Abstract
Objective
To review studies performed in animal models that evaluated therapeutic interventions to inflammatory response and microcirculatory changes after cardiopulmonary bypass.
Methods
It was used the search strategy ("Cardiopulmonary Bypass" (MeSH)) and ("Microcirculation" (MeSH) or "Inflammation" (MeSH) or "Inflammation Mediators" (MeSH)). Repeated results, human studies, non-English language articles, reviews and studies without control were excluded.
Results
Blood filters, system miniaturization, specific primers regional perfusion, adequate flow and temperature and pharmacological therapies with anticoagulants, vasoactive drugs and anti-inflammatories reduced changes in microcirculation and inflammatory response.
Conclusion
Demonstrated efficacy in animal models establishes a perspective for evaluating these interventions in clinical practice.
Keywords: Extracorporeal Circulation; Inflammation; Models, Animal; Microcirculation
Abstract
Objetivo
Revisar estudos realizados em modelos animais avaliando intervenções terapêuticas e resposta inflamatória e alterações da microcirculação após instalação de circulação extracorpórea.
Métodos
Utilizada a estratégia de busca ("Cardiopulmonary Bypass"(MeSH)) AND ("Microcirculation"(MeSH) OR "Inflammation"(MeSH) OR "Inflammation Mediators"(MeSH)). Resultados repetidos, estudos humanos, artigos em língua não inglesa, revisões e estudos sem controle foram excluídos.
Resultados
Filtros sanguíneos, miniaturização do sistema, perfusatos específicos, perfusão regional, fluxo e temperatura adequados e terapias farmacológicas com fármacos anticoagulantes, vasoativos e anti-inflamatórios reduziram alterações em microcirculação e resposta inflamatória.
Conclusão
A eficácia demonstrada em modelos animais estabelece uma perspectiva para avaliação dessas intervenções na prática clínica.
INTRODUCTION
The history of cardiovascular surgery began concretely only in the 1940s, with some procedures that could be performed without cardiopulmonary bypass (CPB). Complex cardiac conditions, however, could not be corrected with the heart beating, or even stopped, during the short time provided by hypothermia. Thus, many surgeons began attempts to put into operation a machine that was able to replace the patient heart and lungs during surgery, allowing more prolonged handling of the arrested heart [1].
After a series of unsuccessful attempts of many scholars, Gibbon successfully performed in 1953, the first cardiac surgery under CPB [2]. Since then, the CPB has become perhaps the most important component of modern cardiac surgery. In these nearly 60 years, the technique has undergone several improvements, coming to current models, which, in essence, does not much differ from the initially proposed by Gibbon. The CPB devices currently consist in a circuit with a pump, which may be centrifugal or roller, an oxygenator, usually membrane, cannulas, tubes and a cardioplegia circuit.
Despite the significant changes and improvements in CPB systems, complications related to tissue damage affecting postoperative morbidity and mortality still persist [3]. The CPB exposes the body to a series of non-physiological conditions, leading to complex changes in normal physiology of the circulatory system. The contact of blood with the artificial surface of the circuit, the phenomenon of ischemia-reperfusion, tissue hypoperfusion and hemolysis may initiate and exacerbate the inflammatory response. CPB induces both humoral and the cellular constituent of the immune system, leading to changes that, at first, are manifested by an exaggerated inflammatory response, but which then lead to a temporary immunodeficiency presentation.
The development of strategies to control the damage caused by CPB in the body is therefore essential in order to reduce these complications and has been the focus of several experimental research and clinical studies. The therapeutic possibilities targeting to reduce the inflammatory process are based on two main pillars: non-pharmacological interventions in the CPB system and administration of drugs in the peri- or intraoperative period.
Although clinical trials may indicate possible routes and general aspects of aggression caused by CPB in the body, new therapies may not be initially assessed by means of this type of study, since they need to be tested on animals to ensure their safety and only then be investigated in clinical studies. Thus, animal models are essential for developing strategies against the inflammatory response to CPB. Furthermore, the use of animal models allows analysis in which the only variable of the study is the studied intervention, since the animals used can be genetically identical, thus reducing possible biases.
The aim of this literature review is therefore to understand how animal models have been used to study the inflammatory response and changes in microcirculation after CPB and possible control strategies, presenting results and prospects for improving CPB systems in its clinical use.
METHODS
Search Strategy
For this review, search in the PubMed databases (MEDLINE, scientific journals and online books) was performed, being used terms from the "Medical Subject Headings" (MeSH), comprising terms controlled by the "National Library of Medicine" of the United States and that is used for indexing articles in PubMed.
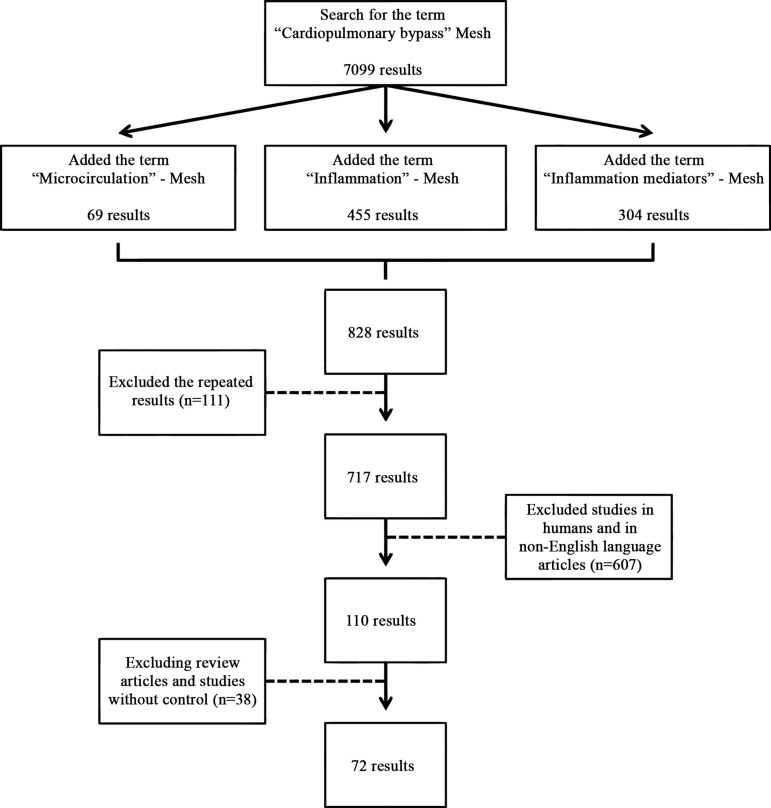
Initially, the term ("Cardiopulmonary Bypass" (MeSH)) was used, and from this search the results published in the last ten years, until May 2012 were selected, yielding 7,099 articles. Subsequently, in addition to the aforementioned term, the search was restricted to results that also contained the terms ("Microcirculation"(MeSH)) or ("Inflammation"(MeSH)) or ("Inflammation Mediators"(MeSH)), being found respectively 69, 455 and 304 results totaling 828. Of these, repeated results were excluded, leading to a total of 717 articles.
