Abstract
Some patients with intellectual disabilities spend longer than others in emergence from ambulatory general anesthesia for dental treatment. Although antiepileptic drugs and anesthetics might be involved, an independent predictor for delay of the emergence remains unclear. Thus, a purpose of this study is to identify independent factors affecting the delay of emergence from general anesthesia. This was a retrospective cohort study in dental patients with intellectual disabilities. Patients in need of sedative premedication were removed from participants. The outcome was time until emergence from general anesthesia. Stepwise multivariate regression analysis was used to extract independent factors affecting the outcome. Antiepileptic drugs and anesthetic parameters were included as predictor variables. The study included 102 cases. Clobazam, clonazepam, and phenobarbital were shown to be independent determinants of emergence time. Parameters relating to anesthetics, patients' backgrounds, and dental treatment were not independent factors. Delay in emergence time in ambulatory general anesthesia is likely to be related to the antiepileptic drugs of benzodiazepine or barbiturates in patients with intellectual disability.
Key Words: Day case, Propofol, Remifentanil, Anticonvulsants, Benzodiazepines, Barbituric acid
Ambulatory general anesthesia is useful for dental treatment of patients with severe intellectual disabilities because it is hard for them to cooperate with dental treatment and to stay in the hospital.1–3 Because controlling the recovery state is important in managing ambulatory general anesthesia, we perform total intravenous anesthesia consisting mainly of propofol and remifentanil, which allows a quick and comfortable recovery.4–7 Midazolam, a short-acting benzodiazepine injection, was shown to be a clear determinant of delayed recovery from general anesthesia in our previous study,8 so we ceased using midazolam injection in ambulatory general anesthesia in our facility. Nevertheless, some patients still spent longer than others in emergence and/or recovery from general anesthesia. Therefore, other independent factors were suspected to affect the recovery state in patients with intellectual disabilities.
Patients with intellectual disabilities use antiepileptic drugs (AEDs) at a higher rate; these are known to interact with other drugs, mainly by affecting drug metabolism, such as CYP and/or uridine diphosphate-glucuronosyl-transferase (UGT).9–13 Because AEDs have a sedative effect, they are considered to enhance the clinical effects of anesthetics. In addition, patients with epilepsy are often controlled with multidrug therapy, and several anesthetics can be used for general anesthesia. It is therefore difficult to identify independent factors affecting recovery from general anesthesia when a patient with epilepsy has a delayed emergence from general anesthesia. We therefore sought to identify factors affecting emergence from general anesthesia. We used multivariate analysis in a retrospective cohort study, in which each AED was included as a predictor variable, in addition to anesthetic parameters.
METHODS
The study was conducted according to the revised Declaration of Helsinki and approved by the Ethics Committee, Okayama University, Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences (approval nos. 433 and 530). Written informed consent was waived as no interventions were conducted and data were anonymized. The design was entirely observational. The study was registered to the UMIN Clinical Trial Registry (intellectual disability: UMIN000006262).
Study Setting
The investigators designed and implemented a retrospective cohort study. The study population was composed of patients presenting for evaluation and management of dental treatment under ambulatory general anesthesia in the clinic of Special Needs Dentistry in Okayama University Hospital from January 2011 to September 2012. For a patient to be included in the study sample, general anesthesia with tracheal intubation had to be maintained with total intravenous anesthesia consisting of remifentanil and propofol. Patients were excluded as study subjects if they were hospitalized or sedative premedication was needed.
Variables
Predictor variables were gender, valproic acid (yes or no), phenytoin (yes or no), clobazam (yes or no), carbamazepine (yes or no), clonazepam (yes or no), phenobarbital (yes or no), zonisamide (yes or no), sevoflurane use for induction (yes or no), tooth extraction (yes or no), age, body mass index, propofol rate (μg/kg body weight/min), remifentanil rate (μg/kg/h), and treatment time (minutes).
The outcome variable was emergence time, which was the duration from the termination of treatment to an extubation of the tracheal tube, which was just after the patient's eyes opened.
