Abstract
Introduction
Most cardiomyocytes do not regenerate after myocardial infarction. Porcine small intestinal submucosa has been shown to be effective in tissue repair.
Objective
To evaluate myocardial tissue regeneration and functional effects of SIS implantation in pigs after left ventriculotomy.
Methods
Fifteen pigs were assigned to two groups: porcine small intestinal submucosa (SIS) (N=10) and control (N=5). The SIS group underwent a mini sternotomy, left ventriculotomy and placement of a SIS patch. The control group underwent a sham procedure. Echocardiography was performed before and 60 days after the surgical procedure. Histological analysis was performed with hematoxylin-eosin stain and markers for actin 1A4, anti sarcomeric actin, connexin43 and factor VIII.
Results
Weight gain was similar in both groups. Echocardiography analysis revealed no difference between groups regarding end diastolic and systolic diameters and left ventricular ejection fraction, both pre (P=0.118, P=0.313, P=0.944) and post procedure (P=0.333, P=0.522, P=0.628). Both groups showed an increase in end diastolic (P<0,001 for both) and systolic diameter 60 days after surgery (P=0.005, SIS group and P=0.004, control group). New cardiomyocytes, blood vessels and inflammatory reactions were histologically identified in the SIS group.
Conclusion
SIS implantation in pigs after left ventriculotomy was associated with angiomuscular regeneration and no damage in cardiac function.
Keywords: Intestinal Mucosa, Regeneration, Myocardium
Abstract
Introdução
A grande maioria dos cardiomiócitos não tem capacidade de regeneração após o infarto do miocárdio. A submucosa do intestino porcino tem-se mostrado eficiente como reparador tecidual.
Objetivo
Analisar a capacidade de regeneração tecidual miocárdica e o efeito funcional do implante da submucosa do intestino porcino após ventriculotomia esquerda em porcos.
Métodos
Quinze porcos foram separados em dois grupos: submucosa (N=10) e controle (N=5). Os animais do grupo submucosa foram submetidos a uma mini esternotomia inferior e ao implante da submucosa porcina na ventriculotomia esquerda. No grupo controle, foi realizada apenas a mini-esternotomia. Foi realizada análise ecocardiográfica no pré-operatório e 60 dias após o procedimento cirúrgico. A análise histológica foi feita com hematoxilina-eosila e marcadores para Actina 1A4, anti-actina sarcomérica, conexina43 e fator VIII.
Resultados
O ganho de peso foi semelhante entre os grupos. Considerando a análise ecocardiográfica, não foi identificada diferença estatisticamente significativa entre os grupos com relação ao diâmetro sistólico final, diâmetro diastólico final e fração de ejeção ventricular esquerda, tanto no pré (P=0.118, P=0.313, P=0.944) quanto no pós-operatório (P=0.333, P=0.522, P=0.628). Ambos os grupos mostraram um aumento no diâmetro sistólico final (P=0.005, grupo submucosa e P=0.004, grupo controle) e diâmetro diastólico final (P<0,001 para ambos) 60 dias após a cirurgia. À histologia, identificou-se a presença de novos cardiomiócitos, fibras musculares lisas, vasos sanguíneos e reação inflamatória no grupo submucosa.
Conclusão
O implante de submucosa intestinal porcina após ventriculotomia esquerda está associado a regeneração angiomuscular, sem prejuízo da função cardíaca.
INTRODUCTION
Although several studies have suggested that mitotic division occurs in the heart, the vast majority of cardiomyocytes do not have the ability to regenerate after myocardial infarction. Myocardial infarction leads to deterioration of contractile element function and, if ventricular remodeling is extensive in the infarcted area, heart failure may occur[1].
Tissue regeneration after transmural fibrosis remains a major concern. Porcine small intestinal submucosa (SIS) is a xenogeneic membrane, classified as biodegradable material since it has natural properties that allow for use as a biomaterial[2,3].
Through extensive research in various areas of medicine, SIS has been shown to be versatile and efficient in tissue repair. It does not trigger an antigenic response and is capable of inducing regeneration of the native tissue in which it was deployed[4,5].
Tissue replacement in the body and development of new grafts using biomaterials are major challenges in regenerative surgery. SIS is an alternative that should be further studied[6].
Thus, the aim of this study is to assess the ability of myocardial tissue regeneration following SIS implant after left ventriculotomy in pigs.
METHODS
All experiments were performed in accordance with the Guiding for the Care and Use of Laboratory Animals approved by the American Physiological Society[7] and the EU Directive 2010/63/EU for animal experiments.
This project was presented to CEUA (Committee of Ethics in Research in Animal Use of the PUCPR) and approved under article number 563 on 02/09/2010.
Submucosa Preparation and Decontamination
SIS was obtained by resection of the proximal jejunum segment of healthy pigs. The mesentery was removed, the intestinal segment was inverted and the mucosa was removed by scraping, taking precautions to not cut the tissue. After reversal of the inverted segment, the seromuscular extract was removed in the same manner. The resulting tissue consisting of the submucosal layer of the small intestine was washed in an isotonic saline solution and stored in a 10% neomycin sulfate solution. Decontamination was performed with a stabilized chlorine dioxide (0.04%) saline solution using a shaker (Bureau 109M, New Ethics Ltda.) for 24 hours[6,8].
Experimental Model
This experimental study included 15 (Landrace) adult pigs, with an average weight of 13.75 kg. The animals were randomized 2:1 and divided into two groups:
• SIS group (N=10): SIS implantation after left ventriculotomy;
• Control group (N=5): Mini lower sternotomy.
The animals were anesthetized with intramuscular administration of pre hydrochloride (1.0 mg/kg), ketamine (20 mg/kg) and acepromazine maleate 1% (0.05 mg/kg) and received prophylactic antibiotics (gentamicin sulfate 5 mg/kg).
The pigs were monitored with an electrocardiogram, pulse oximetry and denitrization with oxygen at 100%, anesthetized with propofol at a dose of 6 mg/kg and intubated. The animals were kept under a mechanical ventilation system with a 50% mix of oxygen and nitrous oxide.
