Abstract
Giardiasis is a common diarrheal disease worldwide caused by the protozoan parasite Giardia intestinalis. It is urgent to develop novel drugs to treat giardiasis, due to increasing clinical resistance to the gold standard drug metronidazole (MTZ). New potential antiparasitic compounds are usually tested for their killing efficacy against G. intestinalis under anaerobic conditions, in which MTZ is maximally effective. On the other hand, though commonly regarded as an ‘anaerobic pathogen,’ G. intestinalis is exposed to relatively high O2 levels in vivo, living attached to the mucosa of the proximal small intestine. It is thus important to test the effect of O2 when searching for novel potential antigiardial agents, as outlined in a previous study [Bahadur et al. (2014) Antimicrob. Agents Chemother. 58, 543]. Here, 45 novel chalcone derivatives with triazolyl-quinolone scaffold were synthesized, purified, and characterized by high resolution mass spectrometry, 1H and 13C nuclear magnetic resonance and infrared spectroscopy. Efficacy of the compounds against G. intestinalis trophozoites was tested under both anaerobic and microaerobic conditions, and selectivity was assessed in a counter-screen on human epithelial colorectal adenocarcinoma cells. MTZ was used as a positive control in the assays. All the tested compounds proved to be more effective against the parasite in the presence of O2, with the exception of MTZ that was less effective. Under anaerobiosis eighteen compounds were found to be as effective as MTZ or more (up to three to fourfold); the same compounds proved to be up to >100-fold more effective than MTZ under microaerobic conditions. Four of them represent potential candidates for the design of novel antigiardial drugs, being highly selective against Giardia trophozoites. This study further underlines the importance of taking O2 into account when testing novel potential antigiardial compounds.
Keywords: chemical synthesis, drug screening, anaerobic protozoa, intestinal disease, microaerobiosis
Introduction
The amitochondriate protozoon Giardia intestinalis is a human parasite, causing extensive morbidity worldwide (Ankarklev et al., 2010). Approximately 6–8% of children and 2% of adults are estimated to be infected in urbanized countries around the world (Craun, 1996). In spite of its recognition as an important human pathogen for a long time, nearly 5,000 people are hospitalized with giardiasis annually in the United States (see (Lengerich et al., 1994; Gardner and Hill, 2001) and references therein). The disease spreads through fecal-oral transmission of the parasite cysts (Adam, 2001). The host is typically infected through ingestion of cyst-contaminated water or food. After exposure to the acidic environment of the stomach lumen, the cyst develops into the trophozoite, the vegetative form of the parasite, that in turn attaches to the mucosa of the proximal small intestine. This is the crucial step in establishing and maintaining the infection. In the small intestine, the trophozoite starts proliferating, causing symptoms like diarrhea, malabsorption, dehydration, weight loss, failure to thrive, and chronic fatigue. Following encystation, the parasite is ready to be expelled into the environment and infect a new host, thus completing the life cycle.
Giardiasis is commonly treated with several approved medications, that include metronidazole (MTZ), tinidazole, furazolidone, albendazole, paromomycin, or nitazoxanide (Ali and Nozaki, 2007; Tejman-Yarden and Eckmann, 2011). This notwithstanding, because of the limited efficacy, heavy side effects, and increasing resistance of the parasite to available treatments, it is mandatory to continue searching for novel antigiardial drug candidates (Upcroft and Upcroft, 2001; Ali and Nozaki, 2007). The gold standard drug against giardiasis is MTZ, (Edwards, 1993), a pro-drug that needs to be activated intracellularly by reduction of the nitro moiety (Edwards, 1993). Relevant to this study, by reaction with O2 the active form of MTZ is converted back to the inactive parent compound. Presence of O2 in the epithelium of the proximal small intestine (Espey, 2013), where Giardia trophozoites adhere with their ventral disks, is therefore expected to significantly decrease the efficacy of MTZ in vivo. Given the fairly aerobic environment inhabited by Giardia in the host, it is important to consider possible effects of O2, when testing novel potential antigiardial drugs. Following this rationale, we have recently carried out a study (Bahadur et al., 2014) in which a set of synthetic compounds has been initially screened for its antigiardial activity under anaerobic condition, and then the compounds with the highest activity were assayed for their efficacy under microaerobic conditions too. This innovative approach allowed us to identify two chalcone derivatives that under microaerobic conditions proved to be selectively active against Giardia trophozoites and more effective than MTZ (Bahadur et al., 2014).
Nowadays, molecular hybridization is a coherent strategy that allows one to design new chemical entities of potential biomedical relevance by fusing two or more recognized active compounds and/or pharmacophoric units of known bioactive molecules (Viegas-Junior et al., 2007; Walsh and Bell, 2009). In this regard, chalcone derivatives linked to a triazolyl-quinolone moiety represent an attractive drug scaffold. Nitrogen containing heterocycles are indeed widely used for the synthesis of compounds of pharmaceutical interest (Syam et al., 2012) and chalcone analogs with their relatively simple structure have a wide variety of pharmacological activities, largely attributed to their α,β unsaturated ketone moiety (Kumar et al., 2003). Moreover, quinoline (1-azanaphthalene) compounds are widely used as “parental” compounds to synthesize molecules with a broad range of biological activities including anti-inflammatory (el-Gazzar et al., 2009), antileishmanial (Palit et al., 2009), antifungal (Kategaonkar et al., 2010), and antituberculosis (Eswaran et al., 2010). Finally, tiazoles represent another important class of heterocycles because of their varied biological activities and, accordingly, triazole-containing ring systems are found in numerous existing drugs, like fluconazole, itraconazole, and voriconazole, commonly used as anti-inflammatories, CNS-stimulants, sedatives, antianxiety, antimicrobials, and antimycotics (Shaker, 2006; Saadeh et al., 2010).
Here, we have synthesized, purified and thoroughly characterized a set of 45 novel chalcone analogs with triazolyl-quinolone scaffold, and comparatively evaluated their antigiardial activity both in anaerobiosis and microaerobiosis. This led to the identification of four compounds poorly toxic against human cells, yet able to affect Giardia trophozoites more effectively than MTZ under both anaerobic and microaerobic conditions.
Materials and Methods
Materials
All chemicals used in the synthesis of chalcones analogs were purchased from Sigma–Aldrich and Fluka and were used as such without any prior purification. MTZ, ATP, penicillin/streptomycin, bovine calf serum, bovine bile, and the chemicals for the Diamond’s TYI-S-33 medium used for Giardia cell cultures were purchased from Sigma–Aldrich. Fetal bovine serum, glutamine, non-essential amino acids, trypsin-EDTA, and the Eagle’s Minimum Essential Medium (EMEM) were purchased from GIBCO (Life technologies). Other chemicals and solvents purchased locally were of analytical grade. Caco-2 cells (ATCC® HTB-37TM) were purchased from Sigma–Aldrich. Incubation bags for anaerobiosis (Anaerocult® A minisystem) and microaerobiosis (Anaerocult® C minisystem) were from Merck. Sterile 96-well white clear-bottom plates were purchased from Perkin Elmer. The ATP one-step luminescence assay systems for microbial (BacTiter-GloTM) and human (ATPliteTM) cells were from Promega and Perkin Elmer, respectively.
