Abstract
Streptococcus pneumoniae (S. pneumoniae), a commensal across the nasal passages, is responsible for the majority of infectious pneumonia cases worldwide. Previous studies have shown that hormonal factors may be influential in regulating S. pneumoniae’s transition from a non-pathogen to a pathogenic state. The current study investigated the effects of corticotropin-releasing hormone (CRH), a peptide hormone involved in stress, on the pathogenicity of S. pneumoniae. Mice were infected with CRH-treated S. pneumoniae via intranasal route, showing an increase in pulmonary bacterial burden. We also quantified S. pneumoniae’s response to CRH through limited serial dilutions and growth curve analysis. We demonstrated that CRH promotes S. pneumoniae titer-dependent proliferation, as well as accelerates log-phase growth. Results also showed an increase in pneumococcal-associated virulence protein A virulence gene expression in response to CRH. These results demonstrate a role for CRH in S. pneumoniae pathogenicity, thus implicating CRH in mediating the transition of S. pneumoniae into a pathogenic state.
Keywords: Corticotropin releasing hormone, Streptococcus pneumoniae, pathogenicity, hormones, virulence, commensal, respiratory
Introduction
Streptococcus pneumoniae (S. pneumoniae) contributes to the highest morbidity and mortality rates worldwide (Laupland and Church, 2014; Oishi et al., 2014). Approximately $4.9 billion dollars in healthcare cost is attributed to pneumonia in the United States (US; Reynolds et al., 2014). The magnitude of this problem is underscored by the significant mortality risks among hospitalized patients ranging between 10 and 25% and increasing to 50% among patients 65 years and older (Reynolds et al., 2014). Although increased antibiotic resistance plays a role, it does not entirely explain the problem. For example, penicillin-resistance accounts for the majority of pneumococcal resistance but attributes to only 4% of the total estimated healthcare costs (Laxminarayan, 2014). Based on these statistics it is clear that a gap in knowledge exists, limiting our understanding of the epidemiology of pneumonia caused by S. pneumoniae infection.
Among the 92 known serotypes of S. pneumoniae, few are considered virulent. Moreover, invasive serotypes are not contagious under conditions within their disease states (e.g., pneumonia, otitis media, and sepsis; Caierão et al., 2014; Del Amo et al., 2014; Feldman and Anderson, 2014). Rather, transmission of disease stems from the reservoir of resident asymptomatic pneumococci along nasal passages, which, through unknown mechanisms, acquires pathogenic status. It is believed that as a commensal, S. pneumoniae’s pathogenicity is largely influenced by complex symbiotic relationships within the host including commensal competition, nutritional status, metabolic stress, mucosal-associated immune responses, and hormonal signals (Alcaide et al., 2012; Bailey, 2014; Cameron et al., 2014; Frydenborg et al., 2014; Ishak et al., 2014). Neuropeptides in particular, have been shown to mediate commensal ecosystems that impact disease susceptibility (Freestone et al., 2008; Schoen et al., 2014). Most of what is understood linking microbial species’ response to stress hormones relates to the neuroendocrine gut mucosal system and its interaction with enteric bacterial species (Freestone and Lyte, 2008; Lyte, 2014). However, mechanisms of disease between hormonal factors and respiratory bacterial commensals remain elusive.
Current knowledge of microorganisms’ response to neuroendocrine factors largely stem from studies defining how dopaminergic, catecholaminergic inotropes and cholinergic pathways impact gut-associated microbes (Freestone and Lyte, 2008). For example, catecholamines have been shown to stimulate toxin release, motility, and attachment by Escherichia coli O157:H7, Vibrio parahaemolyticus and other pathogens (Freestone et al., 2007a). However, far less is known regarding hormonal signals’ impact on infectious disease across the respiratory tract. Two recent studies have examined the role of catecholamine responses by S. pneumoniae. Specifically, Sandrini et al. (2014) demonstrated that norepinephrine (NE) could promote pneumococcal growth and biofilm formation. In addition, Gonzales et al. (2013) showed that NE reduced lung adherence through iron binding as a potential mechanism. This is consistent with the hypothesis that bacterial species along the respiratory tract are responsive to hormonal factors as a potential inducer of pathogenicity. Due to high mortality risks associated with respiratory disease caused by S. pneumoniae, one might expect as a critical need to understand the role of neuroendocrine responses that mediate its pathogenicity and that of other respiratory commensal organisms.
Corticotropin-releasing hormone (CRH) is a 41-amino acid peptide produced in the hypothalamus and throughout the brain where it plays a role in behavior and autonomic responses to stress (Clynen et al., 2014; Gold, 2014; Kolasa et al., 2014). CRH is also defined by its induction of peripheral glucocorticoid (e.g., cortisol in humans and corticosterone in mice) secretion, known to suppress immune function (Quintanar and Guzman-Soto, 2013; Uchoa et al., 2014). In contrast, CRH production also occurs at peripheral sites and appears to be involved in inflammatory conditions associated with diseases such as rheumatoid arthritis, cancer, colitis, and asthma (Vasiadi et al., 2012; Clynen et al., 2014). Using a murine model of aversive stress and S. pneumoniae infection, we have previously demonstrated the expression of CRH in the lung, as well as its impact on host pulmonary cellular immune and inflammatory responses (Gonzales et al., 2008; Kim et al., 2011). The purpose of the current study was to test whether CRH directly impacts S. pneumoniae virulence. Findings presented here demonstrate that CRH directly increases bacterial growth, a key characteristic of invasiveness. In support of the observed increase in bacterial growth, we also demonstrated influences of CRH on the regulation of pavA, a virulence protein expressed by S. pneumoniae associated with the mediation of immune and inflammatory responses. Moreover, infection of mice with CRH-treated S. pneumoniae resulted in greater bacterial carriage in the lung.