Excluding items that were not in English or did not represent experimental studies in animals, 110 results were obtained. These articles received a more detailed analysis, being excluded literature reviews and studies that did not have control group. Thus, in the end, 72 articles were selected for this review (Figure 1).
Fig. 1.
Chart showing the search strategy of the articles until reaching the final number
Studied data
The specific objectives of each study were raised and, after analysis, we determined the main thematic groups that would embrace such goals. In each study the main intervention (independent variable) and the main consequences of them (dependent variables) were highlighted. Thus, it was possible to compare, within each thematic group, the interventions and the results of the selected studies.
It was also determined the number of times that each dependent variable appeared, and for the analysis were selected those that were found in at least three studies. Thus, both for presentation of results and for the sake of argument, only the parameters showing certain frequency have been included.
RESULTS
The studies assessed in this review attempted to assess three main topics: 1) the effects of CPB on the inflammatory response and the microcirculation, 2) non-pharmacological interventions in the CPB system and 3) the administration of drugs in the peri- or intraoperative period.
Effects of CPB on inflammatory response and the microcirculation
Nine studies seek to better understand the consequences of the CPB in microcirculation and inflammatory response, and all showed induction of inflammatory response and microcirculatory changes by CPB. The amount of activated leukocytes adhered to tissues and water in the extracellular space proved to be increased in the groups where CPB was performed, showing, respectively, activation of the inflammatory cascade and increased vascular permeability [4-6]. Moreover, it was found a significant reduction in blood flow and arteriolar endothelium-dependent relaxation [4,5,7-9]. Furthermore, we found increased NF-κB expression and caspase-3 activity [10,11]. With respect to cytokines, both those typically inflammatory, such as TNF-α, IL-6, IL-8 and IL-1β, as anti- inflammatory, such as IL-10, presented increased [4,7,11,12].
Non-pharmacological interventions in the CPB system
Filtration
The use of four different types of filters in the CPB circuit was proposed in five of the studies assessed, with the aim of reducing the deleterious effects of CPB. Darling et al. [13] used zero balance ultrafiltration (Z-BUF), a technique that aims to fix the water balance generated by CPB through infusion of saline solution simultaneously to filtration, demonstrating increased arterial partial pressure of oxygen (PaO2), decreased tissue edema and reduced histologic injury. Alaoja et al. [14] studied the filtration of leukocytes, demonstrating reduction in activated leukocytes adhered to tissues.
The leukocyte depletion was also studied by Tao et al. [15], which showed good results on the inflammatory response and microcirculation, such as decreased neutrophils, IL-8, plasma elastase and myeloperoxidase, in addition to lower pulmonary vascular resistance. The third filter, studied by Ohki et al. [16], was the hemoperfusion with polymyxin B-immobilized cartridge (MPX), which acts as an endotoxin scavenger filter and has demonstrated increased tissue partial pressure of oxygen (PO2), reduction of tissue lesions and diminished IL-8. Finally, the use of the modified ultrafiltration (MUF) technique studied by Atkins et al. [17], in which it keeps filtering the blood for some time after the end of CPB, in addition to reduce pulmonary vascular resistance, reduced levels of IL-6 and IL-8, although it has not changed the values of TNF-α.
Flow
One of the concerns frequently raised by the use of CPB systems is the attempt to bring them closer to the maximum of the physiological functioning of the body, being the characterization of the blood flow a great representative of this concern. Two studies have attempted to assess the effects of different flow regimes on inflammatory response and microcirculation. Voss et al. [18] compared the plasma levels of IL-6 and IL-1 receptor between pulsatile and non-pulsatile flow regimes, but found no significant differences between these two patterns. Anttila et al. [19], in turn, sought to determine the lowest safe performance of CPB flow, assessing the potential adverse changes if CPB was performed under conventional blood flow, and demonstrated that the flow reductions also reduce tissue PO2. Schears et al. [20] showed that even in the presence of a persistent arterial duct, the more dimished the flow, the lower the tissue PO2.
Miniaturization
The miniaturization of CPB systems is very useful for pediatric cardiac surgery, especially in neonates and infants, allowing the reduction of the amount of priming and hence hemodilution. Two studies have attempted to assess the use of miniaturization of the CPB system in order to reduce the inflammatory response and changes in microcirculation. Schnoering et al. [21], using the MiniHLM, a miniaturized heart-lung machine created by the authors, found no significant differences compared to the control group. Ugaki et al. [22], on the other hand, showed a reduction in IL-8, thrombin-antithrombin complex, water content in the extracellular space and pulmonary vascular resistance with the use of Tiny- Pump, an ultra-miniaturized centrifugal pump developed by the group.
Perfusate
One of the most important variables of CPB is the type of perfusate used in the filling of the heart-lung machine circuits. Two studies sought to investigate whether changes in priming would lead to changes in the inflammatory response and the microcirculation after the use of CPB. Ugaki et al. [23] demonstrated that the filtration of blood perfusate prior to the commencement of CPB reduces the formation of IL-8 and thrombin-antithrombin complex, in addition to increase the PaO2. Farstad et al. [24], in turn, investigated the use of iso-oncotic solutions of hetastarch and albumin as perfusate, demonstrating that both significantly reduce tissue edema when compared to commonly used perfusate solution.