Transoperative analgesics included intravenous 2 mg/kg morphine, 2 mg/kg lidocaine, and 2 mg/kg ketamine. The immediate post operatory analgesic administered was 1.1 mg/kg flunixin meglumine, and in the subsequent post operatory periods 50 mg/kg of intramuscular tramadol was administered.
The animals were placed in a dorsal decubitus position. An incision was performed from the sixth intercostal space to the xiphoid appendix in the lower third of the sternum, and subsequently to the lower inferior sternotomy. The pericardium was opened, and the left and right ventricles were visible.
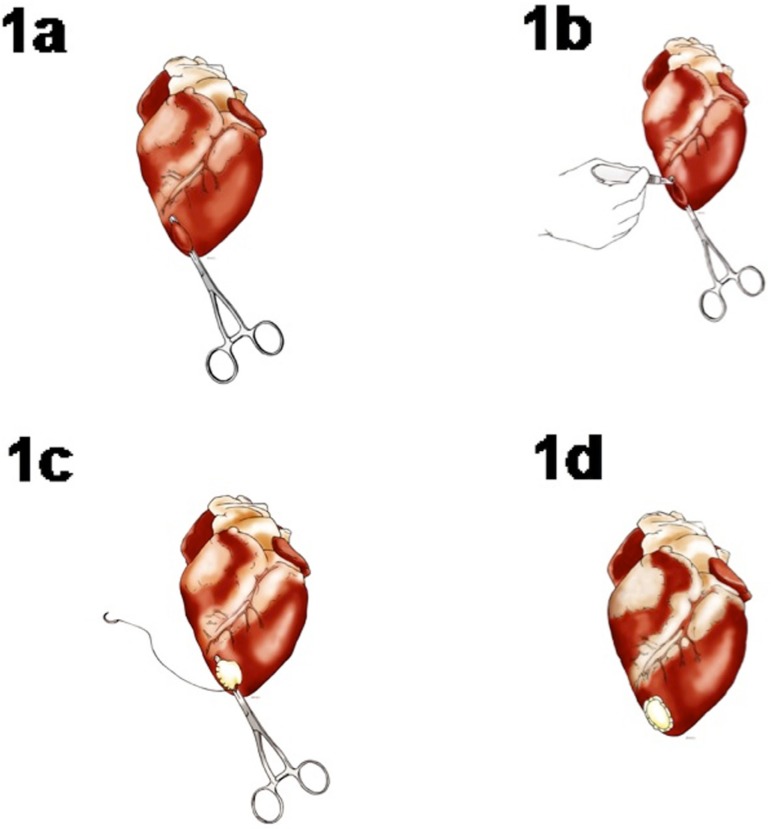
While the heart was beating, partial clamping of the left ventricular apex was performed excluding the anterior interventricular coronary, and a left ventriculotomy of approximately 30 mm in diameter was performed (Figures 1A/B). SIS implantation was performed (approximately 60 mm x 30 mm, double layered) on the apical portion of the left ventricle with a polypropylene 5.0 wire suture (Figures 1C/D). The animals were extubated, monitored in the recovery laboratory, and followed for a period of 60 days.
Fig. 1.
A) Partial clamp of the left ventricular apex; B) Left apical ventriculotomy; C) Implant of the submucosa in the LV apex. Partial suture with polypropylene 5.0 continuous suture; D) Submucosa implanted in the LV apex.
Control group animals underwent mini sternotomy, pericardial opening, and subsequent wound synthesis.
Functional Assessment
The animals underwent echocardiographic analysis at two time points: pre-surgery and 60 days after the procedure, based on the recommendations of the American Society of Echocardiography[9].
The following parameters were assessed (M-mode): cardiac frequency (CF, bpm), left ventricular end systolic diameter (LVESD, mm), left ventricular end diastolic diameter (LVEDD, mm), fractional shortening and left ventricular ejection fraction (LVEF, %). An Agilent echocardiogram equipment (model Sonos 5500; Andover, MA, USA) equipped with high frequency, high resolution transducers (12 MHz and 15 MHz, model 21390A, Agilent, Palo Alto, CA, USA) and a capacity of 120 (Hertz) frames per second was used.
Measurements were taken in M-mode to obtain images of a transversal section of the heart on the short axis. Diameters of the aorta, left atrium, right ventricle at end diastole, septum, and posterior wall at end diastole were assessed. Fractional shortening was obtained using the software provided on the equipment. The LVEF was obtained by LVEDD cubed minus LVESD cubed divided by LVEDD cubed. All measurements were performed three times by the same technician, who was blinded to treatment, and mean values were recorded[10].
Animals were euthanized after the procedure by intraperitoneal injection of Thiopentax (0.5 g sodium pentathol).
Histological Assessment
Hearts were removed, quickly washed in phosphate-buffered saline (PBS) (Gibco, Life Technologies, São Paulo, Brazil) and cryopreserved in liquid nitrogen. Serial transverse sections (8 mm) were obtained from a Leica cryostat (model 1850). Slides were stained with hematoxylin and eosin (H&E) and modified with Gomori's trichrome for morphological assessment. Immunohistochemistry was performed with anti-fast myosin antibody for immunofluorescence (Sigma, St. Louis, MO) at a dilution of 1:400. Slides were then incubated with secondary biotin-labeled affinity-isolated anti-rabbit and anti-mouse immunoglobulins (LSAB® Kit, Peroxidase; DAKO Corp., Carpinteria, CA).
Antigenic recovery of monoclonal mouse anti-human actin (smooth muscle) clone 1A4, monoclonal mouse anti-human von Willebrand factor, and monoclonal mouse anti-actin (sarcomeric) clone alpha-Sr-1 (Dako Cytomation, Denmark) was performed by enzymatic digestion and 0.1% trypsin diluted in PBS, pH 7.4, in a 37ºC oven for one hour.
H&E staining identified inflammatory responses and morphological characteristics. Factor VIII identified endothelial cells and blood vessels, actin 1A4 identified smooth muscle fibers and sarcomeric anti-actin indicated cardiomyocytes.
