Abstract
Introduction
Chagas disease is a major cause of cardiomyopathy and sudden death in our country. It has a high mortality when their patients develop New York Heart Association (NYHA) class IV.
Objective
The objective of this study is to analyze the clinical outcome of patients with Chagas' cardiomyopathy with congestive heart failure with optimized pharmacological therapy, undergoing cardiac resynchronization therapy.
Methods
Between January 2004 and February 2009, 72 patients with Chagas' cardiomyopathy in NYHA class III and IV underwent cardiac resynchronization therapy and were monitored to assess their clinical evolution. We used the t test or the Wilcoxon test to compare the same variable in two different times. A P value < 0.05 was established as statistically significant.
Results
The average clinical follow-up was 46.6 months (range 4-79 months). At the end of the evaluation, 87.4% of patients were in NYHA class I or II (P<0.001). There was response to therapy in 65.3% of patients (P<0.001), with an overall mortality of 34.7%.
Conclusion
In patients with chronic Chagas cardiomyopathy undergoing cardiac resynchronization therapy, we found the following statistically significant changes: improvement in NYHA class and increase of left ventricle ejection fraction, a decrease of the systolic final diameter and systolic final left ventricle volume and improvement of patient survival.
Keywords: Chagas disease; Chagas cardiomyopathy; Myocarditis; Cardiomyopathy, dilated; Death, sudden, cardiac
Abstract
Introdução
A doença de Chagas é a maior causa de miocardiopatia e morte súbita em nosso país. Apresenta alta mortalidade quando seus portadores evoluem para classe funcional IV da New York Heart Association (NYHA).
Objetivo
O objetivo deste trabalho é analisar a evolução clínica dos pacientes portadores de cardiomiopatia chagásica com insuficiência cardíaca avançada e terapia farmacológica otimizada submetido a terapia de ressincronização cardíaca.
Métodos
Entre janeiro de 2004 e fevereiro de 2009, 72 pacientes com cardiomiopatia chagásica em classe funcional III e IV da NYHA foram submetidos à terapia de ressincronização cardíaca e acompanhados para avaliar sua evolução clínica. Para comparar a mesma variável em dois momentos diferentes utilizamos o Teste t pareado ou o Teste de Wilcoxon. Um valor de P<0,05 foi estabelecido como estatisticamente significante.
Resultados
O acompanhamento clínico médio foi de 46,6 meses (variando de 4 a 79 meses). Ao final do seguimento, 87,4% dos pacientes estavam em classe funcional I ou II da NYHA (P<0,001). Houve resposta à terapia em 65,3% dos pacientes (P<0,001), com mortalidade total de 34,7%.
Conclusão
Nos pacientes com cardiomiopatia chagásica crônica submetidos à terapia de ressincronização cardíaca, encontramos as seguintes alterações estatisticamente significativas: melhora da classe funcional segundo NYHA; melhora da fração de ejeção do ventrículo esquerdo; diminuição do diâmetro sistólico final e volume sistólico final do ventrículo esquerdo e maior sobrevida destes pacientes.
INTRODUCTION
Chagas disease is a neglected disease in the world, and in Latin America there are nearly 10 million patients infected with Trypanosoma cruzi [1]. In 2005, it was estimated that, in Brazil, there were approximately 2 million infected [2].
Approximately 30% of these individuals will develop Chagas cardiomyopathy in a period between 10 and 30 years of disease [3].
Chronic Chagas cardiomyopathy is caused by the invasion of Trypanosoma cruzi in the muscular structures and electrical conduction of the heart tissue, leading to destruction of it and replacement by fibrous tissue [3].
It is estimated approximately 3,000 deaths occur each year related to Chagas disease in Brazil [2]. It is the most common cardiomyopathy in Central and South America and, in endemic areas, is the leading cause of cardiovascular death in patients aged between 30 and 50 years old.
The fundamental determinant in the evolution of patients infected by Trypanosoma cruzi is cardiac involvement, due to the occurrence of arrhythmias, cardiac insufficiency in its varying degrees, and thromboembolic phenomena.
Currently, heart failure is the main cause of deaths related to Chagas disease in Brazil [4]. Serious ventricular arrhythmias, especially when associated with severe impairment of ventricular function, are important risk factors for sudden death [4].