Temperature
The effects of hypothermia on the inflammatory response during CPB were assessed in three studies. Qing et al. [25] studying the effects of CPB in liver of pigs, showed decreased production of TNF-α and NF-κB and increased IL-10, confirming the anti-inflammatory effect of hypothermia. Lower leukocyte counts and lower histologic lesions were described by Antilla et al. [26,27] when studying the effects of hypothermia on cerebral territory of pigs on CPB.
Regional perfusion
The use of regional infusion regimes, parallel to the main CPB circuit allows prioritizing the perfusion of vital organs and thus increases the time on cardiac arrest, providing greater freedom to the surgeon. The use of these regimens was assessed in four different studies. In cerebral territory, the techniques used were selective cerebral perfusion, achieved by right carotid artery, and retrograde, performed through the superior vena cava. The first showed increased tissue oxygenation while the second resulted in reduced oxygenation and increased tissue edema [28,29]. In pulmonary system we studied the technique of active perfusion, pulsatile or not, performed through cannulation of the pulmonary artery, showing a decreased expression of cytokines such as IL-1β, IL-6, TNF-α and NF-κB, as well as the caspase-3 activity [30]. In the group on which the perfusion was pulsatile, this difference was even more significant for IL-1β, IL-6 and caspase-3 activity. Finally, DeCampli et al. [31] studied the effect of regional low-flow perfusion, in which the CPB flow is directed exclusively to the brachiocephalic trunk and the left carotid artery, demonstrating increased brain PO2.
Other therapies
Other variables were assessed by least amount of studies, but also showed relevant results to clinical practice. Gabriel et al. [32] sought to investigate the use of a coated synthetic copolymer (methacrylate), showing lower circuit platelet aggregation, but no differences in the number of leukocytes, compared with the control group. The use of mini-sternotomy was studied by Hayashi et al. [33] showing no difference with the traditional technique at the end of procedure. Maintenance of blood pH within a range of equilibrium, by adding carbon dioxide (CO2) during CPB led to increased blood flow, and consequent increase in tissue PO2 in brain [34,35] territory.
Finally, Jiang et al. [36,37] studied the use of partial and full liquid ventilation after CPB, demonstrating lower neutrophil counts and lower expression of IL-6, IL-8 and myeloperoxidase, which resulted in a lower lung injury score, with more significant results with the full than the partial technique. The use of perfluoroctil bromide (PFOB), a perfluorochemical compound used as an artificial blood substitute in perfusate emulsion was studied by Isaka et al. [38], but no significant differences in the traditional perfusate were found.
Administration of drugs in peri- or intraoperative period
The drugs assessed by the studies reviewed herein could be divided into 3 main classes, according to their actions: 1) drugs with effects on coagulation, 2) vasoactive drugs and 3) drugs with anti-inflammatory activity.
Drugs with effects on coagulation
The bivalirudin, a direct thrombin inhibitor, was investigated by Welsby et al. [39] and proved to reduce the thrombin-antithrombin complex as well as IL-6 and IL-10. Another drug that showed a decrease in thrombin-antithrombin complex was dextran sulfate (DXS), an antithrombotic studied by Banz et al. [40], that also reduced neutrophil adherence, extravasation of fluid into the extracellular medium and pulmonary arterial pressure. The DXS also reduced the expression of IL-1β, IL-6, IL-8 and TNF-α cytokines. Finally, the effects of antiplatelet eptifibatide, an inhibitor of IIb/IIIa glycoprotein were studied in cerebral territory by Ben Mime et al. [41], demonstrating reduction of histological lesions and increased tissue PO2, effects possibly related to decreased formation of microbubbles.
Vasoactive drugs
Sildenafil, a selective inhibitor of cGMP-specific phosphodiesterase 5 was studied by Aubin et al. [42], showing reduction in pulmonary arterial pressure and arteriolar endothelium-dependent relaxation, both after application of acetylcholine as bradykinin. A drug with effects similar to sildenafil was prostacyclin, which acts as both vasodilator and inhibitor of platelet aggregation, and its only difference to sildenafil was that endothelium-dependent arteriolar relaxation on the specific action of acetylcholine was not altered [43]. Similarly, tetrahydrobiopterin, a cofactor of the synthesis of nitric oxide (NO) studied by Stevens et al. [44], appeared to increase the endothelium-dependent arteriolar relaxation only on the specific action of bradykinin, while the use of magnesium alone increased the endothelium-dependent arteriolar relaxation on the specif action of acetylcholine [45]. Lamarche et al. [46] also demonstrated that milrinone, a selective inhibitor of phosphodiesterase 3, if inhaled, in addition to reduce the heart rate and increase the mean arterial pressure has a positive effect in relaxation of pulmonary arteries in response to acetylcholine and bradykinin.
El Kebir et al. [47,48] have shown, in two studies, that the inhalation of NO prior to CPB reduces neutrophils and IL-8. Aprotinin, a fibrinolysis retardant studied by Liu et al. [49] and Veres et al. [50], also reduced the levels of IL-8, in addition to increase platelet number, although has not changed the endothelium-dependent arteriolar relaxation after administration of acetylcholine or bradykinin, as the authors expected.
Once the literature described the positive effects of NO and aprotinin on the inflammatory response and changes in microcirculation, it was suggested the concomitant use of these two compounds with prostaglandin 1 (PGE1), and this drug regimen was called blood hibernation. Zhou et al. [51] and Du et al. [52] in two studies using this technique showed, paradoxically, lower leukocyte count and increased neutrophil count, respectively, although both observe an increase in the number of platelets. Furthermore, the technique of blood hibernation decreased the plasma elastase, CD11b, myeloperoxidase and IL-8 expression, as well as the count of thrombin-antithrombin complexes and histological lesions. In turn, the use of ruthenium-based NO sequestrant (AMD6221) studied by Mayers et al. [53], demonstrated increase in the mean arterial pressure.