Statistical Analyses
Statistical analyses were performed using SPSS version 14.0. Groups were compared using Student's t-test for independent samples. Comparisons between pre- and post-operative results were performed by Student's t-test for paired samples. The condition of normality was evaluated by the Shapiro-Wilk test. P-values <0.05 were considered statistically significant.
RESULTS
Weight
Between preoperative assessment and 60 days after surgery, the mean weight of the animals in both the control and SIS groups significantly increased (from 13.2±3.6 kg to 54.2±10.9 kg [P<0.001] and 14.3±2.1 kg to 59.6±8.8 kg [P<0.001], respectively). Differences in mean weight were not significantly different between groups at both pre- and post-operative time points (P=0.456 and P=0.320, respectively) (Table 1).
Table 1.
Inter- and intra-group weight analysis (kg) between pre-operative evaluation and 60 days after surgery.
| Group | n | Mean | Standard Deviation | P | |
|---|---|---|---|---|---|
| Weight pre | SIS | 10 | 14.30 | 2.06 | |
| Control | 5 | 13.20 | 3.56 | 0.456 | |
| Weight pos | SIS | 10 | 59.60 | 8.83 | |
| Control | 5 | 54.20 | 10.94 | 0.320 | |
| Group | P (pre x post) | ||||
| SIS | <0.001 | ||||
| Control | <0.001 |
Histology
In the macroscopic analysis, it was identified an integration between the myocardium and SIS, not presenting a clear cleavage plane between them.
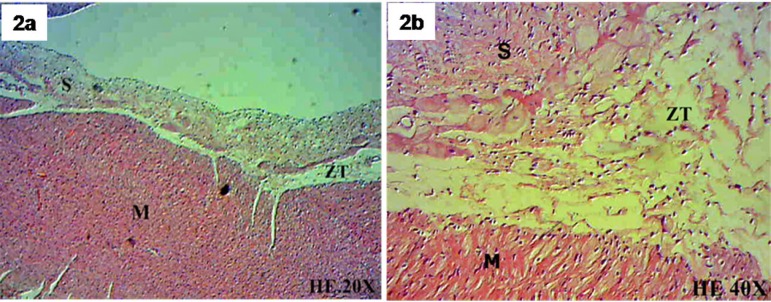
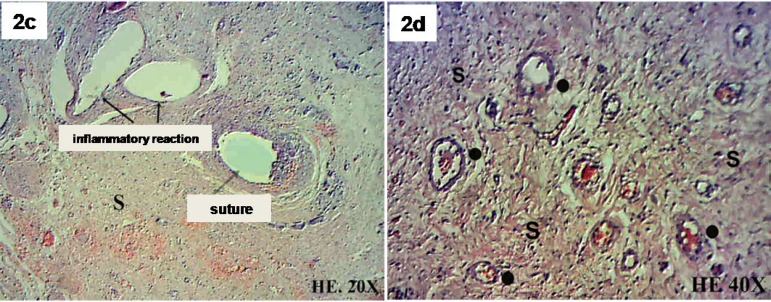
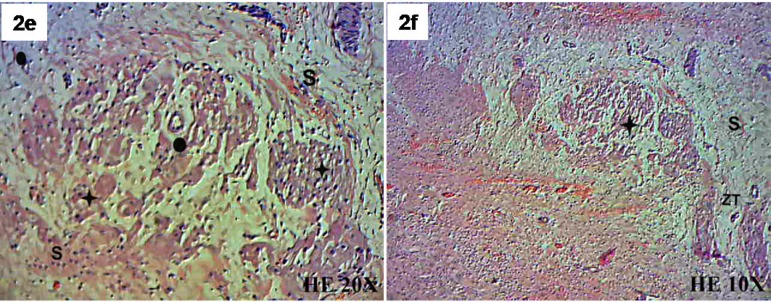
Using H&E, connective tissue in the SIS and in the transition zone between the SIS and the myocardium was identified. The presence of lymphocytes in the transition zone between the SIS and native myocardium were also observed, suggesting signs of a local inflammatory response to the sutures. No lymphocytic cells were identified in the central SIS region. New muscle fibers with a single central nucleus were identified in both the transition zone between the SIS and myocardium and the SIS itself. Endothelial cells and newly formed blood vessels were also observed (Figure 2A/B/C/D/E/F).
Fig. 2 - A,B.
Myocardium (M), transition zone between myocardium and the submucosa (ZT), submucosa (S), H-E (hematoxylin-eosin). 20X and 40X.
Fig. 2 - C,D.
Submucosa (S), blood vessels (o) and inflammatory reaction with lymphocytes around the suture, H-E (hematoxylin-eosin). 20X and 40X.
Fig. 2 - E,F.
Submucosa (S), transition zone between the myocardium and the submucosa (ZT), muscle fiber (+) and blood vessel (o), H-E (hematoxylin-eosin). 10X and 20X.
