Abstract
Sarcolipin (SLN) inhibits the cardiac sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2a) by direct binding and is superinhibitory if it binds through phospholamban (PLN). To determine whether overexpression of SLN in the heart might impair cardiac function, transgenic (TG) mice were generated with cardiac-specific overexpression of NF-SLN (SLN tagged at its N terminus with the FLAG epitope). The level of NF-SLN expression (the NF-SLN/PLN expression ratio) was equivalent to that which induces profound superinhibition when coexpressed with PLN and SERCA2a in HEK-293 cells. In TG hearts, the apparent affinity of SERCA2a for Ca2+ was decreased compared with non-TG littermate control hearts. Invasive hemodynamic and echocardiographic analyses revealed impaired cardiac contractility and ventricular hypertrophy in TG mice. Basal PLN phosphorylation was reduced. In isolated papillary muscle subjected to isometric tension, peak amplitudes of Ca2+ transients and peak tensions were reduced, whereas decay times of Ca2+ transients and relaxation times of tension were increased in TG mice. Isoproterenol largely restored contractility in papillary muscle and stimulated PLN phosphorylation to wild-type levels in intact hearts. No compensatory changes in expression of SERCA2a, PLN, ryanodine receptor, and calsequestrin were observed in TG hearts. Coimmunoprecipitation indicated that overexpressed NF-SLN was bound to both SERCA2a and PLN, forming a ternary complex. These data suggest that NF-SLN overexpression inhibits SERCA2a through stabilization of SERCA2a–PLN interaction in the absence of PLN phosphorylation and through the inhibition of PLN phosphorylation. Inhibition of SERCA2a impairs contractility and calcium cycling, but responsiveness to β-adrenergic agonists may prevent progression to heart failure.
Ca2+ enters cardiomyocytes through L-type Ca2+ channels in the transverse tubules and ryanodine receptor-type Ca2+ release channels in the sarcoplasmic reticulum (SR) to initiate muscle contraction and is removed to induce relaxation by the sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2a), which transports Ca2+ into the SR lumen, plasma membrane Ca2+ ATPases, and Na+/Ca2+ exchangers, which discharge Ca2+ to the extracellular space. SERCA2a is regulated by phospholamban (PLN) and can potentially be regulated by a homologous protein, sarcolipin (SLN) (1, 2).
PLN is a 52-aa SR membrane protein expressed abundantly in cardiac muscle (3). In its dephosphorylated form, PLN interacts with SERCA2a to inhibit Ca2+ transport by lowering the apparent affinity of SERCA2a for Ca2+. When PLN is phosphorylated, its inhibitory effect on SERCA2a is relieved. The ability of PLN to regulate SERCA2a activity, thereby regulating the rate of cardiac relaxation and the size of the SR Ca2+ store, makes PLN a crucial regulator of cardiac function (1, 2, 4, 5).
SLN is a 31-aa SR membrane protein, which has no obvious phosphorylation site (6). Studies based on coexpression of PLN, SLN, and SERCA in heterologous cell culture show that PLN and SLN have similar ability to inhibit either SERCA1a or SERCA2a (7–9). PLN and SLN can form a binary complex that is superinhibitory, presumably because of the formation of a ternary PLN–SLN–SERCA2a complex (9, 10). Thus, SLN can regulate SERCA through either direct interaction with SERCA or through stabilization of the interaction between SERCA and PLN (10).
SLN mRNA is expressed abundantly in human fast-twitch skeletal muscle and in small amounts in cardiac and slow-twitch skeletal muscle (6), but, in rats, SLN may be expressed more abundantly in cardiac muscle (11). In mice, SLN mRNA is present in the atrium but is virtually absent from the ventricle (12), whereas PLN mRNA expression is much lower in the atrium than it is in the ventricle (13). The overexpression of SLN in rat soleus muscle impairs skeletal muscle contractility by inhibiting the Vmax of SERCA function without altering its Ca2+ affinity (14). However, the role of SLN in the heart in vivo is still unknown.
In this study, we investigated the biochemical and physiological consequences of cardiac-specific, transgenic (TG) overexpression of NF-SLN (SLN tagged at its N terminus with the FLAG epitope) in mouse hearts expressing endogenous amounts of wild-type (wt) PLN. We report impairment of cardiac contractility, which can be reversed by isoproterenol, and mild progression to cardiac hypertrophy.
Materials and Methods
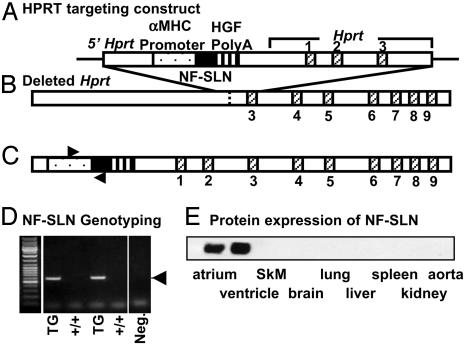
Generation of TG Mice with Cardiac-Specific Overexpression of SLN. Rabbit NF-SLN, a fusion protein of SLN with the FLAG epitope (MDYKDDDDK) at its N terminus, has been described (8). NF-SLN cDNA was incorporated into a targeting vector (the Gateway vector), so that the final construct contained upstream sequences of the hypoxanthine-guanine-phosphoribosyltransferase (Hprt) locus, including part of the Hprt promoter; the α-myosin heavy-chain promoter; NF-SLN cDNA; a polyadenylation signal; and Hprt genomic DNA containing exons 1–3 (Fig. 1A). The targeting vector was transfected into BK4 cells, an HPRT-deficient E14Tg2a embryonic stem cell line, which lacks the promoter and the first two exons of the Hprt gene (Fig. 1B). The construct was then targeted into the Hprt locus by homologous recombination (15). Successful homologous recombination corrects the HPRT-deficiency, restoring the ability of embryonic stem cells to grow in hypoxanthine/aminopterin/thymidine medium. Animals were genotyped by PCR with primers directed against segments of the α-MHC promoter and the SLN coding sequence (Fig. 1D).