Synthesis and Characterization of Chalcones
Chemical Methods
Homogeneity/purity of all the products was analyzed by thin-layer chromatography (TLC) on alumina coated plates (Merck). Product samples in MeOH were loaded on TLC plates and developed in CHCl3–MeOH (9.8:0.2, v/v). On detection of slight impurities by iodine vapor/UV light visualization, compounds were further purified by chromatography on silica gel columns (100–200 mesh size, CDH), using petroleum ether-ethyl acetate (3:2, v/v) as the eluent. Melting points were determined in open glass capillary tubes on a Buchi M-560 instrument and are uncorrected. Infrared (IR) spectra were recorded in KBr medium using a Perkin-Elmer Fourier Transform-IR spectrometer, whereas 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded in CDCl3 medium on a JNM ECX-400P (JEOL, USA) spectrometer with tetramethylsilane (TMS) as internal reference. IR and NMR spectra were recorded at the Department of Chemistry, University of Delhi, India. Absorption frequencies (ν) are expressed in cm-1, chemical shifts in ppm (δ-scale) and coupling constants (J) in Hz. Splitting patterns are described as singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). High resolution mass spectroscopy (HRMS) data were collected with a resolution of 10,000 on a KRATOS MS50TC spectrometer and a Kratos Mach III type at the University of Leuven (KU Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium).
General Procedure for the Synthesis of 2-chloroquinoline-3-carbaldehyde (Ramesh et al., 2008) (2)
2-chloroquinoline-3-carbaldehyde was synthesized from acetanilide (1) via a Vilsmeier–Haack reaction by traditional methods. To a well-stirred mixture of N,N-dimethylformamide (DMF, 3 equiv.) and acetanilide 1 (1 equiv.), POCl3 (12 equiv.) was added dropwise slowly at 0∘C. Afterward the reaction mixture was heated to 100∘C for 16 h and the reaction progress was monitored by TLC. After completion of the reaction, the mixture was allowed to cool down to room temperature and poured into crushed ice under vigorous stirring. The obtained precipitate was filtered, washed with water and re-crystallized from dry EtOH to give the title compound 2 with 62% yield; mp 146–147∘C (lit 148∘C).
General Procedure for the Synthesis of 2-oxoquinoline-3-carbaldehyde (Ramesh et al., 2008) (3)
2-chloroquinoline-3-carbaldehyde 2 was refluxed in 70% acetic acid to obtain 2-oxoquinoline-3-carbaldehyde 3. The reaction mixture was heated to 110∘C for 12 h and the reaction progress was monitored by TLC. After completion of the reaction, the mixture was allowed to cool down to room temperature and poured into crushed ice under vigorous stirring. The obtained precipitate was filtered, washed with water and re-crystallized from dry EtOH to give the title compound 3 with 90% yield; mp 301–302∘C (lit 304∘C).
General Procedure for the Synthesis of 2-oxo-1-(prop-2-ynyl)-1,2-dihydroquinoline-3-carbaldehyde (Pal et al., 2011) (4)
To a solution of 2-oxo-1,2-dihydroquinoline-3-carbaldehyde 3 (1.0 equiv.) in K2CO3 (1.5 equiv.) and DMF, propargyl bromide (1.5 equiv) was added dropwise. The reaction mixture was stirred at room temperature for 12 h. After completion of the reaction, the mixture was poured into ice-cooled water. The solid separated was filtered, washed, dried, and re-crystallized from ethanol to give the title compound 4 with 75% yield; mp 195–197∘C (lit 198∘C).
General Procedure for the Synthesis of Azidobenzene and its Derivatives (Haridas et al., 2011) (5–9)
To a mixture of appropriate aniline (1 equiv.) in 17% HCl stirred at 0∘C, aqueous sodium nitrite (1.2 equiv.) was added dropwise with continued stirring for 10 min at 0∘C. Afterward, aqueous sodium azide (1.2 equiv.) was added dropwise to the reaction mixture at 0∘C with continued stirring for additional 3 h at room temperature. The reaction progress was monitored by TLC [petroleum ether/ethyl acetate (5:1)]. After completion of the reaction, the mixture was subjected to extraction with ethyl acetate (2 × 25 mL). The combined organic layer was dried over Na2SO4 and concentrated under reduced pressure, to obtain an oily brown colored compound.
General Procedure for the Synthesis of 2-oxo-1-((1-phenyl-1H-1,2,3-triazol-4-yl)methyl)-1,2-dihydroquinoline-3-carbaldehyde (10–14)
The mixture of alkyne 4 (1 equiv.), appropriate azide (5–9; 1.5 equiv.), CuSO4⋅5H2O (0.2 equiv.), and sodium ascorbate (0.4 equiv.) was taken in a (3:1) mixture of tetrahydrofuran (THF) and water and stirred at room temperature for 12 h. The reaction progress was monitored by TLC using CHCl3: MeOH (9.7:0.3, v/v) as the solvent system. After completion of the reaction, the mixture was subjected to extraction with ethyl acetate (3 × 30 mL). The combined ethyl acetate layer was dried over Na2SO4 concentrated under reduced pressure and finally purified by silica gel (100–200 mesh size) column chromatography using petroleum ether – ethyl acetate (3:2) as the eluent to yield the desired product.
General Procedure for the Synthesis of (E)-3-(3-oxo-3-phenylprop-1-en-1-yl)-1-((1-phenyl-1H-1,2,3-triazol-4-yl)methyl)quinolin-2(1H)-one (23–67).
To a solution of aryl ketone (15–22; 1 equiv.) and triazolyl-quinaldehyde (10–14; 1 equiv.) in dry MeOH, cat. sodium hydroxide was added. The resulting reaction mixture was stirred at room temperature for 24 h. The reaction progress was monitored by TLC using CHCl3:MeOH (9.8:0.2, v/v) as the solvent system. After completion of the reaction, the mixture was poured into ice-cool water. The obtained colored precipitate was filtered and dried on vacuum. The compounds were finally purified by silica gel (100–200 mesh size) column chromatography using petroleum ether – ethyl acetate (3:2) as the eluent.
Assays on Cells
Cell Cultures
Trophozoites of G. intestinalis strain WB clone C6 (ATCC No. 50803TM) were cultured axenically at 37∘C in 25-cm2 flasks containing Diamond’s TYI-S-33 medium supplemented with 10% bovine calf serum, 1 mg/mL bovine bile, 0.1 g/L streptomycin and 100 U/mL penicillin. Typically, 50 ml medium was inoculated with 25 × 106 cells and after 2 days cells were harvested by chilling the flasks on ice for 30 min for drug susceptibility assays. Human epithelial colorectal adenocarcinoma (Caco-2) cells were grown in 25-cm2 flasks in EMEM supplemented with 1% (v/v) non-essential amino acids, 2 mM glutamine, 5% (v/v) fetal bovine serum, 0.1 g/L streptomycin, and 100 U/mL penicillin.
