the development of a novel anticancer agent, we tested extracts for their capability to inhibit cell growth and viability. Both the extracts showed good inhibition of yeast cell growth [Table 3]. The viable cell count for control solvents chloroform and ethanol were found to be 282 × 103 cells/mL and 283 × 103 cells/mL respectively.
Table 3.
Percentage mitotic index and IC50 value of SEE and SCH in the yeast model
Antiproliferative assay using cell lines
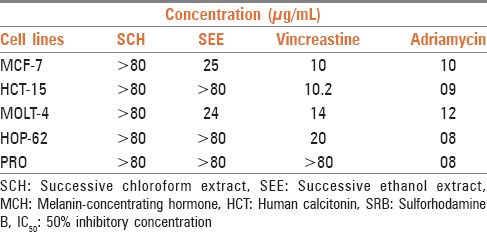
In the present study, we used five human cancer cell lines. It is known that different cell lines might exhibit different sensitivities toward an anticancer activity. Therefore, the use of > 1 cell line is necessary for the detection of anticancer compounds. The results of cytotoxicity screening of the extracts are summarized in Table 4. The SEE was active against MCF-7 and MOLT-4, while ineffective against rest of cell lines. The SCH was found to be ineffective against all the cell lines.
Table 4.
IC50 value of SRB assay on different cell lines
Phytochemical analysis
The phytochemical analysis of T. indicum extracts by HPLC [Figures 2 and 3] revealed the presence of β-sitosterol in SCH. Phenolic compounds, gallic acid and catechin was present in the SEE.
Figure 2.
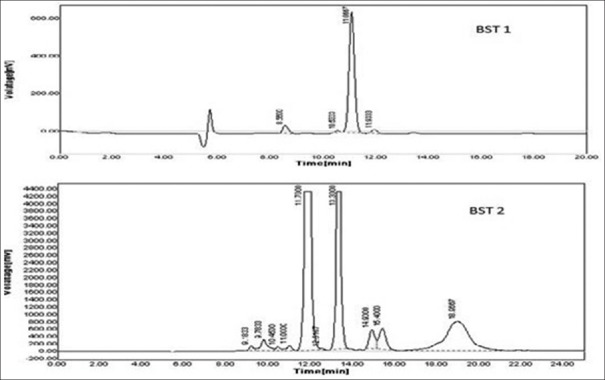
Chromatogram of β-sitosterol in successive chloroform extract. BST 1 - Chromatogram of standard b-sitosterol; BST 2 - Chromatogram of b-sitosterol in sample
Figure 3.
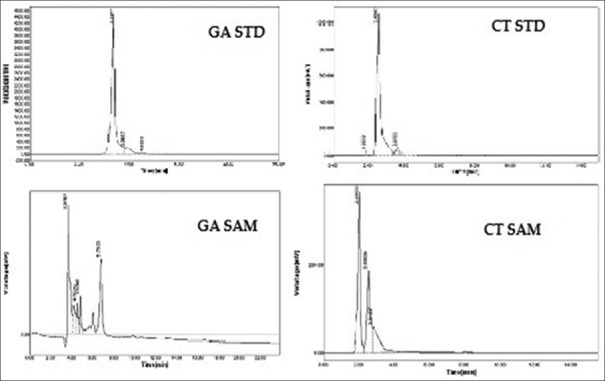
Chromatogram of gallic acid and catechin in successive ethanol extract. CT STD - Chromatogram of standard catechin; CT SAM-Chromatogram of catechin in sample. GA STD - Chromatogram of standard gallic acid; GA SAM - Chromatogram of gallic acid in sample
DISCUSSION
Trichodesma indicum has numerous therapeutic utilities in folk medicine. It is widely reported by many researchers that any compound with strong antioxidant property will also have potential anticancer properties because of the role of free radicals in the development of cancer.[11] Keeping this in mind, the plant extracts were assessed for their in vitro antioxidant properties. The antioxidant and free radical scavenging potential of petroleum ether, chloroform, ethanol and aqueous extracts is more likely to be attributed to different chemical compounds present in them as confirmed by phytochemical analysis. Steroids and other compounds were predominant in petroleum ether extract. Triterpenoids were present in the SCH. The polyphenolic componds were mainly found in SEE and SAE. Phenolic compounds have been reputed for their antioxidant ability.[7]
The antimitotic and antiproliferative effects are the important in vitro assays for the screening of anticancer compounds. In the present study mitotic index of extracts clearly indicates the efficiency in the inhibition of growth of cancer cells either by affecting microtubules or encouraging microtubule formation, and thus stopping the microtubules from being broken down. This makes the cells become so clogged with microtubules that they cannot continue to grow and divide. As a result of this cells arrest in mitosis and eventually, die by apoptosis.[12]
The antiproliferative property of the plant was ascertained using the yeast model as well as human cell lines. It is known that different cell lines exhibit different sensitivities toward anticancer compounds. It is for the reason that >1 cell line is necessary for the detection of anticancer activity.
