Abstract
Introduction
In spite of its importance as an experimental model, the information on the left coronary artery in pigs is sparse.
Objective
To determine the morphologic features of the left coronary artery in pigs.
Methods
We evaluated 158 pig hearts. The left coronary artery was perfused with synthetic resin after their ostia had been catheterized. Diameters and courses of the vascular beds were measured with an electronic caliper (Mitutoyo®).
Results
The diameter of left coronary artery was 6.98 ± 1.56 mm and its length was 3.51±0.99 mm. It was found to end up by bifurcating itself into the anterior interventricular artery and the circumflex artery in 79% of the cases, and by trifurcating in 21% of the cases, with the presence of the diagonal artery. The anterior interventricular artery ended up at the apex in 79.7% of the cases, and the circumflex artery at the posterior aspect of the left ventricle in 64% of the case, this artery never reached the posterior interventricular sulcus. An anastomosis between the terminal branches of the anterior interventricular artery and the posterior interventricular artery was found in 7.6% of the specimens. The antero-superior branch of the anterior interventricular artery occurred in 89.9% of the hearts. A left marginal branch was observed in 87.9% of the cases with a diameter of 2.25±0.55 mm.
Conclusion
Compared with humans, pigs have shorter left coronary artery trunks and branches; even the circumflex artery never reaches the posterior interventricular sulcus. Our findings are useful for the design of experimental hemodynamic and procedural models.
Keywords: Coronary Vessels, Coronary Circulation, Heart
Abstract
Introdução
Apesar de sua importância como modelo experimental, a informação sobre a artéria coronária esquerda em suínos é escassa.
Objetivo
Determinar as características morfológicas da artéria coronária esquerda em suínos.
Métodos
Foram avaliados 158 corações de porcos. A artéria coronária esquerda foram perfundidos com resina sintética após a sua óstios foram cateterizados. Diâmetros e cursos dos leitos vasculares foram medidos com um paquímetro eletrônico (Mitutoyo®).
Resultados
O diâmetro da artéria coronária esquerda foi 6,98±1,56 mm e seu comprimento era de 3,51±0,99 mm. Verificou-se que acabam bifurcando-se no interior da artéria descendente anterior e da artéria circunflexa em 79 % dos casos, e dividindo-se em três em 21% dos casos, com a presença da artéria diagonal. A artéria interventricular anterior acabou no ápice em 79,7% dos casos, sendo a artéria marginal na face posterior do ventrículo esquerdo em 64% dos casos, esta artéria nunca chegou ao sulco interventricular posterior. Uma anastomose entre os ramos terminais da artéria interventricular anterior e artéria interventricular posterior foi encontrado em 7,6% das amostras. O ramo ântero-superior da artéria interventricular anterior ocorreu em 89,9% dos corações. Um ramo marginal esquerda foi observado em 87,9% dos casos, com diâmetro de 2,25±0,55 mm.
Conclusão
Em comparação com os seres humanos, os porcos têm troncos das artérias coronárias esquerdas mais curtos e ramos; até a artéria circunflexa nunca atinge o sulco interventricular posterior. Nossos resultados são úteis para a concepção de hemodinâmica experimental e modelos processuais.
INTRODUCTION
The left coronary artery (LCA) in pigs is the main vessel that provides irrigation to the heart. The LCA irrigates most of the left ventricle and atrium, including the interventricular septum. It emerges from the left aortic sinus, runs to the left behind of the pulmonary trunk, and ends up by bifurcating into the anterior interventricular artery (AIA) and the circumflex artery (CXA). Occasionally, the LCA trifurcates itself, also giving origin to the left diagonal artery (LDA), characteristic which has been described in humans with a frequency of 9-55% and in pigs at 20%[1-3].
The AIA descends through the homonym sulcus and emits septal branches, left branch of the cone (LBC), and right and left ventricular branches. The first left ventricular branch is called anterosuperior branch, and it usually has a good diameter and is projected towards the mid and lower thirds of the obtuse margin of the heart, with a frequency of 81-82% in humans. This branch has not been described in pigs. The AIA extends to the cardiac apex and commonly sends terminal branches to the posterior interventricular sulcus, which may anastomose with the posterior interventricular artery (PIA)[3]. The AIA ending in the apex has been described in a range of 6-33%, while at the posteroinferior aspect has been described in human hearts with a frequency of 42-80%[4-6].
The CXA presents two segments: The first one runs along the left atrioventricular sulcus to the obtuse margin of the heart, whereas the second segment continues through the posterior atrioventricular sulcus and may occasionally ends up at the crux cordis. The main branches of the CXA are the left marginal branch (LMB), left atrial branches and left ventricular branches[3]. In human hearts the CXA has been described as ending up as the PIA (left dominance), with an incidence of 5-20%[7,8] whereas in pigs this morphologic feature occurs rarely[3,9,10]. The sparse studies referring to the LCA in pigs have been conducted with small samples and are limited to qualitative descriptions[2,3,9].