Assays on Giardia trophozoites
The assays were performed following the procedure described in Bahadur et al. (2014), using sterile 96-well white clear-bottom plates. In each well, 50 μL of Giardia trophozoites at a density of 1 × 105 cells/mL was added to 50 μL medium containing either the compound to be tested, serially diluted from a stock solution in dimethyl sulfoxide (DMSO), or the same amount of DMSO as a control; this yielded a final density of 5.000 cells/100 μL in each well. Each drug concentration was tested in at least six replicates. MTZ was used as an internal positive control in the assay. The microtiter plates were then incubated at 37∘C under anaerobic or microaerobic conditions, ensured by the Anaerocult® A or the Anaerocult® C minisystem (Merck), respectively. According to the manufacturer instructions, the Anaerocult® A minisystem produces anaerobic conditions within ∼1 h, whereas the Anaerocult® C minisystem generates microaerobic conditions (∼5% O2) within 24 h. Following 48 h incubation with the compound to be tested, 100 μL of the BacTiter-GloTM Microbial Cell Viability Assay System reagent (Promega) was added to each well for one-step lysis and ATP level detection. Plates were then incubated at room temperature for 15 min and ATP levels finally detected by luminescence on a plate reader (Wallac Victor3 1420 Multilabel Counter, PerkinElmer).
Assays on Caco-2 Cells
Cells were detached with 0.5% trypsin-EDTA and seeded in sterile 96-well white clear-bottom plates at the same density of Giardia trophozoites and at increasing concentration of the compound to be tested, as described above. The assays were carried out exactly as reported for the Giardia trophozoites, except that the plates were incubated (still at 37∘C) at atmospheric O2 level, 5% CO2, and 95% humidity. Each drug concentration was tested in at least seven replicates. MTZ was used as an internal negative control in the assay. Following 48 h incubation with each compound, according to the manufacturer instructions, 100 μL of the ATPliteTM luminescence assay system (Perkin Elmer) was added to each well for one-step lysis and ATP level detection by luminescence.
Determination of Half-Maximal Inhibitory Concentration (IC50) and Selectivity Index (SI)
Luminometric data were calibrated using ATP standard curves and normalized to the ATP level measured in control DMSO-treated cells (taken as 100%). The measured ATP level percentage was plotted as a function of the compound concentration and the half-maximal inhibitory concentration (IC50) was obtained by fitting the resulting titration profile to the Hill equation (Goutelle et al., 2008). The selectivity index (SI) of the compounds was then calculated as the ratio between the IC50 value measured on human cells over the value determined on Giardia trophozoites (SI = IC50,Caco-2/IC50,Giardia).
Results
Synthesis of Novel Triazolyl-Quinolone Based Chalcones
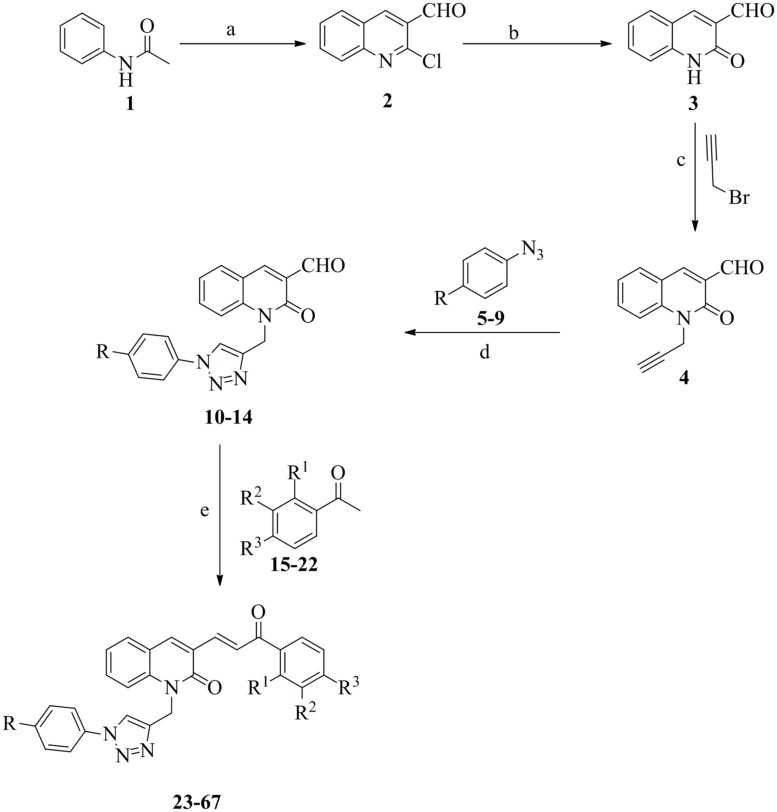
Forty-five novel triazolyl-quinolone-based chalcone derivatives were synthesized based on previously described Claisen-Schmidt condensation (Li et al., 1995), according to the synthetic route outlined in Figure 1. Intermediate triazolyl-quinolone compounds 10–14 were synthesized at room temperature in THF:water with 50–75% yield, by “click chemistry” (Barral et al., 2007) of the synthesized alkyne 4 with appropriate aromatic azides (5–9), using CuSO4⋅5H2O and sodium ascorbate as catalysts. As shown in Figure 1, compounds 23–67 were obtained at room temperature by reaction of the intermediate compounds 10–14 with commercially available aromatic acetophenones 15–22, in the presence of NaOH in dry MeOH. After purification, the yield of products 23–67 ranged from 60 to 95%.
FIGURE 1.
Synthetic route to compounds 23–67. (a) DMF, POCl3, 0–100∘C, 16 h; (b) 70% acetic acid, 110∘C, 12 h; (c) K2CO3, DMF, rt, 12 h; (d) CuSO4⋅5H2O, Na-ascorbate, THF:H2O (3:1), rt, 12 h; (e) NaOH, MeOH, rt, 24 h.
The newly synthesized triazolyl-quinolone-based chalcone derivatives were characterized by HRMS, 1H and 13C NMR and IR spectroscopy, and relevant data are reported in Supplementary Material.
