Secondary messengers associated with oxidative stress are involved in osmosensory signal transduction.
The intracellular milieu, including solute composition and concentration of important electrolytes, is carefully tuned to support metabolism and other vital cell functions. In addition, cell volume is tightly regulated. A change in extracellular osmolality disturbs the delicate balance that maintains intracellular solute composition and cell volume. To counteract deleterious consequences of such disturbance, cells have evolved a universal mechanism to respond to osmotic stress by cell volume regulation (1) and adaptive adjustment of compatible organic osmolyte levels (2). However, these adaptive responses are not instantaneous, and osmotic stress causes significant damage to proteins (3) and DNA (4). Such damage can persist until hyperosmotic stress diminishes (5). In this issue of PNAS, Zhang et al. (6) demonstrate that at least part of the hyperosmotic damage to proteins and DNA is caused by secondary oxidative stress. Zhang et al. report the thought-provoking observation that hyperosmolality increases reactive oxygen species (ROS) and protein carbonylation levels in renal inner medullary (IM) cells in vitro and in vivo. This observation significantly extends our understanding of the molecular nature of the hyperosmotic threat to cells. The work by Zhang et al. also identifies ROS and protein carbonylation as potential signaling intermediates for osmosensory signal transduction.
Consequences of Hyperosmolality
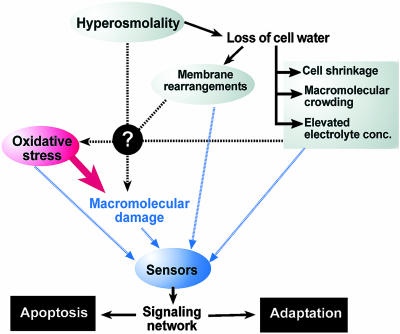
Renal IM cells of mammals are routinely exposed to osmotic fluctuations as a result of the renal concentrating mechanism. Changes in renal urinary concentration and excretion reflect the degree of systemic hydration and salt load. They are based on adjustments of NaCl and urea concentrations in the renal IM. Thus, hyperosmolality in the renal IM is mainly a result of increased concentrations of NaCl and urea. These two major osmolytes have very different effects on cells. First, NaCl does not readily permeate the cell membrane, and hyperosmolality in the form of elevated extracellular NaCl leads to hypertonicity. Hypertonicity results from passive water loss due to osmosis and causes cell shrinkage, macromolecular crowding, and elevation of intracellular electrolyte concentration (Fig. 1). In contrast, urea penetrates cell membranes with similar efficiency as water. For this reason, elevated urea does not cause significant water loss from cells and is nonhypertonic. However, hyperosmotic urea accumulates inside cells, where it is a strong protein denaturant.
Fig. 1.
Effects of hyperosmolality on cell function are monitored by multiple sensors that integrate signals and activate a signaling network that promotes either cell adaptation and survival or programmed cell death (apoptosis). When hyperosmolality exceeds cellular tolerance limits, apoptosis is induced. Oxidative stress and macromolecular damage are consequences of hyperosmolality that provide input into such sensors. However, the initial mechanisms by which hyperosmolality generates oxidative stress and some forms of macromolecular damage are not known. A major difference between hyperosmotic urea and NaCl stress concerns the passive loss of cell water that only occurs during NaCl stress and other forms of hypertonicity. Thus, hypertonicity generates additional avenues of sensory input that are missing during hyperosmotic urea stress.
Another difference between NaCl and urea concerns their effect on genomic integrity. Hypertonic NaCl causes DNA double-strand breaks (dsb), whereas hyperosmotic urea does not (4). Because of these different effects on macromolecules, it is not surprising that the signaling pathways activated in response to hyperosmotic NaCl differ considerably from pathways turned on during elevation of urea (7). Zhang et al. (6) report that another fundamental difference between hyperosmotic NaCl and urea consists in the induction of oxidative nucleotide damage by elevated urea but not NaCl. In addition, they find that formation of 8-oxoguanine (8-oxoG) in response to urea-mediated hyperosmolality is accompanied by an increased number of DNA single-strand breaks (ssb), which are intermediary states of nucleotide excision repair (6). These results suggest that the different types of DNA damage caused by hyperosmotic urea and NaCl could contribute to the activation of solute-specific mechanisms of signaling and adaptation.