Materials and Methods
Bacterial Strains
Streptococcus pneumoniae strain #6301 (ATCC, Manassas, VA, USA) was used in all experiments. Prior to use, S. pneumoniae was maintained in 30% glycerol frozen stock solutions (–80∘C).
Corticotropin-Releasing Hormone (CRH)
Human/rat recombinant CRH (Sigma–Aldrich, St. Louis, MO, USA) was used in all experiments. CRH stock solutions were stored at –20∘C in 20 μl aliquots until use.
Urocortin (UCN)
Urocortin (Sigma–Aldrich, St. Louis, MO, USA), a related CRH homolog peptide, was used as a relevant negative control. UCN stock solutions were stored at –20∘C in 2 ml aliquots until use.
Mice
Female CD1 strain (Harlan Laboratories, Houston, TX, USA) between six and 8 weeks of age were used in all in vivo experiments. Mice were maintained in sterile conditions and provided food and water ad libitum. For infection studies, mice were anesthetized by intraperitoneal injection of ketamine/xylazine solution (50 mg/kg ketamine and 16 μg/kg xylazine for a total volume of 0.2 ml). The University of North Texas Health Science Center’s Institutional Animal Use Committee (IACUC) approved all studies.
In Vivo Determination of CRH-Treated S. pneumoniae Pathogenesis
Briefly, frozen stock cultures were spread onto blood agar plates and incubated for 18 h at 37∘C and 5% CO2 to achieve mid-log phase growth. Cultures were suspended in a 50/50 Brain–Heart Infusion (BHI; EMD Chemicals Inc., Darmstadt, Germany) and Phosphate Buffered Saline (PBS; Life Technologies, Carlsbad, CA, USA) broth mixture. Subsequent bacterial suspensions were adjusted to an optical density (OD) of 1.0 containing approximately 1 × 108 bacterial cells.
Prior to infection, bacterial cells were diluted to 1 × 105 cells of S. pneumoniae and were exposed to 2.1 × 10-4 mM/μl of CRH overnight at 37∘C and 5% CO2. S. pneumoniae was collected and diluted to an infection dose of 2.0 × 105 CFUs (LD50). Subsequently, anesthetized mice (N = 5/group) were administered CRH-treated (CRH-Sp) or untreated S. pneumoniae (Sp; LD50) by intranasal route. Eighteen hours following infection, anesthetized mice were euthanized to compare bacterial carriage in lungs of CRH-Sp versus Sp-infected mice as previously described. Specifically, lungs were harvested and homogenized in sterile cold PBS (Life Technologies, Carlsbad, CA, USA). Ten-fold serial dilutions of lung homogenates were plated in duplicates onto blood agar plates and incubated at 37∘C overnight. Colonies on plates were enumerated, and the results were expressed as log10 CFU per μl.
Quantitation of S. pneumoniae in Response to CRH by Limited Dilution CFU Analysis
Streptococcus pneumoniae strain #6301 (ATCC, Manassas, VA, USA) was grown overnight to achieve mid-log phase cultures on Blood Agar plates. S. pneumoniae was collected and suspended in a 50/50 BHI and PBS broth mixture. Ten-fold dilutions of a starting cell number of 1.3 × 106 were seeded in sterile 96-well flat bottom plates in the presence or absence of CRH at concentrations of 2.1 × 10-4mM/μl and 4.0 × 10-4 mM/μl, respectively. In addition, 4.0 × 10-4 mM/μl UCN was introduced to bacterial cultures to serve as a negative control. Aliquots (8 μl) of bacterial suspensions with or without CRH or UCN were plated on blood agar plates and incubated at 37∘C overnight. Colonies on plates were enumerated, and the results were expressed as log10 CFU per μl. All experiments were performed in duplicate.
Growth Curve Determination in Response to CRH
Bacterial stock of S. pneumoniae was prepared by adding 50 μl of bacteria from frozen stock to 1950 μl of sterile BHI broth. The mixture was incubated overnight at 37∘C (5% CO2). Twenty-four hours later absorbance of the overnight culture was determined and adjusted to 0.2 at OD600 in BHI. A series of duplicate tubes were prepared containing 1950 μl and 50 μl of S. pneumoniae in the presence or absence of 2.1 × 10-4mM/μl CRH and incubated at 37∘C (5% CO2). Duplicate absorbance readings (OD600) of bacterial cultures were taken every 30 min. over a 9 h time period. Absorbance readings were plotted against time to generate bacterial growth curves.
Gene Expression Profiling by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Streptococcus pneumoniae from freezer stock was plated overnight at 37∘C (5% CO2). After an 18 h of incubation, bacteria were collected and suspended in BHI and the absorbance was read and adjusted to 1 (OD600 = 1.0). Bacterial cells were incubated overnight at 37∘C (5% CO2) in presence or absence of 2.1 × 10-4 mM/μl CRH.
Bacterial RNA Extraction
Eighteen hours after incubation, S. pneumoniae from representative experimental conditions were collected in separate labeled tubes using sterile BHI broth. Absorbance of each sample was read and adjusted to an OD600 = 1.0, representing 108–109 cell density. RNA extraction from bacterial cells was performed using a RiboPure RNA Purification Kit (Life Technologies, Carlsbad, CA, USA).
Reverse Transcription and qRT-PCR
cDNA was generated using a starting Total RNA concentration of 1 μg per reaction and MLV (Molony murine leukemia virus) reverse transcriptase (Promega Corp., Madison, WI, USA). After cDNA synthesis, real-time PCR was performed using SYBR green-based amplification techniques. PCR was performed in a 20 μl reaction volume using the StepOne system (Applied Biosystems Inc., Foster City, CA, USA). The expression of the housekeeping gene 16s rRNA was used as an internal control to normalize target gene expression between samples. Pneumococcal adherence and virulence factor A (pavA) gene expression in bacterial cells was calculated using the following formula:
Data was calculated by subtracting δδCT of control group from the CRH-treated group for each target gene. Data was expressed as the fold difference between pavA by normalizing the expression of untreated target genes as 1. Selected target and house keeping gene primer sets; pavA and 16s rRNA were purchased from Life Technologies, Carlsbad, CA, USA (Table 1).