Anesthetic Procedure
Preoperative fasting times were 6 hours for food and 2 hours for water. Medicines in daily use were taken as usual. General anesthesia was started with insertion of an intravenous line. When it was difficult to place, sevoflurane was inhaled as induction for general anesthesia, followed by insertion of an intravenous line. Remifentanil was started at 0.25 μg/kg/min and propofol was started using target-controlled infusion, with the target concentration initially set at 4.0 μg/mL. The infusion rate of remifentanil was based on body weight. In patients under 16 years old, propofol was infused at 10 mg/kg/h (167 μg/kg/min) as target-controlled infusion cannot be used because of the basic settings of the infusion pump. After loss of consciousness, rocuronium was injected to induce muscle relaxation, and an endotracheal tube was inserted, usually through the nose.
Patients were continuously monitored with an electrocardiogram. Blood pressure, SpO2 (noninvasive oxygen saturation of hemoglobin in arterial blood), bispectral index, and partial pressure of CO2 in the anesthetic circuit were also monitored. Body temperature was measured every 30 minutes at the axilla. After intubation, the infusion rate of remifentanil was reduced to 0.10–0.15 μg/kg/min and the target concentration of propofol set at 3.0 μg/mL. During treatment, the bispectral index value was maintained between 40 and 60 by adjusting the target concentration of propofol.14–16 During treatment, local anesthetic containing 2% lidocaine and 1 : 80,000 adrenaline was used if considered necessary. Intravenous or suppository nonsteroidal anti-inflammatory drugs were used after tooth extraction. At the end of surgery, infusion of both remifentanil and propofol was terminated and the effect of the muscle relaxant reversed with sugammadex. The tracheal tube was removed when the patient's eyes opened and spontaneous breathing recovered.
Data analysis
Data were analyzed using JMP 9.0.0 (SAS Institute Inc, Cary, NC). Student's t test was used between each outcome variable and the nominal variables, and a linear regression was applied to examine the bivariate regression between each outcome variable and continuous variables. P < .05 was considered significant. To extract independent variables affecting the outcome, possible predictive variables were selected with stepwise regression, for which the cutoff was a P value <.20, followed by a multiple regression analysis. Confounding factors were examined by Fisher's exact test for nominal variables and by Student's t test for continuous variables.
RESULTS
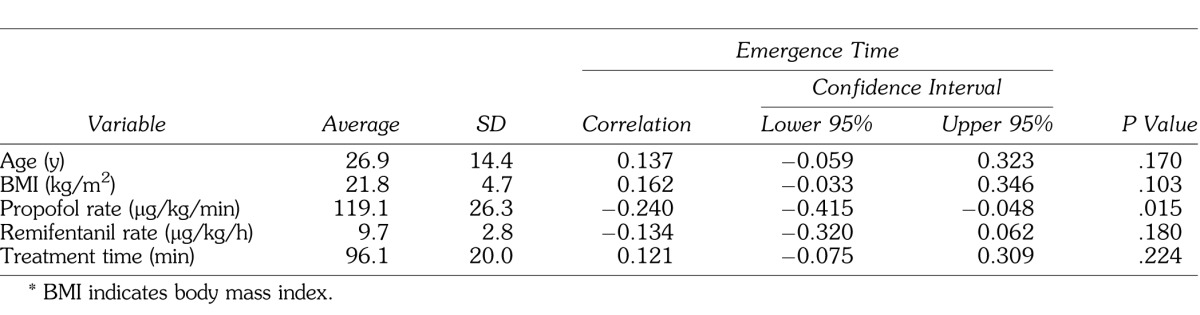
The study group comprised 102 cases (69 male, 33 female). Emergence time was significantly longer in males and with use of valproic acid, phenytoin, clobazam, clonazepam, phenobarbital, and sevoflurane, using bivariate regression (Table 1). Examining the relationship of continuous variables to emergence time, a significant negative correlation with propofol rate was observed (Table 2).
Table 1.