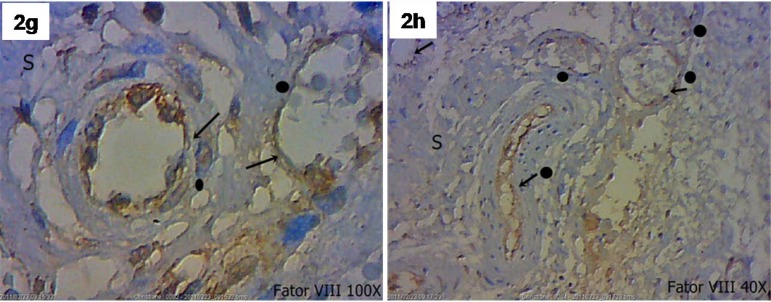
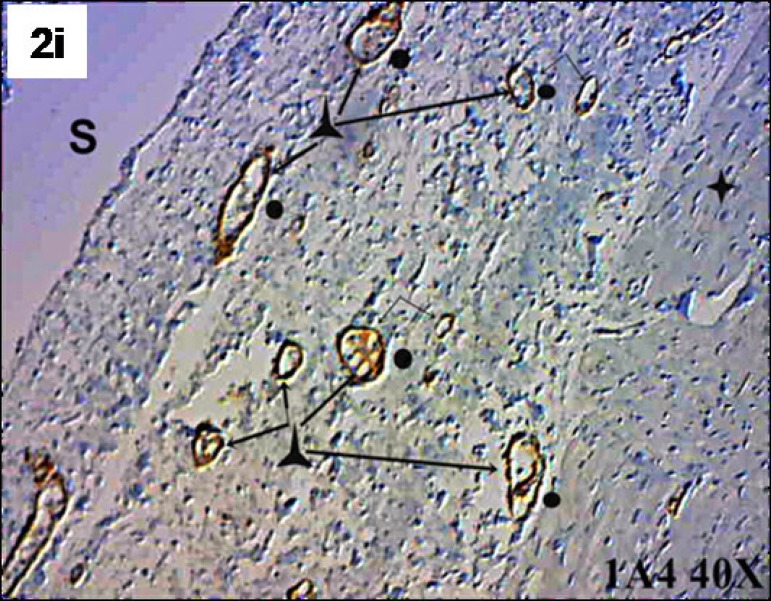
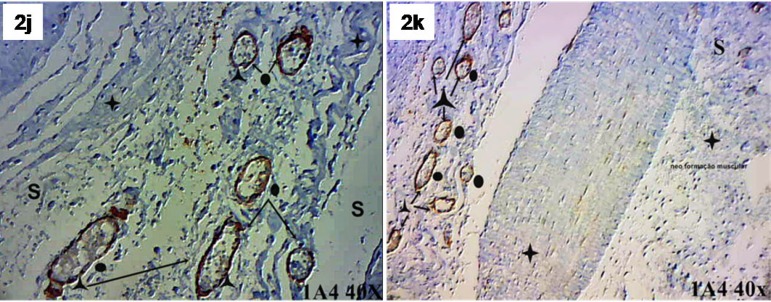
Factor VIII identified the presence of new blood vessels in both the transition zone between the SIS and myocardium and the SIS (Figure 2G/H). Actin 1A4 identified smooth muscle fibers in the walls of the blood vessels marked by factor VIII. Actin 1A4 negatively stained muscle fibers in the implanted SIS (Figure 2I/J/K).
Fig. 2 - G,H.
Submucosa (S), endothelial cells and blood vessels(●). Factor VIII, 40X and 100X.
Fig. 2 - I.
Submucosa (S), smooth muscle fiber (λ), muscle fiber negative for actin 1A4 (+) and blood vessels (o). Actin 1A4, 40X.
Fig. 2 - J,K.
Submucosa (S), blood vessels (o), smooth muscle fiber (λ) and muscle fiber negative for actin 1A4 (+). Actin 1A4, 40X.
Sarcomeric anti actin antibody positively marked new cardiomyocytes in both the transition zone as well as in the implanted SIS (Figure 2L/M).
Fig. 2 - L,M.
Submucosa (S). Cardiomyocyte positively marked (■). Sarcomeric anti-actin, 10X and 40X.
Echocardiography
The SIS and control groups had similar mean left ventricular ejection fraction (LVEF) at the preoperative assessment (68.18±7.7% vs. 68.43±2.9%, P=0.944). At the post-implant assessment, mean LVEF were also similar (73.78±9.6% vs. 71.55±3.4%, P=0.628). Intra-group assessment identified a variation from 68.18±7.7% to 73.78±9.6% (P=0.240) in the SIS group between the preoperative period and 60 days after surgery. In the control group for the same time period, a variation of 68.43±2.9% to 71.55±3.4% (P=0.262) was observed (Table 2).
Table 2.
Inter- and intra-group left ventricular ejection fraction (EF) analysis (%) between pre-operative evaluation and 60 days after surgery.
| Group | n | Mean | Standard Deviation | P | |
|---|---|---|---|---|---|
| EF pre | SIS | 10 | 68.18 | 7.67 | |
| Control | 5 | 68.43 | 2.92 | 0.944 | |
| EF pos | SIS | 10 | 73.78 | 9.60 | |
| Control | 5 | 71.55 | 3.43 | 0.628 | |
| Group | P (pre x post) | ||||
| SIS | 0.240 | ||||
| Control | 0.262 |
SIS - Porcine small intestinal submucosa
In the SIS and control groups, preoperative mean left ventricular end systolic diameter (LVESD) were 23.95±2.7 mm and 22.4±2.6 mm, respectively (P=0.313). In post-operative assessment, mean LVESDs were 30±5.6 mm in the SIS group and 28.2±3.3 mm in the control group (P=0.522). Increases from 23.95±2.7 mm to 30±5.6 mm (P=0.005) in the SIS group and 22.4±2.6 mm to 28.2±3.3 mm (P=0.004) in the control group were identified between pre- and post-operative time periods (Table 3).
Table 3.
Inter- and intra-group left ventricular end systolic diameter analysis (mm) between pre-operative evaluation and 60 days after surgery.
| Group | n | Mean | Standard Deviation | P | |
|---|---|---|---|---|---|
| LVESD pre | SIS | 10 | 23.95 | 2.73 | |
| Control | 5 | 22.40 | 2.61 | 0.313 | |
| LVESD post | SIS | 10 | 30.00 | 5.60 | |
| Control | 5 | 28.20 | 3.27 | 0.522 | |
| Group | P (pre x post) | ||||
| SIS | 0.005 | ||||
| Control | 0.004 |
LVESD - Left ventricular end systolic diameter; SIS - Porcine small intestinal submucosa
With respect to end diastolic diameter (LVEDD) inter-group preoperative assessment identified mean values of 36.43±3.2 mm in the SIS group and 33.2±3.1 mm in the control group (P=0.118). In post-operative assessment, 48.4±7.5 mm was observed in the SIS group and 44.8±3.3 mm in the control group (P=0.333). In intra-group analysis between the pre- and post-operative periods, we identified an increase in LVEDD in both the SIS and control groups (36.43±3.2 mm to 48.4±7.5 mm [P<0.001] and 33.2±3.1 mm to 44.8±3.3 mm [P<0.001], respectively) (Table 4).
Table 4.