Antigiardial Activity of the Synthesized Chalcones
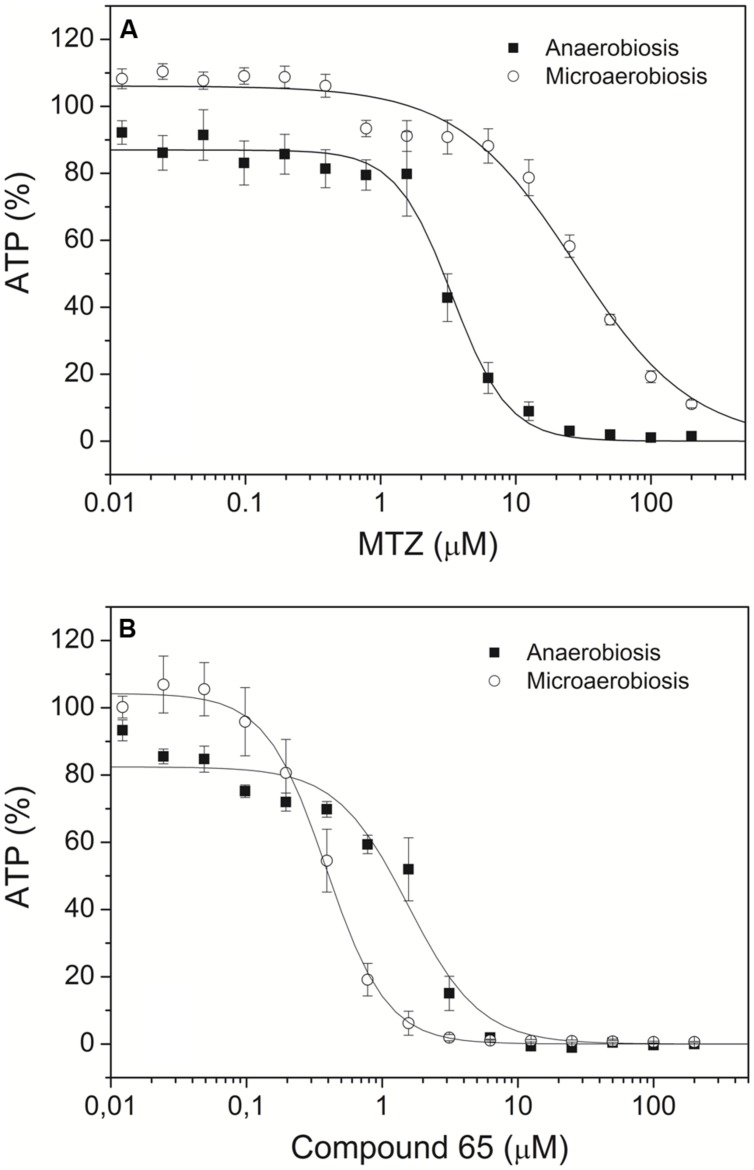
The antigiardial activity of the novel compounds 23–67 was tested under both anaerobic and microaerobic conditions. According to (Dunn et al., 2010; Bahadur et al., 2014), susceptibility of Giardia trophozoites to increasing concentrations of each compound was assessed based on ATP level determination by luminescence. Dose-response curves for each compound were obtained after 48 h-incubation and compared to the data collected under identical conditions with MTZ, the drug of choice for treatment of giardiasis. In these assays, the ATP level measured in control Giardia trophozoites grown under microaerobic conditions was found to be approximately 25% lower than in the same cells grown under anaerobic conditions, and DMSO at concentrations ≤2%v/v caused only marginal effects on cell ATP levels. Typical dose-response curves are shown in Figure 2, whereas the IC50 values measured for the synthetic compounds and MTZ under anaerobic and microaerobic conditions are reported in Table 1. In agreement with the literature (Müller et al., 2006), under the experimental conditions of the assay, MTZ proved to be highly effective (IC50 = 3.4 μM) against Giardia parasites in the absence of O2, but remarkably less (IC50 ≥ 25 μM) under microaerobic conditions. Under anaerobic conditions, 18 out of the 45 synthetic compounds were as effective as MTZ or more under identical conditions (see Table 1). Among them, compounds 41, 43, and 45 displayed the highest activity, being three to fourfold more efficient than MTZ.
FIGURE 2.
Effect of metronidazole (MTZ) and compound 65 on Giardia trophozoites. Dose-response curves of MTZ (A) and the newly synthesized compound 65 (B), measured on Giardia trophozoites cultured under anaerobic (closed symbol) or microaerobic (open symbol) conditions. Data are expressed as mean ± SEM (n ≥ 6).
Table 1.
Chemical identity, IC50 values and selectivity index (SI) of the novel synthetic compounds.
|
Giardia (anaerobiosis) |
Giardia (microaerobiosis) |
Caco-2 |
Selectivity (IC50,Caco/IC50,Giardia) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| S. No | R | R1 | R2 | R3 | IC50 | IC50 | IC50 | Anaerobiosis | Microaerobiosis |
| 23 | OMe | F | H | H | ≥100 | ≥20 | ≥100 | n.d. | n.d. |
| 24 | OMe | H | F | H | ≥100 | 8,4 | ≥50 | n.d. | ≥6 |
| 25 | OMe | H | H | F | 3,9 | 1,3 | ≥20 | ≥ 5 | ≥15 |
| 26 | OMe | H | H | Cl | 5,0 | 2,6 | ≥20 | ≥4 | ≥7 |
| 27 | OMe | H | H | Br | 9,6 | 2,0 | ≥20 | ≥2 | ≥10 |
| 28 | OMe | H | H | Me | 5,4 | 1,5 | ≥80 | ≥14 | ≥53 |
| 29 | OMe | OMe | H | H | 2,2 | 0,4 | ≥40 | ≥18 | ≥100 |
| 30 | OMe | H | OMe | H | 5,9 | 1,3 | ≥200 | ≥33 | ≥153 |
| 31 | OMe | H | H | OMe | ≥50 | 2,0 | ≥80 | n.d. | ≥40 |
| 32 | Me | F | H | H | 3,2 | 0,6 | 17,6 | 5,5 | 29,3 |
| 33 | Me | H | F | H | 4,0 | 0,7 | 13,4 | 3,4 | 19,1 |
| 34 | Me | H | H | F | 3,8 | 1,2 | 9,4 | 2.5 | 7.8 |
| 35 | Me | H | H | Cl | 2,4 | 0,8 | 7,0 | 2.9 | 8.8 |
| 36 | Me | H | H | Br | 10,7 | 2,3 | ≥50 | ≥4 | ≥21 |
| 37 | Me | H | H | Me | 6,3 | 1,6 | ≥50 | ≥7 | ≥31 |
| 38 | Me | OMe | H | H | 2,0 | 0,4 | 13,7 | 6,9 | 34,3 |
| 39 | Me | H | OMe | H | 2,9 | 1,3 | ≥100 | ≥34 | ≥76 |
| 40 | Me | H | H | OMe | ≥15 | 2,4 | ≥60 | n.d. | ≥25 |
| 41 | F | F | H | H | 0,9 | 0,2 | 5,2 | 5,8 | 26 |
| 42 | F | H | F | H | 3,3 | 0,7 | 9,6 | 2,9 | 13,7 |
| 43 | F | H | H | F | 1,2 | 0,3 | 7,0 | 5,8 | 23,3 |
| 44 | F | H | H | Cl | 1,5 | 1,0 | 7,2 | 4.8 | 7,2 |
| 45 | F | H | H | Br | 1,1 | 0,5 | 16,7 | 15,2 | 33,4 |
| 46 | F | H | H | Me | 2,5 | 0,4 | 6,3 | 2,5 | 15,8 |
| 47 | F | OMe | H | H | 2,7 | 0,3 | ≥20 | ≥7 | ≥66 |
| 48 | F | H | OMe | H | 7,0 | 1,6 | ≥ 60 | ≥8 | ≥37 |
| 49 | F | H | H | OMe | 13,0 | 1,3 | ≥50 | ≥3 | ≥38 |
| 50 | Cl | F | H | H | 4,7 | 0,9 | ≥ 50 | ≥10 | ≥55 |
| 51 | Cl | H | F | H | 10,0 | 0,9 | ≥100 | ≥10 | ≥111 |
| 52 | Cl | H | H | F | 8,9 | 1,2 | 23,9 | 2,7 | 19,9 |
| 53 | Cl | H | H | Cl | 9,0 | 1,1 | ≥30 | ≥3 | ≥27 |
| 54 | Cl | H | H | Br | 9,2 | 1,9 | ≥20 | ≥ 2 | ≥10 |
| 55 | Cl | H | H | Me | 8,7 | 1,1 | 20 | 2,3 | 18,2 |
| 56 | Cl | OMe | H | H | 3,2 | 0,6 | 8,4 | 2,6 | 14,0 |
| 57 | Cl | H | OMe | H | 6,6 | 1,1 | 14,7 | 2,2 | 11,3 |
| 58 | Cl | H | H | OMe | ≥100 | ≥30 | 19,7 | ≤0,2 | ≤0,7 |
| 59 | H | F | H | H | 1,9 | 0,4 | 11,2 | 5,9 | 28,0 |
| 60 | H | H | F | H | 3,5 | 0,7 | ≥50 | ≥14 | ≥71 |
| 61 | H | H | H | F | 5,3 | 0,8 | 18,2 | 3,4 | 22,8 |
| 62 | H | H | H | Cl | 2,1 | 0,7 | 5,9 | 2.8 | 8,4 |
| 63 | H | H | H | Br | 6,3 | 1,0 | ≥50 | ≥ 7 | ≥ 50 |
| 64 | H | H | H | Me | 2,8 | 0,5 | 17,8 | 6,4 | 35,6 |
| 65 | H | OMe | H | H | 1,6 | 0,4 | ≥50 | ≥31 | ≥125 |
| 66 | H | H | OMe | H | 3,2 | 1,6 | ≥50 | ≥15 | ≥31 |
| 67 | H | H | H | OMe | 5,9 | 0,8 | ≥100 | ≥16 | ≥125 |
| MTZ | 3,4 | ≥25 | ≥100 | ≥29.0 | n.d. |
Bold, IC50 < IC50,MTZ in anaerobiosis; Compounds 29, 39, 65 and 66 display IC50 < IC50,MTZ in both anaerobiosis and microaerobiosis, low toxicity against Caco-2 cells (IC50 ≥ 40.0) and SI = 10.
