Abstract
In this issue of Cell Stem Cell, Greco et al. (2009) characterize the hair germ as a novel stop between bulge stem cell and transient amplifying cells during hair regeneration. The work implies stem cell states can be regulated to form different numbers of intermediate stops, depending on physiological requirements.
Ectodermal organs are fascinating because they can undergo either continual turn over or episodic regeneration and yet are able to regulate their size, topology, and ratio of differentiated cell types, depending on physiological needs or in response to injury. Understanding this mechanism is central to the progress of regenerative medicine. In the current concept (Potten, 1981), the system is regulated by the equilibrium among three major cell groups: stem cells, transit-amplifying (TA) cells, and differentiated cells. Because hair follicles undergo cyclic regeneration throughout the life of an organism, hair cycling has become a major model for stem cell research. Each hair cycle consists of a period of growth (anagen), regression (catagen), and quiescence (telogen). The hair follicle offers an advantage in research because different populations along the course of stem cell progression have distinct spatial localizations, which facilitate their analyses.
Using long-term label retention, Cotsarelis et al. (1990) discovered slow cycling cells within the hair bulge. These bulge cells were found to give rise to future hair follicles and considered to be the main sites of hair stem cells. Matrix at the follicle base contains rapid proliferating TA cells. These cells generate different differentiated cell types of hair filaments. Based on morphology, a second population of cells surrounding the dermal papilla, called the hair germ, was identified and thought to be involved in hair follicle regeneration (Dry, 1926). Further studies led Panteleyev et al. (2001) and Ito et al. (2004) to propose that these secondary hair germs represent cells that directly give rise to the next hair follicle. They also showed that these hair germ cells can dedifferentiate to form a bulge if the original bulge is damaged. However, despite of the progress in molecular and cellular characterization of bulge stem cells, the properties of hair germ cells have not been clearly characterized.
In this issue, Greco et al. (2009) did a thorough characterization on hair germ cells through rigorous experiments based on genetic lineage analyses, in vitro cultures, molecular profiling, etc. They show that both hair germ and bulge stem cells are quiescent in early telogen. Hair germ cells start to proliferate in late telogen, while bulge cells do not proliferate until early anagen.
Using a combination of hair cycle stages, collagenase digestion, and K14 H2B GFP FACS cell sorting, they were able to isolate a cellular fraction enriched with HG and bulge stem cells. These were separated based on P-cadherin (high in hair germ cells) and CD34 expression (high in bulge cells). This isolation paradigm allows the authors to compare properties of distinct populations in culture and with microarray profiling. Upon culturing, they found hair germ cells proliferate more rapidly than bulge cells but have more limited proliferation potential and do not survive beyond 3 to 4 passages in vitro. In contrast, bulge stem cells can grow for at least 9 passages.
Molecularly, hair germ cells are distinct from bulge stem cells and matrix (TA) cells. Hair germ cells do not express bulge stem cell markers NFATc1, S100A6, and CD34, but both populations express Sox9, Tcf3, Lhx2, and Lgf5 expression. Hair germ cells express Lef1 and P-cadherin, which bulge cells do not. Unlike matrix cells, hair germ cells do not express Msx2 or Shh, among others.
Since the activation of hair stem cells is based on epithelial-mesenchymal interactions, Greco et al. also characterize dermal papilla. They found dermal papilla exhibit significantly different molecular expression profiles during the transition from early to late telogen: FGF7, -10, and BMP antagonists Sostdc1 and Bambi increase while FGF18 decreases. The result is an increase of FGF and Wnt activity accompanied by a decrease in BMP signaling in the hair germ epithelia. This event is a prelude to the beginning of anagen. Bead implantation experiments verify the functional involvement of these pathways. Thus, these analyses significantly increase our understanding of the molecular profiles of epidermal cells during stem cell progression and their interactions with dermal papilla.
In addition, the Fuchs study shows that the dogma of stem, TA, and differentiated cells breaks down, given that an additional step between the stem and TA cell stages is now fully characterized. On the other hand, a recent study analyzing the homeostasis of mouse tail epidermis concludes that it can do away with TA cells. Statistical analyses of in vivo lineage tracing data showed that the proliferative behavior of basal cells does not fit those predicted by the concept of traditional stem/TA/differentiated cell model. Instead, proliferation of progenitor cells is simply regulated stochastically. Thus, Jones et al. (2007) propose TA cells do not exist, at least in mouse tail epidermis. The authors do acknowledge that they do not have data for mouse dorsal skin epidermis or human skin in which the existence of rete pegs may require more complex stem cell organization and cellular interactions. Should we be bothered by the inconsistent presence of these stem cell group entities? Before we answer this, let us take a look at another recent elegant study.
A different way to approach the dynamics of stem cells in epithelial homeostasis is to focus on the control of the flow of cellular states (Zipori, 2004) rather than cell groups. With this perspective, Lander et al. (2009) analyze how the thickness of olfactory mucosa and the number of differentiated olfactory neurons are regulated. Using a combination of mathematical modeling and laboratory experiments, they showed that GDF11 and activin negatively regulate mucosa thickness by suppressing the frequency of TA and stem cell proliferation, respectively. The amount of GDF11 and activin is proportional to the amount of mucosa tissue present. These negative regulators decrease when part of the tissue is lost, leading to in-recovery of mucosal thickness. Follistatin-expressing adjacent stroma works as a sink for both GDF11 and activin and can modulate regeneration. With these dynamic regulatory loops in place, all the progenitor cells may be viewed as having the ability to respond to input signals. Authors argue articulately that it is possible: “typical stem and transit-amplifying behavior are observed, solely as a consequence of feedback control.”