Fig. 1.
Strategy for generation of TG mice with cardiac–specific overexpression of NF-SLN. (A) HPRT targeting construct. The targeting vector contains the promoter and the first two exons of Hprt, the α-myosin heavy-chain promoter, NF-SLN, and the human growth factor (HGF) polyadenylation signal sequence. (B) Depleted Hprt. The mutant Hprt gene in the HPRT-deficient E14Tg2a embryonic stem cell line, BK4, is shown with the sites of targeting vector insertion. (C) Transfection of this vector into E14Tg2a cells, together with successful homologous recombination, corrects the HPRT deficiency, restoring the ability of the cells to grow in hypoxanthine/aminopterin/thymidine medium, allowing for rapid screening of positive clones. (D) PCR genotyping was performed by using a 5′ primer within the α-MHC promoter sequence and a 3′ primer within the NF-SLN sequence (forward and reverse arrows in C). Neg., negative control. (E) Protein expression of NF-SLN. The expression levels of NF-SLN were determined in various tissues by Western blot analysis with the anti-FLAG antibody, M2.
Analysis of the Level of NF-SLN Overexpression. Because the lack of a mouse SLN-specific antibody prevented quantification of SLN expression and because endogenous SLN levels in the heart were believed to be low, a functional approach to measurement of NF-SLN expression was devised. The coexpression in HEK-293 cells of NF-SLN with PLN and either SERCA1a or SERCA2a led to superinhibition of Ca2+ transport activity (8, 9). Optimal superinhibition was achieved when NF-SLN (or SLN), PLN, and SERCA2a cDNAs were transfected in a ratio of 6:6:8 μg per 100-mm plate. The ≈1:1 ratio between the molar ratios of NF-SLN and PLN cDNAs transfected into HEK-293 cells and the prediction that NF-SLN and PLN form a 1:1 binary complex that is superinhibitory (2) suggests that expression of NF-SLN and PLN was approximately equimolar under these conditions.
Semiquantitative Western blotting was carried out with the 1D11 antibody against PLN to obtain a measure of PLN expressed per mg of protein in microsomal fractions from HEK-293 cells and from TG mouse hearts. Aliquots of microsomal fractions from the two different sources were adjusted to achieve approximately equal staining in an experimental gel (n = 3). A duplicate blot was then stained with the anti-FLAG antibody (M2, Sigma) against NF-SLN and developed to achieve staining approximately equal to that observed with the 1D11 antibody. The band intensity of PLN was designated 100%, and a ratio was calculated between the band intensity of NF-SLN and the band intensity of PLN in microsomes from HEK-293 cells. From the same blots, it was then possible to obtain the ratio between the band intensity of NF-SLN expressed in TG ventricles and the band intensity of endogenous PLN expressed in the same sample. A similar experiment to quantify SERCA2a was carried by using duplicate experimental blots and the 2A7-A1 antibody (Affinity BioReagents, Golden, CO).
Ca2+ Transport. ATP and oxalate-dependent Ca2+ uptake activity in whole-heart homogenates was measured by using a Millipore filtration method (16, 17). The Ca2+ concentration required for half maximal velocity for Ca2+ uptake (EC50) was determined by nonlinear curve fitting by using prism 3.0 (GraphPad, San Diego).
In Vivo Echocardiographic and Hemodynamic Assessment of Cardiac Function. Experiments were performed as described in refs. 18 and 19. Some animals were treated with 2 μg/g isoproterenol injected directly into the jugular vein. Following maximal responses to the drug as assessed by continuous hemodynamic recording, hearts were snap-frozen in liquid nitrogen for immunoblot analyses.
Measurement of Ca2+ Transients and Tension in Left Ventricular (LV) Papillary Muscles. The Ca2+ sensitive photoprotein, aequorin, was used to measure Ca2+ transients, as described in ref. 20. Briefly, aequorin was microinjected into 50–70 superficial cells of the preparation. The aequorin light signal was detected with a photo-multiplier and was recorded simultaneously with recordings of tension at 30°C, with a stimulation frequency of 0.2 Hz. The light signals were converted to [Ca2+]i by using an in vitro calibration curve. For evaluation of the effects of SLN, the following parameters were measured: peaks of light and tension; time-to-peak light and tension (the time measured from the onset of stimulus to the peak of light and tension, respectively), decay time of light (the time for the light signal to decay from 75% to 25% of the peak), and relaxation time (the time required for tension to decay from 75% to 25% of the peak). Papillary muscles were also treated with 100 nM isoproterenol, and maximum changes in contractile and Ca2+ transients were measured. Coimmunoprecipitation of SERCA and PLN from microsomal fractions was carried out as described in ref. 21.
Results
Generation of NF-SLN TG Mice. We generated a TG mouse line expressing a modified form of SLN (NF-SLN), which retains the full inhibitory properties of wt SLN (8). The NF-SLN cDNA construct, under the control of the cardiac muscle-specific α-MHC promoter, was targeted into the Hprt locus by homologous recombination. Because the Hprt locus is on the X chromosome, only a single copy of the transgene was expressed. Confocal microscopy with the anti-FLAG antibody, M2, showed uniform expression of NF-SLN in male hearts but a mosaic of NF-SLN expression in female hearts, in line with random X chromosome inactivation (data not shown). Male and female TG mice were born at the expected Mendelian frequency and were indistinguishable in appearance from non-TG littermate controls (wt). Western blot analysis of homogenates from TG tissues with the M2 antibody indicated that expression of NF-SLN protein was cardiac-specific (Fig. 1E).