In Chagas cardiomyopathy, NYHA functional class (the New York Heart Association - NYHA) and left ventricular systolic dysfunction assessed by ejection fraction (LVEF) are predictors of mortality, and patients with optimal medical treatment in functional class IV (NYHA) and LVEF less than 35% have only 16% survival at 36 months [5].
In a systematic review of observational studies, Rassi et al. [5] claim that chagasic patients with impaired ventricular function, cardiomegaly, functional class III and IV (NYHA) and episodes of non-sustained ventricular tachycardia have a poor prognosis in 12 months.
The potential hemodynamic benefit of biventricular pacing in humans was first demonstrated in 1983, but its clinical application occurred only in 1994, when Cazeau et al. [6] reported the case of a 54 year old patient with congestive heart failure functional class IV (NYHA), with electrocardiogram showing left bundle branch block with a QRS interval of 200 ms. The authors believe that the ventricular dyssynchrony was treated, and it was caused by the delay of the electrical impulse in left bundle branch block, and that the stimulation of the four chambers promotes a sequence of ventricular activation close to normal.
In 2000, Cazeau et al. [7], in an editorial of the journal Heart, assessed the concept of cardiac dyssynchrony, defined as a heterogeneous spread of electrical activity of the heart that occurs as a consequence of myocardial progressive focal or global degradation. Such a change in heart electrical propagation provides levels of atrioventricular asynchrony, interventricular and intraventricular [7,8].
Also in 2000, Leclercq et al. [9] present a pilot experience with biventricular pacemaker to treat advanced heart failure, mentioning the indications for the procedure: delayed dilated cardiomyopathy, NYHA functional class III or IV (NYHA) intraventricular conduction delay of the electrical stimulus.
From these original studies by Cazeau and Leclercq, several randomized clinical trials on cardiac resynchronization therapy [10-15] emerged. Since then, studies have shown benefit in morbidity and mortality of non-chagasic patients with chronic systolic heart failure undergoing cardiac resynchronization therapy. However, few studies related to cardiac resynchronization therapy were performed in patients with chronic systolic heart failure due to Chagas' cardiomyopathy. These studies led the Brazilian Society of Cardiology to publish, in 2007, together with the Department of Cardiac Pacing and Brazilian Society of Cardiac Arrhythmias, the Brazilian Guidelines for Cardiac Implantable Electronic Devices, normalizing the recommendations for cardiac resynchronization therapy.
In the literature, it is observed that these studies that supported the creation of the Brazilian guideline were performed in patients with dilated cardiomyopathy of ischemic or idiopathic etiology. Against the scenario of the large number of patients with Chagas cardiomyopathy and advanced heart failure in our country and the excellent results obtained mainly in Europe in the treatment of terminal dilated cardiomyopathy by cardiac resynchronization therapy, the Department of Cardiovascular Surgery and Electrophysiology Service of Santa Casa de São Paulo, together with the Department of Cardiology of Salinas, Minas Gerais, resolved in 2003, to initiate the study of cardiac resynchronization therapy in patients with Chagas' disease.
The aim of this study is to assess the clinical long-term outcome of patients with Chagas cardiomyopathy with advanced heart failure undergoing cardiac resynchronization therapy by assessing the functional class and echocardiographic parameters in 5 years.
METHODS
This study was approved by its Research Ethics Committee in Human Beings of the Irmandade da Santa Casa de Misericórdia de São Paulo, protocol No. 026/2011, on 28/01/2011. In the period between January 2004 and February 2009, were selected by the same cardiologist in Salinas, Minas Gerais, 72 patients with positive serology for Chagas cardiomyopathy and heart failure, receiving optimal dose of the following drugs, according to the characteristics of their disease and specific indications: furosemide, spironolactone, hydrochlorothiazide, captopril, losartan, carvedilol, digoxin, warfarin, aspirin and amiodarone.
The inclusion criteria for the study were patients with the following characteristics: age over 18 years, positive serology for Chagas' disease, severe heart failure despite optimal medical therapy, electrocardiogram with QRS interval greater than 120 ms, LVEF less than 35% and left ventricle end-diastolic diameter greater than 55 mm assessed by Doppler echocardiography.
Patients with the following conditions were excluded: atrial arrhythmias, neoplastic disease, acquired valvular heart disease other than mitral regurgitation secondary to Chagas cardiomyopathy, thoracic aortic aneurysm and cerebral vascular disease.
Once filled the criteria, patients who agreed to participate in this study signed a written informed consent.