We may view hair germ and TA cells as possible transit states during stem cell progression, and their presence depends on the context of the environment. Analyzing representative cell groups and their molecular profiles is still valuable, because it helps us understand the molecular basis of their functional properties. The molecular differences between related populations are often found to be relative rather than absolute, because cellular states transit along a continuum rather than as distinct entities. We should define cell groups first by functional states, then by molecular markers, as performed here by Greco et al. (2009). Chasing molecular signatures without functional validation could be an exercise in futility. Knowing that conditions regulating cell flow can change and that stem cells can be flexible to accommodate these changes, we will then not be bothered to find extra or missing cellular groups in different stem cell homeostasis scenarios in the future. This may occur when an additional control step is required to ensure success, or when an existing control step is no longer required.
Imagine the journey of a progenitor cell traveling along a river toward the ocean of terminal differentiation (Figure 1). The landscape of the river can be shaped as required by the different topology of ectodermal organs. Reservoirs can form and flow rates can vary as the river is modulated by positive/negative regulators set up by feedback dams or physiological macroenvironments (Plikus et al., 2008). Combining the analytical approach of molecular/cellular characterization and the systemic approach to cellular flow strategies is the way to make sure we understand how the flow of stem cells can achieve different tissue architectures under different physiological states.
Figure 1. Landscape Concept of Stem Cell “Rivers”.
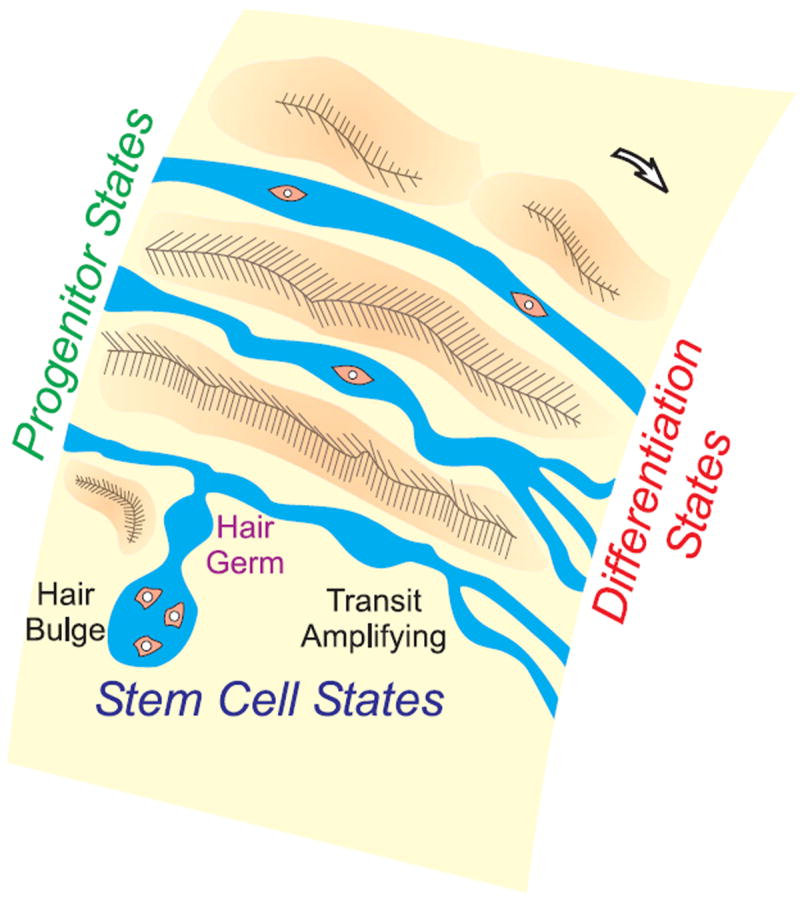
Stem cells progress toward differentiation states via different modes or routes. Intermediate stops may be added or omitted, depending on the topology of different ectodermal organs and physiological needs. Orange particles in the “river” represent cells.
References
- Cotsarelis G, Sun TT, Lavker RM. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Dry FW. J Genet. 1926;16:288–340. [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, de la Cruz-Racelis J, Fuchs E. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Kizawa K, Hamada K, Cotsarelis G. Differentiation. 2004;72:548–557. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- Jones PH, Simons BD, Watt FM. Cell Stem Cell. 2007;1:371–381. doi: 10.1016/j.stem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Lander AD, Gokoffski KK, Wan FYM, Nie Q, Calof AL. PLoS Biol. 2009;7:e15. doi: 10.1371/journal.pbio.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteleyev AA, Jahoda CA, Christiano AM. J Cell Sci. 2001;114:3419–3431. doi: 10.1242/jcs.114.19.3419. [DOI] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. Int Rev Cytol. 1981;69:271–318. doi: 10.1016/s0074-7696(08)62326-8. [DOI] [PubMed] [Google Scholar]
- Zipori D. Nat Rev Genet. 2004;5:873–878. doi: 10.1038/nrg1475. [DOI] [PubMed] [Google Scholar]