Table 1.
List of primer sequences and target gene.
| Sequence | |
| House keeping Gene1 | |
| 16S rRNA-Forward (5′–3′) | TGAGTTAACCGTAAGGAGCCA |
| 16S rRNA-Reverse (3′–5′) | TCACCCCAATCATCTATCCCA |
| pavA (Pneumococcal adherence and virulence factor A)2 | |
| Forward (5′–3′) | GGTCGCATCCAGAAAATC |
| Reverse (3′–5′) | AGAAAGGAGCAGGCGATG |
Statistical Analysis
Statistical analysis was performed using GraphPad Prism Version 6.0 (GraphPad Software, San Diego, CA, USA). For multi-experimental group analysis, data were subjected to one-way and two-way ANOVA (analysis of variance) followed by post hoc tests (Newman–Keuls and Bonfferoni) for group differences. Other results were analyzed using t-test where indicated. All data are expressed as means ± SEM. A P-value of ≤ 0.05 was considered significant.
Results
CRH-Treated S. pneumoniae Increases Lung Carriage
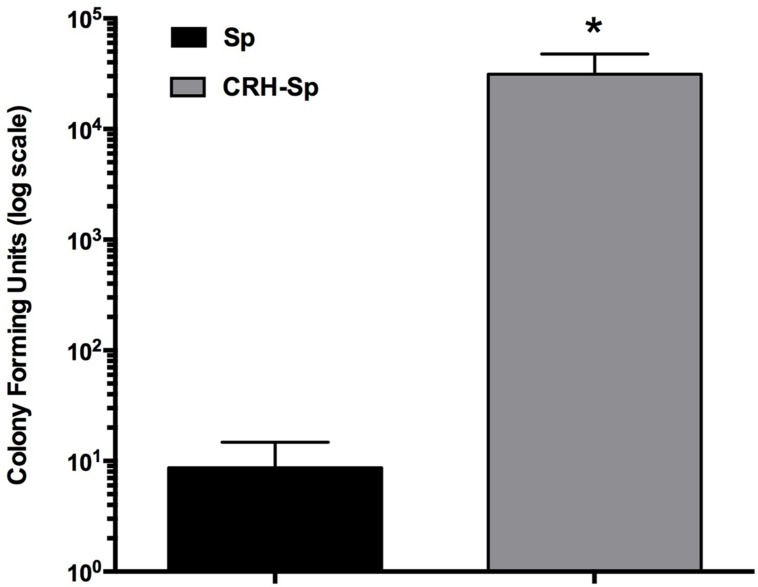
We determined whether exposing S. pneumoniae to CRH would impact its propensity to induce pulmonary infection. S. pneumoniae cultures were exposed to CRH (2.1 × 10-4mM/μl) and used for nasal infection. Bacterial colonization in the lungs of mice was significantly higher (P ≤ 0.05) in mice exposed CRH-treated S. pneumoniae (CRH-Sp) compared to untreated S. pneumoniae (Sp; Figure 1).
FIGURE 1.
Corticotropin-releasing hormone (CRH)-treated Streptococcus pneumoniae increases mice lung carriage. We assessed whether or not exposing mice to S. pneumoniae (105 cells), previously treated or untreated with CRH, would result in bacterial burden for the mice. Mice (N = 5) treated with previously exposed to CRH bacteria (2.0 × 105 cells) showed an increase in lung carriage compared to the unexposed group. Bar graphs represent the mean ± SE of (N = 5) the number of CFUs in lungs. Asterisks (∗) indicate significant (P ≤ 0.05) differences between experimental groups.
CRH Promotes S. pneumoniae Titer-Dependent Proliferation
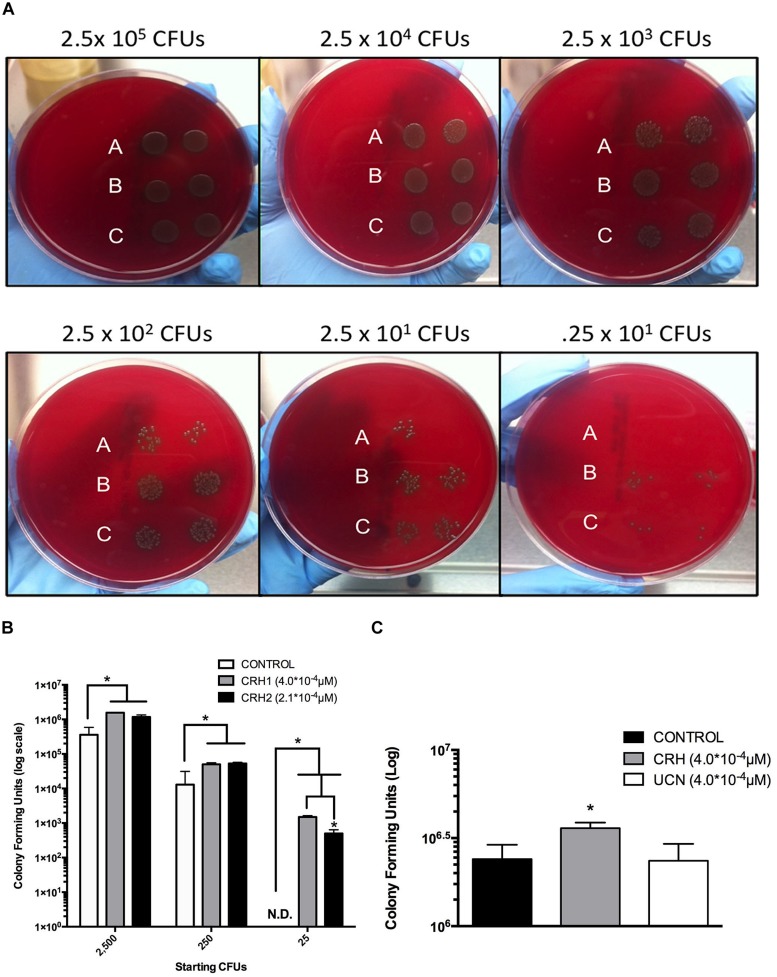
The above results suggested that CRH directly promotes the pathogenicity of S. pneumoniae by increased colonization in the lung. We assessed the effect of CRH on S. pneumoniae proliferation controlling for bacterial titer and variation in CRH concentration. S. pneumoniae colony forming units (CFUs) were significantly higher (P ≤ 0.05) given exposure to both concentrations of CRH at each titer of S. pneumoniae. In contrast to un-treated cultures of S. pneumoniae, CRH significantly sustained detection of CFUs at the lowest titer (8th-fold dilution; Figures 2A,B). To confirm specificity of CRH’s effect, additional experiments were performed whereby S. pneumoniae was exposed to the CRH homolog UCN. Lower concentrations of UCN (2.1 × 10-4mM/μl) were also used in testing. No differences in CFUs were observed in the presence of UCN at different concentrations compare to untreated S. pneumoniae (Figure 2C).
