Abstract
The calcium paradox was first mentioned in 1966 by Zimmerman et al. Thereafter gained great interest from the scientific community due to the fact of the absence of calcium ions in heart muscle cells produce damage similar to ischemia-reperfusion. Although not all known mechanisms involved in cellular injury in the calcium paradox intercellular connection maintained only by nexus seems to have a key role in cellular fragmentation. The addition of small concentrations of calcium, calcium channel blockers, and hyponatraemia hypothermia are important to prevent any cellular damage during reperfusion solutions with physiological concentration of calcium.
Keywords: Heart Arrest, induced; Myocardial Ischemia; Calcium
Abstract
O paradoxo do cálcio foi pela primeira vez citado em 1966 por Zimmerman et al. A partir daí, ganhou grande interesse por parte da comunidade científica internacional devido ao fato da ausência do íon cálcio produzir na célula muscular cardíaca dano semelhante à lesão de isquemia-reperfusão. Apesar de não serem conhecidos todos os mecanismos envolvidos no processo da lesão celular no paradoxo do cálcio, a conexão intercelular mantida somente pelo nexus parece ter papel chave na fragmentação celular. A adição de pequenas concentrações de cálcio, bloqueadores de canal de cálcio, hiponatremia ou hipotermia são importantes para evitar que haja lesão celular no momento da reperfusão com soluções com concentração fisiológica de cálcio.
INTRODUCTION
In 1960 Zimmerman et al.[1,2] described massive lysis of cardiomyocytes after administration of cardioplegia solution without calcium followed by reperfusion with saline solution with calcium physiological concentration in isolated rat heart. This event was called "calcium paradox".
Unlike what would be expected, the complete absence of calcium not only caused the cardiac arrest, but also altered the cell membranes of cardiac myocytes, culminating in the reperfusion phase with their necrosis, explaining the term "paradox"[1].
In the following years many researchers have studied possible physiological mechanisms of paradox, culminating with a significant amount of studies on the subject, many being presented in 1983 at the IX World Congress of the Society for Heart Research, held in London[3].
In this event was compiled much of what we knew at the time about the lack of calcium in cardioplegic solution, the extensive myocardial damage that this solution causes and alternative ways for developing a safe hypocalcemic cardioplegia[4-6].
After nearly 50 years of the discovery of this paradox, this study aims to discuss some harmful effects of calcium paradox in the heart, considering its importance, molecular mechanisms, cellular ultrastructural changes, additive protective or harmful effect when placed in combination with other solutions and some ways to avoid it.
Importance of calcium paradox
In the 1980s, the calcium metabolism in the heart has been extensively studied. At that time, there was consensus on the consequences of the succession of a medium without calcium followed by another filled of it to heart muscle cells, which rapidly internalizes this ion, leading it to lysis and heart failure. This phenomenon is similar to reperfusion injury[7]. Another key aspect is the understanding of mechanisms involved, as cardioplegic solutions should not cause cellular damage. Hypocalcemic cardioplegic solutions are effective to induce cardiac arrest[8]. However, substances that interrupt or mitigate undesirable side effects must be present to prevent ventricular dysfunction after cardiopulmonary bypass [7].
Studies on metabolic pathways that promote or disrupt the process[9,10], as well as their relationship to heart failure[11,12]have been published, which we will discuss briefly below.
Causal mechanism
Several hypotheses were formulated to explain the calcium paradox as increased permeability of calcium in the sarcolemma[13], the glycocalyx[14] and separation of intercalated discs[15,16], but no further clarified the whole mechanism of the calcium paradox.
It is also possible that intracellular hypercalcemia is not the primary cause of the calcium paradox. Its increase may occur as a result of damage to sarcolemma accompanied by an entry of moderate amount of calcium to structurally altered[17] cells.
Isolated absence of calcium can cause cell damage, but its deleterious effect is potentiated in media with anoxia, caffeine, 2,4-dinitrophenol (DNF), ventricular balloon (mechanical strain), etc.. All these conditions cause injury to the myocardium even in the absence of extracellular calcium[18-22]. Another mechanism accepted is that calcium comes into the cell in a massive way, causing damage and cell death[23].