In the US National Cancer Institute plant screening program, a crude extract is generally considered to have in vitro cytotoxic activity if the IC50 value below 30 μg/mL and for pure compounds, below 4 μg/mL.[13] Results of the present study show that the SEE had good cytotoxic activity only against leukemia, MOLT-4 and breast cancer cell line, MCF-7 and was not effective against the rest of human cell lines tested. SCH was ineffective against all the cell lines screened. The observed antiproliferative effect may be due to the inhibition of cell growth during the cell cycle, as they reduces the rate of cell division by preventing the entry of cell into the prophase and subsequent phases, which confirmed the results of antimitotic and antiproliferative activity.
Phytochemically SCH and SEE were rich in triterpenoid and polyphenolic constituents. The major polyphenolic and triterpenoid in SEE were identified as gallic acid, catechin and β-sitosterol. Polyphenol and triterpenoids are well known for their anticancer activity. These molecules might act as cancer-blocking agents, preventing initiation of the carcinogenic process and as cancer-suppressing agents, inhibiting cancer promotion and progression.[14] The anticancer property of gallic acid[14] and catechin[15] on breast cancer and other cancers was previously reported. They act by decreasing the mitochondrial membrane potential and intracellular reactive oxygen species along with inhibition of topoisomerase II and angiogenic inhibition.[16] Thus, we can assume the possible mechanism of the anticancer activity of T. indicum may be due to the presence of phenolic compounds in the extracts. It may be further concluded that the presence of gallic acid, catechin and other similar phytoconstituents present in the extract may contribute to cytotoxicity and cancerous cell growth inhibition.
CONCLUSION
Our findings support the reported therapeutic use of this plant as an anticancer agent in the traditional system of medicine. Further experiments are needed, both in vitro and in vivo to obtain more detailed mechanisms of action. Positive outcomes from the study in breast cancer and in leukemia cell lines introduce this plant as a new anticancer drug.
Editor's note:
Images as sent by author were of low resolution and the Editors have made a one time exception.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 3.Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67:2141–53. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 4.Kirtikar KR, Basu BD. 1st ed. New Delhi: International Book Publisher and Distributors; 1935. Indian Medicinal Plants. [Google Scholar]
- 5.Ali M. 1st ed. India: CBS Publication; 2008. Pharmacognosy. [Google Scholar]
- 6.Durick F, King JS, Jr, Ware PA, Cronheim G. A colorimetric method for the estimation of some tropine alkaloids. J Am Pharm Assoc Am Pharm Assoc. 1950;39:680–2. doi: 10.1002/jps.3030391209. [DOI] [PubMed] [Google Scholar]
- 7.Kalaskar MG, Surana SJ. Free radical scavenging and hepatoprotective potential of Ficus microcarpa L. fil. bark extracts. J Nat Med. 2011;65:633–40. doi: 10.1007/s11418-011-0532-z. [DOI] [PubMed] [Google Scholar]
- 8.Sehgal R, Roy S, Kumar VL. Evaluation of cytotoxic potential of latex of Calotropis procera and podophyllotoxin in Allium cepa root model. Biocell. 2006;30:9–13. [PubMed] [Google Scholar]
- 9.Simon JA. Yeast as a model system for anticancer drug discovery. Expert Opin Ther Targets. 2001;5:177–95. doi: 10.1517/14728222.5.2.177. [DOI] [PubMed] [Google Scholar]
- 10.Fouche G, Cragg GM, Pillay P, Kolesnikova N, Maharaj VJ, Senabe J. In vitro anticancer screening of South African plants. J Ethnopharmacol. 2008;119:455–61. doi: 10.1016/j.jep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Kaur C, Kapoor HC. Antioxidants in fruits and vegetables – The millennium's health. Int J Food Sci Tech. 2001;36:703–25. [Google Scholar]
- 12.Roberge M, Cinel B, Anderson HJ, Lim L, Jiang X, Xu L, et al. Cell-based screen for antimitotic agents and identification of analogues of rhizoxin, eleutherobin, and paclitaxel in natural extracts. Cancer Res. 2000;60:5052–8. [PubMed] [Google Scholar]
- 13.Alonso-Castro AJ, Villarreal ML, Salazar-Olivo LA, Gomez-Sanchez M, Dominguez F, Garcia-Carranca A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J Ethnopharmacol. 2011;133:945–72. doi: 10.1016/j.jep.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 14.Maurya DK, Nandakumar N, Devasagayam TP. Anticancer property of gallic acid in A549, a human lung adenocarcinoma cell line, and possible mechanisms. J Clin Biochem Nutr. 2011;48:85–90. doi: 10.3164/jcbn.11-004FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Indap MA, Radhika S, Motiwale L, Rao K. Anticancer activity of phenolic antioxidants against breast cancer cells and a spontaneous mammary tumor. Indian J Pharm Sci. 2006;68:470–4. [Google Scholar]
- 16.Gallo MB, Sarachine MJ. Biological activities of Lupeol. Int J Biomed Pharm Sci. 2009;3:46–79. [Google Scholar]