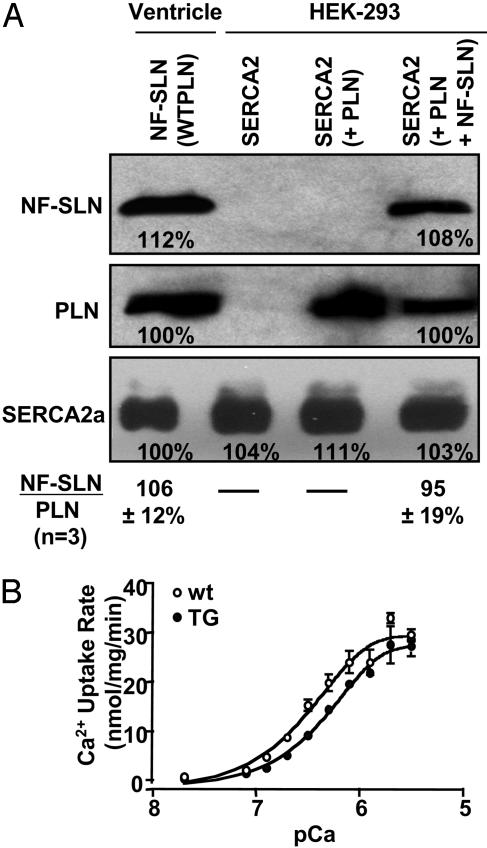
Quantification of NF-SLN Expression. Measurement of the level of expression of NF-SLN protein in microsomes from TG hearts was not absolute but was designed to determine whether sufficient amounts of NF-SLN were expressed to cause superinhibition of SERCA2a in association with endogenous PLN (2). Semiquantitative Western blotting with PLN antibody 1D11 was used to compare the amount of PLN expressed per mg of protein in microsomal fractions from HEK-293 cells, in which superinhibition was optimal, with that from TG mouse hearts. Protein sample sizes were then adjusted to achieve approximately equal band intensity for PLN in the TG ventricle and HEK-293 cell lanes, and these levels were designated as 100% (Fig. 2A). Duplicate blots were then stained with the M2 antibody against the FLAG epitope in NF-SLN. The band intensity for NF-SLN from HEK-293 cells, at 108% of PLN band intensity, was almost identical to the NF-SLN level of 112% of PLN for TG ventricles (Fig. 2A). These results indicate that the amount of NF-SLN expressed in HEK-293 cells, relative to PLN coexpression (average = 95%, n = 3), was almost identical to the amount of NF-SLN expression in TG ventricles (average = 106%, n = 3), relative to endogenous PLN expression. Thus, we deduce that the level of NF-SLN expression in TG hearts was approximately equimolar with the level of PLN expression and at a level that should yield optimal superinhibition, on the basis of our studies with HEK-293 cells.
Fig. 2.
Relative quantification of NF-SLN expression in microsomal fractions from transfected HEK-293 cells and TG hearts. HEK-293 cells were grown to confluence in a 100-cm plate and transfected with 6 μgofPLN,6 μg of NF-SLN, and 8 μg of SERCA1a cDNAs. (A) After 48 h, microsomal fractions were isolated, and 7.5 μg of total protein was separated by SDS/PAGE and stained separately with the M2 antibody against NF-SLN and the 1D11 antibody against PLN. The ratio of NF-SLN band intensity to PLN band intensity averaged 95% (n = 3). Microsomal fractions were also isolated from hearts from 10-week-old TG mice, and Ca2+ transport activity was shown to be superinhibited (data not shown). Microsomal proteins (12.5 μg) were separated by SDS/PAGE and stained separately with antibody M2 against NF-SLN and with antibody 1D11 against PLN. The ratio of NF-SLN band intensity to PLN band intensity averaged 106% (n = 3). In the bottom row, identical aliquots were stained with the antibody 2A7-A1 against SERCA2a. The fact that the band intensity in each of the four lanes varied between 100% and 111% shows that SERCA2a content was comparable in all four samples. (B) Ca2+ uptake activity in cardiac ventricular homogenates prepared from TG (n = 3) or wt (n = 3) mice. Data are expressed as mean ± SEM.
A similar experiment with duplicate experimental blots was carried out with SERCA2a antibody 2A7-A1 (Fig. 2A). The band intensity for endogenous mouse ventricular SERCA2a was virtually the same as the band intensity for rabbit recombinant DNA expressed in HEK-293 cells. Within the limits imposed by the crossreaction of this antibody (raised against canine SERCA2a) between mouse and rabbit proteins, it appeared that the balance among SERCA2a, PLN, and NF-SLN was almost the same in TG ventricles as it was in HEK-293 cells, where superinhibition of SERCA activity can be attributed to NF-SLN expression.
Ca2+ Uptake Activity in TG Hearts. In analysis of the initial rate of Ca2+ uptake in cardiac homogenates (Fig. 2B), a shift to the right in the sigmoid curve measuring Ca2+ dependence of Ca2+ uptake in TG mice showed a reduced apparent Ca2+ affinity in TG hearts. The EC50 value for Ca2+ increased significantly in TG mice compared to wt mice (0.60 ± 0.06 μM for TG mice vs. 0.38 ± 0.03 μM for wt mice). The Vmax of Ca2+ uptake, however, was not significantly different between TG mice and wt mice (29.6 ± 1.2 nmol of Ca2+ per mg·min-1 for SLN TG vs. 32.2 ± 2.3 nmol of Ca2+ per mg·min-1 for wt). This result contrasts with studies in HEK-293 cells (9) and in rat skeletal muscle (14), where Vmax was reduced by the expression of NF-SLN.