The importance of the study of the LCA in pigs lies in the utilization of this species as an experimental model for cardiovascular surgery, hemodynamics, and for compared anatomy teaching - learning processes[3,9,11]. Similarly, given the large similarity existing between the hearts of humans and pigs, this work intends to enrich the knowledge on the coronary circulation in pigs to improve the applications using this animal species.
METHODS
This cross-over descriptive study assessed the LCA of 158 hearts of commercial stock pigs (Pietrain, Landrace Belga and Large White mixed breeds) destined to sacrifice with a mean age of 5 months, obtained from the Vijagual Refrigerating Plant of Bucaramanga - Colombia. The organs were subjected to an exsanguination process in a water source for 6 hours. After the LCA was marked with a silk suture at its origin, it was catheterized through its ostium and perfused with polyester resin (80% palatal GP41L and 20% styrene) impregnated with red mineral. Then, the hearts were subjected to a partial corrosion process with a 15% KOH (potassium hydroxide) in order to remove the subepicardial fat located at the interventricular and atrioventricular sulci.
The diameters of the LCA and its branches were measured at their origins using a digital caliper (Mitutoyo®); lengths were determined, and the finalization of the AIA was typified at the lower third of the anterior interventricular sulcus, at the apex, or at the posterior aspect of the heart. Similarly, the finalization of the CXA was assessed at the obtuse margin of the heart, at the posterior aspect of the left ventricle, at the crux cordis, or at the posterior interventricular sulcus. The branches anterosuperior (ASB) and LMB were characterized with respect to diameters, lengths, and irrigated ventricular segments.
The data were annotated in a physical matrix and recorded in a digital medium using Excel tables. Photographs of all of the pieces analyzed were taken to support the observations.
The continuous variables were analyzed using the t test, whereas the discrete variables were analyzed using Pearson's χ2 test and Fisher's exact test. The results were evaluated using the "Epi - Info 3.5.3" statistical program. The significance level used for this research was (P<0,05).
RESULTS