Differences in Emergence Time From General Anesthesia by Nominal Variables
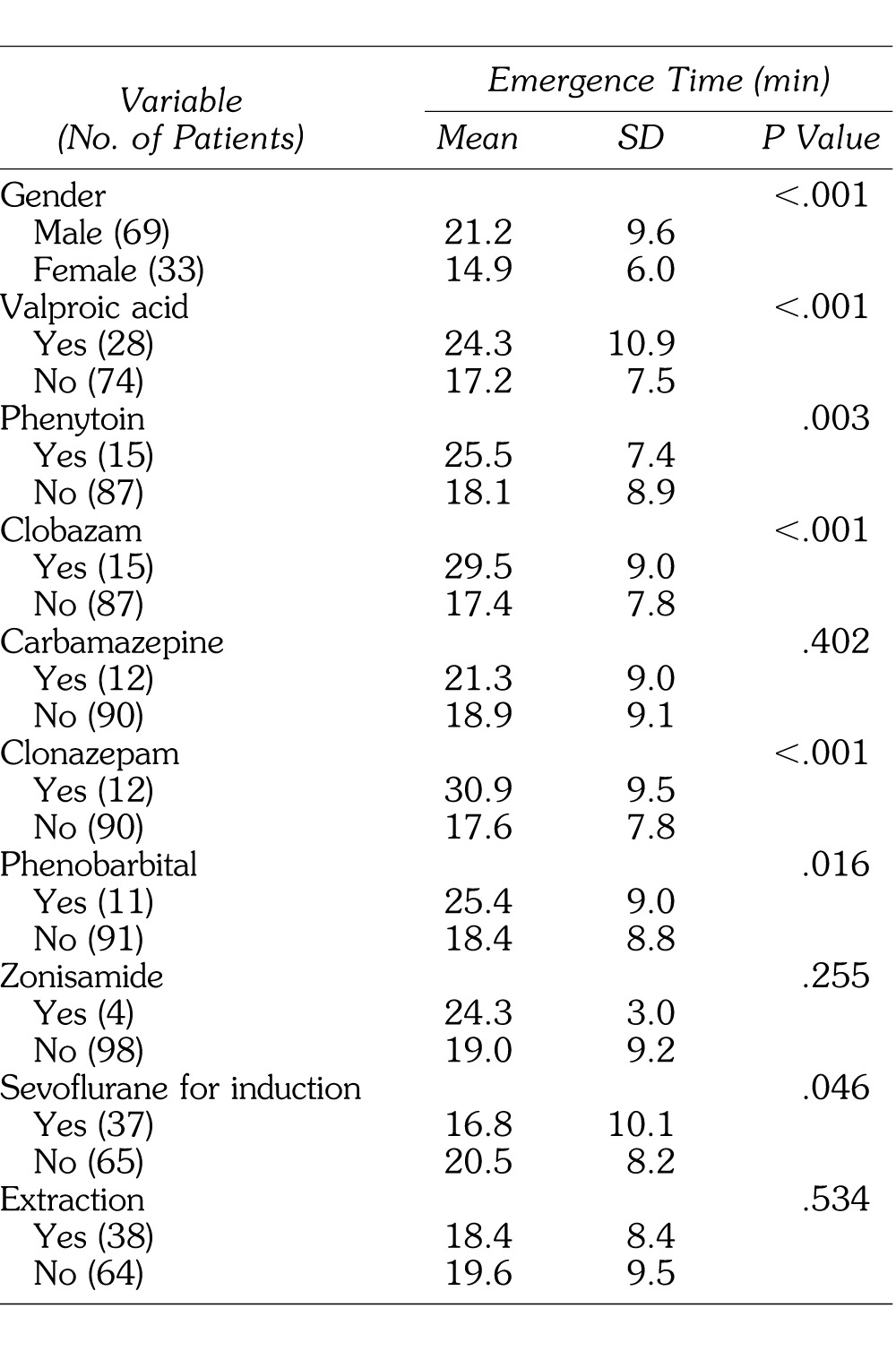
Table 2.
Continuous Variables and Their Relationship to Emergence Time From General Anesthesia*
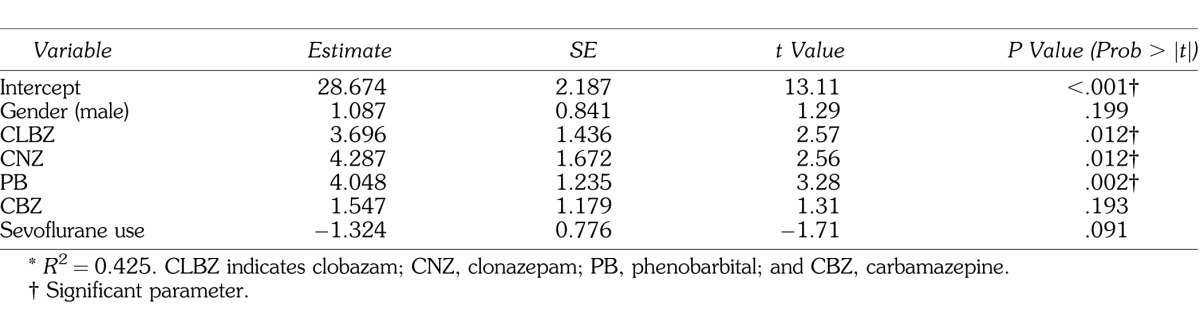
Male gender and use of clobazam, clonazepam, phenobarbital, carbamazepine, and sevoflurane were selected with stepwise regression analysis. In a multiple regression analysis, use of clobazam, clonazepam, and phenobarbital were independent determinants of the delay of emergence (Table 3).