Physical, Hemodynamic, and Echocardiographic Characteristics of TG Hearts. Significant differences between TG and wt hearts were observed in the ratios of LV weight to both tibial length and body weight at 10 weeks of age (Table 1). Cardiac contractility was impaired significantly in the TG mice as the first derivative of the minimum LV pressure (-dp/dt) and the first derivative of maximum LV pressure (+dp/dt) were significantly lower in TG mice compared with those of wt mice. Similar reductions were observed in the fractional shortening, velocity of circumferential shortening corrected, and peak early-filling transmitral diastolic wave velocity. The Doppler analysis of the peak early-filling transmitral diastolic wave velocity provides an accurate estimate of LV relaxation (22), and its reduction in TG mice suggests impaired diastolic function (Table 1). There were no significant differences in LV systolic pressure or end-diastolic pressure between TG and wt mice. No significant progression of cardiac dysfunction was observed with age in 8-month-old TG mice.
Table 1. Echocardiographic and hemodynamic parameters in TG mice with cardiac-specific overexpression of SLN.
| 10 weeks
|
8 months
|
|||
|---|---|---|---|---|
| Parameters | wt (n = 9) | NF-SLN TG (n = 5) | wt (n = 11) | NF-SLN TG (n = 5) |
| H/BW, mg/g | 3.93 ± 0.11 | 4.51 ± 0.14* | 4.1 ± 0.1 | 4.1 ± 0.16 |
| H/TL, mg/cm | 6.1 ± 0.14 | 7.1 ± 0.3† | 7.5 ± 0.53 | 7.6 ± 0.15 |
| HR, bpm | 572 ± 8 | 568 ± 6 | 542 ± 13.1 | 529 ± 19.9 |
| AW, mm | 0.711 ± 0.008 | 0.721 ± 0.007 | 0.77 ± 0.012 | 0.83 ± 0.03 |
| PW, mm | 0.717 ± 0.007 | 0.724 ± 0.009 | 0.78 ± 0.012 | 0.83 ± 0.024 |
| LVEDD, mm | 4.21 ± 0.04 | 4.2 ± 0.06 | 4.46 ± 0.03 | 4.57 ± 0.027† |
| LVESD, mm | 1.98 ± 0.03 | 2.43 ± 0.05* | 2.051 ± 0.05 | 2.67 ± 0.115* |
| FS, % | 52.9 ± 1.1 | 42.1 ± 0.9* | 53.9 ± 0.93 | 41.66 ± 2.36* |
| ETc, ms | 48.7 ± 1.0 | 52.5 ± 1.3† | 49.33 ± 1.163 | 51.274 ± 1.61 |
| VCFc, circ/s | 10.86 ± 0.19 | 7.98 ± 0.11* | 10.98 ± 0.293 | 8.12 ± 0.35* |
| PAVc, cm/s | 112 ± 2.7 | 96 ± 2.4* | 116.11 ± 4.8 | 90.498 ± 6.23† |
| E-wave, cm/s | 86.7 ± 1.8 | 70.5 ± 1.5* | 81.79 ± 3.0 | 68.5 ± 3.0† |
| LVESP, mmHg | 120.13 ± 5.91 | 117.87 ± 4.2 | 120.13 ± 5.23 | 122.96 ± 2.79 |
| LVEDP, mmHg | 4.6 ± 2.2 | 6.25 ± 2.1 | 4.4 ± 1.17 | 5.2 ± 1.5 |
| +dP/dTmax, mmHg/s | 10,156.12 ± 487.1 | 8,182.4 ± 487.13† | 9,181.6 ± 740.4 | 6,605.5 ± 834.8‡ |
| —dP/dTmax, mmHg/s | 9,527.022 ± 350.9 | 8,330.589 ± 349.6† | 9,052.5 ± 655.54 | 7,526.6 ± 1044.9 |
Values are mean ± SEM. HR, heart rate measured in beats per minute (bpm); H/BW, heart/body weight ratio; H/TL, heart/tibial length ratio; AW and PW, anterior and posterior (respectively) wall thickness (LV); LVEDD and LVESD; LV end diastolic and systolic dimension, respectively; FS, fractional shortening calculated as (LVEDD — LVESD)/LVEDD × 100% ETc, ejection time corrected for HR; VCFc, velocity of circumferential shortening corrected for HR = FS/ETc measured in circumferences (circ.) per second; PAVc, peak aortic velocity corrected for HR; E-wave, early-filling transmittal diastolic wave; LVESP and LVEDP, LV end systolic and diastolic pressure, respectively, measured in mmHg (1 mmHg = 133 Pa); +dP/dtmax, maximum positive first derivative of the LV pressure; —dP/dtmax, maximum negative first derivative of the LV pressure. *, P < 0.01 compared with controls. †, P < 0.05 compared with controls. ‡, P = 0.054.
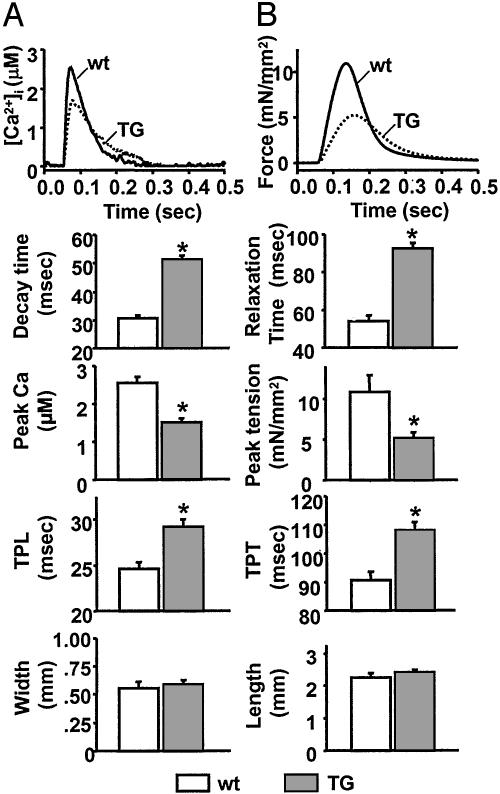
Measurement of Ca2+ Transients and Tension in LV Papillary Muscles. Intracellular Ca2+ kinetics and contractile properties were examined in aequorin-loaded papillary muscle in 2 mM [Ca2+]o at a stimulation frequency of 0.2 Hz (Fig. 3). The diameter and length of the papillary muscle preparations were not significantly different between TG and wt mice. Isolated papillary muscle from TG hearts exhibited a significant decrease in peak Ca2+ amplitude relative to wt hearts, suggesting that the size of the Ca2+ store was diminished and the time to peak amplitude was increased in TG animals. After reaching a peak, the Ca2+ transients declined more slowly in TG mice than in wt mice, in line with diminished SERCA2a activity. Isometric tension measurements on the papillary muscles showed an increase in time to peak tension and a decrease in peak tension. Relaxation time increased in TG mice compared with wt mice, in line with diminished SERCA2a activity.