The effect of bradykinin, a vasodilator and inflammatory mediator, was investigated by Yeh et al. [54], showing decreased levels of IL-6, IL-8, TNF-α, NF-kB, myeloperoxidase and caspase-3 activity, besides having less histological damage of brain tissue. Clark et al. [55] sought to establish the effect of xenon in a CPB scenario, but found no significant results regarding the variables considered in this review.
Anti-inflammatory drugs
The use of peroxynitrite was studied by Hayashi et al. [56] who demonstrated a reduction of IL-6 and IL-8 interleukins. The action of glutamine studied by the same authors showed the same results, in addition to reduced number of adherent neutrophils [57]. In another study, Hamamoto et al. [58] demonstrated that rolipram, a selective phosphodiesterase 4 inhibitor, reduces the expression of plasma elastase, TNF-α and CD11b, similarly to the use of activated protein C, which still increased PaO2 and decreased the expression of IL-1β interleukin and water content in the extracellular space [59].
Flurbiprofen, an inhibitor of prostaglandin synthetase studied by Takewa et al. [60] and Sato et al. [61], although it has not shown significant effects on the inflammatory response, improved blood flow in the mesenteric tissue. Administration of atrial natriuretic peptide (ANP) resulted in lower myeloperoxidase activity and increased tissue blood flow in renal territory in the study by Ohno et al. [62]. Also in renal territory, the use of n-acetylcysteine was studied by Zhu et al. [63], demonstrating lower expression of TNF-α and NF-κβ.
Goebel et al. [64] studied the effects of inhaled carbon monoxide (CO) before and after CPB. The inhalation prior to CPB decreased the expression of IL-1β and TNF-α, in addition to increase the expression of IL-10 and attenuate the activity of caspase-3, reducing the occurrence of pulmonary apoptosis induced by CPB. The use of therapy with CO after CPB has also demonstrated anti-inflammatory effect, with reduced expression of IL-6 and TNF-α, besides reduction of caspase-3 activity [65].
The use of curcumin, a natural dye with anti-inflammatory properties, studied by Liu K et al. [66] showed reduced levels of IL-8, TNF-α, NF-kB, as well as the lung injury score. The PPAR-alpha agonist effects studied by Yeh et al. [67] was similar to those of curcumin on IL-8, TNF-α and NF-κβ interleukins. The PPAR-alpha agonist also reduced the expression of IL-10, myeloperoxidase, caspase-3 activity and histological lesions in cardiac tissue, in addition to increase hemodynamic variables such as heart rate and blood pressure.
Cai et al. [68] demonstrated lower histological lesion in liver tissue after using penehyclidina hydrochloride (PHC), an anticholinergic medication. Still in hepatic territory, An et al. [69] suggested that the use of growth hormone may have anti-inflammatory effect as demonstrated by lower expression of IL-1β and TNF-α, although the values of interleukins with anti-inflammatory action (IL-6 and IL-10) showed no difference when compared to the control group.
Simvastatin was studied by Shao et al. [70] and Shen et al. [71] in lung and heart territories, respectively. Both studies showed reduced expression of IL-6, TNF-α, NF-κB and myeloperoxidase, which resulted in lower lung injury score in the study by Shao et al. [70]. In a study by Kellermann et al. [72], the use of moxifloxacin, an antibiotic of broad-spectrum, also led to lower expression of TNF-α and NF-κB cytokines. In pulmonary territory, sivelestat, an inhibitor of neutrophil elastase used for Wakayama et al. [73], led to reduction of IL-8, myeloperoxidase and plasma elastase, besides increasing PO2 and reducing histological lesions.
De Lange et al. [74] demonstrated an increased inflammatory response to CPB with the use of perfluorocarbon, having found increased expression of IL-1β, IL-6, IL-10 and TNF-α, and higher histological lesion in brain tissue. The sulfide oxygen, in turn, was studied by Osipov et al. [75] and showed no significant effects on any of the main indicators of inflammatory response or changes in microcirculation effects.
CONCLUSION
Studies in animal models have proved to be adequate to demonstrate the effects of CPB on inflammatory response and the microcirculation. Still, it was demonstrated the primary efficacy of various interventions, pharmacological or not, against the activation and maintenance of the inflammatory response and changes in microcirculation caused by CPB. Now, prospect studies to assess these interventions in clinical practice are needed in order to reduce the morbidity and mortality of cardiovascular surgery with CPB.
| Abbreviations, acronyms and symbols | |
|---|---|
| ANP | atrial natriuretic peptide |
| CPB | cardiopulmonary bypass |
| CO | Carbon Monoxide |
| CO2 | Carbon dioxide |
| DXS | Dextran Sulfate |
| MUF | Modified ultrafiltration |
| NO | Nitric oxide |
| PaO2 | Arterial partial pressure of oxygen |
| PFOB | Bromide perfluoroctil |
| PGE1 | Prostaglandin 1 |
| PHC | Hydrochloride penehyclidine |
| PO2 | Partial pressure of oxygen |
| Authors’ roles and responsibilities | |
|---|---|
| GRL | Articles search and literature review |
| AFK | Articles search and literature review |
| LFPM | Idealization and coordination |
ACKNOWLEDGEMENTS
We would like to thank Valéria de Vilhena Lombardi and other employees of the Central Library of the Faculty of Medicine, University of São Paulo Medical School, for their assistance during the literature review.
Footnotes
No financial support.
This study was carried out at the Clinics Hospital of the Faculty of Medicine, University of São Paulo (InCor -FMUSP), São Paulo, SP, Brazil.