The mean weight of the 158 hearts obtained from pigs sacrificed when weighing 90 kilograms was 360±61.21 grams. The LCA had a diameter of 6.98±1.37 mm and a length of 3.51±0.96 mm. This artery bifurcated into the AIA and CXA in 125 hearts (79%) and trifurcated with an additional LDA in 33 specimens (21%), 22% of which were males and 20% females. (Table 1).
Table 1.
Division of the left coronary artery in anterior interventricular artery (AIA), circumflex artery (CXA) and left diagonal artery (LDA), by gender discrimination.
| Total sample | % | Males | % | Females | % | |
|---|---|---|---|---|---|---|
| Bifurcation (AIA y CXA) | 125 | 79 | 69 | 78 | 56 | 80 |
| Trifurcation(AIA, CXA Y LDA) | 33 | 21 | 19 | 22 | 14 | 20 |
| Total | 158 | 100 | 88 | 100 | 70 | 100 |
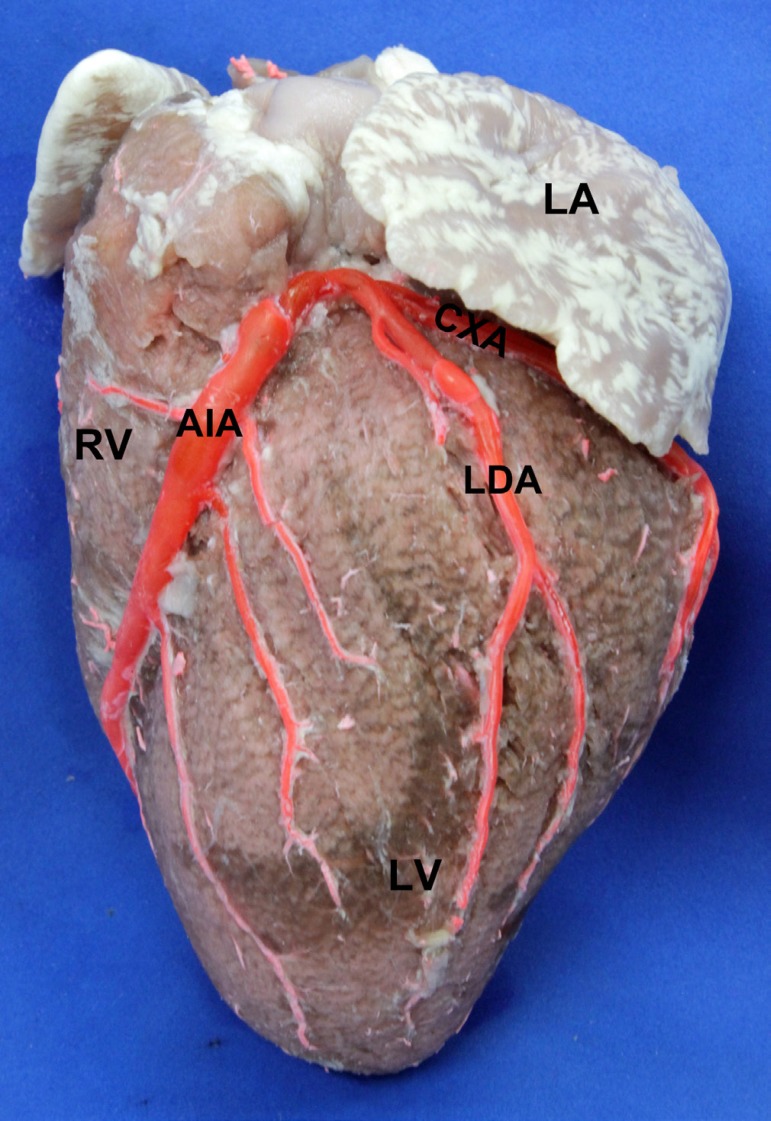
The LDA had a diameter of 2.7±0.72 mm and a length of 72.1±20.48 mm. It ended up at the lower third of the obtuse margin of the heart in 16 specimens (61.5%), whereas in 7 (27%) it ended up at the mid third. In 3 specimens (11.5%) it was short and only reached the upper third of the said margin. The LBC was observed in 109 specimens (69%), with a diameter of 1.25±0.39 mm; with the distance from its emergence to the left coronary ostium measuring 18.54±5.34 mm (Figure 1).
Fig. 1.
Obtuse edge of the heart. LA: Left Atrium. LV: Left Ventricle. RV: Right Ventricle AIA: Anterior Interventricular Artery. CXA: Circumflex Artery. LDA: Left Diagonal Artery
The AIA length was 95.6±15.25 mm. The AIA ended up at the apex in the majority of the cases (79.7%) both in males and in females, whereas its termination at the lower segment of the posterior aspect was 12.7%, a non-significant statistical difference (P=0.59) (Table 2).
Table 2.
Finish of anterior interventricular artery in homonym groove, cardiac apex and posterior surface, by gender discrimination.
| Total sample | % | Males | % | Females | % | |
|---|---|---|---|---|---|---|
| Middle third | 3 | 1.9 | 3 | 3.4 | - | - |
| Lower third | 9 | 5.7 | 4 | 4.4 | 5 | 7.1 |
| Cardiac apex | 126 | 79.7 | 71 | 81 | 55 | 78.6 |
| Posterior surface | 20 | 12.7 | 10 | 11.2 | 10 | 14.3 |
| Total | 158 | 100 | 88 | 100 | 70 | 100 |
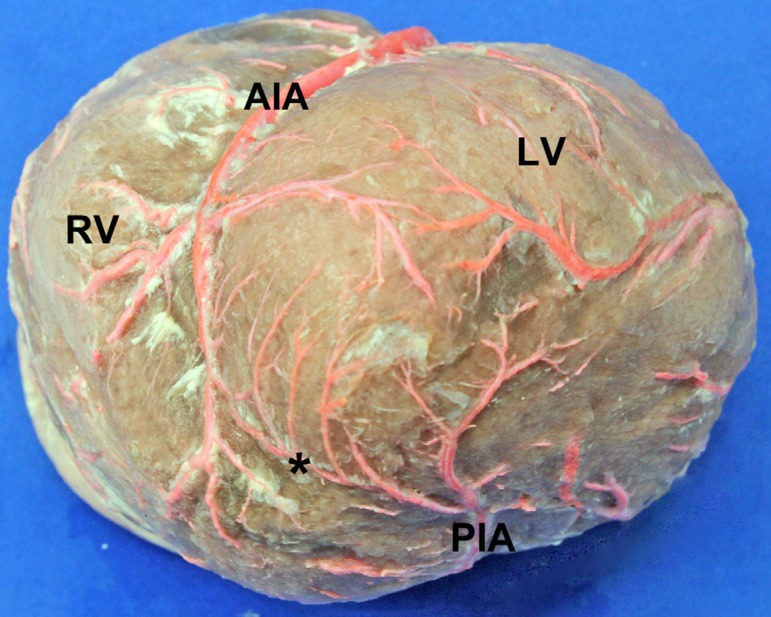
The AIA had on average 5±2.05 right ventricular branches and 5±2.11 left ventricular branches. Its proximal diameter was 4.06±0.73 mm; the mid diameter was of 2.82±0.54 mm, and its distal diameter was of 1.61±0.36 mm. The presence of an anastomosis between the terminal branches of the posterior interventricular artery and the AIA was found in 12 specimens (7.6%) (Figure 2).