Interestingly, at variance from MTZ, all the tested compounds displayed a higher efficacy against Giardia under microaerobic conditions than in the absence of O2. Notably, apart from a few exceptions (namely, compounds 23 and 58), due to their enhanced efficacy and the notably lower activity of MTZ, all the screened compounds were found to be more effective than MTZ under microaerobic conditions. In many cases, the effect of O2 was remarkable, as it can be seen from the IC50 values reported in Table 1. Under microaerobic conditions approximately half of the tested compounds proved to be at least fivefold more active against Giardia than in the absence of O2. The effect of O2 was particularly evident in the case of compounds 24, 31, 49, and 51, which displayed ≥10-fold higher activities under microaerobic conditions than measured in anaerobiosis. Interestingly, up to 10 compounds displayed IC50 ≤ 0.5 μM under microaerobic conditions, thereby becoming >50-fold more active than MTZ under identical conditions.
Selectivity of the Synthetic Compounds
Selectivity of the newly synthesized compounds toward Giardia was assessed by running a counter-screen on human epithelial Caco-2 cells. Similarly to the screen carried out on Giardia trophozoites, dose-response curves and corresponding IC50 values for each compound were obtained after 48 h-incubation, as reported above. This allowed us to calculate the compounds SI, defined as the ratio between the IC50 value measured on human cells over the value determined on Giardia trophozoites. In the assays on human cells, DMSO affected ATP cell levels only slightly (up to 10% at ≤ 2%v/v, not shown) and, as expected, MTZ exhibited a high IC50 value (>100 μM). Interestingly, under the same experimental conditions, 19 compounds were only poorly effective against human cells, being characterized by IC50 values≥40 μM (Table 1). Among these, compounds 29, 39, 65, and 66 were particularly interesting in that they proved to be (i) more effective than MTZ against Giardia trophozoites both in anaerobiosis (IC50 ranging from 2.2 to 3.2 μM) and microaerobiosis (IC50 ranging from 0.4 to 1.6 μM), and (ii) much less effective on human cells (IC50 ≥ 40 μM), thereby exhibiting a high selectivity toward Giardia trophozoites (with SI values exceeding 15 and 30 under anaerobiosis and microaerobiosis, respectively).
Discussion
Compared to other more distal tracts of the gut, the proximal small intestine is a fairly aerobic environment, where O2 is supplied mostly by the submucosal vascular network and in part with swallowed air (Dawson et al., 1965; Levitt, 1970; Sheridan et al., 1990; He et al., 1999). In this tract of the gut, O2 concentration is relatively high and subjected to sudden oscillations, particularly in the postprandial period when the metabolic demand increases upon transit of partly processed food (Espey, 2013). Relevant to the present study, O2 concentration is particularly high at the level of the intestinal epithelium, where trophozoites of G. intestinalis adhere with their ventral disks. O2 tension indeed decreases along a steep gradient from 80–100 to nearly 0 mm Hg, moving inward from the submucosa toward the luminal midpoint (Espey, 2013). Therefore, though commonly regarded as an anaerobic protozoon, Giardia is likely exposed to fairly high and variable O2 levels in vivo. The parasite is amitochondriate and thus unable to utilize O2 to sustain energy metabolism. This notwithstanding, μM O2 concentrations have been shown to produce profound changes in the metabolism of Giardia trophozoites, leading to stimulation of both ethanol and CO2 production, elicited oxidation of the intracellular NAD(P)H pool and reduced alanine production (Paget et al., 1993). In addition to these adaptive metabolic changes, in order to survive oxidative stress conditions, Giardia needs to activate the antioxidant defense system (Brown et al., 1995; Mastronicola et al., 2011; Raj et al., 2014). In this regard, it has been established that, though lacking bona fide catalases or superoxide dismutases, Giardia is endowed with several alternative antioxidant enzymes that have been recently identified and partly characterized. These include: a NADH oxidase (Brown et al., 1996a) and a flavodiiron protein (Di Matteo et al., 2008; Vicente et al., 2009) detoxifying O2 to H2O, a flavohemoglobin converting nitric oxide into nitrate (Mastronicola et al., 2010, 2011; Rafferty et al., 2010), a superoxide reductase promptly degrading superoxide anion to H2O2 (Testa et al., 2011), a thioredoxin reductase (Brown et al., 1996b) and two peroxiredoxins (Mastronicola et al., 2014) implicated in peroxide detoxification and repair of oxidatively damaged molecules. Exposure to O2 is therefore expected to cause notable changes in the metabolic and proteomic profile of G. intestinalis, an aspect that has not been studied in detail thus far.
