Abstract
Anchored periplasmic expression (APEx) is a technology for the isolation of ligand-binding proteins from combinatorial libraries anchored on the periplasmic face of the inner membrane of Escherichia coli. After disruption of the outer membrane by Tris-EDTA-lysozyme, the inner-membrane-anchored proteins readily bind fluorescently labeled ligands as large as 240 kDa. Fluorescently labeled cells are isolated by flow cytometry, and the DNA of isolated clones is rescued by PCR. By using two rounds of APEx, the affinity of a neutralizing antibody to the Bacillus anthracis protective antigen was improved >200-fold, exhibiting a final KD of 21 pM. This approach has several technical advantages compared with previous library screening technologies, including the unique ability to screen for ligand-binding proteins that bind endogenously expressed ligands fused to a short-lived GFP. Further, APEx is able to display proteins either as an N-terminal fusion to a six-residue sequence derived from the native E. coli lipoprotein NlpA, or as a C-terminal fusion to the phage gene three minor coat protein of M13. The latter fusions allow hybrid phage display/APEx strategies without the need for further subcloning.
Recombinant antibodies are increasingly being used as therapeutic and diagnostic tools over a broad spectrum of applications, ranging from cancer treatment to microbial infections. Currently, 13 antibodies are FDA approved, and 30 more are in late-stage clinical trials (1). At the heart of the new generation of antibody therapeutics is protein engineering to reduce immunogenicity and increase antigen affinity (2). For example, we have recently reported studies with a series of antibody fragments produced by directed evolution in which toxin neutralization efficacy correlated with antitoxin antibody affinity in an animal model of anthrax intoxication (3).
Directed evolution involves first the generation of a recombinant library of protein-expressing clones with randomized sequences using molecular biology techniques, and second, the use of screening technologies for the isolation of the protein variants that exhibit the most enhanced activity. The screening of large libraries requires a physical link among a gene, the protein it encodes, and the desired function. Such a link can be established by using a variety of in vivo display technologies that have proven to be invaluable mechanistic studies, biotechnological purposes, and proteomics research (4–6).
Display on M13 bacteriophage represents the oldest and currently most widely used protein library-screening method (7, 8). Phage display has been used successfully for the isolation of antibodies from human and animal repertoire libraries. An alternative approach utilizes the anchoring of protein libraries on the surface of bacteria or yeast cells, most commonly Escherichia coli and Saccharomyces cerevisiae, respectively. Unlike phage, the relatively large size of bacteria and yeast allows screening by flow cytometry (FC) (9, 10). FC combines high-throughput with real-time quantitative multiparameter analysis of each library member. For FC screening of antibody libraries, microorganisms displaying the library are incubated with a limiting amount of a fluorescently labeled antigen, and cells exhibiting a desired level of fluorescence are isolated. With sorting rates of >400 million cells per hour, commercial FC machines can be used to screen libraries of the size accessible within the constraints of microbial transformation efficiencies. Furthermore, multiparameter FC can provide valuable information regarding the function of each and every library clone in real time, thus helping to guide the library construction process and optimize sorting conditions (11, 12).
E. coli offers facile expression of recombinant protein and high DNA transformation efficiencies that allow for efficient large library production and increased coverage of protein library sequence space. Previously, we have shown that the outer membrane of E. coli can be selectively permeabilized, allowing the diffusion of fluorescently conjugated antigens into the cell where they can bind soluble proteins localized within the periplasmic space (13). In addition, others have demonstrated that association of single-chain variable fragment (scFv) with the peptidoglycan layer can allow selection of fluorescently labeled antigen via FC (14). However, these approaches are limited to small molecule and peptide antigens. Large antigens such as proteins cannot be used because conditions that allow the accessibility of high molecular weight species to the recombinant scFv also result in the destruction of the scFv linkage to the cell.