Structural cellular changes
The first description of structural changes in myocyte perfused in calcium-free medium was performed by Muir et al.[16], who observed changes in glycocalyx and intercalated disks of myocytes in isolated rat hearts. Intercalated disks are complex structures divided into several regions, the major one being occupied by adherens fascia. These are the places of greatest tension between the cells at the time of myocardial contraction. Desmosomal junctions, called macula adherens, are present and serve to unite the cells. Nexus or gap junctions are focal points of intimate cell contact, being local with electrical signaling between cells[24].
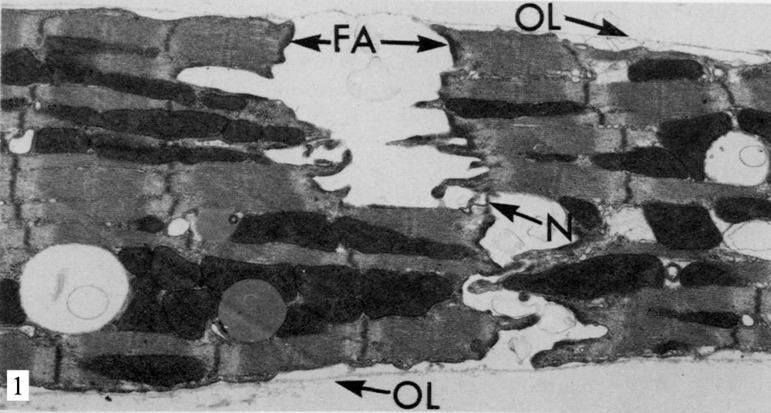
Muir et al.[16] noted that cardiac myocytes which underwent perfusion showed no calcium from 30 minutes of clear separation in regions of the fascia adherens and macula adherens, while the nexus remained intact (Figure 1). Ashraf[13] and Yates & Dhalla[25] observed similar changes in 10 to 15 minutes of exposure to the same environment. Shorter periodes of 3-5 minutes generally do not cause physical separation in the ultrastructure of the intercalated discs[23].
Fig. 1.
Electron micrograph of rat heart after 12 minutes of perfusion without calcium at 37ºC. The intercalated disks are separated in the regions of the fascia adherens (FA), but are still interconnected by the nexus joints (N). The outer layer of the sarcolemma (OL) or glycocalyx is detached from the plasma membrane of the myocytes. Reproduced from Ganote et al., 1985[23]
The calcium-free medium increases the amount of intracellular sodium both in cultured myocytes and in isolated heart. Moving to a medium rich in calcium, this ion rapidly enters cells via antiporter pump Na+/Ca+2, working on a reverse way. The similarities end here, as there is contraction of myocytes in culture, but not cytolysis, whereas in the isolated heart we found mass lysis of the cells. This phenomenon was described as intolerance to calcium[26-28].
Lysis at the time the myocyte contacts reperfusion solution rich in calcium after sensitization in medium without this ion occurs because these cells have intermediate disc only connecting to one another, and there is their avulsion during contraction with exposure of the intracellular medium of each of them explaining the massive cell death of them[16].
Another change that occurs during infusion without calcium is the detachment of glycocalyx, which is usually a focal shift that is not seen until 10 to 15 minutes after infusion of the solution without calcium. Frank et al.[29,30] have shown that despite the detachment of the external layer of glycocalyx, there is an inner membrane that is adhered on the cell membrane.
Ashaf et al.[13] and Frank et al.[29] observed aggregation and anomalous rearrangement of the constituent molecules of the cell membrane when placed in calcium free medium, so that there is irreversible cell damage due to altered membrane permeability. The molecular mechanisms of these changes are not yet known.
Phase of re-entry of calcium into the cell
After 10-15 minutes in medium deprived of calcium, the myocyte is sensitized, and the separation of the intercalated discs between cells is established[23]. The sarcomeres of each cell condense into a single band of contraction. The fascia adherens remain connected to sarcomeres, but are completely separate from the membranes of adjacent cells. Regions of intercalated discs, located between the areas of fascia adherens become fragmented and allow the mitochondria to go into the intercellular space [31]. Ganote et al.[19] mention that hypothermia prevents lysis of the fascia adherens and therefore cytolysis.