Inter- and intra-group left ventricular end diastolic diameter analysis (mm) between pre-operative evaluation and 60 days after surgery.
| Group | n | Mean | Standard Deviation | P | |
|---|---|---|---|---|---|
| LVEDD pre | SIS | 10 | 36.43 | 3.20 | |
| Control | 5 | 33.20 | 4.15 | 0.118 | |
| LVEDD post | SIS | 10 | 48.40 | 7.53 | |
| Control | 5 | 44.80 | 3.35 | 0.333 | |
| Group | P (pre x post) | ||||
| SIS | <0.001 | ||||
| Control | <0.001 |
LVEDD - Left Ventricular End Diastolic Diameter; SIS - Porcine small intestinal submucosa
DISCUSSION
The aim of many clinical and experimental studies is to find ideal materials with all of the fundamental characteristics of biocompatibility. SIS has demonstrated superior results to other collagen-based materials due to its three-dimensional structure and the presence of growth factors and structural proteins, such as glycoproteins and proteoglycans. The combination of these factors promotes migration, cellular matrix interaction, cell differentiation and growth, which are all essential processes for tissue regeneration[3,11].
Decellularized matrices in an injectable form such as a gel have been utilized with the intent to impede progression of cardiac failure after myocardial infarction. These matrices can originate from the intestinal submucosa, as suggested by Chiu & Radisic[12], or even from the pericardium, as Seif-Naraghi et al.[13] suggested. However, Singelyn et al.[14] confirmed that although synthetic material has the capacity to allow neoangiogenesis due to its porous structure, it is not a bioactive substitute for damaged myocardium and does not allow for effective integration between the injected material and the integral myocardium.
The fundamental objective of an injectable biomaterial for post infarction cardiac repair is to form an in situ graft for cellular colonization and to consequently reduce left ventricular wall tension and theoretically stabilize ventricular remodeling. However, these traits were not identified in studies of stem cell injection in the bone marrow[15] or submucosa utilization in a gel form[16]. Implants made of injectable biomaterial may not have the same capacity to reverse ventricular remodeling given that its action is regional and does not affect the integral myocardium despite some degree of benefit.
Synergic effects between growth factors can increase biological effects beyond that of a single isolated factor. The capacity of injectable material to promote effects, such as cell adhesion and migration, in infarction healing can improve the therapeutic response. Components of the SIS include proteoglycans, glycosominoglycans, collagen, fibronectin, vascular growth factors, and fibroblasts. These natural components can promote interactions between host cells and the injected material, creating multiple cellular adhesions[2].
One example of catheter access is stem cell injection, which has been utilized in the peripheral venous system, the intra-coronary venous sinus, and intramuscular endocavity. However, this therapy in not favorable in some cases due to the manner in which the cells are transported to the myocardium. Nakamuta et al.[17] performed a study comparing several forms of stem cell injection into the myocardium and found intramuscular injection to be more efficient. Injection with a fibrin sealant presented an even greater increase in potentiality. Several articles have reported that cellular loss in a single intramuscular injection can be more than 80%, and thus injection of the greatest number of cells possible is necessary. For this reason, use of bioactive grafts is an interesting option because there is less cellular loss and better adhesion of the graft to the host[17].
Although there is an increasing tendency towards use of minimally invasive procedures, the basic causes of a disease cannot be treated using techniques with limited access at the cost of not achieving a thorough therapeutic treatment. Another important aspect is to define the objective of treatment using these new materials (i.e., cellular or mechanical repair) and subsequently define the delivery method into the myocardium. In the present study, the primary objective was mechanical repair, followed by tissue repair.
The technique used for the SIS implant in the present study followed the current tendency of using less invasive procedures. As such, a mini-sternotomy was performed, which can decrease morbidity and mortality given that the pig sternum provides significant support for the animal.
The SIS obtained in development of this study was from the Landrace species, and the animals utilized were from the same species. Although the procedure was not autologous, the use of grafts in animals of the same species reduces susceptibility to immunological responses, despite that fact that many researchers have suggested that the SIS is immunogenic[18].
Immunogenicity is of fundamental importance in using xenogenic grafts. As previously noted, the majority of researchers affirm that SIS is acellular and does not carry immunological information. However, Zhang et al.[19] showed that the SIS presents with porcine characteristics even after decellularization and sterilization based on PCR identification of pig DNA on submucosal plaque after skin implantation, which can trigger a local inflammatory response. These data were obtained after SIS implantation in the rotator cuff in mice and rabbits and calls into question other studies that have suggested the opposite. However, identification of a pig gene on the transplanted membrane and the presence of an inflammatory response can be explained. Tissues that carry DNA and are supposedly non-immunogenic, even without containing whole cells or leftover DNA in acellular tissue, are capable of inducing an innate immune response (inflammatory). Zhang et al.[19] suggested this hypothesis given that mastocytes and lymphocytes were identified in the receptor regions. The lesion itself in these animal models could have triggered the inflammatory process.
In the present study, we identified lymphocytic cells by H&E at the site of the implant, indicating an inflammatory reaction. These cells were next to the suture line that grafted the SIS to native myocardium (in the transition zone). This reaction could have been triggered by a granuloma response to foreign bodies by the suture and not by the SIS. No lymphocytes were identified in the central region of implanted SIS.
Morphological analysis of the SIS using H&E identified new blood vessels and fibers with muscular characteristics (a central single nucleus suggestive of cardiac muscle). This was confirmed by negative actin 1A4 staining of the muscle fibers adjacent to the myocardium and positive specific staining with the sarcomeric anti-actin antibody, corroborating development of new cardiomyocytes.
Factor VIII, which identified endothelial cells and blood vessels, positively stained cells in the SIS, confirming the capacity of the membrane to form new vessels. These vessels were identified both in the transition zone between the SIS and the myocardium and in the internal portion of the implanted membrane.
Actin 1A4 staining, which is specific for smooth muscle fibers, identified muscle fibers in blood vessel walls, while muscle fibers were negatively stained in the region adjacent to the SIS. This suggests that these were not smooth muscle fibers. Because they morphologically presented with a single central nucleus, we can infer that the fibers were striated, or cardiomyocytes.
