Abstract
Introduction:
Gamma amino butyric acid (GABA) is an important ubiquitous four carbon nonprotein amino acid with an amino group attached to gamma carbon instead of beta carbon. It exists in different organisms including bacteria, plants, and animals and plays a crucial role in humans by regulating neuronal excitability throughout the nervous system. It is directly responsible for the regulation of muscle tone and also effective in lowering stress, blood pressure, and hypertension.
Aim and Objective:
The aim of the study was to develop the fingerprint profile of selected medicinally and economically important plants having central nervous system (CNS) activity and to determine the quantity of GABA in the selected plants grown under natural conditions without any added stress.
Materials and Methods:
The high-performance thin layer chromatography analysis was performed on precoated silica gel plate 60F–254 plate (20 cm × 10 cm) in the form of bands with width 8 mm using Hamilton syringe (100 μl) using n-butanol, acetic acid, and water in the proportion 5:2:2 as mobile phase in a CAMAG chamber which was previously saturated for 30 min. CAMAG TLC scanner 3 was used for the densitometric scanning at 550 nm. Specific marker compounds were used for the quantification.
Results and Conclusion:
Among the screened medicinal plants, Zingiber officinale and Solanum torvum were found to have GABA. The percentage of GABA present in Z. officinale and S. torvum were found to be 0.0114% and 0.0119%, respectively. The present work confirmed that among the selected CNS active medicinal plants, only two plants contain GABA. We found a negative correlation with plant having CNS activity and accumulation of GABA. The GABA shunt is a conserved pathway in eukaryotes and prokaryotes but, although the role of GABA as a neurotransmitter in mammals is clearly established, its role in plants is still vague.
KEY WORDS: Central nervous system activity, gamma amino butyric acid, high-performance thin layer chromatography, Solanum torvum, Zingiber officinale
INTRODUCTION
Gamma amino butyric acid (GABA) is an important ubiquitous four carbon nonprotein amino acid with an amino group attached to gamma carbon instead of beta carbon exists in different organisms including bacteria, plants, and animals.[1] It is produced primarily by the decarboxylation of L-glutamic acid catalyzed by the enzyme α-glutamate decarboxylase (GAD).[2] It plays a crucial role in the human body by regulating neuronal excitability throughout the nervous system. It is also directly responsible for the regulation of muscle tone[3] and also effective in lowering stress, blood pressure, and hypertension.[4]
Gamma amino butyric acid was discovered in plants more than half a century ago,[5] but interest in GABA shifted to animals when it was revealed that GABA is found at high levels in the brain, playing a major role in neurotransmission. Thereafter, research on GABA in vertebrates focused mainly on its role as a signaling molecule, particularly in neurotransmission. In plants and animals, GABA is mainly metabolized via a short pathway composed of three enzymes, called the GABA shunt because it bypasses two steps of the tricarboxylic acid (TCA) cycle. The pathway is composed of the cytosolic enzyme GAD and the mitochondrial enzymes GABA transaminase and succinic semialdehyde dehydrogenase. The regulation of this conserved metabolic pathway seems to have particular characteristics in plants. Indeed, interest in the GABA shunt in plants emerged mainly from experimental observations that GABA is largely and rapidly produced in response to biotic and abiotic stresses.[6,7,8] The GABA shunt has since been associated with various physiological responses, including the regulation of cytosolic pH, carbon fluxes into the TCA cycle, nitrogen metabolism, and deterrence of insects, protection against oxidative stress, osmoregulation, and signaling. Accumulation of GABA is a metabolic response of plant systems to stress such as salinity, anoxia, hypoxia, drought, heat, and chilling.[9,10,11,12,13,14]
In this article, we attempt to trace the presence of GABA in selected medicinal plants having central nervous system (CNS) activity reports and also those which are ingredients of Ayurvedic brain tonics. We also seek to know if GABA is present in the above plants if not subjected to stress. We also intend to link these and other findings that have accumulated during the half-century since the discovery of GABA in plants with recent evidence, mainly from Arabidopsis functional genomics approaches, pointing toward the possible role of GABA as a signal molecule in plants, as well as roles in plant responses to stress and in the carbon-nitrogen (C: N) balance. The objective of this study was to determine whether the accumulation of GABA occurs in the selected medicinal plants having CNS activity grown under natural condition without any added stress.