The contractions of the sarcomere and cell necrosis are identical to those observed in other types of injury as catecholamine necrosis and ischemia/reperfusion injury. However, it should be emphasized that the cellular ultrastructure when in medium without calcium differs from all previous separation of the intermediate disks and the presence of a single central shrink band[23].
Traces of calcium
Rebeyka et al.[32]found in CPB model that dog hearts perfused with cold cardioplegic solution without calcium showed a worse recovery of ventricular function and greater area of necrosis than those in which the solution was only 70 μmol/L calcium, showing that even small concentrations of calcium are sufficient to protect the heart of calcium paradox.
Glycocalyx
The separation of the external layer of the cell membrane glycocalyx from myocyte occurs after exposure of the cell to calcium-poor medium[23]. Frank et al.[14] believed that this separation would be responsible for the increase in the membrane calcium permeability. However, Nayler et al. [17] using 2 mM calcium instead of magnesium in the private period of calcium showed that despite a detachment of the glycocalyx, there was no increase in membrane permeability to calcium and Slade et al.[33] have also observed that myocytes placed in buffer medium without calcium also lose glycocalyx, without, however, observing a change from the influx of calcium ion.
With the use of neuraminidase there is complete separation of the glycocalyx of myocytes with increased cell permeability to calcium. To explain this phenomenon, Ganote et al.[23] postulate that in this case the membrane glycoproteins would also be damaged, losing control of the calcium flow.
ATP
Ruigrok et al.[34] showed that the massive release of enzymes which occurs during reperfusion of calcium is dependent on energy. This conclusion is based on experiments that consumed the intracellular ATP from myocyte with anoxic perfusion or the inclusion of this cell in medium without glucose. The non-cardiac cells did not release enzymes during phase of normal calcium after 5 minutes of perfusion without calcium due to depletion of ATP[34].
Calcium channel blockers
Baker & Hearse[35] observed that the effect of calcium channel blockers is best demonstrated when there is low extracellular calcium concentration during reperfusion. Under these conditions, calcium entry occurs preferentially through the slow channels of the membrane. The limitation of this entry enables the recovery from injury of intercalated discs and sarcolemma. In solutions with physiological concentration of calcium in the reperfusion solution, the calcium channel blockers offer little protection to the paradox, suggesting that more than one route is important for the entry of calcium into the cell[36].
Sodium
Dhalla et al.[37] showed that when the concentration of sodium is reduced to 35 mm in phase without calcium, the magnitude of tissue damage is reduced during reperfusion with calcium. This is due to the low concentration of sodium, which slows the entry of calcium into the membrane by antiport pump Na+/Ca+2, facilitating intracellular ionic rebalancing and preventing contracture that would lead to cell death[37].
During the period without calcium, low sodium concentration reduces the transmembrane gradient and delays the calcium efflux and sodium influx. This would slow down the removal of both intracellular calcium and the cell damage caused by the absence of this ion. In the period with normal calcium low sodium would also be beneficial because it reduces calcium influx via antiporter pump Na+/Ca+2, working in a reverse way. The slow elimination of calcium when the cell is without it and slower internalization during reperfusion provides the cell conditions to reestablish its ionic balance before any structural damage[37].
Hypothermia
Hypothermia protects the myocyte from calcium paradox[38,39]. It prevents the separation of the intercalated disc and detachment the glycocalyx[40]. In addition, it reduces the Na+/Ca+2, and may decrease the loss of Ca+2 ions in calcium-free perfusion time[41]. The ideal temperature found for myocardial protection of calcium paradox was 22ºC[42-44].
Mechanical injury
Ganote et al.[20] states that when isolated rat hearts are placed in anoxia in medium with physiological concentration of calcium, the distension of the volumetric balloon in the left ventricle (LV) occurs with a small increase resulting from enzymatic cell lysis, but the LV distension is difficult. When the medium is free of calcium and normoxia, distension is easy and enzyme release is also small. However when we have anoxia and calcium-free medium, there is massive release of cellular enzymes.
This is because anoxic hearts can withstand the wall tension that the balloon prints due to the intercalated disks were incomplete. The cavity is distended by elongation of the sarcomeres, with their lesion. In calcium-free medium and normoxia, the muscle fibers are now relaxed and the voltage produced by the balloon is not sufficient to cause avulsion of weakened intercalated disks. But when anoxia and medium without calcium are superimposed, the cell maintains the stiffness of the sarcomeres to the fragility of the intercalated disks. The pressure of the inflated balloon adversely affects directly the region, causing release of intracellular enzymes[20].