The sarcomeric anti-actin antibody positively stained muscle fibers in both the transition zone and the SIS, effectively confirming the presence of new cardiomyocytes.
The presence of new blood vessels, new smooth muscle fibers, and cardiomyocytes in both the transition zone and the body of the SIS suggests angiomuscular regeneration and supports the hypothesis of cardiac tissue regeneration.
Evidence of new cardiomyocytes was supported by three points: anti sarcomeric antibody staining (specific for cardiomyocytes), morphological characteristics of the muscle fibers with H&E (central single nucleus), and negative staining with the actin 1A4 antibody.
One hypothesis regarding substitution of the extracellular matrix by host tissue, as suggested in some articles, states that a combination of cartilaginous tissue, fibrotic connective tissue, and adipose tissue is present in addition to cardiomyocytes and blood vessels. The presence of connective collagen in these studies was not unexpected given that it is well established that damaged or absent adult myocardial tissue can be substituted by scar tissue. However, according to Badylak et al.[20], simultaneous presence of other tissue types, including adipose, connective and cartilaginous tissue should not occur. Neither cartilaginous nor adipose tissue were identified in the implanted SIS in the present study, only loose connective tissue.
Upon macroscopic analysis, a suture was identified in the middle of the thickened tissue after implant of the SIS. This wire used to perform the implant was situated on the external surface of the myocardium above the epicardium, indicating integration of the membrane with the myocardium as suggested by Cayan et al.[5].
The manner in which these cells colonized the implanted SIS could have been a consequence of direct colonization of the submucosal edge, which was in direct contact with the myocardium. Growth factors such as VEGF may have stimulated development of endothelial cells, which are precursors of blood vessels, to consequently generate new blood vessels. Development of new cardiomyocytes could be explained by the fact that cardiac resident cells, also called cardiac stem cells, may have undergone hyperplasia and, under the stimuli of growth factors such as PDGF and fibrinectin to recruit host cells, proliferated toward the membrane.
Stimulus from neoangiogenesis may be another explanation for cardiomyocyte formation in the transition zone. Growth factors in the SIS could have stimulated proliferation of cardiac cells that colonized on the SIS toward the extremities and the center of the membrane.
One important detail was thickening of the SIS observed at the time of euthanasia without calcification, suggesting bioactivity. In other words, the membrane grew together with the heart, integrating with the host tissue.
Echocardiographic functional analysis showed that SIS and control groups were similar with respect to LVESD, LVEDD, and LVEF in both the pre-and post-operative periods. These data are comparable with each other without methodological bias. The SIS implanted after left ventriculotomy did not cause deterioration of cardiac function or ventricular remodeling.
Within groups, there was an increase in left ventricular end systolic and diastolic diameters between the pre implant period and 60 days after the procedure, although the LVEF was maintained. This could be a result of animal growth given that weight increased significantly after 60 days. It is important to point out that the two groups presented gains proportional to their weight.
Although in the present study we did not identify functional improvement in the SIS group, the proposed experimental model did not present ventricular dysfunction and showed development of new vessels in the transition zone. Thus, we demonstrated improved vascularization of this tissue in support of the hypothesis of Lionetti et al.[21]. These authors defend the theory that the autocrine and paracrine mechanisms, mediated by factors released by resident cells, perform essential roles in the repair process following heart failure. Such signs can influence the function of cardiac stem cells through several mechanisms, including survival of cardiomyocytes and neogenesis. In addition to promoting cytoprotection and angionesis, paracrine factors released by resident cardiac cells can alter cardiac and local extracellular matrix metabolism, interfering in post lesion favorable remodeling. Intracellular signs may be activated and modulated temporally and spatially with several effects in general, depending on the microenvironment as a result of the alteration in the lesioned myocardium. Chemical, mechanical or genetic activation of cardiac cells has been demonstrated to release peptides that protect the tissue against ischemic lesions. These mechanisms can help in the process of supplying specific proteins produced by these cells for new pharmacological therapy in cardiac regeneration. Although medication-based therapy to treat heart failure can lead to better cardiac function, it cannot induce tissue repair or regeneration.
Angiogenesis is fundamental for vascular supply to the grafted tissue in formation. Proliferation and migration of endothelial cells induced by chemotactic factors present in the extracellular matrix and adjacent cells promotes neoangiogenesis[22-25]. These endothelial cells give origin to vascular tubes, stems and capillaries. This process occurs in the first weeks (2-6 weeks) after the graft implant. In the present study, we identified cell groupings stained in the graft and formation of new blood vessels morphologically characterized by H&E and specifically marked by factor VIII and smooth muscle fibers stained by the 1A4 antibody on the neovessel wall in the SIS.
A contractile graft was proposed by Hata et al.[26] using fetal cardiomyocyte cultures in the SIS. However, this technique can transfer the immunogenicity of fetal cardiomyocytes, which may interfere in the colonization process of the host tissue in the short and long term and can present the same collateral effects as synthetic grafts. The ideal mechanism for contractile graft formation is development by the organism's own muscle fibers.
Although Dar et al.[27] cultivated cardiac cells in an alginate membrane, it was not possible to stimulate formation of new cardiomyocytes. In contrast, Matsubayashi et al.[28] performed cell culture in vascular smooth muscle of the aorta in rats on sponge polymer plates reinforced with poly-L lactic acid for two weeks and observed tissue formation. The use of cardiomyocytes in some types of culture does not yield positive results, as shown by Guarita-Souza et al.[29] who reported that differentiated cells significantly reduced their capacity for hyperplasia, contrary to fetal cardiomyocytes, smooth and skeletal muscle cells.
The choice of the left ventriculotomy in the present study originated from Nakamuta et al.[17], in which an experimental model of a lesion in the right ventricle implanted with SIS is described. There was doubt as to the membrane's resistance when implanted into a system with greater pressure. From a mechanical and cellular repair standpoint, the authors identified 70% recovery in contractile force of muscular fibers formed on the SIS and identified not only muscular and blood vessels, but also cartilage, fibrotic and adipose tissue. In the present study, we identified endothelial cells, blood vessels and cardiomyocytes in the transition zone and in the body of the SIS, however, cartilaginous cells were not observed.