MATERIALS AND METHODS
Plant materials
Clitoria ternatea (Root), Zingiber officinale (Rhizome), Baccopa monieri (Whole plant), Terminalia bellerica (Pericarp), Punica granatum (fruit), Berberis aristata (Stem), Blechnum occidentale (Rhizome), Solanum torvum (Whole plant), Jasminium malabaricum (Leaf), Pterocarpus sandalinus (Heartwood), were collected from their natural sources. The collected fresh plant materials were washed, and air dried at room temperatures.
Chemicals
Standard GABA was purchased from Sigma Chemical Co., St Louis, MO, USA. All other chemicals and reagents used were of analytical grade.
Extraction
About 5 g of powdered drug was refluxed with 50 ml of methanol for 5 h. The extract was filtered and concentrated under reduced pressure to get a residue. The residue was dissolved in 10 ml of methanol for high-performance thin-layer chromatography (HPTLC) analysis.
Identification of gamma amino butyric acid by high-performance thin-layer chromatography
The GABA content from the selected medicinal plants was identified by HPTLC using a mixture of n-butanol, acetic acid, and water in the proportion 5:2:2 as mobile phase in a CAMAG chamber which was previously saturated for 30 min. Samples solution of the extract (0.1 g/ml) was used for further analysis and standard solution of GABA was prepared by taking 1 mg GABA dissolved in 10 ml of methanol to get a concentration of 0.1 μg/μl and they were analyzed using CAMAG automatic TLC sampler 4 attached to CAMAG HPTLC system. The samples, 5 μl each were spotted on aluminum backed precoated silica gel plate 60F–254 plate (20 cm × 10 cm) in the form of bands with a width of 8 mm using Hamilton syringe (100 μl). Various concentrations of standard GABA solution, viz., 0.02 μg/μl, 0.04 μg/μl, 0.06 μg/μl and 0.08 μg/μl were also applied to the plate in the same manner. The plates were developed in CAMAG automatic developing chamber 2 using twin trough chamber which was previously saturated with selected solvent system. The development of the plate was carried out up to 8 cm. The developed plate was dried in a hot air oven to evaporate solvents. After drying, the plate was sprayed with 2% ninhydrin in ethanol (particular spraying reagent for the detection for amino acids) and dried at 110°C in hot air oven for 5 min. Photographs of the derivatized plates were captured using CAMAG TLC visualizer. Densitometric scanning of the derivatized plate was performed with a CAMAG TLC scanner 3 equipped with WIN CATS software. Scanning was performed at 550 nm. Rf value and color of the bands in samples and standard were compared for the detection of GABA. Scanned areas of samples were matched with the scan area of standard GABA.[15,16]
RESULTS AND DISCUSSION
Gamma amino butyric acid content in selected medicinal plants
We had selected 10 medicinal plants that show prominent CNS activity according to Ayurvedic system of medicine. The selected plants were screened for the presence of GABA, beneficial amino acid in humans.
Identification of gamma amino butyric acid by high-performance thin layer chromatography
In this study, the GABA content of different samples was screened by HPTLC using silica coated aluminum plates. Different extracts (0.1 g/ml) were spotted on silica plates along with standard GABA at the end for the easy identification of the GABA. It shows that the HPTLC chromatogram of colored spots of GABA and the extracts after derivatization. GABA was identified on the basis of the retention factor (Rf = 0.57).
From the 10 plant extracts, GABA was identified in two extracts [Figure 1]. The presence of GABA in those two extracts was further confirmed by spotting the two extracts along with four different concentrations, viz., 0.2, 0.4, 0.6, and 0.8 μl of standard GABA [Figures 2 and 3]. GABA quantification was also done using this plate, and it shows desirable amount of GABA in the extracts. The percentage amount of GABA present in Z. officinale and S. torvum was found to be 0.0114% and 0.0119%, respectively. In the present analysis, the calibration curve of standard GABA was found to be linear (Y = 147.284 + 33.300X, r = 0.99844) which is presented in Figure 4. The interpretation of results suggests that the samples contained considerable amount of GABA.
Figure 1.