Table 3.
Stepwise Logistic Regression Models for Emergence Time From General Anesthesia*
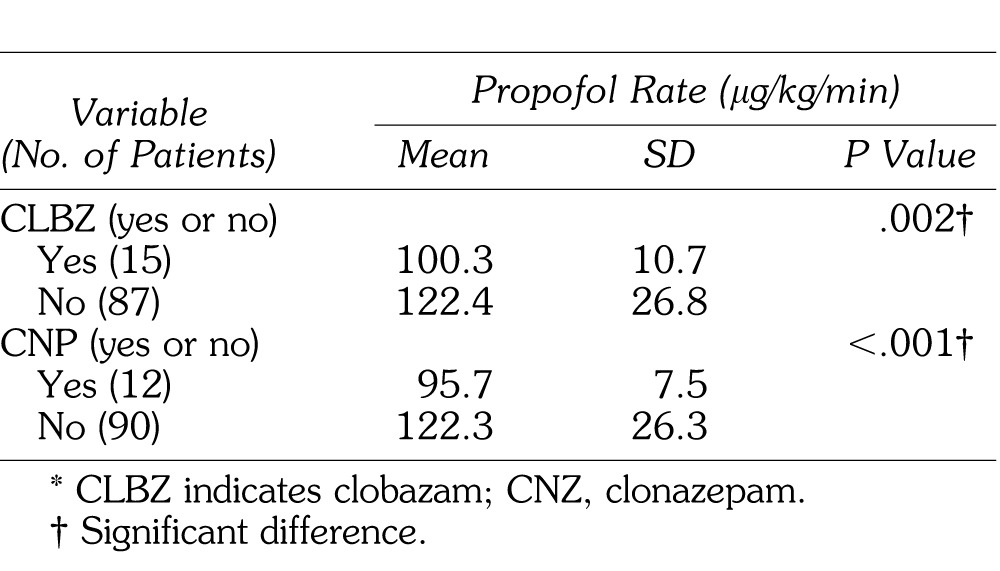
Because valproic acid, phenytoin, sevoflurane use, and propofol rate may be confounding factors, their relationship with clobazam, clonazepam, and phenobarbital was examined. Clobazam was taken by 35.7% of patients also taking valproic acid compared with only 6.8% of patients not taking valproic acid. Phenobarbital was taken by 66.7% of patients also taking phenytoin but only 1.2% of those not taking phenytoin. Finally, in patients in need of sevoflurane, phenobarbital was used in only 2.7%, compared with 15.4% of those not in need of sevoflurane. These differences were statistically significant. Propofol rate was significantly decreased in patients taking clobazam or clonazepam (Table 4).
Table 4.
Differences in Propofol Rate by CLBZ and CNP*
DISCUSSION
Independent factors associated with a delay in emergence from ambulatory general anesthesia in patients with intellectual disabilities were clobazam, clonazepam, and phenobarbital, but not parameters related to general anesthesia. This result suggests that daily medication with benzodiazepine and/or a barbiturate enhances the clinical pharmacological effect of anesthetics. Among them, the effect of propofol is considered to be enhanced by these drugs because its mechanism is mainly mediated via activation of gamma-aminobutyric acid,17 which is the same mechanism as both benzodiazepine and barbiturates. This induces sedation and hypnosis.18,19
Propofol rate was negatively correlated with emergence time, and was significantly decreased in patients using clobazam or clonazepam in this study. Because the depth of the general anesthesia was adjusted according to bispectral index, which is a reliable monitor of conscious level during general anesthesia,20,21 it is considered that the effect of propofol was enhanced by clobazam and clonazepam, and that a lower propofol rate was enough to maintain the depth of general anesthesia in patients using these drugs. Despite a lower propofol rate, both clobazam and clonazepam were still independent predictors of delay of emergence, suggesting strongly that both drugs enhance the anesthetic effect of propofol.