Despite being physiologically exposed to O2 in vivo, Giardia trophozoites are commonly assayed in vitro for their drug susceptibility under anaerobic conditions. This may bias the results of a screening. Leading to changes in cell metabolism, O2 may indeed modify the susceptibility of the parasite to specific drugs, possibly up- or down-regulating molecular targets. Moreover, by directly reacting with the tested drugs, it may alter their mechanism of action, possibly leading to enhanced or reduced efficacy. This is the case of MTZ, the gold standard drug against giardiasis, whose efficacy is abolished upon reaction of its nitro-radical active form with O2 (Edwards, 1993). When testing new potential antigiardial drugs, it is thus important to take O2 into account also to attempt identifying antiparasitic compounds that could be fully effective under the more physiological microaerobic conditions in which MTZ efficacy is reduced due to O2 presence. This issue has been highlighted in a recent study by our groups (Bahadur et al., 2014) in which O2, while making MTZ expectedly less effective, was reported to enhance the efficacy of three piperidine/piperazine chalcone derivatives, pre-selected in a screen conducted under anaerobic conditions.
In the present study, 45 novel chalcone derivatives with triazolyl-quinolone scaffold were synthesized, purified, and characterized by HRMS, 1H and 13C NMR and IR spectroscopy. As an innovative approach, all the compounds were comparatively tested for their efficacy against G. intestinalis trophozoites under both anaerobic and microaerobic conditions. Compared to anaerobic conditions, the presence of O2 was found to increase the IC50 of MTZ from 3.4 to ≥25 μM. This result agrees with the notion that O2 interferes with MTZ, by converting its nitro-radical active form into the parental inactive form (Edwards, 1993). However, noteworthy, in spite of the reduced efficacy of MTZ, all the tested compounds proved to be more effective in the presence of O2, though to different extent. For most of the compounds the IC50 was four to sevenfold smaller than measured under anaerobic conditions, but in the case of four compounds (24, 31, 49, and 51) O2 was particularly effective decreasing their IC50 by more than 10-fold. While under anaerobic conditions only 18 out of the 45 tested compounds proved to be as active as MTZ or more, in the presence of O2 almost all the tested compounds (with the exception of compounds 23 and 58) were more effective than MTZ. Under microaerobic conditions, up to 10 compounds showed notably low IC50 values (≤0.5 μM), thereby becoming >50-fold more effective than MTZ under the same microaerobic conditions, yet less effective than the alternative antigiardial drug albendazole that under anaerobic conditions shows very low IC50 values (<0.1 μM, Cedillo-Rivera and Munoz, 1992). Interestingly, 19 out of the 45 tested compounds displayed very poor toxicity against human Caco-2 cells (IC50≥40 μM), and by comparing their efficacy on human and Giardia cells 10 out of these 19 compounds proved to be highly selective toward the parasite, being ≥10-fold more effective against Giardia under both anaerobic and microaerobic conditions. Among these 10 compounds highly selective against Giardia, four can be considered as hits to develop potential antigiardial drugs, namely compounds 29, 39, 65, and 66, being more effective than MTZ under both anaerobic and aerobic conditions.
The compounds characterized here carry substitutions in two phenyl rings: ring A (attached to the triazole moiety) and ring B (attached to the α,β unsaturated moiety). By inspecting #x1-21000doc, it can be noted that on average the compounds with the highest antigiardial activity have either unsubstituted (compounds 59–67) or fluoro-substituted (compounds 41–49) ring A. Comparatively, less antigiardial activity is observed when the same ring is substituted with chloro (compounds 50–58), methoxy (compounds 23–31) or methyl (compounds 32–40) groups. Compared to other types of substitutions in ring A, the fluoro-substitution seems to result into higher toxicity of the compounds against human cells. The occurrence of substitutions at ring B and their position in the ring also appear to modulate the antigiardial activity. Comparison of compounds 29, 30, and 31 shows that the position of a methoxy substituent in ring B has great effects on the antiparasitic activity of the molecules. The ortho substituted compound 29 is more effective than the meta substituted compound 30, that in turn displays a higher antigiardial activity compared to the para substituted analog (compound 31). Interestingly, the same trend (ortho > meta > para) is invariably observed by comparing the compound triplets 38–40, 47–49, 56–58, and 65–67, all having methoxy-substituted ring B. Finally, it is worth noticing that the compounds optimally combining high antigiardial activity and low toxicity against human cells (compounds 29, 39, 65, and 66) have ring B invariably methoxy-substituted at ortho or meta positions, and ring A either unsubstituted or substituted with methoxy or methyl groups.
To the best of our knowledge, this is the first study in which a complete set of new synthetic compounds has been screened for its ability to affect Giardia trophozoites under both anaerobic and microaerobic conditions, providing unequivocal evidence for an effect of O2. The results show that, as compared to anaerobic conditions, the presence of low, more physiological O2 concentrations elicits the antiparasitic activity of the tested compounds, while having opposite effects on MTZ. The molecular mechanism underlying the observed effects of O2 has yet to be established. A possibility is that O2, by directly affecting Giardia trophozoites, on the one hand impairs MTZ activation and on the other makes the cells more susceptible to the novel synthetic drugs here described. A more intriguing possibility is that, in response to O2 exposure, the parasite activates specific metabolic pathways that are selectively targeted by the compounds tested in the present study, but not MTZ. To address this issue, it would important in future studies to acquire more detailed information on the pathways that are activated in Giardia in the metabolic transition from anaerobic to microaerobic conditions.
Conclusion
In this study we have identified four new compounds that under both anaerobic and more physiological microaerobic conditions are highly effective against Giardia trophozoites, targeting the parasite selectively and more efficiently than MTZ. These four synthetic chalcone derivatives represent potential candidates for the design of novel antigiardial drugs. This work further highlights that it is important to take O2 into account when screening new potential antigiardial drugs.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was partially supported by Ministero dell’Istruzione, dell’Università e della Ricerca of Italy (PNR-CNR Aging Program 2012-2014 AG, FIRB RBIN06E9Z8 and PRIN 20107Z8XBW_005 to PS), Ministry of Science and Technology, Department of Science and Technology (DST/INT/UKR/2012/P-10), India, Council of Scientific and Industrial Research and University of Delhi under the Strengthening R&D Doctoral Research Programme, Delhi, India.