FIGURE 2.
Titer-dependent effects of CRH on S. pneumoniae colony formation. Ten-fold serial dilutions of S. pneumoniae were incubated in the absence (A) or presence of two concentrations of CRH, 2.1 × 10-4 μM (B) and 4.0 × 10-4 μM, (C) and plated on blood agar plates. Data shown is representative of two independent experiments (A). (B) Represents quantitation of the aforementioned results in (A). Bars represent mean (N = 3) ± SE. Asterisks (∗) indicate significant (P ≤ 0.05) differences between experimental groups. White bars denote unexposed S. pneumoniae, gray and black bars denote S. pneumoniae exposed to two different CRH concentrations, 4.0 × 10-4 μM and 2.0 × 10-4 μM, respectively (B). (C) Highlights the specificity of CRH compared to its homolog UCN. Bars represent mean (N = 3) ± SE. Asterisks (∗) indicate significant (P ≤ 0.05) differences between experimental groups.
CRH Accelerates Log-Phase Growth of S. pneumoniae Growth
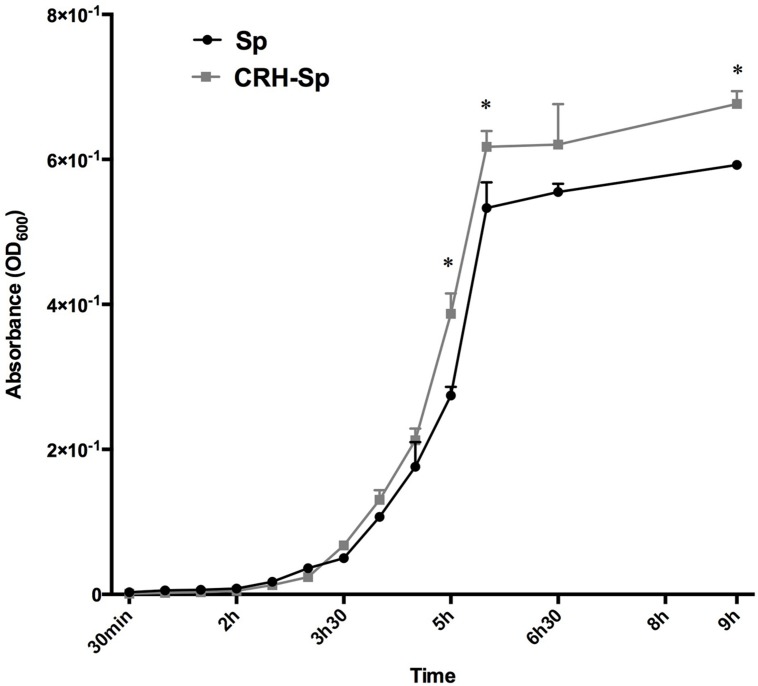
We compared the bacterial growth curve in presence and absence of CRH. A significant difference (P ≤ 0.05) was observed in bacteria exposed to CRH during log phase growth. In addition, significant differences in the final bacterial mass were observed during stationary growth phase (Figure 3).
FIGURE 3.
Corticotropin-releasing hormone accelerates log-phase growth of S. pneumoniae. S. pneumoniae, CRH exposed (2.1 × 10-4 μM/μl) vs. non-exposed, growth analysis was performed over a period of 9 h. Asterisks (∗) indicate significant (P ≤ 0.05) differences observed in the final bacterial mass during stationary phase. All experiments were performed in duplicates.