The actions of remifentanil and rocuronium were also possibly enhanced by AEDs. Remifentanil is metabolized rapidly by nonspecific cholinesterase in plasma and leads to quick recovery in patients with any condition.6,7 Besides, because its context-sensitive half-life is 5–10 minutes,22,23 interaction with other drugs in the recovery state is unlikely. Rocuronium, a nondepolarizing neuromuscular blocking agent, can be reversed by sugammadex, a specific reversal agent for rocuronium neuromuscular blockade.24 In addition, the mechanisms of action of both anesthetics are completely different from those of AEDs. Thus, the effect of both remifentanil and rocuronium on the delay of emergence is considered to be negligible.
Valproic acid inhibits cytochrome P450 2C9 and CYP2C19,9 and is also believed to suppress UGT1A9 and UGT2B7.10,25,26 Because propofol is metabolized by CYP2C9 and UGT1A9,27 it was expected that valproic acid would be an independent predictor of delayed emergence. On the other hand, clobazam is a weak inducer of CYP3A4 and has the potential to induce UGT1A1, but it does not have a significant induction or inhibition effect on CYPs at clinically meaningful concentrations in vitro.28 Clonazepam has also been shown to have no induction effect on CYP1A2 and CYP3A4 in vitro,29,30 and is reportedly clinically safe in drug interactions.30 Although phenobarbital induces CYP2B2, CYP2B6, CYP2C, CYP3A, and UGT1A-like mRNAs,11–13 a direct effect on propofol metabolism has never been examined. Thus, although these 3 drugs are suggested to have an induction effect on CYPs, any effect of changes in the mechanism of drug metabolism induced by AEDs seems to be less than a direct pharmacological effect on the clinical enhancing effect of propofol.
In this study, valproic acid and phenytoin were not independent predictors of delayed emergence. However, this does not mean they have no effect on emergence. One major adverse effect of antiepileptics is sedation/fatigue/tiredness.31,32 In epilepsy patients with genetic polymorphisms in CYP2C9 and CYP2C19, excessive sedation was observed as a clear clinical symptom of a higher plasma phenytoin level.33 In our previous report, valproic acid was related to a decrease in propofol dose for sedation.25 Thus, an enhancing sedative effect of anesthetics may be common among AEDs with side effects of sedation/fatigue/tiredness. Furthermore, in multivariate analyses, only stronger factors can be extracted. Our previous report showed midazolam was an independent determinant of recovery from general anesthesia where epilepsy was not detected.8 Taken together, although not significant in this study, other AEDs such as valproic acid and phenytoin may enhance the anesthetic effect of propofol.
This study is a retrospective observational analysis with a small sample size, so further study is necessary to confirm these results. However, it is difficult to analyze the individual effect of each AED on clinical pharmacology because patients with epilepsy are often controlled with polytherapy. A prospective analysis of combinations of AEDs in a larger sample size may be useful for assessing the clinical situation.
ACKNOWLEDGMENTS
This study was approved by the Ethics Committee, Okayama University, Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, and funded by the Japanese Ministry of Health, Labour, and Welfare (H22-shintai/chiteki-ippan-008).