Patients were referred to the Irmandade da Santa Casa de São Paulo, at the Cardiovascular Surgery Unit and, after surveying the clinical history, laboratory tests, medications, and complementary tests (blood count, thrombin time, activated partial thromboplastin time, serum sodium, potassium, urea and creatinine) underwent implantation of cardiac resynchronization device, placing an electrode in the right atrium and an electrode in the right ventricle (both intravenous), and an electrode in the lateral side of the left ventricle through a left anterior mini-thoracotomy in the 4th intercostal space, with placement of an epicardial lead. When possible, the electrode placement of the left ventricle was performed through the coronary venous sinus, moving the endocardial electrode on the left side of the left ventricular coronary vein.
Preoperative clinical parameters were:
Functional class: 60 (83.8%) patients were in functional class III and 12 (16.2%), functional class IV;
Medication used: 59 (81.9%) patients used amiodarone, 39 (54.1%), captopril, 72 (100%), carvedilol, 16 (22.2%), digoxin, 71 (98.6%), spironolactone, 68 (94.4%), furosemide and 11 (15.2%), losartan;
Electrocardiographic parameters: 34 (47.2%) patients had left bundle branch block, 11 (15.3%), pacemaker-induced left bundle branch block, 26 (36.2%), right bundle branch block + left anterior hemiblock and 1 (1.3%), complete atrioventricular block. The average width of the QRS interval was 148.1 ± 17.5 ms;
Doppler echocardiographic parameters: mean LVEF calculated by Teicholz method was 27.3 ± 7.7%, the average left ventricular end systolic diameter was 57.5 ± 7.2 mm, the end left ventricular diastolic diameter was 66.2 ± 7.6 mm, the mean left ventricular end-systolic volume was 167.8 ± 50.6ml and end-diastolic volume of the left ventricle average was 230±63.3ml;
Average dose of carvedilol 20±16.2mg;
Mean QRS interval before cardiac resynchronization: 140±38.2ms.
From January 2004 to November 2010, when the study was completed, patients underwent clinical and quarterly electrocardiographic control and echocardiographical control; also, assessment of cardiac resynchronization semiannually was performed, observing the command analysis and sensitivity and statistical biventricular command.
In clinical management, in addition to the adequacy of medication treatment, we assessed the functional class according to the NYHA classification.
The electrocardiogram assessed the rhythm and biventricular command.
Doppler echocardiography assessed: LVEF, left ventricular end-systolic diameter, left ventricular end-diastolic diameter, left ventricular end-systolic volume and left ventricular end-diastolic volume.
Performance assessment of resynchronization was performed at the Irmandade da Santa Casa de São Paulo, and the other exams in Salinas, Minas Gerais.
The criteria used for patients considered to be responders to cardiac ressincronization therapy were: being in functional class I or II (NYHA) or alive at the end of follow-up.
In statistical analysis and the construction of the graphs we used the GraphPad Prism software version 6.00 (GraphPad Software, San Diego, California, USA, www.graphpad.com), to make graphical comparisons of samples used in the study.
For analysis of the same variable of the same individual at two different times, we used the t test for paired samples (variable of parametric distribution) and Wilcoxon test (non-parametric variable distribution). In all tests, we used a significance level of 5% (P<0.05).
RESULTS
The follow-up after implantation of resynchronization device occurred until November 2010, ranging from 4 to 79 months (mean follow-up of 46.6 months). The implantation of resynchronization was performed through mini-thoracotomy in 48 (66.7%) patients and via venous sinus in 24 (33.3%).
At the final follow-up, 45.8% of patients were in functional class I, 41.6% in functional class II, 7% in functional class III and 5.6% in functional class IV (NYHA). Regarding the response to cardiac resynchronization therapy, it was observed that 47 (65.3%) patients responded to cardiac resynchronization therapy and 24 (33.3%) did not respond with loss to follow-up of 1 (1.4%) patient. The overall mortality was 34.7 % (25 patients), and the causes of death were worsening heart failure in 15 (60%) cases, ischemic stroke in 1 (4%), sudden death in 2 (8%), endocarditis in 1 (4%), chronic obstructive pulmonary disease in 1 (4%), pneumonia in 1 (4%) and 4 (16%) patients had unknown cause of death.