CRH Increases Pneumococcal Adherence and Virulence Factor A (pavA) mRNA Gene Expression by S. pneumoniae
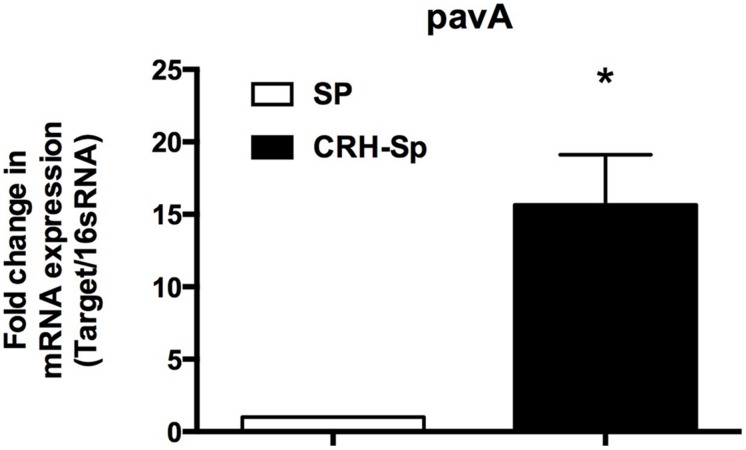
PavA gene expression is found to modulate bacterial adhesion, invasion, and inflammation associated with septicemia (Pracht et al., 2005). Figure 4 demonstrates a significant (P ≤ 0.05) increase in pavA mRNA expression by S. pneumoniae exposed to CRH, compared to untreated controls as determined by quantitative realtime PCR.
FIGURE 4.
Streptococcus pneumoniae adhesion and virulence factors increase in the presence of CRH. pavA gene expression was determined by quantitative realtime PCR analysis of S. pneumoniae’s mRNA exposed to CRH (N = 3). The analysis showed overexpression of the bacterium’s adhesion and virulence gene (pavA) when compared to control genes. Asterisks (∗) indicate significant (P ≤ 0.05) difference between CRH treated (CRH-Sp) and non-CRH treated (Sp) bacterial groups.
Discussion
This study expands upon an emerging area of research that seeks to define hormonal signals as determinants for pathogenic transformation of commensal bacterial species (Freestone, 2013; Lyte, 2014). We demonstrate how CRH, a hormonal mediator of central and peripheral neuro-immune axes, works in mediating virulence-associated factors of the upper respiratory tract commensal S. pneumoniae. Moreover, our previous studies established the role of CRH in regulating experimental pneumonia and sepsis caused by S. pneumoniae (Gonzales et al., 2008; Kim et al., 2011), implicating it in mediation of host cellular immune and inflammatory responses. Research defining CRH and other hormonal factors as direct modifiers of respiratory commensal species remain unknown.
A commensal organism’s ability to sense a change in its environment is essential for its survival. Prevalent hormonal stress signals identified to influence bacterial phenotype and function include catecholamines, NE and epinephrine (Freestone and Lyte, 2010). Studies demonstrated that in vitro, NE and epinephrine promote microorganism’s growth, metabolic potential, nutritional status, and biofilm formation (Neal et al., 2001; Lyte et al., 2003; Freestone et al., 2007b; Yang et al., 2014b) furthermore suggesting that catecholamines, and possibly other hormonal stress factors, are influential regulators of microbial virulence (Li et al., 2009; Lucas et al., 2012; Pande et al., 2014). However, aforementioned hormonal effects have been primarily a focus of bacterial species resident in gut mucosa.
Previous studies have been associated with the activation of stress factors with disease pathogenesis along respiratory tissues (Lim et al., 2014; Runeson-Broberg and Norback, 2014; Tomljenovic et al., 2014; Yang et al., 2014a). Utilizing an experimental model of aversive stress and S. pneumoniae infection, we demonstrated CRH’s ability to regulate disease severity by controlling cellular immune and inflammatory responses (Kim et al., 2011). Notably, our studies demonstrated that inhibiting CRH from binding its target receptor CRHR1 caused increased pneumonia and sepsis. We thus hypothesized that increasing peripheral CRH levels allows for increased exposure to S. pneumoniae, potentially impacting its pathogenicity. In the current study, we tested the possible direct effects imparted by CRH on the propensity of S. pneumoniae to cause disease, to influence bacterial growth and to modulate pavA gene expression. As shown in Figure 1, exposing S. pneumoniae cultures to CRH prior to experimental infection produced greater pulmonary bacterial burden compared to infection with untreated cultures. This finding suggested that independent of CRH’s impact on host cellular immune function, disease severity could be elicited from S. pneumoniae being directly exposed to CRH; thus supporting the potential for S. pneumoniae to recognize and respond to CRH for purposes of modulating its phenotype.
Possible mechanisms through which CRH could be imparting its function were determined by testing its effects on key indicators of bacterial virulence (e.g., microorganisms’ growth). We determined that CRH enhanced the growth phase kinetics of S. pneumoniae (Figure 3), reinforcing earlier studies demonstrating hormonal factors’ ability to modulate bacterial growth (Freestone et al., 2007b; Seeley et al., 2013; Pande et al., 2014). The ability of CRH to promote colony formation further substantiated its role in growth-associated virulence at significantly lower bacterial titers (Figures 2A-C). Ongoing studies using a clinical invasive serotype 3 pneumococcal strain were more invasive in the presence of CRH interpreted by its ability to reestablish secondary colony formation from primary biofilms (Thapa et al., unpublished data). These findings suggest a role for CRH in regulating mechanisms of invasiveness associated with high risk for pneumonia and sepsis.
The studies presented here highlight for the first time the ability of CRH to serve as a catalyst for S. pneumoniae virulence. Mechanisms of action have been identified as basis for S. pneumoniae’s response to stress hormonal signals. Catecholamines play a role in the process of Fe-transport across bacterial membranes and making Fe valence available for enriched metabolic processes (Freestone et al., 2000, 2003). NE, a known siderophore, enhances iron availability to S. pneumoniae, thus promoting bacterial growth and virulence (Kinney et al., 2000; Gonzales et al., 2013). Whereas siderophore processing cascades of Gram-negative species have been understood (Seeley et al., 2013), they remain unknown for the Gram-positive bacterial species. Future studies should address how CRH is sensed and processed by S. pneumoniae and other bacterial species.