Fig. 2.
Anastomosis between branches of the anterior and posterior interventricular arteries. LV: Left Ventricle. RV: Right Ventricle. AIA: Anterior Interventricular Artery. PIA: Posterior Interventricular Artery. (*): Anastomosis near the apex of the heart
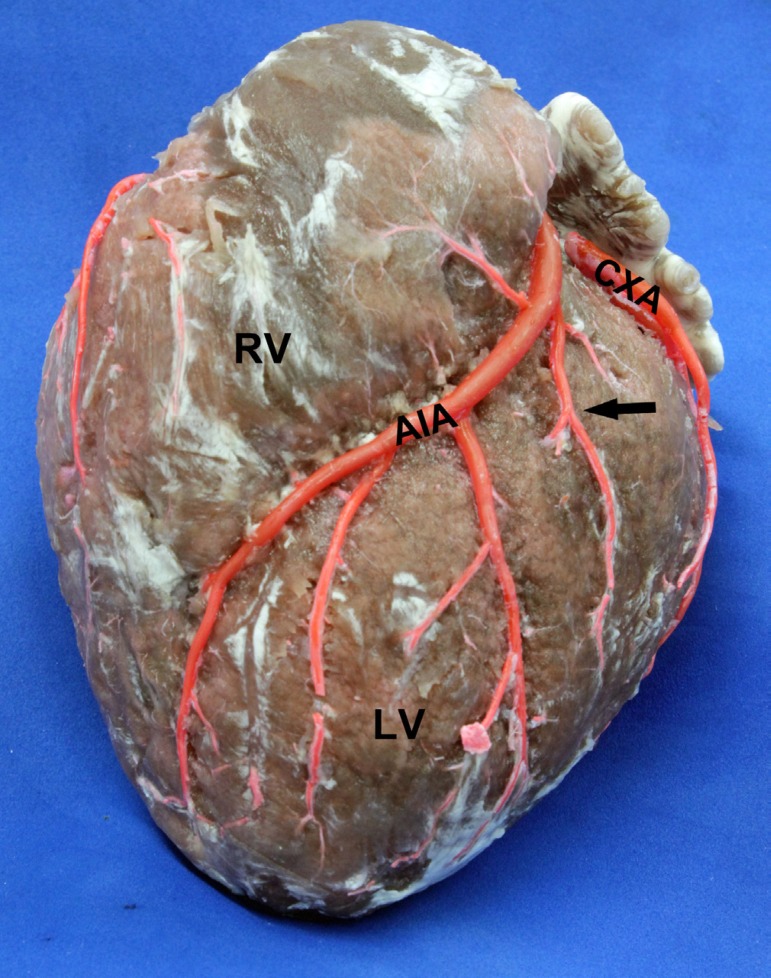
The ASB of the AIA was found in 142 hearts (89.9%), with a diameter of 1.84±0.68 mm and a length of 45.75±21.39 mm. The distance between its emergence and the left coronary ostium was of 24.63±7.42 mm (Figure 3). This branch ended up more frequently (39.4%) at the mid third of the obtuse margin of the heart (Table 3).
Fig. 3.
Anterior view of the heart. LV: Left Ventricle. RV: Right Ventricle. AIA: Anterior Interventricular Artery. CXA: Circumflex Artery. Arrow: Antero Superior Branch, finishing in the middle third of heart obtuse edge
Table 3.
Finish of anterosuperior branch (ASB) of anterior interventricular artery (AIA) on the obtuse side of the heart, by gender discrimination.