REFERENCES
- 1.Konig MW, Varughese AM, Brennen KA, et al. Quality of recovery from two types of general anesthesia for ambulatory dental surgery in children: a double-blind, randomized trial. Paediatr Anaesth. 2009;19:748–755. doi: 10.1111/j.1460-9592.2009.03054.x. [DOI] [PubMed] [Google Scholar]
- 2.Jinzenji A, Maeda S, Higuchi H, et al. Partial laryngospasms during general anesthesia with a laryngeal mask airway for dental treatment: a report of 5 cases. J Oral Maxillofac Surg. 2010;68:2554–2557. doi: 10.1016/j.joms.2009.09.083. [DOI] [PubMed] [Google Scholar]
- 3.Maeda S, Kita F, Miyawaki T, et al. Assessment of patients with intellectual disability using the International Classification of Functioning, Disability and Health to evaluate dental treatment tolerability. J Intellect Disabil Res. 2005;49:253–259. doi: 10.1111/j.1365-2788.2005.00644.x. [DOI] [PubMed] [Google Scholar]
- 4.Abdallah FW, Morgan PJ, Cil T, et al. Ultrasound-guided multilevel paravertebral blocks and total intravenous anesthesia improve the quality of recovery after ambulatory breast tumor resection. Anesthesiology. 2014;120:703–713. doi: 10.1097/ALN.0000436117.52143.bc. [DOI] [PubMed] [Google Scholar]
- 5.Reves JG, Glass P, Lubarsky D, McEvoy M, Martinez-Ruiz R. Intravenous anesthetics. In: Miller R, editor. Miller's Anesthesia. 7th ed. Philadelphia, Pa: Churchill Livingstone Elsevier;; 2010. pp. 719–768. In. ed. [Google Scholar]
- 6.Bergmann I, Gohner A, Crozier TA, et al. Surgical pleth index-guided remifentanil administration reduces remifentanil and propofol consumption and shortens recovery times in outpatient anaesthesia. Br J Anaesth. 2013;110:622–628. doi: 10.1093/bja/aes426. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda K. Opioids. In: Miller R, editor. Miller's Anesthesia. 7th ed. Philadelphia, Pa: Churchill Livingstone Elsevier;; 2010. pp. 769–824. In. ed. [Google Scholar]
- 8.Maeda S, Tomoyasu Y, Higuchi H, Mori T, Egusa M, Miyawaki T. Midazolam is associated with delay in recovery and agitation after ambulatory general anesthesia for dental treatment in patients with disabilities: a retrospective cohort study. J Oral Maxillofac Surg. 2012;70:1315–1320. doi: 10.1016/j.joms.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Nadkarni A, Oldham MA, Howard M, Berenbaum I. Drug-drug interactions between warfarin and psychotropics: updated review of the literature. Pharmacotherapy. 2012;32:932–942. doi: 10.1002/j.1875-9114.2012.01119. [DOI] [PubMed] [Google Scholar]
- 10.Langdon G, Davis J, Layton G, Chong CL, Weissgerber G, Vourvahis M. Effects of ketoconazole and valproic acid on the pharmacokinetics of the next generation NNRTI, lersivirine (UK-453,061), in healthy adult subjects. Br J Clin Pharmacol. 2012;73:768–775. doi: 10.1111/j.1365-2125.2011.04136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Audet-Walsh E, Auclair-Vincent S, Glucocorticoids Anderson A. and phenobarbital induce murine CYP2B genes by independent mechanisms. Expert Opin Drug Metab Toxicol. 2009;5:1501–1511. doi: 10.1517/17425250903234709. [DOI] [PubMed] [Google Scholar]
- 12.Zancanella V, Giantin M, Lopparelli RM, Nebbia C, Dacasto M. Constitutive expression and phenobarbital modulation of drug metabolizing enzymes and related nuclear receptors in cattle liver and extra-hepatic tissues. Xenobiotica. 2012;42:1096–109. doi: 10.3109/00498254.2012.694493. [DOI] [PubMed] [Google Scholar]
- 13.Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- 14.Mashour GA, Shanks A, Tremper KK, et al. Prevention of intraoperative awareness with explicit recall in an unselected surgical population: a randomized comparative effectiveness trial. Anesthesiology. 2012;117:717–725. doi: 10.1097/ALN.0b013e31826904a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishiyama T, Komatsu K. Cerebral state index versus bispectral index during propofol-fentanyl-nitrous oxide anesthesia. J Anesth. 2010;24:380–385. doi: 10.1007/s00540-010-0906-5. [DOI] [PubMed] [Google Scholar]
- 16.Onuki K, Onuki N, Imamura T, et al. Pentazocine increases bispectral index without surgical stimulation during nitrous oxide-sevoflurane anesthesia. J Anesth. 2011;25:946–949. doi: 10.1007/s00540-011-1224-2. [DOI] [PubMed] [Google Scholar]