REFERENCES
- 1.Braile DM, Godoy MF. History of heart surgery in the world. Rev Bras Cir Cardiovasc. 2012;27(1):125–136. doi: 10.5935/1678-9741.20120019. [DOI] [PubMed] [Google Scholar]
- 2.Gibbon JH., Jr Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37(3):171–185. [PubMed] [Google Scholar]
- 3.Moura HV, Pomerantzeff PMA, Gomes WJ. Síndrome da resposta inflamatória sistêmica na circulação extracorpórea: papel das interleucinas. Rev Bras Cir Cardiovasc. 2001;16(4):376–387. [Google Scholar]
- 4.Bierbach B, Meier M, Kasper-König W, Heimann A, Alessandri B, Horstick G, et al. Emboli formation rather than inflammatory mediators are responsible for increased cerebral water content after conventional and assisted beating-heart myocardial revascularization in a porcine model. Stroke. 2008;39(1):213–219. doi: 10.1161/STROKEAHA.107.496620. [DOI] [PubMed] [Google Scholar]
- 5.Dong GH, Wang CT, Li Y, Xu B, Qian JJ, Wu HW, et al. Cardiopulmonary bypass induced microcirculatory injury of the small bowel in rats. World J Gastroenterol. 2009;15(25):3166–3172. doi: 10.3748/wjg.15.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan TA, Bianchi C, Araujo EG, Ruel M, Voisine P, Sellke FW. Activation of pulmonary mitogen-activated protein kinases during cardiopulmonary bypass. J Surg Res. 2003;115(1):56–62. doi: 10.1016/s0022-4804(03)00236-1. [DOI] [PubMed] [Google Scholar]
- 7.Doguet F, Litzler PY, Tamion F, Richard V, Hellot MF, Thuillez C, et al. Changes in mesenteric vascular reactivity and inflammatory response after cardiopulmonary bypass in a rat model. Ann Thorac Surg. 2004;77(6):2130–2137. doi: 10.1016/j.athoracsur.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Khan TA, Bianchi C, Ruel M, Feng J, Sellke FW. Differential effects on the mesenteric microcirculatory response to vasopressin and phenylephrine after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2007;133(3):682–688. doi: 10.1016/j.jtcvs.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Glavind-Kristensen M, Brix-Christensen V, Toennesen E, Ravn HB, Forman A, Sorensen K, et al. Pulmonary endothelial dysfunction after cardiopulmonary bypass in neonatal pigs. Acta Anaesthesiol Scand. 2002;46(7):853–859. doi: 10.1034/j.1399-6576.2002.460716.x. [DOI] [PubMed] [Google Scholar]
- 10.Fischer UM, Klass O, Stock U, Easo J, Geissler HJ, Fischer JH, et al. Cardioplegic arrest induces apoptosis signal-pathway in myocardial endothelial cells and cardiac myocytes. Eur J Cardiothorac Surg. 2003;23(6):984–990. doi: 10.1016/s1010-7940(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 11.Jungwirth B, Eckel B, Blobner M, Kellermann K, Kochs EF, Mackensen GB. The impact of cardiopulmonary bypass on systemic interleukin-6 release, cerebral nuclear factor-kappa B expression, and neurocognitive outcome in rats. Anesth Analg. 2010;110(2):312–320. doi: 10.1213/ANE.0b013e3181bbc42e. [DOI] [PubMed] [Google Scholar]
- 12.Homi HM, Jones WL, de Lange F, Mackensen GB, Grocott HP. Exacerbation of systemic inflammation and increased cerebral infarct volume with cardiopulmonary bypass after focal cerebral ischemia in the rat. J Thorac Cardiovasc Surg. 2010;140(3):660-6, 666.e1. doi: 10.1016/j.jtcvs.2009.10.063. [DOI] [PubMed] [Google Scholar]
- 13.Darling E, Searles B, Nasrallah F, Robins M, You X, Gatto L, et al. High-volume, zero balanced ultrafiltration improves pulmonary function in a model of post-pump syndrome. J Extra Corpor Technol. 2002;34(4):254–259. [PubMed] [Google Scholar]
- 14.Alaoja H, Niemelä E, Anttila V, Dahlbacka S, Mäkelä J, Kiviluoma K, et al. Leukocyte filtration to decrease the number of adherent leukocytes in the cerebral microcirculation after a period of deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2006;132(6):1339–1347. doi: 10.1016/j.jtcvs.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Tao K, An Q, Lin K, Lui RC, Wu X, Zhou J, et al. Which is better to preserve pulmonary function: short-term or prolonged leukocyte depletion during cardiopulmonary bypass? J Thorac Cardiovasc Surg. 2009;138(6):1385–1391. doi: 10.1016/j.jtcvs.2009.07.059. [DOI] [PubMed] [Google Scholar]
- 16.Ohki S, Oshima K, Takeyoshi I, Matsumoto K, Morishita Y. Endotoxin removal with a polymyxin B-immobilized hemoperfusion cartridge improves cardiopulmonary function after cardiopulmonary bypass. J Surg Res. 2008;145(1):74–79. doi: 10.1016/j.jss.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Atkins BZ, Danielson DS, Fitzpatrick CM, Dixon P, Petersen RP, Carpenter AJ. Modified ultrafiltration attenuates pulmonary-derived inflammatory mediators in response to cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2010;11(5):599–603. doi: 10.1510/icvts.2010.234344. [DOI] [PubMed] [Google Scholar]
- 18.Voss B, Krane M, Jung C, Brockmann G, Braun S, Günther T, et al. Cardiopulmonary bypass with physiological flow and pressure curves: pulse is unnecessary! Eur J Cardiothorac Surg. 2010;37(1):223–232. doi: 10.1016/j.ejcts.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 19.Anttila V, Hagino I, Zurakowski D, Iwata Y, Duebener L, Lidov HG, et al. Specific bypass conditions determine safe minimum flow rate. Ann Thorac Surg. 2005;80(4):1460–1467. doi: 10.1016/j.athoracsur.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Schears G, Schultz SE, Creed J, Greeley WJ, Wilson DF, Pastuszko A. Effect of perfusion flow rate on tissue oxygenation in newborn piglets during cardiopulmonary bypass. Ann Thorac Surg. 2003;75(2):560–565. doi: 10.1016/s0003-4975(02)04342-4. [DOI] [PubMed] [Google Scholar]