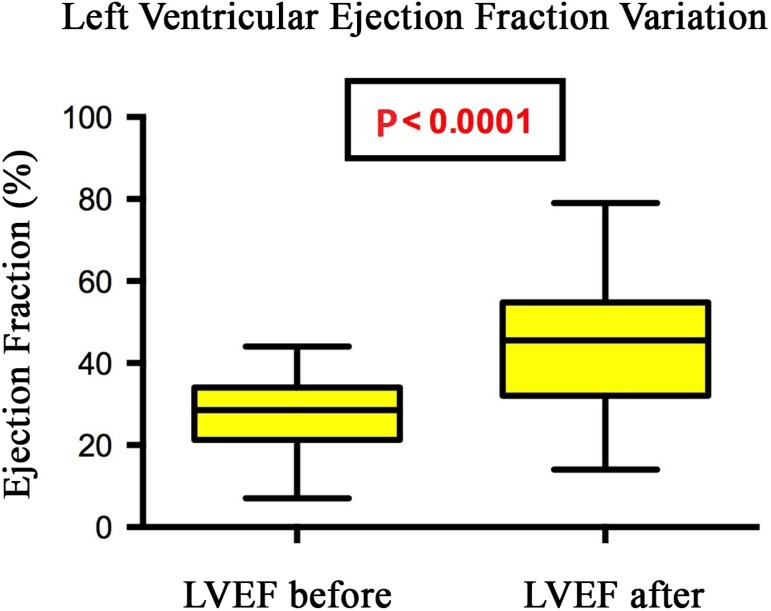
LVEF ranged from 27.3% to 44.2%, on average, after implantation of the cardiac resynchronization device (P<0.0001) (Figure 1).
Fig. 1.
Changes in ejection fraction in patients undergoing cardiac resynchronization therapy, Santa Casa de São Paulo, 2013
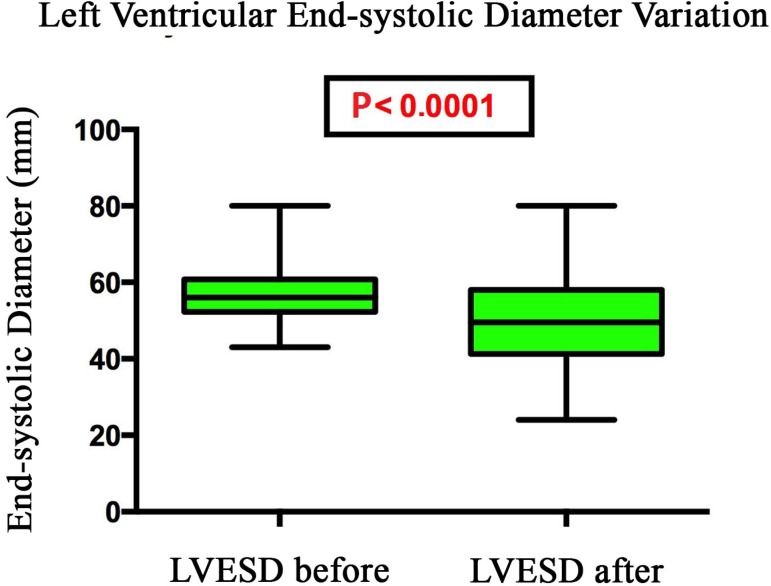
The left ventricular end-systolic diameter decreased from 57.5 mm to 50.8 mm on average after implantation of cardiac resynchronization therapy (P< 0.0001) (Figure 2).
Fig. 2.
Variation of left ventricular end-systolic diameter of patients undergoing cardiac resynchronization therapy, Santa Casa de São Paulo, 2013
The left ventricular end-diastolic diameter decreased from 66.2 mm to 65.4 mm on average after implantation of cardiac resynchronization device (P=0.295).