Fig. 3.
Ca2+ transients and tension in left ventricular papillary muscles. Dissected papillary muscles (n = 8 for TG mice and 10 for wt mice) were microinjected with aequorin. (A and B) Aequorin light signals and tensions (stimulation frequency, 0.2 Hz) were recorded simultaneously at 30°C. Shown are traces representing light signal (A) and tension (B). The light signals were converted to [Ca2+]i by using an in vitro calibration curve. Decay time is the time for the light signal to decay from 75% to 25% of the peak. Relaxation time is the time required for the tension to decay from 75% to 25% of the peak. Time-to-peak light (TPL) and time-to-peak tension (TPT) are the times measured from the onset of stimulus to the peaks of light and tension, respectively. Width and length are those of the isolated papillary muscle preparation. *, P < 0.05 vs. wt.
Quantification of SR Proteins and PLN Phosphorylation by Western Blot Analysis. To rule out compensatory changes in the expression of other Ca2+ signaling proteins, levels of the major Ca2+ signaling proteins in TG hearts were measured by semiquantitative Western blotting with appropriate antibodies. The expression of SERCA2a, ryanodine receptor 2, and calsequestrin 2 was not significantly different between TG and wt mice (data not shown).
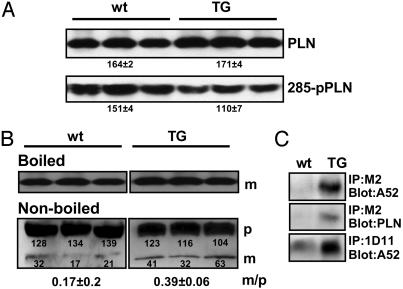
A comparison of total PLN levels in TG and wt hearts showed no obvious difference (in arbitrary densitometric units: wt, 164 ± 2; TG, 171 ± 4), but the level of phosphorylated PLN in TG hearts was reduced by ≈40% (wt, 151 ± 4; TG, 110 ± 7; Fig. 4A). Because NF-SLN can change the monomer/pentamer ratio of PLN when NF-SLN and PLN are coexpressed in HEK-293 cells (9), homogenates from TG or wt hearts were dissolved in SDS sample buffer, and samples were either boiled for 10 min before application to SDS/PAGE to depolymerize PLN pentamers or subjected directly to SDS/PAGE and immunoblotting (Fig. 4B). In nonboiled samples, monomeric PLN in TG hearts was increased, resulting in an increase in the monomer/pentamer ratio (wt, 0.17 ± 0.2 vs. TG, 0.39 ± 0.06). There was no significant difference between TG and wt mice in the levels of total monomeric PLN protein in the boiled samples (96.1 ± 3.5 vs. 98.4 ± 1.6, respectively). These observations support the view that NF-SLN interacts with and depolymerizes PLN in TG hearts.
Fig. 4.
Properties of PLN in NF-SLN TG mice. (A) Western blot analyses of ventricular samples to detect PLN and phospho-PLN (285-pPLN) and PLN from wt and TG hearts. Numbers represent the mean ± SEM in arbitrary densitometric units. Note the reduced level of PLN phosphorylation in TG samples. (B) Monomer/pentamer ratio (m/p) of PLN in TG hearts. Boiled or nonboiled microsomal fractions were subjected to immunoblotting with anti-PLN antibody. p, Pentameric PLN; m, monomeric PLN. (C) Physical interaction of PLN with SERCA2a in TG hearts. Microsomal fractions were subjected to immunoprecipitation with anti-PLN antibody, 1D11, or anti-FLAG antibody, M2. Precipitates were separated by SDS/PAGE, and SERCA2a or PLN were detected by immunoblotting.
Physical Interaction of PLN with SERCA in TG Hearts. The M2 antibody coimmunoprecipitated both SERCA2a and PLN in homogenates from TG hearts, suggesting that NF-SLN interacts with both SERCA2a and PLN (Fig. 4C). When the cardiac homogenates were immunoprecipitated with PLN antibody 1D11, the amount of SERCA2a in the precipitates was greater in TG mice than in wt mice, indicating that NF-SLN stabilized the interaction between SERCA2a and PLN in vivo. Given that physical and functional assays for measurement of the interaction of PLN with SERCA are well correlated (23), it can be deduced that stabilization of the physical interaction between SERCA and PLN results in a reduction in SERCA2a activity. These data suggest that SLN overexpression inhibits the activity of SERCA2a through the stabilization of the SERCA2a/PLN interaction, thereby impairing cardiac function in mice.