Supplementary material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fmicb.2015.00256/abstract
References
- Adam R. D. (2001). Biology of Giardia lamblia. Clin. Microbiol. Rev. 14 447–475 10.1128/CMR.14.3.447-475.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali V., Nozaki T. (2007). Current therapeutics, their problems, and sulfur-containing-amino-acid metabolism as a novel target against infections by “amitochondriate” protozoan parasites. Clin. Microbiol. Rev. 20 164–187 10.1128/CMR.00019-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankarklev J., Jerlstrom-Hultqvist J., Ringqvist E., Troell K., Svärd S. G. (2010). Behind the smile: cell biology and disease mechanisms of Giardia species. Nat. Rev. Microbiol. 8 413–422 10.1038/nrmicro2317 [DOI] [PubMed] [Google Scholar]
- Bahadur V., Mastronicola D., Tiwari H. K., Kumar Y., Falabella M., Pucillo L. P., et al. (2014). O(2)-dependent efficacy of novel piperidine- and piperazine-based chalcones against the human parasite Giardia intestinalis. Antimicrob. Agents Chemother. 58 543–549 10.1128/AAC.00990-913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral K., Moorhouse A. D., Moses J. E. (2007). Efficient conversion of aromatic amines into azides: a one-pot synthesis of triazole linkages. Org. Lett. 9 1809–1811 10.1021/ol070527h [DOI] [PubMed] [Google Scholar]
- Brown D. M., Upcroft J. A., Upcroft P. (1995). Free radical detoxification in Giardia duodenalis. Mol. Biochem. Parasitol. 72 47–56 10.1016/0166-6851(95)00065-9 [DOI] [PubMed] [Google Scholar]
- Brown D. M., Upcroft J. A., Upcroft P. (1996a). A H2O-producing NADH oxidase from the protozoan parasite Giardia duodenalis. Eur. J. Biochem. 241 155–161 10.1111/j.1432-1033.1996.0155t.x [DOI] [PubMed] [Google Scholar]
- Brown D. M., Upcroft J. A., Upcroft P. (1996b). A thioredoxin reductase-class of disulphide reductase in the protozoan parasite Giardia duodenalis. Mol. Biochem. Parasitol. 83 211–220 10.1016/S0166-6851(96)02776-4 [DOI] [PubMed] [Google Scholar]
- Cedillo-Rivera R., Munoz O. (1992). In-vitro susceptibility of Giardia lamblia to albendazole, mebendazole and other chemotherapeutic agents. J. Med. Microbiol. 37 221–224 10.1099/00222615-37-3-221 [DOI] [PubMed] [Google Scholar]
- Craun G. F. (1996). “Waterborne outbreaks of giardiasis: current status,” in Giardia and Giardiasis: Biology, Pathogenesis, and Epidemiology, eds Erlandsen S. L., Meyer E. A. (New York, NY: Plenum Press; ), 243–261. [Google Scholar]
- Dawson A. M., Trenchard D., Guz A. (1965). Small bowel tonometry: assessment of small gut mucosal oxygen tension in dog and man. Nature 206 943–944 10.1038/206943b0 [DOI] [PubMed] [Google Scholar]
- Di Matteo A., Scandurra F. M., Testa F., Forte E., Sarti P., Brunori M., et al. (2008). The O2-scavenging flavodiiron protein in the human parasite Giardia intestinalis. J. Biol. Chem. 283 4061–4068 10.1074/jbc.M705605200 [DOI] [PubMed] [Google Scholar]
- Dunn L. A., Burgess A. G., Krauer K. G., Eckmann L., Vanelle P., Crozet M. D., et al. (2010). A new-generation 5-nitroimidazole can induce highly metronidazole-resistant Giardia lamblia in vitro. Int. J. Antimicrob. Agents 36 37–42 10.1016/j.ijantimicag.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. I. (1993). Nitroimidazole drugs–action and resistance mechanisms. I. mechanisms of action. J. Antimicrob. Chemother. 31 9–20 10.1093/jac/31.1.9 [DOI] [PubMed] [Google Scholar]
- el-Gazzar A. B., Hafez H. N., Nawwar G. A. (2009). New acyclic nucleosides analogues as potential analgesic, anti-inflammatory, anti-oxidant and anti-microbial derived from pyrimido[4,5-b]quinolines. Eur. J. Med. Chem. 44 1427–1436 10.1016/j.ejmech.2008.09.030 [DOI] [PubMed] [Google Scholar]
- Espey M. G. (2013). Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 55 130–140 10.1016/j.freeradbiomed.2012.10.554 [DOI] [PubMed] [Google Scholar]
- Eswaran S., Adhikari A. V., Ajay Kumar R. (2010). New 1,3-oxazolo[4,5-c]quinoline derivatives: synthesis and evaluation of antibacterial and antituberculosis properties. Eur. J. Med. Chem. 45 957–966 10.1016/j.ejmech.2009.11.036 [DOI] [PubMed] [Google Scholar]
- Gardner T. B., Hill D. R. (2001). Treatment of giardiasis. Clin. Microbiol. Rev. 14 114–128 10.1128/CMR.14.1.114-128.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutelle S., Maurin M., Rougier F., Barbaut X., Bourguignon L., Ducher M., et al. (2008). The Hill equation: a review of its capabilities in pharmacological modelling. Fundam. Clin. Pharmacol. 22 633–648 10.1111/j.1472-8206.2008.00633.x [DOI] [PubMed] [Google Scholar]
- Haridas V., Sahu S., Kumar P. P. P. (2011). Triazole-based chromogenic and non-chromogenic receptors for halides. Tetrahedron Lett. 52 6930–6934 10.1016/j.tetlet.2011.10.066 [DOI] [Google Scholar]
- He G., Shankar R. A., Chzhan M., Samouilov A., Kuppusamy P., Zweier J. L. (1999). Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc. Natl. Acad. Sci. U.S.A. 96 4586–4591 10.1073/pnas.96.8.4586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kategaonkar A. H., Pokalwar R. U., Sonar S. S., Gawali V. U., Shingate B. B., Shingare M. S. (2010). Synthesis, in vitro antibacterial and antifungal evaluations of new alpha-hydroxyphosphonate and new alpha-acetoxyphosphonate derivatives of tetrazolo [1, 5-a] quinoline. Eur. J. Med. Chem. 45 1128–1132 10.1016/j.ejmech.2009.12.013 [DOI] [PubMed] [Google Scholar]
- Kumar S. K., Hager E., Pettit C., Gurulingappa H., Davidson N. E., Khan S. R. (2003). Design, synthesis, and evaluation of novel boronic-chalcone derivatives as antitumor agents. J. Med. Chem. 46 2813–2815 10.1021/jm030213+ [DOI] [PubMed] [Google Scholar]