- 21.Schnoering H, Arens J, Terrada E, Sachweh JS, Runge M, Schmitz-Rode T, et al. A newly developed miniaturized heart-lung machine: expression of inflammation in a small animal model. Artif Organs. 2010;34(11):911–917. doi: 10.1111/j.1525-1594.2010.01146.x. [DOI] [PubMed] [Google Scholar]
- 22.Ugaki S, Honjo O, Nakakura M, Douguchi T, Itagaki A, Yokoyama N, et al. Transfusion-free neonatal cardiopulmonary bypass using a TinyPump. Ann Thorac Surg. 2010;90(5):1615–1621. doi: 10.1016/j.athoracsur.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 23.Ugaki S, Honjo O, Kotani Y, Nakakura M, Douguchi T, Oshima Y, et al. Ultrafiltration of priming blood before cardiopulmonary bypass attenuates inflammatory response and maintains cardiopulmonary function in neonatal piglets. ASAIO J. 2009;55(3):291–295. doi: 10.1097/MAT.0b013e31819b00c2. [DOI] [PubMed] [Google Scholar]
- 24.Farstad M, Kvalheim VL, Husby P. Cold-induced fluid extravasation during cardiopulmonary bypass in piglets can be counteracted by use of iso-oncotic prime. J Thorac Cardiovasc Surg. 2005;130(2):287–294. doi: 10.1016/j.jtcvs.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Qing M, Nimmesgern A, Heinrich PC, Schumacher K, Vazquez-Jimenez JF, Hess J, et al. Intrahepatic synthesis of tumor necrosis factor-alpha related to cardiac surgery is inhibited by interleukin-10 via the Janus kinase (Jak)/signal transducers and activator of transcription (STAT) pathway. Crit Care Med. 2003;31(12):2769–2775. doi: 10.1097/01.CCM.0000098858.64868.9C. [DOI] [PubMed] [Google Scholar]
- 26.Anttila V, Hagino I, Zurakowski D, Lidov HG, Jonas RA. Higher bypass temperature correlates with increased white cell activation in the cerebral microcirculation. J Thorac Cardiovasc Surg. 2004;127(6):1781–1788. doi: 10.1016/j.jtcvs.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 27.Anttila V, Christou H, Hagino I, Iwata Y, Mettler BA, Fernandez-Gonzalez A, et al. Cerebral endothelial nitric oxide synthase expression is reduced after very-low-flow bypass. Ann Thorac Surg. 2006;81(6):2202–2206. doi: 10.1016/j.athoracsur.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Schears G, Zaitseva T, Schultz S, Greeley W, Antoni D, Wilson DF, et al. Brain oxygenation and metabolism during selective cerebral perfusion in neonates. Eur J Cardiothorac Surg. 2006;29(2):168–174. doi: 10.1016/j.ejcts.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duebener LF, Hagino I, Schmitt K, Sakamoto T, Stamm C, Zurakowski D, et al. Direct visualization of minimal cerebral capillary flow during retrograde cerebral perfusion: an intravital fluorescence microscopy study in pigs. Ann Thorac Surg. 2003;75(4):1288–1293. doi: 10.1016/s0003-4975(02)04724-0. [DOI] [PubMed] [Google Scholar]
- 30.Siepe M, Goebel U, Mecklenburg A, Doenst T, Benk C, Stein P, et al. Pulsatile pulmonary perfusion during cardiopulmonary bypass reduces the pulmonary inflammatory response. Ann Thorac Surg. 2008;86(1):115–122. doi: 10.1016/j.athoracsur.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 31.DeCampli WM, Schears G, Myung R, Schultz S, Creed J, Pastuszko A, et al. Tissue oxygen tension during regional low-flow perfusion in neonates. J Thorac Cardiovasc Surg. 2003;125(3):472–480. doi: 10.1067/mtc.2003.13. [DOI] [PubMed] [Google Scholar]
- 32.Gabriel EA, Montevilla FM, Chida VV, Dias FN, Montoya CV, Otsubo H, et al. Experimental research with synthetic copolymer-coated cardiopulmonary bypass circuits: inflammatory and thrombogenicity analysis. Artif Organs. 2012;36(1):110–114. doi: 10.1111/j.1525-1594.2011.01291.x. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi Y, Sawa Y, Nishimura M, Satoh H, Ohtake S, Matsuda H. Avoidance of full-sternotomy: effect on inflammatory cytokine production during cardiopulmonary bypass in rats. J Card Surg. 2003;18(5):390–395. doi: 10.1046/j.1540-8191.2003.02046.x. [DOI] [PubMed] [Google Scholar]
- 34.Duebener LF, Hagino I, Sakamoto T, Mime LB, Stamm C, Zurakowski D, et al. Effects of pH management during deep hypothermic bypass on cerebral microcirculation: alpha-stat versus pH-stat. Circulation. 2002;106(12) Suppl 1:I103–I108. [PubMed] [Google Scholar]
- 35.Ye J, Li Z, Yang Y, Yang L, Turner A, Jackson M, et al. Use of a pH-stat strategy during retrograde cerebral perfusion improves cerebral perfusion and tissue oxygenation. Ann Thorac Surg. 2004;77(5):1664–1670. doi: 10.1016/j.athoracsur.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Jiang L, Wang Q, Liu Y, Du M, Shen X, Guo X, et al. Total liquid ventilation reduces lung injury in piglets after cardiopulmonary bypass. Ann Thorac Surg. 2006;82(1):124–130. doi: 10.1016/j.athoracsur.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Jiang L, Wang Q, Liu Y, Du M, Shen X, Xie N, et al. Effect of different ventilation modes with FC-77 on pulmonary inflammatory reaction in piglets after cardiopulmonary bypass. Pediatr Pulmonol. 2007;42(2):150–158. doi: 10.1002/ppul.20510. [DOI] [PubMed] [Google Scholar]
- 38.Isaka M, Sakuma I, Imamura M, Makino Y, Fukushima S, Nakai K, et al. Experimental studies on artificial blood usage for hemodilution during cardiopulmonary bypass. Ann Thorac Cardiovasc Surg. 2005;11(4):238–244. [PubMed] [Google Scholar]