| Total sample | % | Males | % | Females | % | |
|---|---|---|---|---|---|---|
| Upper third | 40 | 28.2 | 17 | 21.8 | 23 | 36 |
| Middle third | 56 | 39.4 | 37 | 47.4 | 19 | 30 |
| Lower third | 43 | 30.3 | 21 | 27 | 22 | 34 |
| Cardiac apex | 3 | 2.1 | 3 | 3.8 | - | - |
| Total | 142 | 100 | 78 | 100 | 64 | 100 |
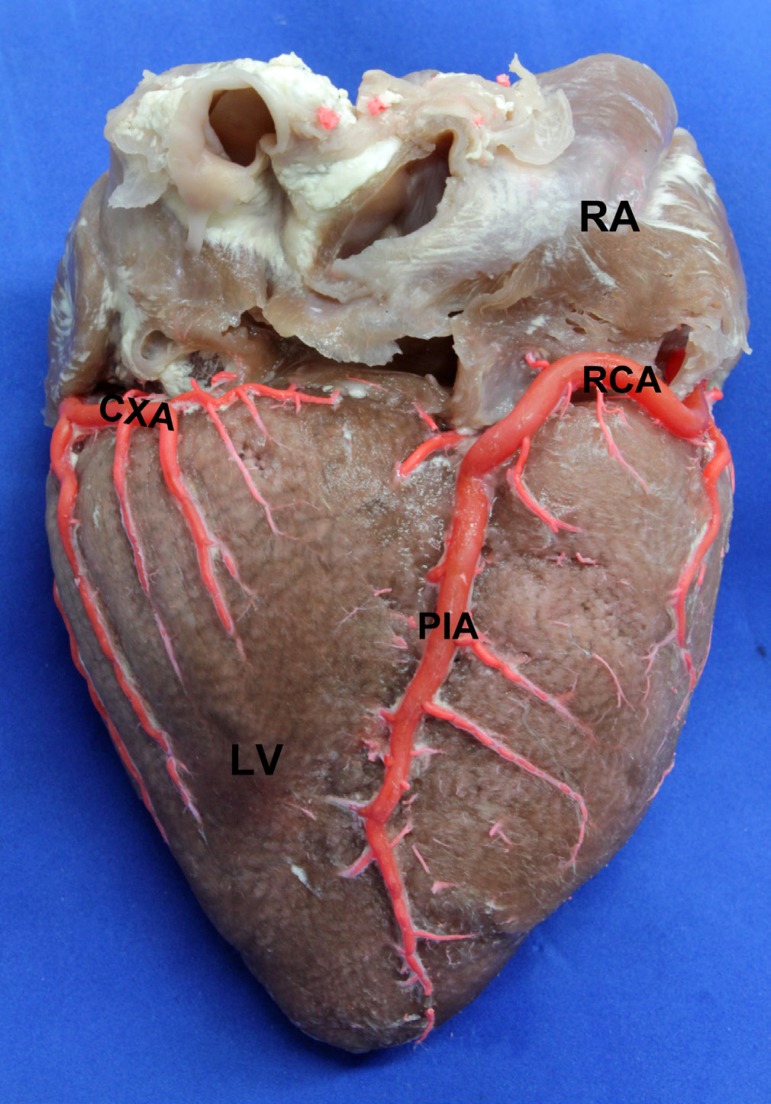
The ACX length was 80±16.26 mm being lower with respect to the length of the AIA (P=0,6317). The CXA ended up at the posterior aspect of the left ventricle in 101 hearts (64%); in 55 samples (34.8%) it reached the crux cordis (Figure 4), whereas in two cases (1.2%) it was short and ended up as the LMB. Its proximal diameter was 3.68±0.79 mm; its mid diameter was 2.75±0.55 mm, and its distal diameter was 1.66±0.55 mm. The smaller proximal diameter of the CXA with respect to the AIA was not statistically significant (P=0.0905).
Fig. 4.
Posterior view of the heart. LV: Left Ventricle. RA: Right Atrium. RCA: Right Coronary Artery. PIA: Posterior Interventricular Artery. CXA: Circumflex Artery, ending near the crux cordis
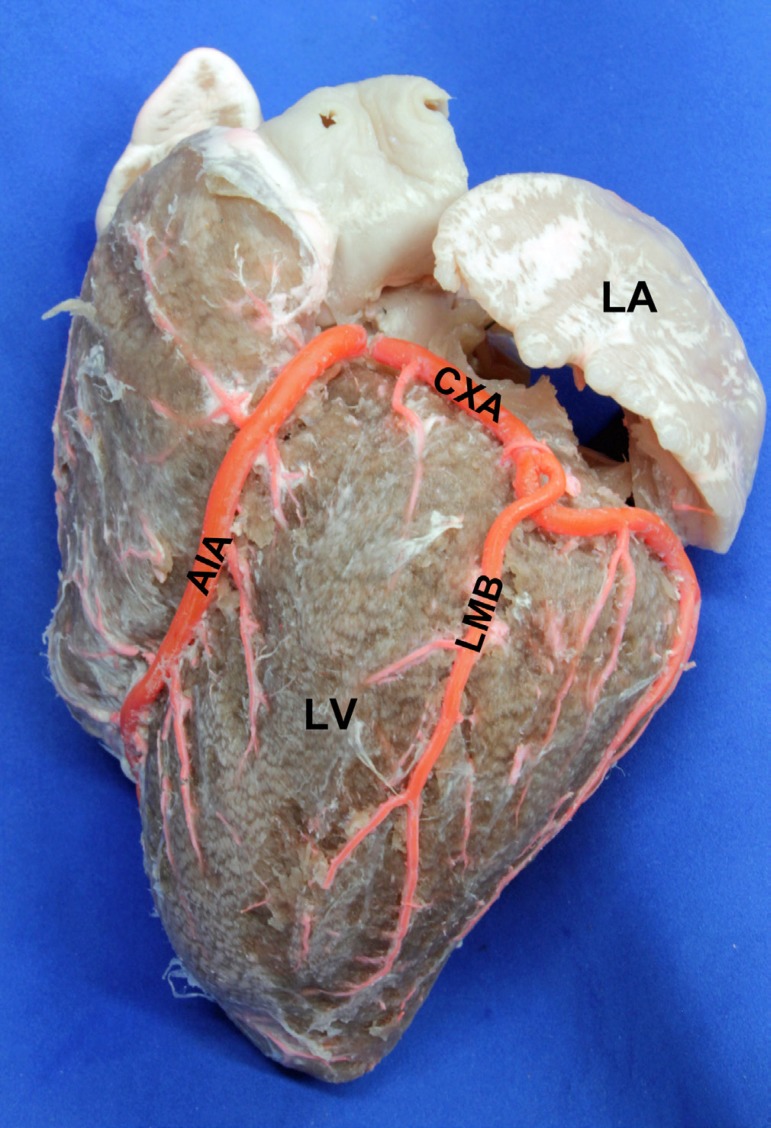
The LMB was found in 139 hearts (87.9%), ending up at the mid third of the obtuse margin of the heart in 72 specimens (51.7%). In 46 (33%) specimens ended up at the lower third, whereas in 15 specimens (11%) they ended up at the level of the apex. It was very short, reaching the upper third in 6 cases (4.3%). Its proximal diameter was 2.25±0.55 mm, and its distal diameter was 1.23±0.32 mm. It has a length of 63.13±14.97 mm and the distance from its point of finalization to the apex was 36.59±13.84 mm (Figure 5).
Fig. 5.
Obtuse edge of the heart. LA: Left Atrium. LV: Left Ventricle. AIA: Anterior Interventricular Artery. CXA: Circumflex Artery. LMB: Left Marginal Branch, ending at the apex of the heart
DISCUSSION
The length of the LCA was 4-5 mm in pigs[3,12] and in humans of 6-15 mm[13-16] reported in prior studies is longer than that found in this study (3.51 mm), an important issue to bear in mind for the training in the passing of catheters for experimental cardiovascular surgery models. LCAs shorter than 6 mm have been recorded in humans with an incidence of 7-15%[16-18]. Short coronary arteries are considered as risk factors for an appropriate coronary perfusion during aortic valve replacements, because the catheter may be inserted into one of the terminal branches and thus cause ischemic areas with potential arrhythmias, myocardial infarctions, or both[16,19].