The inability to tailor inflammatory responses hinders significant advances to improve disease outcomes associated with infectious pneumonia. Despite the current use of systemic corticosteroids as standard adjunctive treatment to reduce inflammatory reactions in cases of pneumonia (Steel et al., 2013; Sibila et al., 2014), mortality and morbidity rates remain high (Lin et al., 2010; Moon et al., 2012; Peterkovic et al., 2012), raising questions of their benefit (Ramsey and Gorman, 2014; Sibila et al., 2014). Stress-induced release of endogenous glucocorticoids or other hormones may negatively impact adjunctive corticosteroid use. Currently there are few studies demonstrating the potential relationships between glucocorticoids and bacterial pathogenicity. Pseudomonas aeruginosa was found to produce a protease factor that inhibits cortisol-binding globulin as a mechanism of blocking plasma cortisol transport (Simard et al., 2014). In addition, Verbrugghe et al. (2011) showed that cortisol can directly impact bacterial functioning. They demonstrated that Salmonella typhimurium’s proliferation was increased within macrophages exposed to cortisol in pigs, but not in the presence of catecholamines (Verbrugghe et al., 2011). In the current study, we found that CRH increased pavA (modulator of bacterial adherence and inflammation) gene expression by S. pneumoniae (Figure 4), thus highlighting the role of CRH in bacterial-associated inflammatory responses. While mechanisms defining interactions between the central nervous and immune systems have been proved important for host susceptibility and resolution of disease (Dhabhar, 2014), few therapies take into account the direct influence of stress factors on bacterial physiology. In total, our results provide evidence in support of CRH to directly influence S. pneumoniae virulence. However, limitations requiring further investigation such as the direct abrogation of CRH-mediated responses will be key to understanding the mechanistic relationships between CRH and its pathogenicity. Such studies could lead to novel approaches to reduce the onset of disease.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Natsumi Nemoto, Rutika Kokate, Brittney Burnley, and Santosh Thapa for the help provided during the execution of these studies.
References
- Alcaide M., Messina E., Richter M., Bargiela R., Peplies J., Huws S. A., et al. (2012). Gene sets for utilization of primary and secondary nutrition supplies in the distal gut of endangered Iberian lynx. PLoS ONE 7:e51521 10.1371/journal.pone.0051521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M. T. (2014). Influence of stressor-induced nervous system activation on the intestinal microbiota and the importance for immunomodulation. Adv. Exp. Med. Biol. 817 255–276 10.1007/978-1-4939-0897-4_12 [DOI] [PubMed] [Google Scholar]
- Caierão J., Hawkins P., Sant’anna F. H., Rosa da Cunha G., d’Azevedo P. A., McGee L., et al. (2014). Serotypes and genotypes of invasive Streptococcus pneumoniae before and after PCV10 implementation in southern Brazil. PLoS ONE 9:e111129 10.1371/journal.pone.0111129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron E. A., Kwiatkowski K. J., Lee B. H., Hamaker B. R., Koropatkin N. M., Martens E. C. (2014). Multifunctional nutrient-binding proteins adapt human symbiotic bacteria for glycan competition in the gut by separately promoting enhanced sensing and catalysis. MBio 5 e01441-14. 10.1128/mBio.01441-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynen E., Swijsen A., Raijmakers M., Hoogland G., Rigo J. M. (2014). Neuropeptides as targets for the development of anticonvulsant drugs. Mol. Neurobiol. 50 626–646 10.1007/s12035-014-8669-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope E. K., Goldstein-Daruech N., Kofonow J. M., Christensen L., McDermott B., Monroy F., et al. (2011). Regulation of virulence gene expression resulting from Streptococcus pneumoniae and nontypeable Haemophilus influenzae interactions in chronic disease. PLoS ONE 6:e28523 10.1371/journal.pone.0028523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Amo E., Selva L., de Sevilla M. F., Ciruela P., Brotons P., Triviño M., et al. (2014). Estimation of the invasive disease potential of Streptococcus pneumoniae in children by the use of direct capsular typing in clinical specimens. Eur. J. Clin. Microbiol. Infect. Dis. 34 705–711 10.1007/s10096-014-2280-y [DOI] [PubMed] [Google Scholar]
- Dhabhar F. S. (2014). Effects of stress on immune function: the good, the bad, and the beautiful. Immunol. Res. 58 193–210 10.1007/s12026-014-8517-0 [DOI] [PubMed] [Google Scholar]
- Feldman C., Anderson R. (2014). Recent advances in our understanding of Streptococcus pneumoniae infection. F1000Prime Rep. 6 82 10.12703/P6-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone P. (2013). Communication between bacteria and their hosts. Scientifica 2013 361073 10.1155/2013/361073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone P. P., Haigh R. D., Lyte M. (2007a). Blockade of catecholamine-induced growth by adrenergic and dopaminergic receptor antagonists in Escherichia coli O157:H7 Salmonella enterica and Yersinia enterocolitica. BMC Microbiol. 7:8 10.1186/1471-2180-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone P. P., Haigh R. D., Lyte M. (2007b). Specificity of catecholamine-induced growth in Escherichia coli O157:H7 Salmonella enterica and Yersinia enterocolitica. FEMS Microbiol. Lett. 269 221–228 10.1111/j.1574-6968.2006.00619.x [DOI] [PubMed] [Google Scholar]