Dinitrophenol
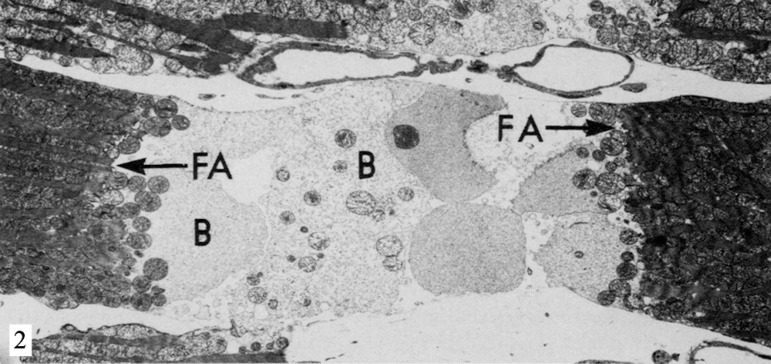
The dinitrophenol (DNP) is a fat-soluble weak acid that acts as protonophore (translocator protons) entering the mitochondria positively charged and leaves it negatively charged, creating electron transport out of the mitochondria, preventing the conversion of ADP to ATP[45]. It also causes rapid ventricular contraction both in hearts which underwent calcium-free medium as in those with normal concentration of this ion. However, those in medium without calcium causes massive cell lysis[22] (Figure 2).
Fig. 2.
Electron micrograph of rat heart after 5 minutes in calciumfree perfusion, followed by 15 minutes with the addition of DNP at the first perfusate. The sarcomeres are contracted herein, pulling the fascia adherens (FA) and causing damage to the cell membrane of the myocyte in the nexus area. The cytosol is exteriorized in the form of blebs (B) in the intercellular region. Reproduced from Ganote et al., 1985[23]
This observation is consistent with the hypothesis that contraction physically separates the cells, causing cell lysis in those in free-calcium medium. The DNP alone causes contraction of cells, and it is not necessary to add calcium to the medium. Intracellular calcium present in mitochondria and sarcolemma would not be sufficient to generate environment that simulates medium with normal calcium[22].
Caffeine
Caffeine causes the sarcolemma calcium release but not in mitochondria[46]. Thus, intracellular calcium increases slightly, but without its overhead[31]. The persistent contraction produced by caffeine is dependent on calcium and in its absence there is support for only 20 to 30 seconds, followed by relaxation[18].
Hearts perfused with solution containing caffeine, but without calcium at 22ºC do not manifest increased enzymes, but those kept at 37ºC have similar injury to the calcium paradox[18].
Whereas the increase in intracellular calcium concentration is not significant because there was no reperfusion with calcium, it is unlikely that the lesion is originally from poisoning by calcium, but by direct action of ventricular contraction on sarcolemma[18].
CONCLUSION
We should fear the phenomenon known as "calcium paradox" because it irreversibly damages the membrane of the myocyte, causing extrusion of cellular contents. However, despite its biomolecular mechanisms are not fully understood, measures such as hypothermia, hyponatremia, and the presence of traces of calcium in the perfusion solution decreases the risk of this injury, enabling the recovery of ventricular function after induced cardiac arrest.
| Abbreviations, acronyms & symbols | |
|---|---|
| DNP | Dinitrophenol |
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| LV | Left ventricle |
| Authors’ roles & responsibilities | |
|---|---|
| MABO | Main Author |
| ACB | Help in bibliographic survey and translated articles |
| CAS | Help in literature review and translation of articles |
| PHHB | Help in correcting the manuscript |
| JLLC | Help in correcting the manuscript |
| GG | Co-supervisor |
| DMB | Advisor |
Footnotes
This study was carried out at São José do Rio Preto Medical School (FAMERP), São José do Rio Preto, SP, Brazil.
No financial support.