NaCl and urea contribute to about an equal extent to the hyperosmotic milieu in the renal IM. Although osmotic tolerance limits of renal IM cells are lower than physiologically necessary when exposed to either NaCl or urea increases, a mixture of NaCl and urea significantly increases osmotic tolerance limits (8). A potential reason for this beneficial effect of mixing NaCl and urea on renal IM cells is that each solute causes somewhat different lesions that are not additive and, therefore, expands the tolerance range during hyperosmolality.
Interestingly, despite the lack of oxidative DNA damage in NaCl-stressed renal IM cells, both forms of hyperosmolality (NaCl and urea) cause oxidative stress and increase levels of glutathione and protein carbonylation (6). Glutathione is the most abundant low-molecular-weight thiol synthesized de novo in animal cells. It represents the major redox couple in animal cells and a useful bioindicator of oxidative stress (9). Protein carbonylation is a nonenzymatic protein modification that occurs during oxidative stress. Protein carbonyl groups (CO) are relatively stable once formed, which enables accurate quantification. Therefore, protein carbonyl content is increasingly being used as a biomarker of severe oxidative protein damage. Several common renal diseases, including diabetic nephropathy and chronic renal failure, are associated with elevated levels of protein carbonylation (10). Thus, the link between hyperosmolality, which is a common condition in the renal medulla, and increased levels of protein carbonylation provides an interesting avenue for studying molecular processes that are disturbed in such renal diseases.
Osmosensing
Because ROS have been proposed as second messengers of environmental stress they represent a potent avenue of input for osmosensory signal transduction (11). Oxidative stress activates many stress signaling pathways by direct modification of signaling proteins, including mitogen-activated protein kinases, AP-1, and NF-κB transcription factors (12–14). Alternatively, ROS activate stress signaling pathways indirectly via damaging effects on macromolecules (Fig. 1). However, at present, it is unknown how hyperosmolality leads to oxidative stress. Although membrane rearrangements, cell shrinkage, macro-molecular crowding, and elevated electrolyte levels may cause oxidative stress in cells exposed to hypertonicity, such effects are not observed during hyperosmotic urea stress. Because elevations in both NaCl and urea cause oxidative stress, it is more likely that other, as-yet-unknown mechanisms are responsible for increasing ROS levels in response to hyperosmolality. Nevertheless, down-stream events resulting from hypertonicity and loss of cell water may be required for preventing oxidative nucleotide damage during hypertonic stress. How renal IM cells achieve this protection from oxidative DNA damage during hypertonicity is not clear. Future research addressing the origin of oxidative stress during hyperosmolality and the different effects of hypertonicity and urea stress regarding oxidative DNA damage should provide interesting insight into fundamental cellular processes affected by hyperosmolality.
The signals emanating from oxidative damage contribute to sensory input that is perceived by stress sensors during hyperosmolality. Such sensors include generic stress sensors that monitor macromolecular damage (15) as well as osmosensors that are specifically activated in response to either hypertonicity or urea stress. Much work has focused on DNA damage sensors, and phosphatidyl-inositol-3 kinase-like kinases (ATM, ATR, DNA-PK) have emerged as central early transducers of various types of DNA damage, including hyperosmotically induced DNA damage (4, 16–19). Protein damage sensors are less well understood, but proteases, molecular chaperones, and sensors associated with the unfolded protein response in the endoplasmic reticulum are potential candidates. Paradoxically, we still know little about the function of specific osmosensors in mammalian cells, although the list of potential candidates is quite extensive (20). However, it is clear that multiple sensors perceive different types of signals generated during hyperosmotic stress. The integration of information flow through such sensors is critical for appropriate signal transduction events that coordinate the cellular response to different forms of hyperosmolality (Fig. 1). The results reported by Zhang et al. (6) suggest that secondary messengers associated with oxidative stress, e.g., H2O2, are involved in osmosensory signal transduction. This information significantly extends our knowledge about upstream signals for regulatory networks controlling osmotic stress adaptation.