- Lengerich E. J., Addiss D. G., Juranek D. D. (1994). Severe giardiasis in the United States. Clin. Infect. Dis. 18 760–763 10.1093/clinids/18.5.760 [DOI] [PubMed] [Google Scholar]
- Levitt M. D. (1970). Oxygen tension in the gut. N. Engl. J. Med. 282 1039–1040 10.1056/NEJM197004302821814 [DOI] [PubMed] [Google Scholar]
- Li R., Kenyon G. L., Cohen F. E., Chen X., Gong B., Dominguez J. N., et al. (1995). In vitro antimalarial activity of chalcones and their derivatives. J. Med. Chem. 38 5031–5037 10.1021/jm00026a010 [DOI] [PubMed] [Google Scholar]
- Mastronicola D., Falabella M., Testa F., Pucillo L. P., Teixeira M., Sarti P., et al. (2014). Functional characterization of peroxiredoxins from the human protozoan parasite Giardia intestinalis. PLoS Negl. Trop. Dis. 8:e2631 10.1371/journal.pntd.0002631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronicola D., Giuffrè A., Testa F., Mura A., Forte E., Bordi E., et al. (2011). Giardia intestinalis escapes oxidative stress by colonizing the small intestine: a molecular hypothesis. IUBMB Life 63 21–25 10.1002/iub.409 [DOI] [PubMed] [Google Scholar]
- Mastronicola D., Testa F., Forte E., Bordi E., Pucillo L. P., Sarti P., et al. (2010). Flavohemoglobin and nitric oxide detoxification in the human protozoan parasite Giardia intestinalis. Biochem. Biophys. Res. Commun. 399 654–658 10.1016/j.bbrc.2010.07.137 [DOI] [PubMed] [Google Scholar]
- Müller J., Ruhle G., Muller N., Rossignol J. F., Hemphill A. (2006). In vitro effects of thiazolides on Giardia lamblia WB clone C6 cultured axenically and in coculture with Caco2 cells. Antimicrob. Agents Chemother. 50 162–170 10.1128/AAC.50.1.162-170.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget T. A., Kelly M. L., Jarroll E. L., Lindmark D. G., Lloyd D. (1993). The effects of oxygen on fermentation in Giardia lamblia. Mol. Biochem. Parasitol. 57 65–71 10.1016/0166-6851(93)90244-R [DOI] [PubMed] [Google Scholar]
- Pal S., Durgadas S., Nallapati S. B., Mukkanti K., Kapavarapu R., Meda C. L., et al. (2011). Novel 1-alkynyl substituted 1,2-dihydroquinoline derivatives from nimesulide (and their 2-oxo analogues): a new strategy to identify inhibitors of PDE4B. Bioorg. Med. Chem. Lett. 21 6573–6576 10.1016/j.bmcl.2011.08.033 [DOI] [PubMed] [Google Scholar]
- Palit P., Paira P., Hazra A., Banerjee S., Gupta A. D., Dastidar S. G., et al. (2009). Phase transfer catalyzed synthesis of bis-quinolines: antileishmanial activity in experimental visceral leishmaniasis and in vitro antibacterial evaluation. Eur. J. Med. Chem. 44 845–853 10.1016/j.ejmech.2008.04.014 [DOI] [PubMed] [Google Scholar]
- Rafferty S., Luu B., March R. E., Yee J. (2010). Giardia lamblia encodes a functional flavohemoglobin. Biochem. Biophys. Res. Commun. 399 347–351 10.1016/j.bbrc.2010.07.073 [DOI] [PubMed] [Google Scholar]
- Raj D., Ghosh E., Mukherjee A. K., Nozaki T., Ganguly S. (2014). Differential gene expression in Giardia lamblia under oxidative stress: significance in eukaryotic evolution. Gene 535 131–139 10.1016/j.gene.2013.11.048 [DOI] [PubMed] [Google Scholar]
- Ramesh E., Sree Vidhya T. K., Raghunathan R. (2008). Indium chloride/silica gel supported synthesis of pyrano/thiopyranoquinolines through intramolecular imino Diels–Alder reaction using microwave irradiation. Tetrahedron Lett. 49 2810–2814 10.1016/J.telet.2008.02.128 [DOI] [Google Scholar]
- Saadeh H. A., Mosleh I. M., Al-Bakri A. M., Mubarak M. S. (2010). Synthesis and antimicrobial activity of new 1,2,4-triazole-3-thiol metronidazole derivatives. Monatsh. Chem. 141 471–478 10.1007/s00706-010-0281-289 [DOI] [Google Scholar]
- Shaker R. M. (2006). The chemistry of mercapto- and thione- substituted 1,2,4-triazoles and their utility in heterocyclic synthesis. ARKIVOC 2006 59–112 10.3998/ark.5550190.0007.904 [DOI] [Google Scholar]
- Sheridan W. G., Lowndes R. H., Young H. L. (1990). Intraoperative tissue oximetry in the human gastrointestinal tract. Am. J. Surg. 159 314–319 10.1016/S0002-9610(05)81226-7 [DOI] [PubMed] [Google Scholar]
- Syam S., Abdelwahab S. I., Al-Mamary M. A., Mohan S. (2012). Synthesis of chalcones with anticancer activities. Molecules 17 6179–6195 10.3390/molecules17066179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejman-Yarden N., Eckmann L. (2011). New approaches to the treatment of giardiasis. Curr. Opin. Infect. Dis. 24 451–456 10.1097/QCO.0b013e32834ad401 [DOI] [PubMed] [Google Scholar]
- Testa F., Mastronicola D., Cabelli D. E., Bordi E., Pucillo L. P., Sarti P., et al. (2011). The superoxide reductase from the early diverging eukaryote Giardia intestinalis. Free Radic. Biol. Med. 51 1567–1574 10.1016/j.freeradbiomed.2011.07.017 [DOI] [PubMed] [Google Scholar]
- Upcroft P., Upcroft J. A. (2001). Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin. Microbiol. Rev. 14 150–164 10.1128/CMR.14.1.150-164.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente J. B., Testa F., Mastronicola D., Forte E., Sarti P., Teixeira M., et al. (2009). Redox properties of the oxygen-detoxifying flavodiiron protein from the human parasite Giardia intestinalis. Arch. Biochem. Biophys. 488 9–13 10.1016/j.abb.2009.06.011 [DOI] [PubMed] [Google Scholar]
- Viegas-Junior C., Danuello A., Da Silva Bolzani V., Barreiro E. J., Fraga C. A. (2007). Molecular hybridization: a useful tool in the design of new drug prototypes. Curr. Med. Chem. 14 1829–1852 10.2174/092986707781058805 [DOI] [PubMed] [Google Scholar]
- Walsh J. J., Bell A. (2009). Hybrid drugs for malaria. Curr. Pharm. Des. 15 2970–2985 10.2174/138161209789058183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.