The diameter of the LCA has not been reported in pigs, and we consider that our finding (6.98 mm) is considerably greater than what has been reported in humans[8,13,15,20], a trend that is maintained with the diameters of all of the branches, taking into account that the hearts evaluated were obtained from pigs with a mean weight of 90 kilograms. The bifurcated expression of the LCA (AIA and CXA) observed in 79% of the cases and trifurcated (AIA, CXA, and LDA) in the 21% of the samples evaluated, is consistent with the report by Jordão et al.[1]; but not with the works by Kato et al.[2], Sahni et al.[3], and Crick et al.[9] who reported the bifurcate expression in the 100% of the pig hearts they evaluated.
This disagreement may be understood by the different criteria utilized to characterize and name the branches that irrigate the obtuse aspect the pig hearts. The bifurcation of the LCA has been reported in humans with a frequency of between 40-70%, the trifurcate expression with a frequency of 9-55%, and a tetrafurcate expression between 5-7% of the heart specimens studied[13,15,16,21]. In agreement with prior studies in pig hearts, our work failed to observe the tetrafurcate division of the LCA. This morphologic expression differs slightly from the left coronary irrigation of humans.
The LDA ending up at the lower third of the obtuse margin of the heart in 61.5% of the specimens is in disagreement with the findings of Jordão et al.[1] who reported a short LDA (22.4 mm) ending up at the upper third in 95% of the cases. Some studies in human hearts indicate a predominance of LDA penetrating the myocardium at the level of the mid third of the ventricular surface[21,22], whereas some other works report a predominance of the LDA ending up at the upper third[15]. Length variations of the diagonal branches are particularly relevant in cardiac surgery because their subepicardial portion is commonly used to implant a bypass graft, so the existence of a short LDA limits the likelihood to perform this type of procedures[23].
ASB have not been described in studies with pigs. The occurrence of this vessel in our series (89.9%) is slightly more common than that reported by works conducted in humans[15,22], with which there is concordance in the finalization at the mid third of the left ventricle. Our findings concerning the length and diameter of the ASB (45.7 and 1.84 mm) are similar to reports in humans[15,22]. The LBC has only been reported before by Sahni et al.[3] who described it as a short branch with no subepicardial anastomosis with the right branch of the cone, a morphologic feature that is consistent with what was observed in our work.