Osmosensory signal transduction stimulates cell adaptation via repair of macromolecular damage and reestablishment of cell volume and electrolyte homeostasis. However, severe hyperosmolality that exceeds cellular tolerance limits leads to activation of a cell death program (apoptosis). At present, it is not known how cells recognize that their osmotic tolerance limits are exceeded and apoptotic programs need to be activated. Potential cellular mechanisms of assessing the severity of hyperosmolality include quantification of macromolecular damage or oxidative stress. It will be interesting to study the significance of such potential mechanisms for hyperosmotic stress quantification. Future research in this area promises to shed light on the pathology of renal and systemic diseases associated with abnormal osmoregulation. The link between hyperosmotic and oxidative stress demonstrated by Zhang et al. (6) is particularly intriguing with regard to renal proliferative diseases, including renal cancers. It breaks ground for studying molecular mechanisms of such diseases and will stimulate approaches of disease prevention and treatment.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-59470.
See companion article on page 9491.
References
- 1.Wehner, F., Olsen, H., Tinel, H., Kinne-Saffran, E. & Kinne, R. K. (2003) Rev. Physiol. Biochem. Pharmacol. 148, 1-80. [DOI] [PubMed] [Google Scholar]
- 2.Yancey, P. H., Clark, M. E., Hand, S. C., Bowlus, R. D. & Somero, G. N. (1982) Science 217, 1214-1222. [DOI] [PubMed] [Google Scholar]
- 3.Bolen, D. W. (2001) Methods Mol. Biol. 168, 17-36. [DOI] [PubMed] [Google Scholar]
- 4.Kültz, D. & Chakravarty, D. (2001) Proc. Natl. Acad. Sci. USA 98, 1999-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dmitrieva, N. I., Cai, Q. & Burg, M. B. (2004) Proc. Natl. Acad. Sci. USA 101, 2317-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang, Z., Dmitrieva, N. I., Park, J.-H., Levine, R. L. & Burg, M. B. (2004) Proc. Natl. Acad. Sci. USA 101, 9491-9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian, W. & Cohen, D. M. (2002) Am. J. Physiol. 283, F388-F398. [DOI] [PubMed] [Google Scholar]
- 8.Santos, B. C., Chevaile, A., Hebert, M. J., Zagajeski, J. & Gullans, S. R. (1998) Am. J. Physiol. 274, F1167-F1173. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson, D. A. & Forman, H. J. (2002) Ann. N.Y. Acad. Sci. 973, 488-504. [DOI] [PubMed] [Google Scholar]
- 10.Dalle-Donne, I., Giustarini, D., Colombo, R., Rossi, R. & Milzani, A. (2003) Trends Mol. Med. 9, 169-176. [DOI] [PubMed] [Google Scholar]
- 11.Reth, M. (2002) Nat. Immunol. 3, 1129-1134. [DOI] [PubMed] [Google Scholar]
- 12.Adler, V., Yin, Z., Tew, K. D. & Ronai, Z. (1999) Oncogene 18, 6104-6111. [DOI] [PubMed] [Google Scholar]
- 13.Toone, W. M., Morgan, B. A. & Jones, N. (2001) Oncogene 20, 2336-2346. [DOI] [PubMed] [Google Scholar]
- 14.Wang, T., Zhang, X. & Li, J. J. (2002) Int. Immunopharmacol. 2, 1509-1520. [DOI] [PubMed] [Google Scholar]
- 15.Kültz, D. (2003) J. Exp. Biol. 206, 3119-3124. [DOI] [PubMed] [Google Scholar]
- 16.Stewart, G. S., Wang, B., Bignell, C. R., Taylor, A. M. & Elledge, S. J. (2003) Nature 421, 961-966. [DOI] [PubMed] [Google Scholar]
- 17.Horejsi, Z., Falck, J., Bakkenist, C. J., Kastan, M. B., Lukas, J. & Bartek, J. (2004) Oncogene 23, 3122-3127. [DOI] [PubMed] [Google Scholar]
- 18.Lee, J. H. & Paull, T. T. (2004) Science 304, 93-96. [DOI] [PubMed] [Google Scholar]
- 19.Bradbury, J. M. & Jackson, S. P. (2003) Biochem. Soc. Trans. 31, 40-44. [DOI] [PubMed] [Google Scholar]
- 20.Kültz, D. & Burg, M. B. (1998) Contrib. Nephrol. 123, 94-109. [DOI] [PubMed] [Google Scholar]