With respect to tissue resistance of the SIS, left ventricular pressure in both the immediate and late post-operative periods was maintained. A double layer of membrane could have aided in the process. Long term resistance was satisfactory given that while the SIS was differentiating through angiomuscular regeneration, it was integrating with the adjacent myocardium and creating greater adherence and resistance.
Another reason for using a left ventriculotomy and SIS implant was the lack of studies using this experimental model (integral myocardium without infarction or induced myocardiopathy). The aim of this study was to perform mechanical repair and subsequently assess the capacity of cellular regeneration in the transition zones between the SIS and myocardium. In models using myocardial infarction, fibrosis impedes direct contact with the myocardium and can block access to growth factors. Some authors may have prioritized utilizing the extracellular matrix in a gel form because this type of graft can remain between layers with fibrosis.
Design of the present study with only two groups was based on the fact that SIS implanted in the left ventricle has not been previously described in the literature, and therefore we do not have initial results to compare with other types of grafts. The inclusion of a control group without intervention in the myocardium was supported by the fact that if we had performed left ventriculotomy and subsequent suture of the ventricular apex, we may have induced fibrosis, which could have interfered in comparison with the study group and created a methodological bias. We would have been comparing the SIS group with fibrosis rather than native myocardium. A third group with PTFE and/or bovine pericardium was not proposed because the aim was to define what would happen using SIS implanted in the left ventricle and not to define which graft was better.
It is important to differentiate experimental animal models of myocardial infarct from transmural myocardial infarction given that several studies have reported the use of submucosa injection in a gel form for myocardial infarct without identification of compromise in the ventricular wall. Physiopathologically, the behavior of the two models is different from a tissue repair standpoint. Infarction models with thick myocardium have better regeneration potential because cardiomyocytes are stunned and could benefit from neoangiogenesis induced by the SIS itself, which has been shown in similar studies using mononuclear stem cells. However, transmural infarction presents a thin ventricular wall without cardiomyocytes, and thus the repair mechanism by the graft should be mechanical and not cellular.
With respect to post left ventriculotomy assessment, it is important to confirm preservation of cardiac contractile function even after SIS implantation. There was neither ventricular dysfunction nor remodeling despite an identified increase in systolic and diastolic diameters.
Based on these results, we suggest that the SIS can be utilized as repair in aneurysmectomy surgery of the left ventricle and acts in two different ways: actively by correcting a mechanical defect, reducing ventricular dilatation and minimizing ventricular remodeling as suggested by Hsu et al.[30]; and passively by forming new blood vessels and muscle fibers around the implanted membrane in the transition zone between the SIS and the integral myocardium. These two processes could reduce late development of congestive heart failure.
CONCLUSION
We conclude that 60 days after surgical procedure, the SIS integrated with the myocardium and new blood vessel were formed, as well as new cardiomyocytes, suggesting a angiomuscular regeneration.
| Abbreviations, acronyms & symbols | |
|---|---|
| CF | Cardiac frequency |
| CEUA | Committee of Ethics in Research in Animal Use of the PUCPR |
| H&E | Hematoxylin and Eosin |
| LVEF | Left ventricular ejection fraction |
| LVEDD | Left Ventricular End Diastolic Diameter |
| LVESD | Left Ventricular End Systolic Diameter |
| PBS | Phosphate-buffered Saline |
| SIS | Porcine small intestinal submucosa |
| Authors' roles & responsibilities | |
|---|---|
| CMGR | Analysis and/or interpretation of data; final approval of the manuscript; conception and design of the study; implementation of operations and/or experiments; writing of the manuscript or revising it critically for its content |
| JCF | Conception and design of the study; implementation of operations and/or experiments; writing of the manuscript or revising it critically for its content |
| MO | Analysis and/or interpretation of data; statistical analysis |
| KATC | Final approval of the manuscript; conception and design of the study; writing of the manuscript or revising it critically for its content |
| RC | Final approval of the manuscript; conception and design of the study; implementation of operations and/or experiments |
| BOE | Final approval of the manuscript; writing of the manuscript or revising it critically for its content |
| LFJ | Aid in surgery |
| CPB | Paper discussion |
| VFA | Aind in surgery |
| LN | Analysis of slides in pathological anatomy |
| RMM | Paper review |
| JRFN | Analysis and/or interpretation of data; final approval of the manuscript; writing of the manuscript or revising it critically for its content |
| LCGS | Analysis and/or interpretation of data; final approval of the manuscript; conception and design of the study; implementation of operations and/or experiments; writing of the manuscript or revising it critically for its content |
Footnotes
DISCLOSURE STATEMENT
The authors declare no conflicts of interest.
This study was carried out at Pontifícia Universidade Católica do Paraná (PUCPR), Curitiba, PR, Brazil
No financial support.