The AIA ending up at the level of the apex in 79% of the cases is consistent with the findings by Sahni et al.[3] with only minor differences as to the number of left ventricular branches emitted by this artery (3-4), in comparison with our work (5 branches). In humans, it has been reported to end up at the lower segment of the posterior interventricular sulcus in 42-80% of the cases, and at the apex in the 6-33% of the subjects[5,6,13,24,25].
The site where the AIA ends up can be considered as a difference in the cardiac irrigation of humans and pigs. The presence of anastomosis between the terminal branches of the AIA and the posterior interventricular artery has been described by Lumb et al.[12] in a qualitative manner, and was observed in 7.6% of the specimens of our series, with this morphologic expression being relevant as protective of the coronary irrigation. The distal segment of the AIA that, after overcoming the apex, is distributed into the neighboring territory of the diaphragmatic aspect, irrigates the area not reached by the posterior interventricular artery. This anatomic expression should be taken into account when assessing myocardial infarctions located in the lower segment of the posterior wall of the heart, because the arterial obstruction could compromise the distal portion of the AIA instead of the posterior interventricular branch as it is commonly believed.
In agreement with most prior studies[3,9,12] the CXA did not end up at the posterior interventricular sulcus in any of our specimens; it mostly ended up at the posterior aspect of the left ventricle (64%) and to a lower percentage at the crux cordis. The study by Weaver et al.[10], who reported the CXA ending up as the posterior interventricular artery in 5% of the cases should be highlighted. The CXA has been reported as ending up at the posterior interventricular sulcus with a frequency of 7 - 23% in human hearts, with this being the feature of the left coronary dominance[5,8,13,14,21].
The LMB has not been sufficiently characterized in either humans or pigs. The occurrence of this vessel as reported by Sahni et al.[3] is similar to our finding (87.9%). Short CXAs have been described as ending up as the LMB with a frequency of 13-25.3% in human hearts[5,8,13,21]. In the majority of the cases the marginal branches are seen to irrigate segments of the obtuse aspect of the heart through collateral branches with horizontal or oblique courses.
CONCLUSION
Compared with humans, pigs have shorter LCA trunks and branches; even the CXA never reaches the posterior interventricular sulcus.
The greater diameter of the LCA and its branches found in the present study in comparison with what has been reported in humans is probably due to the greater weight of the hearts of the pigs evaluated.
The incidence of a trifurcate division of the LCA in pigs is situated in a mid range with respect to what has been reported in human hearts.
The ASB, LMB, and LDA with their good length and diameter, are relevant players for the irrigation of the obtuse aspect and posterior wall of the left ventricle in pig hearts.
The qualitative and morphometric knowledge of the LCA and its branches in pigs supports the utilization of this species for experimental models.
| Abbreviations, acronyms & symbols | |
|---|---|
| LCA | Left coronary artery |
| CXA | Circumflex artery |
| AIA | Anterior interventricular artery |
| LDA | Left diagonal artery |
| LBC | Left branch of the cone |
| PIA | Posterior interventricular artery |
| LMB | Left marginal branch |
| Authors’ roles & responsibilities | |
|---|---|
| FAG | Characterization of the left coronary artery in pigs, coronary vessels injection, records of findings, and writing the paper |
| LEB | Characterization of the left coronary artery in pigs, coronary vessels injection, records of findings, and writing the paper |
ACKNOWLEDGEMENTS
To Vijagual Refrigerating Plant in the city of Bucaramanga - Colombia and to Dr. Luz Stella Cortés, DMV for donating the pieces to conduct this investigation.
Footnotes
Work carried out at Universidad Industrial de Santander Bucaramanga, Colombia.
No financial support.