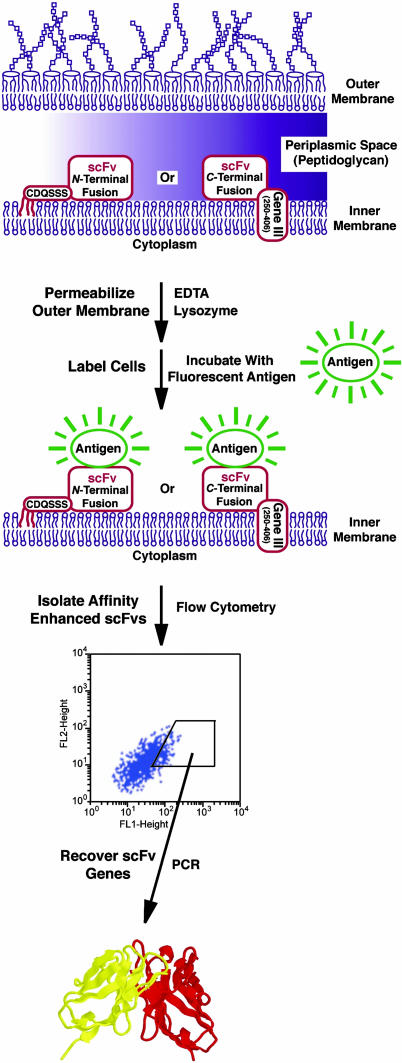
Here, we report a protein library-screening technology, based on anchored periplasmic expression (APEx). In APEx, proteins are expressed in the periplasm, tethered to the inner membrane of E. coli via lipidation of a small N-terminal 6-aa fusion or as a C-terminal fusion to the N terminus of the M13 phage gene 3 minor coat protein (g3p), for FC analysis of clones selected by phage display without further subcloning. After chemical/enzymatic permeabilization of the bacterial outer membrane, E. coli cells expressing anchored scFv antibodies can be specifically labeled with fluorescent antigens, ranging in size up to at least 240 kDa, and analyzed by FC (Fig. 1). By using APEx, we have demonstrated the efficient isolation of well expressed antibodies with markedly improved ligand affinities, including an antibody fragment to the protective antigen (PA) of Bacillus anthracis with an affinity that was increased >200-fold. Further, we show that fusions between GFP and antigen can be expressed endogenously and captured by perplasmically anchored scFv. Thus, after a washing step, cells that express both the fluorescent antigen and an APEx-anchored scFv are highly fluorescent and can be readily sorted from cells that express either only an scFv or GFP-antigen fusion alone. This feature should be particularly useful for high-throughput antibody selections in proteomics applications, epitope mapping, or when searching genomes for interacting pairs of proteins.
Fig. 1.
A schematic diagram showing the principle of APEx for the FC-based isolation of high-affinity antibody fragments.
Materials and Methods
Recombinant DNA Techniques. The leader peptide and first six amino acids of the mature NlpA protein flanked by NdeI and SfiI sites were amplified by whole-cell PCR of XL1-Blue (Stratagene) by using primers BRH#08 (5′-GAAGGAGATATACATATGAAACTGACAACACATCATCTA-3′) and BRH#09 (5′-CTGGGCCATGGCCGGCTGGGCCTCGCTGCTACTCTGGTCGCAACC-3′). The resulting NlpA fragment was used to replace the pelB leader sequence of pMoPac1 (15) via NdeI and SfiI to generate pAPEx1. scFv specific for digoxin (16), B. anthracis PA (PA) (3), and methamphetamine (Meth) (B.R.H., A. Shanafelt, B.L.I., and G.G., unpublished work) were inserted downstream of the NlpA fragment in pAPEx1 via the noncompatible SfiI sites. Corresponding g3p fusions of the scFv were made by cloning the same genes into phage display vector pAK200 (17).
Growth Conditions. E. coli ABLE C (Stratagene) was the host strain used throughout. E. coli transformed with the pAPEx1 or pAK200 derivatives were inoculated in terrific broth (12 g of pancreatic digest of casein/24 g of yeast extract/9.4 g of dipotassium phosphate/2.2 g of monopotassium phosphate, pH 7.2) supplemented with 2% glucose and chloramphenicol at 30 μg/ml to an OD600 of 0.1. Cell growth and induction were performed as described (13). After induction, the cellular outer membrane was permeabilized as described (18). Briefly, cells (equivalent to ≈1 ml of 20 OD600) were pelleted and resuspended in 350 μl of ice-cold solution of 0.75 M sucrose/0.1 M Tris·HCl, pH 8.0/100 μg/ml hen egg lysozyme. We gently added 700 μl of ice-cold 1 mM EDTA, and the suspension was left on ice for 10 min; 50 μl of 0.5 M MgCl2 was added, and the mix was left on ice for a further 10 min. The resulting cells were gently pelleted and resuspended in 1× PBS with 200 nM probe at room temperature for 45 min before evaluation by FC.
Fluorescent Probe. The synthesis of digoxigenin (Dig)–4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-propionyl ehtylenediamine (BODIPY) has been described (19). Meth–fluorescein conjugate was a gift from Roche Diagnostics. Purified PA protein, kindly provided by S. Leppla (National Institutes of Health, Bethesda), was conjugated to BODIPY plus FL SE D-2184 at a 1:7 molar ratio according to the manufacturer's instructions. Unconjugated BODIPY was removed by dialysis.
To synthesize Dig-phycoerythrin, R-phycoerythrin, 3-amino-3-dioxy-Dig hemisuccinamide, and succinimidyl ester (Molecular Probes) were conjugated at a 1:5 molar ratio according to the manufacturer's instructions. Free Dig was removed by dialysis in excess PBS.
Affinity Maturation of scFv Libraries with FC. Libraries were made from the 14B7 parental scFv by using error-prone PCR with standard techniques (20) and cloned into the pAPEx1 expression vector. Upon transformation, induction, and labeling, the cells were then stained with propidium iodide (PI) (emission 617 nm) to monitor inner membrane integrity. Cells were analyzed on a MoFlo (Cytomation, Fort Collins, CO) droplet deflection flow cytometer by using a 488-nm Argon laser for excitation. Cells were selected based on improved fluorescence in the fluorescein/BODIPY fluorescein emission spectrum detecting through a 530/40 band-pass filter and for the absence of labeling in PI emission detecting through a 630/40 band-pass filter.