- 39.Welsby IJ, Jones WL, Arepally G, De Lange F, Yoshitani K, Phillips-Bute B, et al. Effect of combined anticoagulation using heparin and bivalirudin on the hemostatic and inflammatory responses to cardiopulmonary bypass in the rat. Anesthesiology. 2007;106(2):295–301. doi: 10.1097/00000542-200702000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Banz Y, Rieben R, Zobrist C, Meier P, Shaw S, Lanz J, et al. Addition of dextran sulfate to blood cardioplegia attenuates reperfusion injury in a porcine model of cardiopulmonary bypass. Eur J Cardiothorac Surg. 2008;34(3):653–660. doi: 10.1016/j.ejcts.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Ben Mime L, Arnhold S, Fischer JH, Addicks K, Rainer de Vivie E, Bennink G, et al. Pharmacologic cerebral capillary blood flow improvement after deep hypothermic circulatory arrest: an intravital fluorescence microscopy study in pigs. J Thorac Cardiovasc Surg. 2005;130(3):670–676. doi: 10.1016/j.jtcvs.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 42.Aubin MC, Laurendeau S, Mommerot A, Lamarche Y, Denault A, Carrier M, et al. Differential effects of inhaled and intravenous sildenafil in the prevention of the pulmonary endothelial dysfunction due to cardiopulmonary bypass. J Cardiovasc Pharmacol. 2008;51(1):11–17. doi: 10.1097/FJC.0b013e3181598279. [DOI] [PubMed] [Google Scholar]
- 43.Fortier S, DeMaria RG, Lamarche Y, Malo O, Denault A, Desjardins F, et al. Inhaled prostacyclin reduces cardiopulmonary bypass-induced pulmonary endothelial dysfunction via increased cyclic adenosine monophosphate levels. J Thorac Cardiovasc Surg. 2004;128(1):109–116. doi: 10.1016/j.jtcvs.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 44.Stevens LM, Fortier S, Aubin MC, El-Hamamsy I, Maltais S, Carrier M, et al. Effect of tetrahydrobiopterin on selective endothelial dysfunction of epicardial porcine coronary arteries induced by cardiopulmonary bypass. Eur J Cardiothorac Surg. 2006;30(3):464–471. doi: 10.1016/j.ejcts.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Volpe MA, Carneiro JJ, Magna LA, Viaro F, Origuela EA, Evora PR. The role of magnesium in the endothelial dysfunction caused by global ischemia followed by reperfusion: in vitro study of canine coronary arteries. Scand Cardiovasc J. 2003;37(5):288–296. doi: 10.1080/14017430310014939. [DOI] [PubMed] [Google Scholar]
- 46.Lamarche Y, Malo O, Thorin E, Denault A, Carrier M, Roy J, et al. Inhaled but not intravenous milrinone prevents pulmonary endothelial dysfunction after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005;130(1):83–92. doi: 10.1016/j.jtcvs.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 47.El Kebir D, Hubert B, Taha R, Troncy E, Wang T, Gauvin D, et al. Effects of inhaled nitric oxide on inflammation and apoptosis after cardiopulmonary bypass. Chest. 2005;128(4):2910–2917. doi: 10.1378/chest.128.4.2910. [DOI] [PubMed] [Google Scholar]
- 48.El Kebir D, Taha R, Hubert B, Gauvin D, Gangal M, Blaise G. The anti-inflammatory effect of inhaled nitric oxide on pulmonary inflammation in a swine model. Can J Physiol Pharmacol. 2005;83(3):252–258. doi: 10.1139/y05-008. [DOI] [PubMed] [Google Scholar]
- 49.Liu JL, Stammers AH, Zheng H, Mills NJ, Nichols JD, Kmiecik SA, et al. The effect of controlled aprotinin administration through cardiotomy suction during cardiopulmonary bypass. J Extra Corpor Technol. 2002;34(3):203–208. [PubMed] [Google Scholar]
- 50.Veres G, Radovits T, Schultz H, Lin LN, Hütter J, Weigang E, et al. Effect of recombinant aprotinin on postoperative blood loss and coronary vascular function in a canine model of cardiopulmonary bypass. Eur J Cardiothorac Surg. 2007;32(2):340–345. doi: 10.1016/j.ejcts.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 51.Zhou J, Wu XD, Lin K, Lui RC, An Q, Tao KY, et al. Blood hibernation: a novel strategy to inhibit systemic inflammation and coagulation induced by cardiopulmonary bypass. Chin Med J (Engl) 2010;123(13):1741–1747. [PubMed] [Google Scholar]
- 52.Du L, Zhou J, Tang J, An Q, Lin K, Wu X, et al. Aprotinin combined with nitric oxide and prostaglandin E1 protects the canine kidney from cardiopulmonary bypass-induced injury. Eur J Cardiothorac Surg. 2010;38(1):98–103. doi: 10.1016/j.ejcts.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 53.Mayers I, Hurst T, Radomski A, Johnson D, Fricker S, Bridger G, et al. Increased matrix metalloproteinase activity after canine cardiopulmonary bypass is suppressed by a nitric oxide scavenger. J Thorac Cardiovasc Surg. 2003;125(3):661–668. doi: 10.1067/mtc.2003.38. [DOI] [PubMed] [Google Scholar]
- 54.Yeh CH, Chen TP, Wang YC, Lin YM, Fang SW. Cardiomyocytic apoptosis limited by bradykinin via restoration of nitric oxide after cardioplegic arrest. J Surg Res. 2010;163(1):e1–e9. doi: 10.1016/j.jss.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Clark JA, Ma D, Homi HM, Maze M, Grocott HP. Xenon and the inflammatory response to cardiopulmonary bypass in the rat. J Cardiothorac Vasc Anesth. 2005;19(4):488–493. doi: 10.1053/j.jvca.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Hayashi Y, Sawa Y, Nishimura M, Fukuyama N, Ichikawa H, Ohtake S, et al. Peroxynitrite, a product between nitric oxide and superoxide anion, plays a cytotoxic role in the development of post-bypass systemic inflammatory response. Eur J Cardiothorac Surg. 2004;26(2):276–280. doi: 10.1016/j.ejcts.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 57.Hayashi Y, Sawa Y, Fukuyama N, Nakazawa H, Matsuda H. Preoperative glutamine administration induces heat-shock protein 70 expression and attenuates cardiopulmonary bypass-induced inflammatory response by regulating nitric oxide synthase activity. Circulation. 2002;106(20):2601–2607. doi: 10.1161/01.cir.0000035651.72240.07. [DOI] [PubMed] [Google Scholar]