- 17.Kotani Y, Shimazawa M, Yoshimura S, Iwama T, Hara H. The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther. 2008;14:95–106. doi: 10.1111/j.1527-3458.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saari TI, Uusi-Oukari M, Ahonen J, Olkkola KT. Enhancement of GABAergic activity: neuropharmacological effects of benzodiazepines and therapeutic use in anesthesiology. Pharmacol Rev. 2011;63:243–267. doi: 10.1124/pr.110.002717. [DOI] [PubMed] [Google Scholar]
- 19.Sieghart W, Ramerstorfer J, Sarto-Jackson I, Varagic Z, Ernst M. A novel GABA(A) receptor pharmacology: drugs interacting with the alpha(+) beta(−) interface. Br J Pharmacol. 2012;166:476–485. doi: 10.1111/j.1476-5381.2011.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kertai MD, Whitlock EL, Avidan MS. Brain monitoring with electroencephalography and the electroencephalogram-derived bispectral index during cardiac surgery. Anesth Analg. 2012;114:533–546. doi: 10.1213/ANE.0b013e31823ee030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villafranca A, Thomson IA, Grocott HP, Avidan MS, Kahn S, Jacobsohn E. The impact of bispectral index versus end-tidal anesthetic concentration-guided anesthesia on time to tracheal extubation in fast-track cardiac surgery. Anesth Analg. 2013;116:541–548. doi: 10.1213/ANE.0b013e31827b117e. [DOI] [PubMed] [Google Scholar]
- 22.Futier E, Chanques G, Cayot Constantin S, et al. Influence of opioid choice on mechanical ventilation duration and ICU length of stay. Minerva Anestesiol. 2012;78:46–53. [PubMed] [Google Scholar]
- 23.Scott LJ, Perry CM. Spotlight on remifentanil for general anaesthesia. CNS Drugs. 2005;19:1069–1074. doi: 10.2165/00023210-200519120-00010. [DOI] [PubMed] [Google Scholar]
- 24.Lien CA. Development and potential clinical impairment of ultra-short-acting neuromuscular blocking agents. Br J Anaesth. 2011;107((suppl 1)):i60–i71. doi: 10.1093/bja/aer341. [DOI] [PubMed] [Google Scholar]
- 25.Ishii M, Higuchi H, Maeda S, Tomoyasu Y, Egusa M, Miyawaki T. The influence of oral VPA on the required dose of propofol for sedation during dental treatment in patients with mental retardation: a prospective observer-blinded cohort study. Epilepsia. 2012;53:e13–e16. doi: 10.1111/j.1528-1167.2011.03328.x. [DOI] [PubMed] [Google Scholar]
- 26.de Leon J, Diaz FJ, Spina E. Pharmacokinetic drug-drug interactions between olanzapine and valproate need to be better studied. J Clin Psychiatry. 2010;71:957–958. doi: 10.4088/JCP.09lr05902yel. [DOI] [PubMed] [Google Scholar]
- 27.Mikstacki A, Skrzypczak-Zielinska M, Tamowicz B, Zakerska-Banaszak O, Szalata M, Slomski R. The impact of genetic factors on response to anaesthetics. Adv Med Sci. 2013;58:9–14. doi: 10.2478/v10039-012-0065-z. [DOI] [PubMed] [Google Scholar]
- 28.Walzer M, Bekersky I, Blum RA, Tolbert D. Pharmacokinetic drug interactions between clobazam and drugs metabolized by cytochrome P450 isoenzymes. Pharmacotherapy. 2012;32:340–353. doi: 10.1002/j.1875-9114.2012.01028.x. [DOI] [PubMed] [Google Scholar]
- 29.Vrzal R, Kubesova K, Pavek P, Dvorak Z. Benzodiazepines medazepam and midazolam are activators of pregnane X receptor and weak inducers of CYP3A4: investigation in primary cultures of human hepatocytes and hepatocarcinoma cell lines. Toxicol Lett. 2010;193:183–188. doi: 10.1016/j.toxlet.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Gaertner J, Ruberg K, Schlesiger G, Frechen S, Voltz R. Drug interactions in palliative care—it's more than cytochrome P450. Palliat Med. 2012;26:813–825. doi: 10.1177/0269216311412231. [DOI] [PubMed] [Google Scholar]
- 31.St Louis EK. Minimizing AED adverse effects: improving quality of life in the interictal state in epilepsy care. Curr Neuropharmacol. 2009;7:106–114. doi: 10.2174/157015909788848857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siniscalchi A, Gallelli L, Russo E, De Sarro G. A review on antiepileptic drugs-dependent fatigue: pathophysiological mechanisms and incidence. Eur J Pharmacol. 2013;718:10–16. doi: 10.1016/j.ejphar.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Dorado P, Lopez-Torres E, Penas-Lledo EM, Martinez-Anton J, Llerena A. Neurological toxicity after phenytoin infusion in a pediatric patient with epilepsy: influence of CYP2C9, CYP2C19 and ABCB1 genetic polymorphisms. Pharmacogenomics J. 2013;13:359–361. doi: 10.1038/tpj.2012.19. [DOI] [PubMed] [Google Scholar]