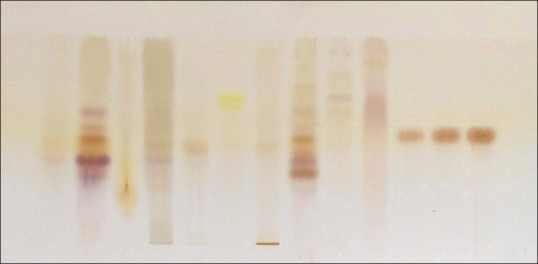
High-performance thin layer chromatography profile of standard gamma amino butyric acid (GABA) and extracts. 1–10 plants extracts and 11–13 standard GABA with different concentration
Figure 2.
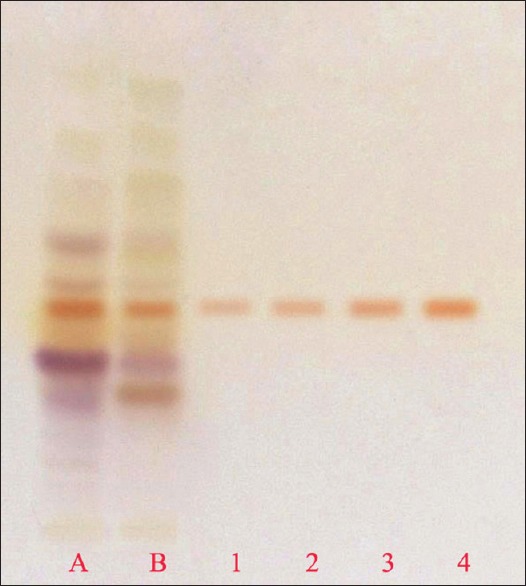
High-performance thin layer chromatography Profile of standard gamma amino butyric acid (GABA) and extracts. (a) Zingiber officinale and (b) Solanum torvum. Standard GABA with different concentration: 1, 2, 3 and 4
Figure 3.
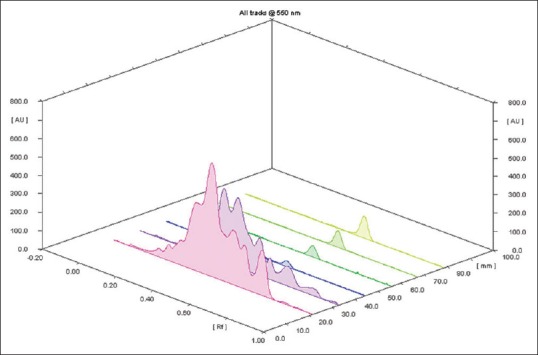
Three-dimensional display of high-performance thin layer chromatography chromatogram of standard gamma amino butyric acid and extracts
Figure 4.
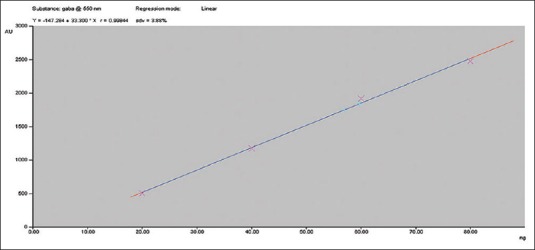
Calibration curve of gamma amino butyric acid
It is well-known, GABA plays an important beneficial role in the CNS as an inhibitory neurotransmitter.[3] It also accelerates the metabolic activity in the brain of growing children, prevents or minimizes the risk of headache, colon cancer, heart disease, Alzheimer's disease, constipation, regulates blood sugar level, lowers blood pressure, hypertension, and promotes sleep and rejuvenates the memory.[4] The results obtained show absence of GABA in plants like B. monniera, C. ternatea, P. granatum, B. aristata, B. occidentale, S. torvum having CNS activity.
In the mammalian CNS, GABA is the principal neurotransmitter mediating inhibitory synaptic currents by binding to receptors localized in the pre- or post-synaptic membranes. Reviews[8,17] have discussed the possible presence of GABA receptors in plants, which could mediate the action of GABA as a signaling molecule.