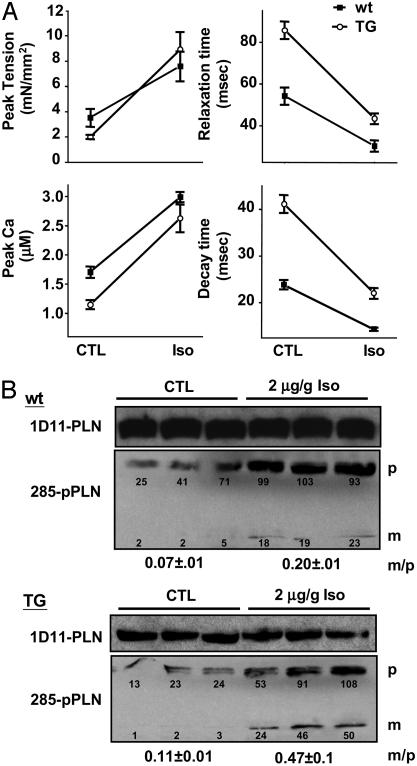
Reversal of Cardiac Impairment in NF-SLN TG Mice by Isoproterenol. Papillary muscle and whole hearts were treated with 100 nM or 2 μg/g isoproterenol, respectively, to determine whether β-adrenergic activation could reverse the observed functional impairment of cardiac muscle in TG muscle. Analysis of isolated wt papillary muscle shows that isoproterenol enhanced the force of cardiac contraction and the rate of relaxation, as assessed by measurements of peak tension and relaxation time (Fig. 5A). The addition of isoproterenol also resulted in a significant increase in peak Ca2+ amplitude and a decrease in time-to-decay of the Ca2+ transient. These indicators of contractility were all significantly impaired in the TG samples but were largely restored by application of the β-adrenergic agonist.
Fig. 5.
Response to isoproterenol. (A) Isolated papillary muscles from wt (n = 7) and TG (n = 9) mice were isolated and analyzed as described in the legend to Fig. 3 in the absence (CTL) and presence (Iso) of 100 nM isoproterenol applied to the surface of the muscles. (B) Hemodynamic measurements on whole hearts were taken after the administration of 2 μg/g isoproterenol through the jugular vein of anesthetized animals. Under resting conditions (CTL) and at the point of maximal changes as assessed by continuous hemodynamic recordings (Iso), ventricular tissue (n = 3 in each condition) was isolated, snap-frozen, and analyzed by immunoblotting with antibodies to PLN (1D11) and phosphorylated PLN (285). Numbers represent the mean ± SEM in arbitrary densitometric units. p, Pentameric PLN; m, monomeric PLN; m/p, the ratio of monomeric to pentameric PLN.
Based on the observation that both wt and TG muscles showed similar levels of peak tension after isoproterenol treatment, we speculated that under these conditions PLN phosphorylation would be increased in both samples. To confirm this postulate, isoproterenol was injected into the jugular vein and the response to the drug was monitored by continuous hemodynamic recording. At the time of peak response to isoproterenol, assessed by maximal changes in blood pressure and heart rate, cardiac muscle was snap-frozen and later subjected to immunoblotting with antibodies to phosphorylated PLN (Ab 285) and PLN (Ab 1D11). PLN phosphorylation was increased several fold in both wt and TG samples after the application of isoproterenol. Although the levels of phosphorylated PLN were initially lower in TG samples (Figs. 4B and 5B), there was a substantial increase in PLN phosphorylation to levels similar to those observed in the wt mouse, a finding consistent with our functional data (Fig. 5A). We also observed greater levels of phosphorylated monomeric PLN under these conditions, coherent with the higher levels of monomeric PLN in these animals. We determined that the monomer/pentamer ratio in wt mice was 0.07 ± 0.01 under control conditions and 0.20 ± 0.08 with isoproterenol; in TG mice the ratios were 0.11 ± 0.03 and 0.47 ± 0.01, respectively.
Discussion
In this study, we generated TG mice with cardiac-specific overexpression of NF-SLN as a first step in the investigation of a potential role for SLN in cardiac function. PLN is expressed abundantly in the ventricle (24), whereas SLN is expressed abundantly in the atrium (12) and in skeletal muscle (6, 11). This finding probably means that these homologous molecules are designed to function in isolation from each other through the tissue specificity of their promoters. However, we cannot assume that SLN expression is confined to the atrium in other species. In particular, the expression of SLN in rat ventricles appears to occur at relatively high levels (11). Moreover, barriers to coexpression might break down under abnormal conditions, such as the elevation of thyroid hormone (T3), which has dramatic effects on the expression of a number of muscle proteins (25–27). The key impetus for our investigation of the effects of overexpression of SLN in the heart was our finding that a ternary complex of PLN, SLN, and SERCA2a is highly superinhibitory and could have serious consequences for cardiac contractility and progression to failure (9).
A single copy of the NF-SLN transgene was integrated into the Hprt locus in embryonic stem cells, and the cells were used to generate TG mice. Advantages of this method over conventional protocols are, first, that selection is rapid and highly efficient (given that repair of the Hprt locus permits selection with hypoxanthine/aminopterin/thymidine medium), which permits rapid generation of TG mice and, second, that the site of insertion is known, obviating not only the possibility of disruption of another functional gene or location-sensitive suppression or activation of additional genes but also the need to generate more than one TG line; successive lines generated by insertion of mutant cDNAs at the same site will be directly comparable. Disadvantages of the method are the inability to regulate expression through an increase in copy number and the fact that females must be homozygous for the transgene before they can be compared with males.
Our earlier studies of NF-SLN expression in heterologous cell culture showed that NF-SLN can inhibit the activity of either SERCA1a or SERCA2a by lowering its apparent Ca2+ affinity, and we showed that equimolar expression of NF-SLN with PLN results in superinhibition (8, 9). In the TG hearts, which express equimolar levels of NF-SLN and PLN, we observed the expected phenotype: The affinity of SERCA2a for Ca2+ was decreased in microsomal fractions isolated from TG hearts; hemodynamic and echocardiographic assessment of TG animals showed that contractility was impaired and relaxation was delayed; and measurement of Ca2+ transients and tension in LV papillary muscles showed that peak Ca2+ amplitudes and peak tension were decreased significantly and decay time for the Ca2+ transient and relaxation time for muscle tension were extended. These results are consistent with the view that SERCA2a inhibition by the NF-SLN–PLN binary complex that was shown to exist in the muscle results in a reduced rate of Ca2+ sequestration, a delay in cardiac muscle relaxation, and a reduced SR Ca2+ store. Our evidence showed that contractility could be restored by the addition of the β-adrenergic agent, isoproterenol. This restoration was accompanied by extensive PLN phosphorylation, presumably leading to dissociation of the NF-SLN-PLN binary complex from SERCA2a.