- Freestone P. P., Haigh R. D., Williams P. H., Lyte M. (2003). Involvement of enterobactin in norepinephrine-mediated iron supply from transferrin to enterohaemorrhagic Escherichia coli. FEMS Microbiol. Lett. 222 39–43 10.1016/S0378-1097(03)00243-X [DOI] [PubMed] [Google Scholar]
- Freestone P. P., Lyte M. (2008). Microbial endocrinology: experimental design issues in the study of interkingdom signaling in infectious disease. Adv. Appl. Microbiol. 64 75–105 10.1016/S0065-2164(08)00402-4 [DOI] [PubMed] [Google Scholar]
- Freestone P., Lyte M. (2010). Stress and microbial endocrinology: prospects for ruminant nutrition. Animal 4 1248–1257 10.1017/S1751731110000674 [DOI] [PubMed] [Google Scholar]
- Freestone P. P., Lyte M., Neal C. P., Maggs A. F., Haigh R. D., Williams P. H. (2000). The mammalian neuroendocrine hormone norepinephrine supplies iron for bacterial growth in the presence of transferrin or lactoferrin. J. Bacteriol. 182 6091–6098 10.1128/JB.182.21.6091-6098.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone P. P., Sandrini S. M., Haigh R. D., Lyte M. (2008). Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 16 55–64 10.1016/j.tim.2007.11.005 [DOI] [PubMed] [Google Scholar]
- Frydenborg B. R., Krediet C. J., Teplitski M., Ritchie K. B. (2014). Temperature-dependent inhibition of opportunistic vibrio pathogens by native coral commensal bacteria. Microb. Ecol. 67 392–401 10.1007/s00248-013-0334-9 [DOI] [PubMed] [Google Scholar]
- Gold P. W. (2014). The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatry 20 32–47 10.1038/mp.2014.163 [DOI] [PubMed] [Google Scholar]
- Gonzales X. F., Castillo-Rojas G., Castillo-Rodal A. I., Tuomanen E., Lopez-Vidal Y. (2013). Catecholamine norepinephrine diminishes lung epithelial cell adhesion of Streptococcus pneumoniae by binding iron. Microbiology 159(Pt 11), 2333–2341 10.1099/mic.0.065607-0 [DOI] [PubMed] [Google Scholar]
- Gonzales X. F., Deshmukh A., Pulse M., Johnson K., Jones H. P. (2008). Stress-induced differences in primary and secondary resistance against bacterial sepsis corresponds with diverse corticotropin releasing hormone receptor expression by pulmonary CD11c+ MHC II+ and CD11c- MHC II+ APCs. Brain Behav. Immun. 22 552–564 10.1016/j.bbi.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak N., Tikhomirova A., Bent S. J., Ehrlich G. D., Hu F. Z., Kidd S. P. (2014). There is a specific response to pH by isolates of Haemophilus influenzae and this has a direct influence on biofilm formation. BMC Microbiol. 14:47 10.1186/1471-2180-14-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. J., Kayembe K., Simecka J. W., Pulse M., Jones H. P. (2011). Corticotropin-releasing hormone receptor-1 and 2 activity produces divergent resistance against stress-induced pulmonary Streptococcus pneumoniae infection. J. Neuroimmunol. 237 57–65 10.1016/j.jneuroim.2011.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney K. S., Austin C. E., Morton D. S., Sonnenfeld G. (2000). Norepinephrine as a growth stimulating factor in bacteria–mechanistic studies. Life Sci. 67 3075–3085 10.1016/S0024-3205(00)00891-2 [DOI] [PubMed] [Google Scholar]
- Kolasa M., Faron-Górecka A., Kuśmider M., Szafran-Pilch K., Solich J., Zurawek D., et al. (2014). Differential stress response in rats subjected to chronic mild stress is accompanied by changes in CRH-family gene expression at the pituitary level. Peptides 61 98–106 10.1016/j.peptides.2014.09.008 [DOI] [PubMed] [Google Scholar]
- Laupland K. B., Church D. L. (2014). Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin. Microbiol. Rev. 27 647–664 10.1128/CMR.00002-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R. (2014). Antibiotic effectiveness: balancing conservation against innovation. Science 345 1299–1301 10.1126/science.1254163 [DOI] [PubMed] [Google Scholar]
- Li W., Lyte M., Freestone P. P., Ajmal A., Colmer-Hamood J. A., Hamood A. N. (2009). Norepinephrine represses the expression of toxA and the siderophore genes in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 299 100–109 10.1111/j.1574-6968.2009.01739.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R., Fedulov A. V., Kobzik L. (2014). Maternal stress during pregnancy increases neonatal allergy susceptibility: role of glucocorticoids. Am. J. Physiol. Lung Cell. Mol. Physiol. 307 L141–L148 10.1152/ajplung.00250.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Sun Y., Lin R. J., Xu J., Li N. (2010). Influence of inhaled corticosteroids on distribution of throat flora in children with bronchial asthma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 45 656–659. [PubMed] [Google Scholar]
- Lucas R., Sridhar S., Rick F. G., Gorshkov B., Umapathy N. S., Yang G., et al. (2012). Agonist of growth hormone-releasing hormone reduces pneumolysin-induced pulmonary permeability edema. Proc. Natl. Acad. Sci. U.S.A. 109 2084–2089 10.1073/pnas.1121075109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M. (2014). The effect of stress on microbial growth. Anim. Health Res. Rev. 15 172–174 10.1017/S146625231400019X [DOI] [PubMed] [Google Scholar]
- Lyte M., Freestone P. P., Neal C. P., Olson B. A., Haigh R. D., Bayston R., et al. (2003). Stimulation of Staphylococcus epidermidis growth and biofilm formation by catecholamine inotropes. Lancet 361 130–135 10.1016/S0140-6736(03)12231-3 [DOI] [PubMed] [Google Scholar]
- Moon S. Y., Chung D. R., Kim S. W., Chang H. H., Lee H., Jung D. S., et al. (2012). Is adjunctive corticosteroid beneficial in pneumococcal meningitis in a region with high rates of resistance to penicillin and ceftriaxone? J. Neurol. 259 1453–1460 10.1007/s00415-011-6373-6 [DOI] [PubMed] [Google Scholar]