E. coli captured after the first sort were immediately resorted through the flow cytometer. Subsequently, the scFv genes in the sorted cell suspension were amplified by PCR. Once amplified, the mutant scFv genes were then recloned into pAPEx1 vector, retransformed into cells, and grown overnight on agar plates at 30°C. The resulting clones were subjected to a second round of sorting plus resorting as described above, before scFv genes were subcloned into pMoPac16 (15) for expression of single-chain antibody fragment (scAb) protein.
Surface Plasmon Resonance (SPR) Analysis. Monomeric scAb proteins were purified by immobilized metal affinity chromatography size-exclusion FPLC as described (15). Affinity measurements were obtained via SPR by using a BIACORE 3000 (Biacore, Uppsala) instrument. We coupled ≈500 response units of PA to a CM5 chip by using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydroxy succinimide chemistry. BSA was similarly coupled and used for in-line subtraction. Kinetic analysis was performed at 25°C in Hepes-buffered saline-EP buffer (Biacore) at a flow rate of 100 μl/min. Five 2-fold dilutions of each antibody beginning at 20 nM were analyzed in triplicate.
Methods and procedures for the endogenously expressed antigen–GFP fusions can be found in Supporting Text, which is published as supporting information on the PNAS web site.
Results
APEx Detection of Ligand Binding. For screening applications, an ideal expression system should minimize cell toxicity or growth abnormalities that can arise from the synthesis of heterologous polypeptides. We have devised an expression system that enables the anchoring of proteins on the periplasmic face of the E. coli inner membrane via a lipoprotein targeting motif, without any of the complications that are associated with transmembrane protein fusions (21, 22) (Fig. 1). Unlike membrane proteins, bacterial lipoproteins are not known to require the signal recognition particle or YidC pathways for membrane anchoring (23). Lipoproteins are secreted across the membrane via the Sec pathway and, once in the periplasm, a diacylglyceride group is attached through a thioether bond to a cysteine residue on the C-terminal side of the signal sequence. The signal peptide is then cleaved by signal peptidase II, the protein is fatty acylated at the modified cysteine residue, and finally the lipophilic fatty acid inserts into the membrane, thereby anchoring the protein (24).
NlpA is a nonessential E. coli lipoprotein that exclusively localizes to the inner membrane (25, 26). A sequence encoding the leader peptide and first six amino acids of the mature NlpA (containing the putative fatty acylation and inner membrane targeting sites) was used for anchoring scFv antibodies to the periplasmic face of the inner membrane (see Materials and Methods). Of particular note is the aspartate residue adjacent to the fatty acylated cysteine residue that is thought to be a consensus residue for inner membrane targeting (25, 27, 28). NlpA fusions to the 26–10 antidigoxin/Dig scFv and to the anti-B. anthracis PA 14B7 scFv were constructed and expressed from a lac promoter in E. coli. Note that the presence of only a 6-aa fusion partner should have minimal influence on scFv folding, so that well expressed antibodies in this fusion format are expected to be well expressed in soluble form in E. coli.
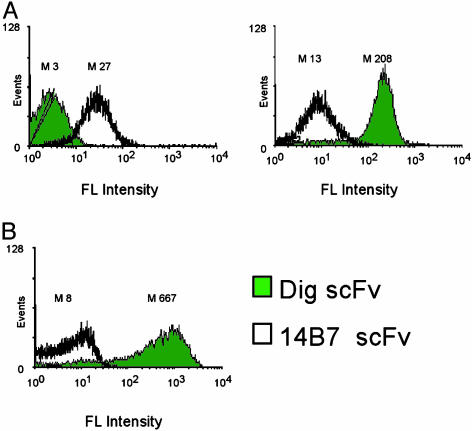
After induction of NlpA-[scFv] synthesis using isopropyl β-d-thiogalactoside, the cells were incubated with EDTA and lysozyme to disrupt the outer membrane and the cell wall. The permeabilized cells were mixed with their respective antigens conjugated to the fluorescent dye BODIPY (200 nM), and the cell fluorescence was determined by FC. Treated cells expressing the NlpA-[14B7 scFv] and the NlpA-[Dig scFv] exhibited ≈9- and 16-fold higher mean fluorescence intensity, respectively, compared to controls (Fig. 2A).
Fig. 2.
Examples of targets visualized by APEx. (A) Fluorescence distribution of ABLEC cells expressing PA-specific (14B7), scFv (white), and Dig-specific (Dig) scFv (green), and labeled with 200 nM BODIPY-conjugated fluorescent antigens. Histograms represent the mean fluorescence intensity (M) of 10,000 E. coli events. (B) Histograms of cells expressing 14B7 scFv (white) or Dig scFv (green) labeled with 200 nM of the 240-kDa Dig-phycoerythrin conjugate.