REFERENCES
- 1.Jordão MT, Bertolini SM, Areas JH, Junior, Prates NH. Anatomic study of the diagonal arteries in hearts of pigs. Rev Chil Anat. 1999;17(1):75–79. [Google Scholar]
- 2.Kato T, Yasue T, Shoji Y, Shimabukuro S, Ito Y, Goto S, et al. Angiographic diference in coronary artery of man, dog, pig and monkey. Acta Pathol Jpn. 1987;37(3):361–373. doi: 10.1111/j.1440-1827.1987.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 3.Sahni D, Kaur GD, Jit H, Jit I. Anatomy and distribution of coronary arteries in pig in comparison with man. Indian J Med Res. 2008;127(6):564–570. [PubMed] [Google Scholar]
- 4.Ilia R, Rosenshtein G, Weinstein J, Cafri C, Abu-Ful A, Gueron M. Left anterior descending artery length in left and right coronary artery dominance. Coron Artery Dis. 2001;12(1):77–78. doi: 10.1097/00019501-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Kalpana RA. A study on principal branches of coronary arteries in humans. J Anat Soc India. 2003;52(2):137–140. [Google Scholar]
- 6.Sahni D, Jit I. Blood supply of the human interventricular septum in north-west Indians. Indian Heart J. 1990;42(3):161–169. [PubMed] [Google Scholar]
- 7.DiDio LJ, Wakefield TW. Coronary arterial predominance or balance on the surface of the human cardiac ventricles. Anal Anz. 1975;137(1-2):147–158. [PubMed] [Google Scholar]
- 8.Baroldi G, Scomazzoni G. Coronary circulation in the normal and the pathologic heart. Washington, U.S.: Government Printing Office; 1967. pp. 5–90. [Google Scholar]
- 9.Crick S, Sheppard M, Ho SY, Gebstein L, Anderson RH. Anatomy of the pig heart: comparisons with normal human cardiac structure. Pt 1J Anat. 1998;193:105–119. doi: 10.1046/j.1469-7580.1998.19310105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver ME, Pantely GA, Bristow JD, Ladely HD. A quantitative study of the anatomy distribution of coronary arteries in swine in comparison with other animals and man. Cardiovasc Res. 1986;20(12):907–917. doi: 10.1093/cvr/20.12.907. [DOI] [PubMed] [Google Scholar]
- 11.López Centeno M, Ruiz Ripstein G, Ramírez Ruíz M, Arce Ruelas A. Cambios fisiológicos en cerdo de cirugía experimental para trasplante cardíaco. Invest Salud. 2004;6(1):11–13. [Google Scholar]
- 12.Lumb G, Singletary H. Blood supply to the atrioventricular node and bundle of His: a comparative study in pig, dog, and man. Am J Pathol. 1962;41:65–75. [PMC free article] [PubMed] [Google Scholar]
- 13.Ballesteros LE, Ramirez LM. Morphological expression of the left coronary artery: a direct anatomical study. Folia Morphol (Warsz) 2008;67(2):135–142. [PubMed] [Google Scholar]
- 14.Mouchet A. Les arteres coronaires du coeur chez l'homme. 2nd ed. Paris: Norbert Maloine; 1933. [Google Scholar]
- 15.Ortale JR, Meciano J, Filho, Paccola AM, Leal JG, Scaranari CA. Anatomia dos ramos lateral, diagonal e ântero-superior no ventrículo esquerdo do coração humano. Rev Bras Cir Cardiovasc. 2005;20(2):149–158. [Google Scholar]
- 16.Reig J, Petit M. Main trunk of the left coronary artery: anatomic study of the parameters of clinical interest. Clin Anat. 2004;17(1):6–13. doi: 10.1002/ca.10162. [DOI] [PubMed] [Google Scholar]
- 17.Leguerrier A, Bourgin T, Marcade E, Duval JM, Rioux C, Logeais Y, et al. Ventricular branches of the circumflex artery of the heart. Bull Assoc Anat (Nancy) 1980;64(186):415–423. [PubMed] [Google Scholar]
- 18.MacAlpine WA. Heart and Coronary Arteries.An Anatomical Atlas for Clinical Diagnosis, Radiological Investigation and Surgical Treatment. New York: Springer; 1975. [Google Scholar]
- 19.Fox C, Davies MJ, Webb-Peploe M. Length of the left main coronary artery. Br Heart J. 1973;35(8):796–802. doi: 10.1136/hrt.35.8.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zindrou D, Taylor KM, Bagger JP. Coronary artery size and disease in UK South Asian and Caucasian men. Eur J Cardiothorac Surg. 2006;29(4):492–495. doi: 10.1016/j.ejcts.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Baptista CA, DiDio LJ, Prates JC. Types of division of the left coronary artery and the ramus diagonalis of the human heart. Jpn Heart J. 1991;32(3):323–335. doi: 10.1536/ihj.32.323. [DOI] [PubMed] [Google Scholar]
- 22.Ballesteros LE, Ramirez LM, Saldarriaga V. Evaluation of the heart diagonal, anterosuperior, right posterolateral and lateral branches. A study in Colombian individuals. Int J Morphol. 2009;27(4):1073–1080. [Google Scholar]
- 23.Acar C, Farge A, Chardigny C, Beyssen B, Pagny JY, Grare P, et al. Use of the radial artery for coronary artery bypass. A new experience after 20 years. Arch Mal Coeur Vaiss. 1993;86(12):1683–1689. [PubMed] [Google Scholar]
- 24.James TN. Anatomy of the coronary arteries. New York: Paul Hoeber; 1961. [Google Scholar]
- 25.Lima R, Junior, Cabral RH, Prates NEVB. Tipos de circulação e predominância das artérias coronárias em corações de brasileiros. Rev Bras Cir Cardiovasc. 1993;8(1):9–19. [Google Scholar]