- Neal C. P., Freestone P. P., Maggs A. F., Haigh R. D., Williams P. H., Lyte M. (2001). Catecholamine inotropes as growth factors for Staphylococcus epidermidis and other coagulase-negative staphylococci. FEMS Microbiol. Lett. 194 163–169 10.1016/S0378-1097(00)00523-1 [DOI] [PubMed] [Google Scholar]
- Oishi K., Tamura K., Akeda Y. (2014). Global control of pneumococcal infections by pneumococcal vaccines. Trop. Med. Health 42(2 Suppl. ) 83–86 10.2149/tmh.2014-S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande G. S., Suong N. T., Bossier P., Defoirdt T. (2014). The catecholamine stress hormones norepinephrine and dopamine increase the virulence of pathogenic Vibrio anguillarum and Vibrio campbellii. FEMS Microbiol. Ecol. 90 761–769 10.1111/1574-6941.12432 [DOI] [PubMed] [Google Scholar]
- Peterkovic V., Trkulja V., Kutlesa M., Krajinovic V., Lepur D. (2012). Dexamethasone for adult community-acquired bacterial meningitis: 20 years of experience in daily practice. J. Neurol. 259 225–236 10.1007/s00415-011-6150-6 [DOI] [PubMed] [Google Scholar]
- Pracht D., Elm C., Gerber J., Bergmann S., Rohde M., Seiler M., et al. (2005). PavA of Streptococcus pneumoniae modulates adherence, invasion, and meningeal inflammation. Infect. Immun. 73 2680–2689 10.1128/IAI.73.5.2680-2689.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanar J. L., Guzman-Soto I. (2013). Hypothalamic neurohormones and immune responses. Front. Integr. Neurosci. 7:56 10.3389/fnint.2013.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey T. D., Gorman S. K. (2014). Corticosteroids in the treatment of severe community-acquired pneumonia. Curr. Infect. Dis. Rep. 16 405 10.1007/s11908-014-0405-1 [DOI] [PubMed] [Google Scholar]
- Reynolds C. A., Finkelstein J. A., Ray G. T., Moore M. R., Huang S. S. (2014). Attributable healthcare utilization and cost of pneumonia due to drug-resistant Streptococcus pneumonia: a cost analysis. Antimicrob. Resist. Infect. Control 3 16 10.1186/2047-2994-3-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runeson-Broberg R., Norback D. (2014). Work-related psychosocial stress as a risk factor for asthma, allergy, and respiratory infections in the Swedish workforce. Psychol. Rep. 114 377–389 10.2466/15.14.PR0.114k20w3 [DOI] [PubMed] [Google Scholar]
- Sandrini S., Alghofaili F., Freestone P., Yesilkaya H. (2014). Host stress hormone norepinephrine stimulates pneumococcal growth, biofilm formation and virulence gene expression. BMC Microbiol. 14:180 10.1186/1471-2180-14-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen C., Kischkies L., Elias J., Ampattu B. J. (2014). Metabolism and virulence in Neisseria meningitidis. Front. Cell. Infect. Microbiol. 4:114 10.3389/fcimb.2014.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley E. J., Barry S. S., Narala S., Matthay M. A., Wolters P. J. (2013). Noradrenergic neurons regulate monocyte trafficking and mortality during gram-negative peritonitis in mice. J. Immunol. 190 4717–4724 10.4049/jimmunol.1300027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibila O., Ferrer M., Agusti C., Torres A. (2014). Corticosteroids as adjunctive treatment in community-acquired pneumonia. Minerva Anestesiol. 80 1336–1344 10.1007/978-3-319-03746-2_5 [DOI] [PubMed] [Google Scholar]
- Simard M., Hill L. A., Underhill C. M., Keller B. O., Villanueva I., Hancock R. E., et al. (2014). Pseudomonas aeruginosa elastase disrupts the cortisol-binding activity of corticosteroid-binding globulin. Endocrinology 155 2900–2908 10.1210/en.2014-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel H. C., Cockeran R., Anderson R., Feldman C. (2013). Overview of community-acquired pneumonia and the role of inflammatory mechanisms in the immunopathogenesis of severe pneumococcal disease. Mediators Inflamm. 2013 490346 10.1155/2013/490346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomljenovic D., Pinter D., Kalogjera L. (2014). Perceived stress and severity of chronic rhinosinusitis in allergic and nonallergic patients. Allergy Asthma Proc. 35 398–403 10.2500/aap.2014.35.3774 [DOI] [PubMed] [Google Scholar]
- Uchoa E. T., Aguilera G., Herman J. P., Fiedler J. L., Deak T., de Sousa M. B. (2014). Novel aspects of glucocorticoid actions. J. Neuroendocrinol. 26 557–572 10.1111/jne.12157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiadi M., Therianou A., Sideri K., Smyrnioti M., Sismanopoulos N., Delivanis D. A., et al. (2012). Increased serum CRH levels with decreased skin CRHR-1 gene expression in psoriasis and atopic dermatitis. J. Allergy Clin. Immunol. 129 1410–1413 10.1016/j.jaci.2012.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugghe E., Boyen F., Van Parys A., Van Deun K., Croubels S., Thompson A., et al. (2011). Stress induced Salmonella typhimurium recrudescence in pigs coincides with cortisol induced increased intracellular proliferation in macrophages. Vet. Res. 42 118 10.1186/1297-9716-42-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. J., Lee S. Y., Suh D. I., Shin Y. H., Kim B. J., Seo J. H., et al. (2014a). The cohort for childhood origin of asthma and allergic diseases (COCOA) study: design, rationale and methods. BMC Pulm. Med. 14:109 10.1186/1471-2466-14-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Anh N. D., Bossier P., Defoirdt T. (2014b). Norepinephrine and dopamine increase motility, biofilm formation, and virulence of Vibrio harveyi. Front. Microbiol. 5:584 10.3389/fmicb.2014.00584 [DOI] [PMC free article] [PubMed] [Google Scholar]