REFERENCES
- 1.Hughes S. Cardiac stem cells. J Pathol. 2002;197(4):468–478. doi: 10.1002/path.1159. [DOI] [PubMed] [Google Scholar]
- 2.Abraham GA, Murray J, Billiar K, Sullivan SJ. Evaluation of the porcine intestinal collagen layer as a biomaterial. J Biomed Mater Res. 2000;51(3):442–452. doi: 10.1002/1097-4636(20000905)51:3<442::aid-jbm19>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67(4):478–491. [PubMed] [Google Scholar]
- 4.Arnaud JP, Eloy R, Adloff M, Grenier JF. Critical evaluation of prosthetic materials in repair of abdominal wall hernias: new criteria of tolerance and resistance. Am J Surg. 1977;133(3):338–345. doi: 10.1016/0002-9610(77)90542-6. [DOI] [PubMed] [Google Scholar]
- 5.Cayan S, Chermansky C, Schlote N, Sekido N, Nunes L, Dahiya R, et al. The bladder acellular matrix graft in a rat chemical cystitis model: functional and histologic evaluation. J Urol. 2002;168(2):798–804. doi: 10.1016/s0022-5347(05)64746-5. [DOI] [PubMed] [Google Scholar]
- 6.Greca FH, de Paula JB, Biondo-Simões ML, da Costa FD, da Silva AP, Time S, Mansur A. The influence of differing pore sizes on the biocompatibility of two polypropylene meshes in the repair of abdominal defects. Experimental study in dogs. Hernia. 2001;5(2):59–64. doi: 10.1007/s100290100001. [DOI] [PubMed] [Google Scholar]
- 7.National Research Council (US) Institute for Laboratory Animal Research . Guidance for the Description of Animal Research in Scientific Publications. Washington: The National Academies Press; 2011. pp. 1–26. [PubMed] [Google Scholar]
- 8.Senior K. Intestinal collagen shows promise as a small-vessel graft. Lancet. 1999;354(9189):1533. [Google Scholar]
- 9.Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, et al. American Society of Echocardiography American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17(10):1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Cosmo S, Francisco JC, Cunha RC, Macedo RM, Faria-Neto JR, Simeoni R, et al. Effect of exercise associated with stem cell transplantation on ventricular function in rats after acute myocardial infarction. Rev Bras Cir Cardiovasc. 2012;27(4):542–551. doi: 10.5935/1678-9741.20120096. [DOI] [PubMed] [Google Scholar]
- 11.Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47(1):74–80. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 12.Chiu LL, Radisic M. Cardiac tissue engineering. Curr Opin Chem Eng. 2013;2(1):41–52. [Google Scholar]
- 13.Seif-Naraghi SB, Salvatore MA, Schup-Magoffin PJ, Hu DP, Christman KL. Design and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineering. Tissue Eng Part A. 2010;16(6):2017–2027. doi: 10.1089/ten.tea.2009.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singelyn JM, DeQuach JA, Christman KL. Injectable myocardial matrix as a scaffold for myocardial tissue engineering. Conf Proc IEEE Eng Med Biol Soc. 2009:2406–2408. doi: 10.1109/IEMBS.2009.5334839. [DOI] [PubMed] [Google Scholar]
- 15.Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361(9351):45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 16.Pope 4th JC, Davis MM, Smith ER, Jr., Walsh MJ, Ellison PK, Rink RC, et al. The ontogeny of canine small intestinal submucosa regenerated bladder. Pt 2J Urol. 1997;158(3):1105–1110. doi: 10.1097/00005392-199709000-00106. [DOI] [PubMed] [Google Scholar]
- 17.Nakamuta JS, Danoviz ME, Marques FL, dos Santos L, Becker C, Goncalves GA, et al. Cell therapy attenuates cardiac dysfunction post myocardial infarction: effect of timing, routes of injection and a fibrin scaffold. PLoS One. 2009;4(6):e6005. doi: 10.1371/journal.pone.0006005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer EM, Beilfuss BA, Nagai T, Semnani RT, Badylak SF, van Seventer GA. Human helper T cell activation and differentiation is suppressed by porcine small intestinal submucosa. Tissue Eng. 2002;8(5):893–900. doi: 10.1089/10763270260424259. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Wang GY, Xiao YP, Fan LY, Wang Q. The biomechanical behavior and host response to porcine-derived small intestine submucosa, pericardium and dermal matrix acellular grafts in a rat abdominal defect model. Biomaterials. 2011;32(29):7086–7095. doi: 10.1016/j.biomaterials.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Badylak S, Obermiller J, Geddes L, Matheny R. Extracellular matrix for myocardial repair. Heart Surg Forum. 2003;6(2):E20–E26. doi: 10.1532/hsf.917. [DOI] [PubMed] [Google Scholar]
- 21.Lionetti V, Recchia FA. New therapies for the failing heart: transgenes versus trans-cells. Transl Res. 2010;156(3):130–135. doi: 10.1016/j.trsl.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Bel A, Messas E, Agbulut O, Richard P, Samuel JL, Bruneval P, et al. Transplantation of autologous fresh bone marrow into infarcted myocardium: a word of caution. Circulation. 2003;108(Suppl 1):II247–II252. doi: 10.1161/01.cir.0000089040.11131.d4. [DOI] [PubMed] [Google Scholar]
- 23.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7(4):430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 24.Scorsin M, Souza LC. Cellular transplantation for the treatment of heart failure. State of the art. Arq Bras Cardiol. 2001;77(2):103–106. doi: 10.1590/s0066-782x2001000800001. [DOI] [PubMed] [Google Scholar]
- 25.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73(6):1919–1925. doi: 10.1016/s0003-4975(02)03517-8. [DOI] [PubMed] [Google Scholar]
- 26.Hata H, Bär A, Dorfman S, Vukadinovic Z, Sawa Y, Haverich A, et al. Engineering a novel three-dimensional contractile myocardial patch with cell sheets and decellularised matrix. Eur J Cardiothorac Surg. 2010;38(4):450–455. doi: 10.1016/j.ejcts.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Dar A, Shachar M, Leor J, Cohen S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol Bioeng. 2002;80(3):305–312. doi: 10.1002/bit.10372. [DOI] [PubMed] [Google Scholar]
- 28.Matsubayashi K, Fedak PW, Mickle DA, Weisel RD, Ozawa T, Li RK. Improved left ventricular aneurysm repair with bioengineered vascular smooth muscle grafts. Circulation. 2003;108(Suppl 1):II219–II225. doi: 10.1161/01.cir.0000087450.34497.9a. [DOI] [PubMed] [Google Scholar]
- 29.Souza LCG, Carvalho RG, Pouzet B, Vilquin JT, Garcin I, Menasché P, et al. The transplant of cardiac cells and myoblast skeletal cells in myocardial infarction. Rev Bras Cir Cardiovasc. 2002;17(4):312–322. [Google Scholar]
- 30.Hsu CC, Chen YW, Hao CL, Chong JT, Lee CI, Tan HT, et al. Comparison of automated 4D-MSPECT and visual analysis for evaluating myocardial perfusion in coronary artery disease. Kaohsiung J Med Sci. 2008;24(9):445–452. doi: 10.1016/S1607-551X(09)70001-4. [DOI] [PubMed] [Google Scholar]