Plants sense their nutrient environment and respond to it by modifying their uptake and metabolism using an intricate system of sensors, receptors, transporters, signal transduction components, and gene expression regulators that collectively lead to changes in growth rates and development.[18] More specifically, the complex mechanisms coordinating the C: N balance involves sensing and regulating sugar levels, nitrate uptake and internal contents of reduced nitrogen (e.g. ammonium and amino acids). The GABA shunt appears to be part of the metabolic pathways involved in the C:N balance and the metabolism of nitrogen, the extent of which is not fully understood.[19]
An earlier finding suggests that the production of GABA is tightly linked to the glutamate content[20] and the authors hypothesized that GABA has a dominant role in buffering the production of glutamate. This might confirm previous observations about the role of GABA as a transient nitrogen storage metabolite.[6,7] Following this idea, it is also tempting to speculate that GAD activity is regulated by the level of glutamate.[6] To control their C:N balance, plants can sense their reduced nitrogen status and regulate the uptake and reduction of nitrate adequately.[21] Still, the identity of the metabolite(s) that plants use as sensor(s) in that process is unclear. The role played by glutamine is still a matter of debate and other metabolites, including amino acids or sugars, are probably involved. All the reported works show that GABA could be one of these metabolites.
More recent studies have shown that aqueous extracts of valerian roots contain appreciable amounts of GABA which could directly cause sedation, but there is some controversy surrounding the bioavailability of this compound.[22] Studies demonstrate that the H+/amino acid cotransporter could provide a potential route for oral absorption of GABA and some related compounds. Experiments demonstrate that GABA transport across the human intestinal epithelial cell membrane is coupled to proton flow into the cell.[23] Comparative pharmacokinetics and bioavailability of two oral formulations of a GABA-mimetic muscle relaxant drug was investigated in 8 healthy male volunteers after administration of single oral 8 mg doses. Plasma samples were assayed by a capillary gas chromatography-mass spectrometry method following enzymatic hydrolysis. Irrespective of the formulation used, the drug was rapidly absorbed from the gastrointestinal tract, peak levels of about 17 ng/ml being detected within 1 h in most subjects. Elimination was rapid, with mean residence time values of 5–6 h. All kinetic parameters showed considerable interindividual variability, but none differed significantly between the two formulations. Relative to the tablet formulation, the oral bioavailability of the capsule formulation was 1.06 ± 0.39.[24] In the present study, we found that the percentage amount of GABA present in Z. officinale and S. torvum was found to be 0.0114% and 0.0119%, respectively. Z. officinale is used as a spice in daily diet and a minimal rate of GABA uptake is possible through the oral absorption and there is no reported adverse effect of the usage of ginger in small quantities in human beings. S. torvum is used as a drug an in Ayurvedic preparations. Further pharmacological and clinical study has to be conducted in these lines to study the pharmacokinetics of GABA absorption.
CONCLUSIONS
High-performance thin layer chromatography fingerprinting profile is an important parameter of herbal drug standardization for the proper identification of the medicinal plants. The present work confirmed that from the selected CNS active medicinal plants, only two of them show presence of GABA. We found a negative correlation with plant having CNS activity and accumulation of GABA. In future, these two plants will help in the study of GABA as there is a significant amount of GABA in them, further it is possible to isolate natural GABA from these source plants. Uncoupling and deciphering the signaling and metabolic functions of GABA will require intensive and exciting research work during the coming years.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Akçay N, Bor M, Karabudak T, Ozdemir F, Türkan I. Contribution of Gamma amino butyric acid (GABA) to salt stress responses of Nicotiana sylvestris CMSII mutant and wild type plants. J Plant Physiol. 2012;169:452–8. doi: 10.1016/j.jplph.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Murakami Y, Kato Y, Kabayama Y, Tojo K, Inoue T, Imura H. Involvement of growth hormone-releasing factor in growth hormone secretion induced by Gamma-Aminobutyric acid in conscious rats. Endocrinology. 1985;117:787–9. doi: 10.1210/endo-117-2-787. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1–47. doi: 10.1016/s0074-7696(02)13011-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Yao HY, Chen F. Accumulation of Gamma-Aminobutyric acid in rice germ using protease. Biosci Biotechnol Biochem. 2006;70:1160–5. doi: 10.1271/bbb.70.1160. [DOI] [PubMed] [Google Scholar]