- 58.Hamamoto M, Suga M, Takahashi Y, Sato Y, Inamori S, Yagihara T, et al. Suppressive effect of phosphodiesterase type 4 inhibition on systemic inflammatory responses after cardiopulmonary bypass. J Artif Organs. 2006;9(3):144–148. doi: 10.1007/s10047-006-0335-2. [DOI] [PubMed] [Google Scholar]
- 59.Yamazaki S, Inamori S, Nakatani T, Suga M. Activated protein C attenuates cardiopulmonary bypass-induced acute lung injury through the regulation of neutrophil activation. J Thorac Cardiovasc Surg. 2011;141(5):1246–1252. doi: 10.1016/j.jtcvs.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 60.Takewa Y, Taenaka Y, Tatsumi E, Sato K, Ohnishi H, Oshikawa M, et al. Prostaglandin synthesis inhibitor affects humoral conditions and oxygen metabolism during normothermic cardiopulmonary bypass. Artif Organs. 2002;26(8):676–681. doi: 10.1046/j.1525-1594.2002.06915.x. [DOI] [PubMed] [Google Scholar]
- 61.Sato K, Takewa Y, Taenaka Y, Tatsumi E, Nishinaka T, Shioya K, et al. Prostaglandin synthesis inhibitor prevents hypotension without impairing gut perfusion during normothermic cardiopulmonary bypass. ASAIO J. 2002;48(5):503–507. doi: 10.1097/00002480-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 62.Ohno M, Omoto T, Fukuzumi M, Oi M, Ishikawa N, Tedoriya T. Hypothermic circulatory arrest: renal protection by atrial natriuretic peptide. Asian Cardiovasc Thorac Ann. 2009;17(4):401–407. doi: 10.1177/0218492309341712. [DOI] [PubMed] [Google Scholar]
- 63.Zhu J, Yin R, Shao H, Dong G, Luo L, Jing H. N-acetylcysteine to ameliorate acute renal injury in a rat cardiopulmonary bypass model. J Thorac Cardiovasc Surg. 2007;133(3):696–703. doi: 10.1016/j.jtcvs.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 64.Goebel U, Siepe M, Mecklenburg A, Stein P, Roesslein M, Schwer CI, et al. Carbon monoxide inhalation reduces pulmonary inflammatory response during cardiopulmonary bypass in pigs. Anesthesiology. 2008;108(6):1025–1036. doi: 10.1097/ALN.0b013e3181733115. [DOI] [PubMed] [Google Scholar]
- 65.Goebel U, Siepe M, Schwer CI, Schibilsky D, Brehm K, Priebe HJ, et al. Postconditioning of the lungs with inhaled carbon monoxide after cardiopulmonary bypass in pigs. Anesth Analg. 2011;112(2):282–291. doi: 10.1213/ANE.0b013e318203f591. [DOI] [PubMed] [Google Scholar]
- 66.Liu K, Shen L, Wang J, Dong G, Wu H, Shao H, et al. The preventative role of curcumin on the lung inflammatory response induced by cardiopulmonary bypass in rats. J Surg Res. 2012;174(1):73–82. doi: 10.1016/j.jss.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Yeh CH, Chen TP, Lee CH, Wu YC, Lin YM, Lin PJ. Cardiomyocytic apoptosis following global cardiac ischemia and reperfusion can be attenuated by peroxisome proliferator-activated receptor alpha but not gamma activators. Shock. 2006;26(3):262–270. doi: 10.1097/01.shk.0000225863.56714.96. [DOI] [PubMed] [Google Scholar]
- 68.Cai DS, Jin BB, Pei L, Jin Z. Protective effects of penehyclidine hydrochloride on liver injury in a rat cardiopulmonary bypass model. Eur J Anaesthesiol. 2010;27(9):824–828. doi: 10.1097/EJA.0b013e32833b650f. [DOI] [PubMed] [Google Scholar]
- 69.An Y, Xiao YB. Growth hormone prevents acute liver injury induced by cardiopulmonary bypass in a rat model. J Thorac Cardiovasc Surg. 2007;134(2):342–350. doi: 10.1016/j.jtcvs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 70.Shao H, Shen Y, Liu H, Dong G, Qiang J, Jing H. Simvastatin suppresses lung inflammatory response in a rat cardiopulmonary bypass model. Ann Thorac Surg. 2007;84(6):2011–2018. doi: 10.1016/j.athoracsur.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 71.Shen Y, Wu H, Wang C, Shao H, Huang H, Jing H, et al. Simvastatin attenuates cardiopulmonary bypass-induced myocardial inflammatory injury in rats by activating peroxisome proliferator-activated receptor γ. . Eur. J Pharmacol. 2010;649(1-3):255–262. doi: 10.1016/j.ejphar.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 72.Kellermann K, Dertinger N, Blobner M, Kees F, Kochs EF, Jungwirth B. Perioperative moxifloxacin treatment in rats subjected to deep hypothermic circulatory arrest: reduction in cerebral inflammation but without improvement in cognitive performance. J Thorac Cardiovasc Surg. 2011;141(3):796–802. doi: 10.1016/j.jtcvs.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 73.Wakayama F, Fukuda I, Suzuki Y, Kondo N. Neutrophil elastase inhibitor, sivelestat, attenuates acute lung injury after cardiopulmonary bypass in the rabbit endotoxemia model. Ann Thorac Surg. 2007;83(1):153–160. doi: 10.1016/j.athoracsur.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 74.de Lange F, Yoshitani K, Proia AD, Mackensen GB, Grocott HP. Perfluorocarbon administration during cardiopulmonary bypass in rats: an inflammatory link to adverse outcome? Anesth Analg. 2008;106(1):24–31. doi: 10.1213/01.ane.0000297439.90773.c7. [DOI] [PubMed] [Google Scholar]
- 75.Osipov RM, Robich MP, Feng J, Chan V, Clements RT, Deyo RJ, et al. Effect of hydrogen sulfide on myocardial protection in the setting of cardioplegia and cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2010;10(4):506–512. doi: 10.1510/icvts.2009.219535. [DOI] [PubMed] [Google Scholar]