These results can be evaluated in relation to results obtained by the overexpression of superinhibitory and other mutant forms of PLN in TG mouse hearts. The 2-fold overexpression of wt PLN reduced cardiac contractility but did not progress to heart disease (28). The overexpression of the superinhibitory mutants PLN L37A and PLN I40A impaired contractility and induced minimal hypertrophy but did not lead to heart failure (29, 30). Impairment was reversible by isoproterenol. Overexpression of the PLN N27A superinhibitory mutant impaired contractility more severely, and function was not fully restored by isoproterenol (31). This mutation led to gender-specific heart failure, with female mice being more resistant to early heart failure (32). By contrast, the PLN R9C mutant (33) was not superinhibitory, but its failure to be phosphorylated resulted in a depressed response to β-adrenergic agonists. This mutation led to dilated cardiomyopathy by 24 weeks. In summary, these results suggest that superinhibition of SERCA2a will induce cardiac hypertrophy but will not progress to dilated cardiomyopathy and heart failure if β-adrenergic stimulation of the heart can reverse SERCA2a inhibition. As a corollary, PLN need not be superinhibitory to induce dilated cardiomyopathy, but it will induce heart failure if it is chronically inhibitory (33). If the heart cannot respond to β-adrenergic stimulation, heart failure will result.
It is of interest that basal phosphorylation of PLN was decreased in TG animals. It is not yet known whether the PLN-SLN binary complex is resistant to phosphorylation, nor is it known whether PLN phosphorylation dissociates the PLN-SLN complex. If basal levels of PLN phosphorylation are reduced, then basal SERCA2a activity should also be reduced. Thus, there may be three mechanisms by which SLN reduces cardiac function: (i) direct inhibition of SERCA2a by NF-SLN; (ii) formation of the highly inhibitory PLN:NF-SLN binary complex, which, when combined in a ternary complex with SERCA2a, stabilizes SERCA2a in the inactive E2 conformation; and (iii) decreased PLN phosphorylation.
Our results show that overexpression of NF-SLN in both the atrium and the ventricle of mouse hearts impairs cardiac contractility, raising the possibility that induction of expression of SLN in human hearts could impair cardiac function. In humans, SLN mRNA is expressed to a minor extent in cardiac muscle (6), suggesting that SLN does not normally play a prominent regulatory role in cardiac muscle. However, because there is often a discrepancy between mRNA and protein levels, the expression of SLN protein in human cardiac muscle needs more thorough investigation. Until more insight into the role of SLN in cardiac function is obtained, mutations in the SLN gene should be investigated as potential causal genes in patients with cardiomyopathies.
Acknowledgments
We thank Ms. Atsuko Nakai for expert technical assistance. This work was supported by Ministry of Education, Culture, Sports, Science, and Technology Grant-in-Aid 13470145 (to K.O.), Heart and Stroke Foundation of Ontario (HSFO) Grant T-5042, a Canadian Genetic Diseases Network of Centers of Excellence grant, Canadian Institutes for Health Research Grants MT-12545 and MOP-49493, and the Neuromuscular Research Partnership Program (to D.H.M.). P.H.B. is a career investigator of the HSFO, T.M. is a postdoctoral fellow of the HSFO, and A.O.G. is a postdoctoral fellow of the Heart and Stroke Foundation of Canada.
Abbreviations: PLN, phospholamban; SLN, sarcolipin; NF-SLN, N-terminal FLAG-tagged SLN; SERCA, sarco(endo)plasmic reticulum Ca2+ ATPase; TG, transgenic; wt, wild type; SR, sarcoplasmic reticulum; Hprt, hypoxanthine-guanine-phosphoribosyltransferase; LV, left ventricular.