To evaluate further the ability of antibody fragments anchored on the cytoplasmic membrane to bind bulky antigens, we examined the ability of the NlpA-[Dig scFv] to recognize Dig conjugated to the 240-kDa fluorescent protein phycoerythrin (PE). The conjugate was mixed with cells expressing NlpA-[Dig scFv] and treated with EDTA-lysozyme. A high cellular fluorescence was observed, indicating binding of Dig-PE conjugate by the membrane anchored antibody (Fig. 2B). Labeling with Dig-PE followed by one round of FC resulted in a >500-fold enrichment of bacteria expressing NlpA-[Dig scFv] from cells expressing a similar fusion with an scFv having unrelated antigen specificity.
Library Screening by APEx. The gene encoding the anti-PA 14B7 scFv was mutagenized by error-prone PCR and fused to the NlpA membrane anchoring sequence in an appropriate expression vector, and the resulting library was transformed into E. coli, giving rise to 1 × 107 independent clones. DNA sequencing of 12 library clones selected at random revealed an average of 2% nucleotide substitutions per gene. After induction of NlpA–scFv synthesis with isopropyl β-d-thiogalactoside, the cells were treated with Tris–EDTA–lysozyme, washed, and labeled with 200 nM PA–BODIPY. Inner membrane integrity was monitored by staining with PI. A total of 2 × 108 bacteria were sorted by using an ultrahigh-throughput Cytomation. MoFlo droplet deflection flow cytometer selectively gating for low PI fluorescence (630-nm emission) and high BODIPY fluorescence. Approximately 5% of the cells sorted with the highest 530-nm fluorescence were collected, immediately restained with PI alone, and resorted as above. Because no antigen was added during the resort, only cells expressing antibodies with slow dissociation kinetics remained fluorescent. The plating efficiency of this population was low, presumably due to a combination of potential scFv expression toxicity (29, 30) and the Tris–EDTA–lysozyme treatment. Therefore, to avoid loss of potentially high-affinity clones, genes encoding scFvs were rescued by PCR amplification of the DNA collected from ≈1 × 104 fluorescent events resorted. It should be noted that the conditions used for PCR amplification result in the quantitative release of cellular DNA from the cells that have partially hydrolyzed cell walls due to the Tris–EDTA–lysozyme treatment during labeling. After 30 rounds of PCR amplification, the DNA was ligated into pAPEx1 and transformed into fresh E. coli. A second round of sorting was performed exactly as above, except that in this case only the most fluorescent 2% of the population was collected and then immediately resorted without relabeling.
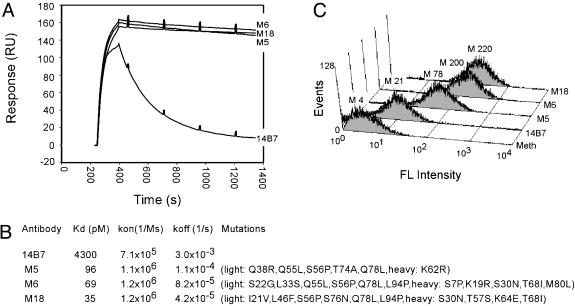
The scFv DNA from the second round was amplified by PCR and ligated into pMoPac16 (15) for expression of the antibody fragments in soluble form in the scAb format. The scAb antibody fragment is comprised of an scFv in which the light chain is fused to a human κ constant region (30, 31). We picked 20 clones in the scAb format at random, and they were grown in small-scale shake-flask cultures. After induction with isopropyl β-d-thiogalactoside, the periplasmic fractions were isolated, and the scAb proteins were rank-ordered with respect to their relative antigen dissociation kinetics using SPR analysis. Of the 20 clones, 11 exhibited slower antigen dissociation kinetics compared to the 14B7 parental antibody. The three scAbs with the slowest antigen dissociation kinetics were produced in large scale and purified as monomers through a combination of Ni chromatography followed by gel-filtration FPLC. All of the library-selected clones exhibited excellent expression characteristics and resulted in yields of 4–8 mg of purified protein per liter in shake-flask culture. Detailed SPR analysis indicated that all three clones exhibit a substantially lower KD value for PA compared with the parental 14B7 antibody (Fig. 3 A and B). The improved KD values result primarily from slower antigen dissociation (i.e., slower koff). The highest-affinity clone, M18, exhibited a KD of 35 pM, with a koff of 4.2 × 10-5·M-1·sec-1, corresponding to an M18-PA half life of 6.6 h. This represents a >120-fold affinity improvement compared to the parental antibody 14B7 (KD = 4.3 nM, as determined by BIACORE 3000).
Fig. 3.
Analysis of anti-PA antibody fragments selected using APEx. (A) SPR analysis of anti-PA scAb binding to PA. (B) Table of affinity data acquired by SPR. (C) FC histograms depicting the mean fluorescence (FL) intensity (M) of E. coli expressing anti-PA scFv clones in pAPEx1 and labeled with 200 nM PA-BODIPY conjugate as compared with those expressing the anti-Meth scFv as a negative control.
The fluorescence intensity of Tris–EDTA–lysozyme permeabilized cells expressing NlpA fusions to the isolated mutant antibodies varied in proportion to their respective antigen-binding affinity in solution (Fig. 3C). After incubation in excess buffer for 1.5 h after labeling with the fluorescent probe, cells expressing the NlpA-[M18 scFv] protein displayed a mean fluorescence of 220, and antibodies with intermediate affinities displayed intermediate fluorescence intensities.