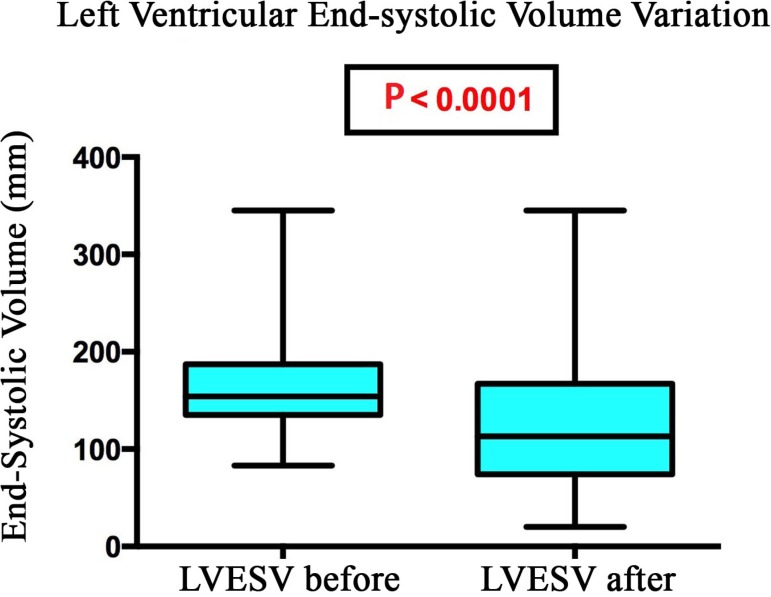
The end-systolic left ventricular volume decreased from 167.8 ml to 130.9 ml on average after implantation of the cardiac resynchronization device (P<0.0001) (Figure 3).
Fig. 3.
Change in left ventricular end-systolic volume of patients undergoing cardiac resynchronization therapy, Santa Casa de São Paulo, 2013
The left ventricular end-diastolic volume decreased from 230.0 ml to 224.5 ml on average after implantation of cardiac resynchronization device (P=0.206).
DISCUSSION
Dilated cardiomyopathy combines primary abnormalities in the heart muscle, with alterations in filling its chambers, neurohormonal activation and molecular adaptations triggered by increasing stress and the endocardial hypertrophy. In conjunction with these changes, affecting the myocardium diffusely, changes in electrical conduction can change the range of atrioventricular conduction or delay in the portions of the left ventricle relative to each other, generating a contractile dyssynchrony. This dyssynchrony is frequently observed in patients with a widened QRS complex and left bundle branch block - the standard of intraventricular conduction disturbance.
In the present study, performed in an uncontrolled way and not randomized, we observed that 87.4% of patients were in functional class I or II at the end of follow-up, and we found only 12.6% in functional class III or IV. We found 33% of patients who did not respond to cardiac resynchronization therapy, given that this is slightly higher than that found in patients with heart failure of ischemic or idiopathic etiology.
The observed increase in LVEF was 61.9 % on average between the beginning and end of follow-up, with a percentage reduction of 11.4% in left ventricular end-systolic diameter and 21.9% in left ventricular end-systolic volume.
A number of randomized studies have demonstrated that cardiac resynchronization therapy leads to reduced dimensions and internal volume of the left ventricle and increased LVEF when compared to drug therapy. Although most of the remodeling occurs between 3 and 9 months after cardiac resynchronization therapy, remodeling still occurs up to 18 months [16]. A decrease of the end-systolic left ventricular volume of more than 10% after cardiac resynchronization therapy was associated with lower mortality in an observational study [17].
At the molecular level, there is a reduction in interstitial fibrosis in the proinflammatory cytokine tumor necrosis factor-alpha and decreased cellular apoptosis [18]. The improvement in ventricular function after cardiac resynchronization therapy is also associated with favorable changes in genes that regulate the contractile apparatus and pathological myocardial hypertrophy [19].
CONCLUSION
In patients with chronic Chagas cardiomyopathy undergoing cardiac resynchronization therapy, we found the following statistically significant changes: the functional class; improvement in LVEF, decrease in left end-systolic diameter and end-systolic volume on Doppler echocardiography.
| Abbreviations, acronyms and symbols | |
|---|---|
| LVEF | Left ventricular ejection fraction |
| NYHA | New York Heart Association |
| Authors' roles and responsibilities | |
|---|---|
| EFA | Design and conduct of the study; writing of the manuscript |
| EGC | Preparation of graphics |
| APP | Review and text orientation |
| WLP | Review and drafting of the text |
| SMAG | Revisão e elaboração do textoReview and drafting of the text |
| LAR | Final review of the text |
Footnotes
No financial support.
This study was carried out at Faculty of Medical Sciences of Santa Casa de São Paulo (FCMSCSP), São Paulo, SP, Brazil.