References
- 1.MacLennan, D. H. & Kranias, E. G. (2003) Nat. Rev. Mol. Cell. Biol. 4, 566-577. [DOI] [PubMed] [Google Scholar]
- 2.Asahi, M., Nakayama, H., Tada, M. & Otsu, K. (2003) Trends Cardiovasc. Med. 13, 152-157. [DOI] [PubMed] [Google Scholar]
- 3.Tada, M. & Kadoma, M. (1989) BioEssays 10, 157-163. [DOI] [PubMed] [Google Scholar]
- 4.Koss, K. L. & Kranias, E. G. (1996) Circ. Res. 79, 1059-1063. [DOI] [PubMed] [Google Scholar]
- 5.Kiriazis, H. & Kranias, E. G. (2000) Annu. Rev. Physiol. 62, 321-351. [DOI] [PubMed] [Google Scholar]
- 6.Odermatt, A., Taschner, P. E. M., Scherer, S. W., Beatty, B., Khanna, V. K., Cornblath, D. R., Chaudry, V., Yee, W. C., Schrank, B., Karpati, G., et al. (1997) Genomics 45, 541-553. [DOI] [PubMed] [Google Scholar]
- 7.Toyofuku, T., Kurzydlowski, K., Lytton, J. & MacLennan, D. H. (1992) J. Biol. Chem. 267, 14490-14496. [PubMed] [Google Scholar]
- 8.Odermatt, A., Becker, S., Khanna, V. K., Kurzydlowski, K., Leisner, E., Pette, D. & MacLennan, D. H. (1998) J. Biol. Chem. 273, 12360-12369. [DOI] [PubMed] [Google Scholar]
- 9.Asahi, M., Kurzydlowski, K., Tada, M. & MacLennan, D. H. (2002) J. Biol. Chem. 277, 26725-26728. [DOI] [PubMed] [Google Scholar]
- 10.Asahi, M., Sugita, Y., Kurzydlowski, K., De Leon, S., Tada, M., Toyoshima, C. & MacLennan, D. H. (2003) Proc. Natl. Acad. Sci. USA 100, 5040-5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gayan-Ramirez, G., Vanzeir, L., Wuytack, F. & Decramer, M. (2000) J. Physiol. 524, 387-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minamisawa, S., Wang, Y., Chen, J., Ishikawa, Y., Chien, K. R. & Matsuoka, R. (2003) J. Biol. Chem. 278, 9570-9575. [DOI] [PubMed] [Google Scholar]
- 13.Kadambi, V. J., Koss, K. L., Grupp, I. L. & Kranias, E. G. (1998) J. Mol. Cell Cardiol. 30, 1275-1284. [DOI] [PubMed] [Google Scholar]
- 14.Tupling, A. R., Asahi, M. & MacLennan, D. H. (2002) J. Biol. Chem. 277, 44740-44746. [DOI] [PubMed] [Google Scholar]
- 15.Bronson, S. K., Plaehn, E. G., Kluckman, K. D., Hagaman, J. R., Maeda, N. & Smithies, O. (1996) Proc. Natl. Acad. Sci. USA 93, 9067-9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker, D. L., Hashimoto, K., Grupp, I. L., Ji, Y., Reed, T., Loukianov, E., Grupp, G., Bhagwhat, A., Hoit, B., Walsh, R., et al. (1998) Circ. Res. 83, 1205-1214. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama, H., Otsu, K., Yamaguchi, O., Nishida, K., Date, M. O., Hongo, K., Kusakari, Y., Toyofuku, T., Hikoso, S., Kashiwase, K., et al. (2003) FASEB J. 17, 61-63. [DOI] [PubMed] [Google Scholar]
- 18.Crackower, M. A., Oudit, G. Y., Kozieradzki, I., Sarao, R., Sun, H., Sasaki, T., Hirsch, E., Suzuki, A., Shioi, T., Irie-Sasaki, J., et al. (2002) Cell 110, 737-749. [DOI] [PubMed] [Google Scholar]
- 19.Oudit, G. Y., Crackower, M. A., Eriksson, U., Sarao, R., Kozieradzki, I., Sasaki, T., Irie-Sasaki, J., Gidrewicz, D., Rybin, V. O., Wada, T., et al. (2003) Circulation 108, 2147-2152. [DOI] [PubMed] [Google Scholar]
- 20.Hongo, K., Tanaka, E. & Kurihara, S. (1993) J. Physiol. 461, 167-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asahi, M., Kimura, Y., Kurzydlowski, K., Tada, M. & MacLennan, D. H. (1999) J. Biol. Chem. 274, 32855-32862. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt, A. G., Gerst, M., Zhai, J., Carr, A. N., Pater, L., Kranias, E. G. & Hoit, B. D. (2002) J. Am. Soc. Echocardiogr. 15, 1065-1073. [DOI] [PubMed] [Google Scholar]
- 23.Asahi, M., McKenna, E., Kurzydlowski, K., Tada, M. & MacLennan, D. H. (2000) J. Biol. Chem. 275, 15034-15038. [DOI] [PubMed] [Google Scholar]
- 24.Boknik, P., Unkel, C., Kirchhefer, U., Kleideiter, U., Klein-Wiele, O., Knapp, J., Linck, B., Luss, H., Muller, F. U., Schmitz, W., et al. (1999) Cardiovasc. Res. 43, 67-76. [DOI] [PubMed] [Google Scholar]
- 25.Lompre, A. M., Nadal-Ginard, B. & Mahdavi, V. (1984) J. Biol. Chem. 259, 6437-6446. [PubMed] [Google Scholar]
- 26.Wickenden, A. D., Kaprielian, R., Parker, T. G., Jones, O. T. & Backx, P. H. (1997) J. Physiol. 504, 271-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dillmann, W. H. (2002) Thyroid 12, 447-452. [DOI] [PubMed] [Google Scholar]
- 28.Kadambi, V. J., Ponniah, S., Harrer, J. M., Hoit, B. D., Dorn, G. W., II, Walsh, R. A. & Kranias, E. G. (1996) J. Clin. Invest. 97, 533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zvaritch, E., Backx, P. H., Jirik, F., Kimura, Y., de Leon, S., Schmidt, A. G., Hoit, B. D., Lester, J. W., Kranias, E. G. & MacLennan, D. H. (2000) J. Biol. Chem. 275, 14985-14991. [DOI] [PubMed] [Google Scholar]
- 30.Pan, Y., Kislinger, T., Gramolini, A. O., Zvaritch, E., Kranias, E. G., MacLennan, D. H. & Emili, A. (2004) Proc. Natl. Acad. Sci. USA 101, 2241-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhai, J., Schmidt, A. G., Hoit, B. D., Kimura, Y., MacLennan, D. H. & Kranias, E. G. (2000) J. Biol. Chem. 275, 10538-10544. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt, A. G., Zhai, J., Carr, A. N., Gerst, M. J., Lorenz, J. N., Pollesello, P., Annila, A., Hoit, B. D. & Kranias, E. G. (2002) Cardiovasc. Res. 56, 248-259. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt, J. P., Kamisago, M., Asahi, M., Li, G. H., Ahmad, F., Mende, U., Kranias, E. G., MacLennan, D. H., Seidman, J. G. & Seidman, C. E. (2003) Science 299, 1410-1413. [DOI] [PubMed] [Google Scholar]