The three clones analyzed in detail, M5, M6, and M18, contained 7-, 12-, and 11-aa substitutions, respectively. In earlier studies using phage display (3), we had isolated a variant of the 14B7 scFv by three cycles, each consisting of (i) mutagenic error prone PCR, (ii) five rounds of phage panning, and (iii) DNA shuffling of the postpanning clones. The best clone isolated in that study, 1H, contained Q55L and S56P substitutions and exhibited a KD of 150 pM. These two mutations likely increase the hydrophobicity of the binding pocket adding to the mounting evidence that an increase in hydrophobic interactions are often a dominant effect in antibody affinity maturation (32). The same amino acid substitutions are also found in the M5 and M6 clones isolated by APEx. However, the presence of the additional mutations beyond Q55L and S56P in these two clones conferred a further increase in affinity, as well as significantly improved expression levels. It is noteworthy that the M5, M6, and M18 were isolated after a single round of asexual PCR, yet they all had higher affinity relative to the best antibody that could be isolated by phage display, even after multiple rounds of sexual mutagenesis and selection.
M18, the highest-affinity clone isolated by APEx, contained the S56P mutation but lacked the Q55L substitution found in 1H, M5, and M6. When the Q55L substitution was introduced into M18 by site-specific mutagenesis, the resultant scAb exhibited a further improvement in antigen binding (KD = 21 pM) with a kon of 1.1 × 106 M-1·sec-1 and a koff of 2.4 × 10-5 sec-1, corresponding to a complex half life of almost 12 h. However, the introduction of this mutation reduced the yield of purified protein >5-fold to 1.2 mg/liter in shake-flask culture.
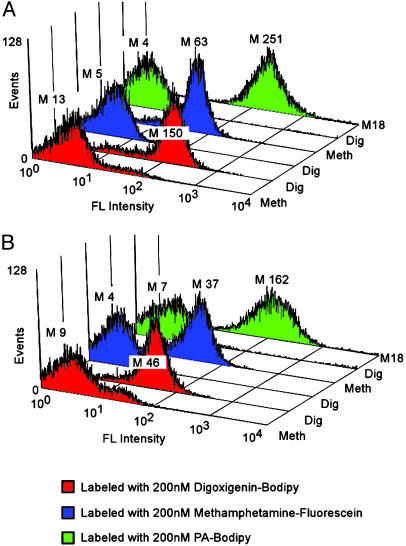
APEx of Phage Displayed scFv Antibodies. Numerous antibody fragments to important therapeutic and diagnostic targets have been isolated from repertoire libraries screened by phage display. Such repertoire libraries are an important resource in antibody discovery efforts. Antibodies are most commonly displayed on filamentous phage via C-terminal fusion to the N-terminus of the g3p (33). During phage morphogenesis, g3p becomes transiently attached to the inner membrane via its extreme C terminus, before it can be incorporated onto the growing virion (34). We evaluated whether g3p fusion proteins can be exploited for antibody library screening purposes using the APEx format. The high-affinity anti-PA M18 scFv discussed above, the antidigoxin/Dig 26–10 scFv, and an anti-Meth scFv (Meth) were cloned in-frame to the N terminus of g3p downstream from a lac promoter in phagemid pAK200 (see Supporting Text), which is widely used for phage display purposes and utilizes a short variant of gene III for g3p display (17). After induction with isopropyl β-d-thiogalactoside, cells expressing scFv-g3p fusions were permeabilized by Tris–EDTA–lysozyme and labeled with the respective fluorescent antigens (Fig. 4). High fluorescence was obtained for all three scFvs only when incubated with their respective antigens. Significantly, the mean fluorescence intensities of the scFvs fused to the N terminus of g3p were comparable to those obtained by fusion to the C terminus of the NlpA anchor. The results in Fig. 4 demonstrate that (i) antibody fragments cloned into phagemids for display on filamentous phage can be readily analyzed by FC using the APEx format, and (ii) scFv antibodies can be anchored on the cytoplasmic membrane either as N- or C-terminal fusions without loss of antigen binding.
Fig. 4.
N-vs. C-terminal anchoring strategy comparison. Data represent the mean fluorescence intensity (M) of 10,000 E. coli events. (A) Anti-Dig scfv, anti-PA M18 scFv, and anti-Meth scFv expressed as N-terminal fusions in the pAPEx1 vector in E. coli specifically label with 200 nM of their respective antigen. (B) C-terminal fusions of same scFv in pAK200 vector specifically labeled with 200 nM of their respective antigen.