REFERENCES
- 1.Kirchhoff LV. Changing epidemiology and approaches to therapy for Chagas disease. Cur Infect Dis Rep. 2003;5(1):59–65. doi: 10.1007/s11908-003-0066-y. [DOI] [PubMed] [Google Scholar]
- 2.Braz SC, Melo MF, Lorena VM, Souza WV, Gomes YM. Chagas disease in the state of Pernambuco, Brazil: analysis of admissions and mortality time series. Rev Soc Med Trop. 2011;44(3):318–323. doi: 10.1590/s0037-86822011005000038. [DOI] [PubMed] [Google Scholar]
- 3.Dias E, Laranja FS, Miranda A, Nobrega G. Chagas' disease; a clinical, epidemiologic, and pathologic study. Circulation. 1956;14(6):1035–1060. doi: 10.1161/01.cir.14.6.1035. [DOI] [PubMed] [Google Scholar]
- 4.Theodoropoulos TA, Bestetti RB, Otaviano AP, Cordeiro JA, Rodrigues VC, Silva AC. Predictors of all-cause mortality in chronic Chagas' heart disease in the current era of heart failure therapy. Int J Cardiol. 2008;128(1):22–29. doi: 10.1016/j.ijcard.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 5.Rassi A, Jr, Rassi A, Rassi SG. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 2007;115(9):1101–1108. doi: 10.1161/CIRCULATIONAHA.106.627265. [DOI] [PubMed] [Google Scholar]
- 6.Cazeau S, Ritter P, Bakdach S, Lazarus A, Limousin M, Henao L, et al. Four chamber pacing in dilated cardiomyopathy. Pt 2Pacing Clin Electrophysiol. 1994;17(11):1974–1979. doi: 10.1111/j.1540-8159.1994.tb03783.x. [DOI] [PubMed] [Google Scholar]
- 7.Cazeau S, Gras D, Lazarus A, Ritter P, Mugica J. Multisite stimulation for correction of cardiac asynchrony. Heart. 2000;84(6):579–581. doi: 10.1136/heart.84.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leclercq C, Cazeau S, Le Breton H, Ritter P, Mabo P, Gras D, et al. Acute hemodynamic effects of biventricular DDD pacing in patients with end-stage heart failure. J Am Coll Cardiol. 1998;32(7):1825–1831. doi: 10.1016/s0735-1097(98)00492-6. [DOI] [PubMed] [Google Scholar]
- 9.Leclercq C, Cazeau S, Ritter P, Alonso C, Gras D, Mabo P, et al. A pilot experience with permanent biventricular pacing to treat advanced heart failure. Am Heart J. 2000;140(6):862–870. doi: 10.1067/mhj.2000.110570. [DOI] [PubMed] [Google Scholar]
- 10.Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, et al. The Pacing Therapies for Congestive Heart Failure Study Group. The Guidant Congestive Heart Failure Research Group Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation. 1999;99(23):2993–3001. doi: 10.1161/01.cir.99.23.2993. [DOI] [PubMed] [Google Scholar]
- 11.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, et al. Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344(12):873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 12.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. MIRACLE Study Group Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 13.Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42(8):1454–1459. doi: 10.1016/s0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 14.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 15.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 16.Ghio S, Freemantle N, Scelsi L, Serio A, Magrini G, Passotti M, et al. Long-term left ventricular reverse remodeling with cardiac resynchronization therapy: results from the CARE-HF trial. Eur J Heart Fail. 2009;11(5):480–488. doi: 10.1093/eurjhf/hfp034. [DOI] [PubMed] [Google Scholar]
- 17.Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE, et al. Left ventricular reverse remodeling but not clinical improvements predicts long-term survival after cardiac resynchronization therapy. Circulation. 2005;112(11):1580–1586. doi: 10.1161/CIRCULATIONAHA.105.538272. [DOI] [PubMed] [Google Scholar]
- 18.D'Ascia C, Cittadini A, Monti MG, Riccio G, Sacca L. Effects of biventricular pacing on interstitial remodeling, tumor necrosis factor-alpha expression, and apoptotic death in failing human myocardium. Eur Heart J. 2006;27(2):201–206. doi: 10.1093/eurheartj/ehi579. [DOI] [PubMed] [Google Scholar]
- 19.Vanderheyden M, Mullens W, Delrue L, Goethals M, Bruyne B, Wijns W, et al. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J Am Coll Cardiol. 2008;51(2):129–136. doi: 10.1016/j.jacc.2007.07.087. [DOI] [PubMed] [Google Scholar]