REFERENCES
- 1.Zimmerman AN, Hulsmann WC. Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature. 1966;211(5049):646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman ANE, Daems W, Hülsmann WC, Snijder J, Wisse E, Durrer D. Morphological changes of heart muscle caused by successive perfusion with calcium-free and calcium-containing solutions (calcium paradox) Cardiovasc Res. 1967;1(3):201–209. doi: 10.1016/s0008-6363(99)00303-x. [DOI] [PubMed] [Google Scholar]
- 3.Poole-Wilson PA, Nayler WG. Preface. J Mol Cell Cardiol. 1984;16(2):111. [Google Scholar]
- 4.Poole-Wilson PA, Harding DP, Bourdillon PD, Tones MA. Calcium out of control. J Mol Cell Cardiol. 1984;16(2):175–187. doi: 10.1016/s0022-2828(84)80706-3. [DOI] [PubMed] [Google Scholar]
- 5.Nayler WG, Dresel PE. Ca2+ and the sarcoplasmic reticulum. J Mol Cell Cardiol. 1984;16(2):165–174. doi: 10.1016/s0022-2828(84)80705-1. [DOI] [PubMed] [Google Scholar]
- 6.Langer GA. Calcium at the sarcolemma. J Mol Cell Cardiol. 1984;16(2):147–153. doi: 10.1016/s0022-2828(84)80703-8. [DOI] [PubMed] [Google Scholar]
- 7.Piper HM. The calcium paradox revisited: an artefact of great heuristic value. Cardiovasc Res. 2000;45(1):123–127. doi: 10.1016/s0008-6363(99)00304-1. [DOI] [PubMed] [Google Scholar]
- 8.Gebhard MM, Bretschneider HJ, Gersing E, Preusse CJ, Schnabel PA, Ulbricht LJ. Calcium-free cardioplegia--pro. Eur Heart J. 1983;4(Suppl H):151–160. doi: 10.1093/eurheartj/4.suppl_h.151. [DOI] [PubMed] [Google Scholar]
- 9.Bi SH, Jin ZX, Zhang JY, Chen T, Zhang SL, Yang Y, et al. Calpain inhibitor MDL 28170 protects against the Ca2+ paradox in rat hearts. Clin Exp Pharmacol Physiol. 2012;39(4):385–392. doi: 10.1111/j.1440-1681.2012.05683.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang JY, Tong W, Wu F, Bi SH, Xu M, Jin ZX, et al. Different roles for contracture and calpain in calcium paradox-induced heart injury. PLoS One. 2012;7(12):e52270. doi: 10.1371/journal.pone.0052270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenzel S, Tastan I, Abdallah Y, Schreckenberg R, Schluter KD. Aldosterone improves contractile function of adult rat ventricular cardiomyocytes in a non-acute way: potential relationship to the calcium paradox of aldosteronism. Basic Res Cardiol. 2010;105(2):247–256. doi: 10.1007/s00395-009-0059-6. [DOI] [PubMed] [Google Scholar]
- 12.Kass RS, Lindegger N, Hagen B, Lederer WJ. Another calcium paradox in heart failure. J Mol Cell Cardiol. 2008;45(1):28–31. doi: 10.1016/j.yjmcc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Ashraf M. Correlative studies on sarcolemmal ultrastructure, permeability, and loss of intracellular enzymes in the isolated heart perfused with calcium-free medium. Am J Pathol. 1979;97(2):411–432. [PMC free article] [PubMed] [Google Scholar]
- 14.Frank JS, Rich TL, Beydler S, Kreman M. Calcium depletion in rabbit myocardium. Ultrastructure of the sarcolemma and correlation with the calcium paradox. Circ Res. 1982;51(2):117–130. doi: 10.1161/01.res.51.2.117. [DOI] [PubMed] [Google Scholar]
- 15.Vander Heide RS, Ganote CE. Caffeine-induced myocardial injury in calcium-free perfused rat hearts. Am J Pathol. 1985;118(1):55–65. [PMC free article] [PubMed] [Google Scholar]
- 16.Muir AR. The effects of divalent cations on the ultrastructure of the perfused rat heart. Pt 2J Anat. 1967;101:239–261. [PMC free article] [PubMed] [Google Scholar]