- 5.Steward GR. g-Aminobutyric acid: A constituent of the potato tuber. Science. 1949;110:439–40. [Google Scholar]
- 6.Shelp BJ, Bown AW, McLean MD. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999;4:446–452. doi: 10.1016/s1360-1385(99)01486-7. [DOI] [PubMed] [Google Scholar]
- 7.Snedden WA, Fromm H. Regulation of the g-aminobutyrate – Synthesizing enzyme, glutamate decarboxylase, by calcium – Calmodulin: A mechanism for rapid activation in response to stress. In: Lerner HR, editor. Plant Responses to Environmental Stresses: From Phytohormones to Genome Reorganization. New York, USA: Marcel Dekker; 1999. pp. 549–74. [Google Scholar]
- 8.Kinnersley AM, Turano FJ. Gamma amino butyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci. 2000;19:479–509. [Google Scholar]
- 9.Streeter JG, Thompson JF. In vivo and In vitro studies on gamma-aminobutyric acid metabolism with the radish plant (Raphanus sativus, L.) Plant Physiol. 1972;49:579–84. doi: 10.1104/pp.49.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace W, Secor J, Schrader LE. Rapid accumulation of gamma-aminobutyric acid and alanine in soybean leaves in response to an abrupt transfer to lower temperature, darkness, or mechanical manipulation. Plant Physiol. 1984;75:170–5. doi: 10.1104/pp.75.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsushida T, Murai T. Conservation of glutamic acid to Gamma Aminobutyric acid in tea leaves under anaerobic conditions. Agric Biol Chem. 1987;51:2865–71. [Google Scholar]
- 12.Mayer RR, Cherry JH, Rhodes D. Effects of heat shock on amino acid metabolism of cowpea cells. Plant Physiol. 1990;94:796–810. doi: 10.1104/pp.94.2.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aurisano N, Bertani A, Reggiani R. Involvement of calcium and calmodulin in protein and amino-acid metabolism in rice roots under anoxia. Plant Cell Physiol. 1995;36:1525–9. [Google Scholar]
- 14.Zushi K, Matsuzoe N. Salt stress-enhanced Gamma amino butyric acid (GABA) in tomato fruit. Acta Hortic. 2007;761:431–5. [Google Scholar]
- 15.Pradeep SR, Malleshi NG, Manisha G. Germinated millets and legumes as a source of gamma-aminobutyric acid. World Appl Sci. 2011;14:108–3. [Google Scholar]
- 16.Saravana BC, Sunil AG, Vasanthi HR, Muthuswamy VS, Ramanadan M. Development and validation of an HPTLC method for simultaneous estimation of excitatory neurotransmitters in rat brain. J Liq Chromatogr. 2007;30:2891–902. [Google Scholar]
- 17.Bouché N, Lacombe B, Fromm H. GABA signaling: A conserved and ubiquitous mechanism. Trends Cell Biol. 2003;13:607–10. doi: 10.1016/j.tcb.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Coruzzi GM, Zhou L. Carbon and nitrogen sensing and signaling in plants: Emerging ‘matrix effects’. Curr Opin Plant Biol. 2001;4:247–53. doi: 10.1016/s1369-5266(00)00168-0. [DOI] [PubMed] [Google Scholar]
- 19.Bouché N, Fromm H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004;9:110–5. doi: 10.1016/j.tplants.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Masclaux-Daubresse C, Valadier MH, Carrayol E, Reisdorf-Cren M, Hirel B. Diurnal changes in the expression of glutamate dehydrogenase and nitrate reductase are involved in the C/N balance of tobacco source leaves. Plant Cell Environ. 2002;25:1451–62. [Google Scholar]
- 21.Forde BG. Local and long-range signaling pathways regulating plant responses to nitrate. Annu Rev Plant Biol. 2002;53:203–24. doi: 10.1146/annurev.arplant.53.100301.135256. [DOI] [PubMed] [Google Scholar]
- 22.Houghton PJ. The Scientific basis for the reputed activity of Velarine. J Pharm Pharmacol. 1999;51:505–12. doi: 10.1211/0022357991772772. [DOI] [PubMed] [Google Scholar]
- 23.Thwaites DT, Basterfield L, McCleave PM, Carter SM, Simmons NL. Gamma-Aminobutyric acid (GABA) transport across human intestinal epithelial (Caco-2) cell monolayers. Br J Pharmacol. 2000;129:457–64. doi: 10.1038/sj.bjp.0703069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perucca E, Poitou P, Pifferi G. Comparative pharmacokinetics and bioavailability of two oral formulations of thiocolchicoside, a GABA-mimetic muscle relaxant drug, in normal volunteers. Eur J Drug Metab Pharmacokinet. 1995;20:301–5. doi: 10.1007/BF03190249. [DOI] [PubMed] [Google Scholar]