Fluorescent Cell Labeling Using Endogenously Expressed Antigen–GFP Fusions. We have developed a strategy that capitalizes on APEx for labeling by antigens expressed endogenously as fusions to short-lived GFP. GFP, tagged on the C terminus with the SsrA peptide, is targeted for rapid degradation by the powerful ClpXP proteolytic machinery of the bacterial cytoplasm (35). Fusion of GFP–SsrA to the ssTorA leader that directs protein export via twin arginine transporter pathway enables export of ssTorA-GFP–SsrA protein into the periplasm where it sequestered away from the ClpXP protease and rescued from proteolytic degradation (36). As a result, cells expressing ssTorA–GFP–SsrA exhibit high fluorescence. However, the fluorescence is lost when the outer membrane is permeabilized and the ssTorA–GFP–SsrA fusion can escape into the extracellular fluid.
We constructed fusions consisting of the sequence of a peptide antigen recognized by a scFv antibody sandwiched between the ssTorA leader peptide and GFP–SsrA (see Supporting Text). The ssTorA peptide antigen–GFP–SsrA protein chimera is exported into the periplasm rendering the cells fluorescent, but after outer membrane permeabilization, the fluorescence is lost. However, in cells that also express an APEx-displayed antibody specific to the peptide antigen, the ssTorA peptide antigen–GFP–SsrA protein chimera becomes associated with the cell via its interaction with the membrane-tethered scFv antibody fragment. Thus, in this case, upon treatment with Tris–EDTA–lysozyme, the cells retain their fluorescence. This concept was demonstrated by using the 7C2 scFv that recognizes the peptide antigen (CFTFKEFQNNPNPRSLVK) from the MacI protein with a KD of 142 nM. After Tris–EDTA–lysozyme treatment, cells expressing NlpA-[7C2 scFv] together with the ssTorA peptide antigen–GFP–SsrA fusion exhibited a 3-fold higher fluorescence compared with control cells expressing (i) the ssTorA peptide antigen–GFP–SsrA fusion alone, (ii) the ssTorA peptide antigen–GFP–SsrA fusion coexpressed with an NlpA-fused irrelevant scFv, or (iii) ssTorA–GFP–SsrA without peptide antigen coexpressed with an NlpA-[7C2 scFv] (Fig. 5). Even though the fluorescence signal obtained was not as high compared with that obtained with exogenous fluorescent antigens, it is sufficient for library screening purposes. A higher signal may be obtained in strains that afford more efficient degradation of GFP–SsrA in the cytoplasm or by using leader peptides optimized for GFP export (36).
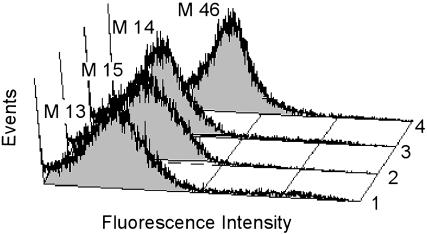
Fig. 5.
Fluorescent cell labeling using endogenously expressed antigen–GFP fusions. Histograms depict 10,000 E. coli events expressing (i) GFP–peptide fusion alone (mean = 13), (ii) GFP–peptide coexpressed with Dig scFv (mean = 15), (iii) GFP without peptide fusion coexpressed with 7C2 antipeptide scFv (mean = 14), and (iv) GFP–peptide coexpressed with 7C2 antipeptide scFv (mean = 46).
Discussion
We have developed a FC-based method using bacterial expression for the efficient selection of high-affinity ligand-binding proteins, and specifically scFv antibodies, from combinatorial libraries. APEx is based on the anchoring of proteins to the periplasmic side of the inner membrane, followed by disruption of the outer membrane before incubation with fluorescently labeled antigen and FC sorting. This strategy offers several advantages over previous bacterial periplasmic and surface display approaches. (i) APEx is an E. coli-based system and therefore provides an easy route to the creation of large libraries by transformation and preparative protein expression of isolated antibodies. (ii) By using a fatty acylated anchor to retain the protein in the inner membrane, a fusion as short as 6 aa is all that is required for display. The short fusion is unlikely to influence the affinity or expression characteristics of the isolated proteins. (iii) The inner membrane lacks molecules such as LPS or other complex carbohydrates that can sterically interfere with large antigen binding to displayed polypeptides. (iv) The fusion must only traverse one membrane before it is displayed, and therefore biosynthetic limitations that might restrict the export of certain sequences to the yeast or bacterial surface may be circumvented. (v) Display is accomplished by using either N- or C-terminal fusion. (vi) APEx can be used directly for proteins expressed from widely used phage display vectors. This latter point is particularly important because it enables the use of the many available phage display antibody fragment libraries, along with hybrid library screening strategies, in which clones from a phage panning experiment can be quantitatively analyzed or sorted further by FC without the need for any subcloning steps. Finally, (vii) APEx provides a means for the simultaneous expression of fluorescent antigen and antibodies within the same cell. This latter approach, which is likely to be particularly important for peptide antigens, circumvents time-consuming processes for synthesis, purification, and conjugation of preparative amounts of probe, as is required when the fluorescent antigen is incubated with the library.
APEx can be used for the detection of antigens ranging from small molecules (e.g., Dig and Meth, <1 kDa) to phycoerythrin conjugates (240 kDa). In fact, the phycoerythrin conjugate used in Fig. 2B is not meant to define an upper limit for antigen detection, because larger proteins have not yet been tested.