- 17.Nayler WG, Elz JS, Perry SE, Daly MJ. The biochemistry of uncontrolled calcium entry. Eur Heart J. 1983;4(Suppl H):29–41. doi: 10.1093/eurheartj/4.suppl_h.29. [DOI] [PubMed] [Google Scholar]
- 18.Ganote CE, Sims MA, VanderHeide RS. Mechanism of enzyme release in the calcium paradox. Eur Heart J. 1983;4(Suppl H):63–71. doi: 10.1093/eurheartj/4.suppl_h.63. [DOI] [PubMed] [Google Scholar]
- 19.Ganote CE, Sims MA. Parallel temperature dependence of contracture-associated enzyme release due to anoxia, 2,4-dinitrophenol (DNP), or caffeine and the calcium paradox. Am J Pathol. 1984;116(1):94–106. [PMC free article] [PubMed] [Google Scholar]
- 20.Ganote CE, Sims MA. Physical stress-mediated enzyme release from calcium-deficient hearts. J Mol Cell Cardiol. 1983;15(7):421–429. doi: 10.1016/0022-2828(83)90262-6. [DOI] [PubMed] [Google Scholar]
- 21.Ganote CE, Liu SY, Safavi S, Kaltenbach JP. Anoxia, calcium and contracture as mediators of myocardial enzyme release. J Mol Cell Cardiol. 1981;13(1):93–106. doi: 10.1016/0022-2828(81)90231-5. [DOI] [PubMed] [Google Scholar]
- 22.Ganote CE, Grinwald PM, Nayler WG. 2,4-Dinitrophenol (DNP)-induced injury in calcium-free hearts. J Mol Cell Cardiol. 1984;16(6):547–557. doi: 10.1016/s0022-2828(84)80641-0. [DOI] [PubMed] [Google Scholar]
- 23.Ganote CE, Nayler WG. Contracture and the calcium paradox. J Mol Cell Cardiol. 1985;17(8):733–745. doi: 10.1016/s0022-2828(85)80035-3. [DOI] [PubMed] [Google Scholar]
- 24.De Mello WC. Intercellular communication in cardiac muscle. Circ Res. 1982;51(1):1–9. doi: 10.1161/01.res.51.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Yates JC, Dhalla NS. Structural and functional changes associated with failure and recovery of hearts after perfusion with Ca2+-free medium. J Mol Cell Cardiol. 1975;7(2):91–103. doi: 10.1016/0022-2828(75)90011-5. [DOI] [PubMed] [Google Scholar]
- 26.Altschuld R, Gibb L, Ansel A, Hohl C, Kruger FA, Brierley GP. Calcium tolerance of isolated rat heart cells. J Mol Cell Cardiol. 1980;12(12):1383–1395. doi: 10.1016/0022-2828(80)90123-6. [DOI] [PubMed] [Google Scholar]
- 27.Haworth RA, Hunter DR, Berkoff HA. The isolation of Ca2+-resistant myocytes from the adult rat. J Mol Cell Cardiol. 1980;12(7):715–723. doi: 10.1016/0022-2828(80)90101-7. [DOI] [PubMed] [Google Scholar]
- 28.Haworth RA, Hunter DR, Berkoff HA. Mechanism of Ca2+ resistance in adult heart cells isolated with trypsin plus Ca2+ J Mol Cell Cardiol. 1982;14(9):523–530. doi: 10.1016/0022-2828(82)90213-9. [DOI] [PubMed] [Google Scholar]
- 29.Frank JS. Ca depletion of the sarcolemma--ultrastructural changes. Eur Heart J. 1983;4(Suppl H):23–27. doi: 10.1093/eurheartj/4.suppl_h.23. [DOI] [PubMed] [Google Scholar]
- 30.Frank JS, Langer GA, Nudd LM, Seraydarian K. The myocardial cell surface, its histochemistry, and the effect of sialic acid and calcium removal on its structure and cellular ionic exchange. Circ Res. 1977;41(5):702–714. doi: 10.1161/01.res.41.5.702. [DOI] [PubMed] [Google Scholar]
- 31.Hunter DR, Haworth RA, Berkoff HA. Cellular calcium turnover in the perfused rat heart: modulation by caffeine and procaine. Circ Res. 1982;51(3):363–370. doi: 10.1161/01.res.51.3.363. [DOI] [PubMed] [Google Scholar]