In the present study, genes encoding scFvs that bind the fluorescently labeled antigen were rescued from the sorted cells by PCR. An advantage of this approach is that it enables the isolation of clones that may not be viable because of the combination of potential scFv toxicity and Tris–EDTA–lysozyme disruption. Yet another advantage of PCR rescue is that the amplification of DNA from pooled cells can be carried out under mutagenic conditions before subcloning. Thus, after each round of selection, random mutations can be introduced into the isolated genes, simplifying further rounds of directed evolution (37). Further, PCR conditions that favor template switching among the protein-encoding genes in the pool may be used during the amplification step to allow recombination among the selected clones.
An important issue with any library screening technology is the ability to express isolated clones at a high level. Existing display formats involve fusion to large anchoring sequences, which can influence the expression characteristics of the displayed proteins. For this reason, scFvs that display well as fusions in phage, yeast, or bacteria may not necessarily be amenable to high expression in soluble form as nonfusion proteins (15). In contrast, the short (6-aa) sequence required for N-terminal tethering of proteins onto the cytoplasmic membrane in APEx is unlikely to affect the expression characteristics of the fusion. Consistent with this hypothesis, all three affinity-enhanced clones to the anthrax PA toxin isolated by APEx exhibited excellent soluble expression characteristics despite having numerous amino acid substitutions. Similarly, well expressing clones have been obtained in the affinity maturation of a Meth antibody (B.R.H., A. Shanafelt, B.L.I., and G.G., unpublished work), suggesting that the isolation of clones that can readily be produced in soluble form in bacteria on a large scale may be an intrinsic feature of APEx selections.
In this study, we used APEx for affinity maturation purposes and engineered scFvs to the B. anthracis PA exhibiting KD values as low as 21 pM. The scFv-binding site exhibiting the highest affinity for PA has been humanized and converted to full-length IgG, and its neutralizing potential to anthrax intoxication is being evaluated in preclinical studies. In addition to affinity maturation, APEx can be exploited for several other protein engineering applications, including the selection of enzyme variants with enhanced function, because the cell envelope provides sites for retention of enzymatic catalytic products, thereby enabling selection based directly on catalytic turnover (38), or for the analysis of membrane protein topology, whereby a scFv antibody anchored in a periplasmic loop is able to bind fluorescent antigen and serves as a fluorescent reporter.
Supplementary Material
Acknowledgments
We thank Dr. Stephen Leppla for providing the purified PA protein and Dr. Armen Shanafelt for providing the anti-Meth hybridoma and Meth–fluorescein conjugate. This work was supported by the U.S. Army Army Research Office/Multidisciplinary University Research Initiative program and, in connection with contract no. DADD17-01-D-0001, by the U.S. Army Research Laboratory. K.J.J. was supported by a postdoctoral fellowship from the Korea Science and Engineering Foundation (KOSEF).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: APEx, anchored periplasmic expression; FC, flow cytometry; g3p, M13 phage gene 3 minor coat protein; PA, protective antigen; Dig, digoxigenin; SPR, surface plasmon resonance; PI, propidium iodide; Meth, methamphetamine; BODIPY, 4,4-difluoro-4-bora-3{α},4{α}-diaza-s-indacene; scFv, single-chain variable fragment; scAb, single-chain Ab fragment.
References
- 1.Hudson, P. J. & Souriau, C. (2003) Nat. Med. 9, 129-134. [DOI] [PubMed] [Google Scholar]
- 2.Brekke, O. H. & Loset, G. A. (2003) Curr. Opin. Pharmacol. 3, 544-550. [DOI] [PubMed] [Google Scholar]
- 3.Maynard, J. A., Maassen, C. B., Leppla, S. H., Brasky, K., Patterson, J. L., Iverson, B. L. & Georgiou, G. (2002) Nat. Biotechnol. 20, 597-601. [DOI] [PubMed] [Google Scholar]
- 4.Hoess, R. H. (2001) Chem. Rev. 101, 3205-3218. [DOI] [PubMed] [Google Scholar]
- 5.Hayhurst, A. & Georgiou, G. (2001) Curr. Opin. Chem. Biol. 5, 683-689. [DOI] [PubMed] [Google Scholar]