- 32.Rebeyka IM, Axford-Gatley RA, Bush BG, del Nido PJ, Mickle DA, Romaschin AD, et al. Calcium paradox in an in vivo model of multidose cardioplegia and moderate hypothermia. Prevention with diltiazem or trace calcium levels. J Thorac Cardiovasc Surg. 1990;99(3):475–483. [PubMed] [Google Scholar]
- 33.Slade AM, Severs NJ, Powell T, Twist VW. Isolated calcium-tolerant myocytes and the calcium paradox: an ultrastructural comparison. Eur Heart J. 1983;4(Suppl H):113–122. doi: 10.1093/eurheartj/4.suppl_h.113. [DOI] [PubMed] [Google Scholar]
- 34.Ruigrok TJ, Boink AB, Spies F, Blok FJ, Maas AH, Zimmerman AN. Energy dependence of the calcium paradox. J Mol Cell Cardiol. 1978;10(11):991–1002. doi: 10.1016/0022-2828(78)90395-4. [DOI] [PubMed] [Google Scholar]
- 35.Baker JE, Hearse DJ. Slow calcium channel blockers and the calcium paradox: comparative studies in the rat with seven drugs. J Mol Cell Cardiol. 1983;15(7):475–485. doi: 10.1016/0022-2828(83)90266-3. [DOI] [PubMed] [Google Scholar]
- 36.Nayler WG, Perry SE, Elz JS, Daly MJ. Calcium, sodium, and the calcium paradox. Circ Res. 1984;55(2):227–237. doi: 10.1161/01.res.55.2.227. [DOI] [PubMed] [Google Scholar]
- 37.Dhalla NS, Alto LE, Singal PK. Role of Na+-Ca2+ exchange in the development of cardiac abnormalities due to calcium paradox. Eur Heart J. 1983;4(Suppl H):51–56. doi: 10.1093/eurheartj/4.suppl_h.51. [DOI] [PubMed] [Google Scholar]
- 38.Baker JE, Bullock GR, Hearse DJ. The temperature dependence of the calcium paradox: enzymatic, functional and morphological correlates of cellular injury. J Mol Cell Cardiol. 1983;15(6):393–411. doi: 10.1016/0022-2828(83)90323-1. [DOI] [PubMed] [Google Scholar]
- 39.Holland CE, Jr., Olson RE. Prevention by hypothermia of paradoxical calcium necrosis in cardiac muscle. J Mol Cell Cardiol. 1975;7(12):917–928. doi: 10.1016/0022-2828(75)90152-2. [DOI] [PubMed] [Google Scholar]
- 40.Rich TL, Langer GA. Calcium depletion in rabbit myocardium. Calcium paradox protection by hypothermia and cation substitution. Circ Res. 1982;51(2):131–141. doi: 10.1161/01.res.51.2.131. [DOI] [PubMed] [Google Scholar]
- 41.Reuter H, Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boink AB, Ruigrok TJ, de Moes D, Maas AH, Zimmerman AN. The effect of hypothermia on the occurrence of the calcium paradox. Pflugers Arch. 1980;385(2):105–109. doi: 10.1007/BF00588688. [DOI] [PubMed] [Google Scholar]
- 43.Hearse DJ, Humphrey SM, Bullock GR. The oxygen paradox and the calcium paradox: two facets of the same problem? J Mol Cell Cardiol. 1978;10(7):641–668. doi: 10.1016/s0022-2828(78)80004-2. [DOI] [PubMed] [Google Scholar]
- 44.Bulkley BH, Nunnally RL, Hollis DP. "Calcium paradox" and the effect of varied temperature on its development: a phosphorus nuclear magnetic resonance and morphologic study. Lab Invest. 1978;39:133–140. [PubMed] [Google Scholar]
- 45.Harper JA, Dickinson K, Brand MD. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes Rev. 2001;2(4):255–265. doi: 10.1046/j.1467-789x.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 46.Blayney L, Thomas H, Muir J, Henderson A. Action of caffeine on calcium transport by isolated fractions of myofibrils, mitochondria, and sarcoplasmic reticulum from rabbit heart. Circ Res. 1978;43(4):520–526. doi: 10.1161/01.res.43.4.520. [DOI] [PubMed] [Google Scholar]