- 6.Wittrup, K. D. (2000) Nat. Biotechnol. 18, 1039-1040. [DOI] [PubMed] [Google Scholar]
- 7.Rodi, D. J. & Makowski, L. (1999) Curr. Opin. Biotechnol. 10, 87-93. [DOI] [PubMed] [Google Scholar]
- 8.Smith, G. P. (1985) Science 228, 1315-1317. [DOI] [PubMed] [Google Scholar]
- 9.Feldhaus, M. J., Siegel, R. W., Opresko, L. K., Coleman, J. R., Feldhaus, J. M., Yeung, Y. A., Cochran, J. R., Heinzelman, P., Colby, D., Swers, J., et al. (2003) Nat. Biotechnol. 21, 163-170. [DOI] [PubMed] [Google Scholar]
- 10.Georgiou, G. (2000) Adv. Protein Chem. 55, 293-315. [DOI] [PubMed] [Google Scholar]
- 11.Boder, E. T. & Wittrup, K. D. (2000) Methods Enzymol. 328, 430-444. [DOI] [PubMed] [Google Scholar]
- 12.Daugherty, P. S., Chen, G., Iverson, B. L. & Georgiou, G. (2000) Proc. Natl. Acad. Sci. USA 97, 2029-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, G., Hayhurst, A., Thomas, J. G., Harvey, B. R., Iverson, B. L. & Georgiou, G. (2001) Nat. Biotechnol. 19, 537-542. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs, P., Weichel, W., Dubel, S., Breitling, F. & Little, M. (1996) Immunotechnology 2, 97-102. [DOI] [PubMed] [Google Scholar]
- 15.Hayhurst, A., Happe, S., Mabry, R., Koch, Z., Iverson, B. L. & Georgiou, G. (2003) J. Immunol. Methods 276, 185-196. [DOI] [PubMed] [Google Scholar]
- 16.Chen, G., Dubrawsky, I., Mendez, P., Georgiou, G. & Iverson, B. L. (1999) Protein Eng. 12, 349-356. [DOI] [PubMed] [Google Scholar]
- 17.Krebber, A., Bornhauser, S., Burmester, J., Honegger, A., Willuda, J., Bosshard, H. R. & Pluckthun, A. (1997) J. Immunol. Methods 201, 35-55. [DOI] [PubMed] [Google Scholar]
- 18.Hayhurst, A. & Harris, W. J. (1999) Protein Expr. Purif. 15, 336-343. [DOI] [PubMed] [Google Scholar]
- 19.Daugherty, P. S., Olsen, M. J., Iverson, B. L. & Georgiou, G. (1999) Protein Eng. 12, 613-621. [DOI] [PubMed] [Google Scholar]
- 20.Fromant, M., Blanquet, S. & Plateau, P. (1995) Anal. Biochem. 224, 347-353. [DOI] [PubMed] [Google Scholar]
- 21.Mingarro, I., von Heijne, G. & Whitley, P. (1997) Trends Biotechnol. 15, 432-437. [DOI] [PubMed] [Google Scholar]
- 22.Miroux, B. & Walker, J. E. (1996) J. Mol. Biol. 260, 289-298. [DOI] [PubMed] [Google Scholar]
- 23.Samuelson, J. C., Chen, M., Jiang, F., Moller, I., Wiedmann, M., Kuhn, A., Phillips, G. J. & Dalbey, R. E. (2000) Nature 406, 637-641. [DOI] [PubMed] [Google Scholar]
- 24.Pugsley, A. P. (1993) Microbiol. Rev. 57, 50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi, K., Yu, F. & Inouye, M. (1988) Cell 53, 423-432. [DOI] [PubMed] [Google Scholar]
- 26.Yu, F., Inouye, S. & Inouye, M. (1986) J. Biol. Chem. 261, 2284-2288. [PubMed] [Google Scholar]
- 27.Seydel, A., Gounon, P. & Pugsley, A. P. (1999) Mol. Microbiol. 34, 810-821. [DOI] [PubMed] [Google Scholar]
- 28.Yakushi, T., Masuda, K., Narita, S., Matsuyama, S. & Tokuda, H. (2000) Nat. Cell Biol. 2, 212-218. [DOI] [PubMed] [Google Scholar]
- 29.Somerville, J. E., Jr., Goshorn, S. C., Fell, H. P. & Darveau, R. P. (1994) Appl. Microbiol. Biotechnol. 42, 595-603. [DOI] [PubMed] [Google Scholar]
- 30.Hayhurst, A. (2000) Protein Expr. Purif. 18, 1-10. [DOI] [PubMed] [Google Scholar]
- 31.McGregor, D. P., Molloy, P. E., Cunningham, C. & Harris, W. J. (1994) Mol. Immunol. 31, 219-226. [DOI] [PubMed] [Google Scholar]
- 32.Li, Y., Li, H., Yang, F., Smith-Gill, S. J. & Mariuzza, R. A. (2003) Nat. Struct. Biol. 10, 482-488. [DOI] [PubMed] [Google Scholar]
- 33.Barbas, C. F., 3rd, Kang, A. S., Lerner, R. A. & Benkovic, S. J. (1991) Proc. Natl. Acad. Sci. USA 88, 7978-7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boeke, J. D. & Model, P. (1982) Proc. Natl. Acad. Sci. USA 79, 5200-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottesman, S., Roche, E., Zhou, Y. & Sauer, R. T. (1998) Genes Dev. 12, 1338-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeLisa, M. P., Samuelson, P., Palmer, T. & Georgiou, G. (2002) J. Biol. Chem. 277, 29825-29831. [DOI] [PubMed] [Google Scholar]
- 37.Hanes, J. & Pluckthun, A. (1997) Proc. Natl. Acad. Sci. USA 94, 4937-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen, M. J., Stephens, D., Griffiths, D., Daugherty, P., Georgiou, G. & Iverson, B. L. (2000) Nat. Biotechnol. 18, 1071-1074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.