Abstract
Herbivores induce plants to undergo diverse processes that minimize costs to the plant, such as producing defences to deter herbivory or reallocating limited resources to inaccessible portions of the plant. Yet most plant tissue is consumed by decomposers, not herbivores, and these defensive processes aimed to deter herbivores may alter plant tissue even after detachment from the plant. All consumers value nutrients, but plants also require these nutrients for primary functions and defensive processes. We experimentally simulated herbivory with and without nutrient additions on red alder (Alnus rubra), which supplies the majority of leaf litter for many rivers in western North America. Simulated herbivory induced a defence response with cascading effects: terrestrial herbivores and aquatic decomposers fed less on leaves from stressed trees. This effect was context dependent: leaves from fertilized-only trees decomposed most rapidly while leaves from fertilized trees receiving the herbivory treatment decomposed least, suggesting plants funnelled a nutritionally valuable resource into enhanced defence. One component of the defence response was a decrease in leaf nitrogen leading to elevated carbon : nitrogen. Aquatic decomposers prefer leaves naturally low in C : N and this altered nutrient profile largely explains the lower rate of aquatic decomposition. Furthermore, terrestrial soil decomposers were unaffected by either treatment but did show a preference for local and nitrogen-rich leaves. Our study illustrates the ecological implications of terrestrial herbivory and these findings demonstrate that the effects of selection caused by terrestrial herbivory in one ecosystem can indirectly shape the structure of other ecosystems through ecological fluxes across boundaries.
Keywords: trophic interactions, indirect effects, induced defences, cross-system effects, ecosystem subsidies, CNP stoichiometry
1. Introduction
Plant–herbivore interactions are a well-studied example of antagonistic coevolutionary arms races [1,2]. Plant defences are varied and can include reduction in tissue quality, production of mechanical and chemical defences, and signals to attract predators of the herbivores to the source of infestation [3,4]. Some of these defences are produced constitutively and others plastically, in response to herbivory, with the type and quantity of defence often dependent on available resources. These strong plant–herbivore relationships are an ideal system to study cascading trophic linkages [5,6] because of the extensive intraspecific variability in the plant resource, and the obvious effects this variability should have at other trophic levels, such as decomposer communities.
With 80–90% of annual plant production ending up as detritus, understanding the cascading effects of plant–herbivore interactions on decomposition is essential to understanding vital ecosystem processes in both aquatic and terrestrial systems [7,8]. Decomposers play no direct role in the interactions between terrestrial plants and their arboreal herbivores. Yet, decomposers in both aquatic and terrestrial systems are dependent on the mosaic of nutritionally diverse leaf litter whose composition is shaped by herbivores.
Plant responses to herbivory may have varied effects on decomposers [9]. Decomposers and terrestrial herbivore preferences may be positively correlated if they both favour leaves of high-nutrient quality (though nutrient requirements may differ: often nitrogen limits terrestrial systems while phosphorus usually limits freshwater systems [10,11]). Conversely, decomposer and terrestrial herbivore preferences may be negatively correlated if feeding by terrestrial herbivores induces the production of defences that deter decomposition. If the constitutive properties and the induced properties of leaves drive feeding preferences in different directions, a relationship between herbivore and decomposer feeding may be difficult to detect without manipulating defence production and/or nutrient content.
Here, we ask how aquatic and terrestrial decomposition processes are affected by terrestrial herbivores and nutrients in the streams and forests of the Pacific Northwest. In forested regions, aquatic insect and microbial decomposers residing in small streams rely heavily on fallen organic matter from terrestrial vegetation [12,13] especially where vegetation in these forests shades the stream, limiting primary production in the stream's water column. Fresh green leaves fall into Pacific Northwest streams in abundance and throughout the season, and are an important food source for aquatic decomposers, particularly during the summer growing season for macroinvertebrates [14]. Aquatic leaf decomposition varies substantially depending on the species of the source tree [15] and even among individuals within a single species of tree [14]. Two potential contributors to this within species variation in leaf decomposition rates could be individual tree differences in leaf defensive properties and leaf nutrient content. Herbivory stress and the availability of nutrients both influence the production of plant defences [16,17] and nutrient content often drives diet selection independent of its effect on defence production [18,19]. Varying nutrient stress may change the trade-off balance for the costs of defence, that is, surplus nutrients may mitigate defence induction [20]; or conversely, plants with surplus nutrients can afford a greater defence response [21]. We experimentally studied the potential for the cascading effects of terrestrial herbivory, independently and in combination with nutrient additions, across the ecosystem boundary between the terrestrial and stream ecosystem.
Our response variables to these experimental manipulations are measured changes in ecosystem processes. To date, we know of no comprehensive studies of either induced or constitutive defence compounds in red alder. Identifying chemical structures and unravelling the physiology behind induced defence compounds is an extensive task. Instead, we report here the broad-scale ecological consequences of herbivory and nutrients on ecosystem function.
2. Material and methods
(a). Study sites
We experimentally studied herbivore effects on aquatic and terrestrial decomposition on the Olympic Peninsula of Washington State, USA, using leaves of a numerically and structurally dominant deciduous tree species (red alder, Alnus rubra) commonly found in riparian zones and disturbed forests of the Pacific Northwest. Alders have an unusual capacity to fix atmospheric nitrogen due to symbiotic root associations with the Frankia alni bacterium. Red alder is thus an abundant pioneer species that enriches soil for further plant succession [22]. Alder trees experience ubiquitous insect damage to leaves with 45–100% (mean 74.3% ± 3.1 s.e.) of leaves having visible signs of damage (such as defoliation and skeletonization, rolled and folded leaves, leaf miner scars and galls), including up to 16% of leaf area consumed by chewing insects (mean 6.2% ± 0.65 s.e., determined from a regional survey of 30 trees across the study region, with 20 leaves per tree imported into ImageJ to measure area consumed). Alder trees used in the experiment were growing at 21 locations along roadsides of the Merrill & Ring Pysht Tree Farm (48.09°N, 124.12°W). Leaf packs were deployed in the soil of each of these 21 locations and at seven locations along the South Fork of the Pysht River, which runs through the tree farm. The riparian zones along the South Fork Pysht comprise early successional forest dominated by red alder with occasional bigleaf maple (Acer macrophyllum), western hemlock (Tsuga heterophylla) and other conifers. Understory vegetation included mostly salmonberry (Rubus spectabilis), thimbleberry (Rubus parviflorus), vine maple (Acer cinereus), sword fern (Polystichum munitum) and salal (Gautheria shallon). In surveys of collected leaves that fell naturally in the Pysht during the summer season, we found 78% of alder leaves were green as opposed to brown senescent litter. We document a more extensive survey of leaf litter (g m−2) entering small streams in the Olympic Peninsula elsewhere [14]. Location, morphology, nutrient and environmental characteristics for our study sites on the Pysht river have been published previously [14,23].
(b). Experimental design
Leaf defensive traits could inhibit leaf decomposers as well as the terrestrial herbivores these components are meant to deter. Nutrients could affect decomposers directly via nutrition or indirectly by providing resources for plants to generate a more complete defence response. Therefore, we implemented a 2 × 2 experimental design to test the independent and interactive effects of herbivory and fertilizer treatments. Our experimental units were 84 young to moderately aged individuals (15.7 ± 1.4 cm s.e. trunk diameter at 1.5 m above ground, which correlates on average to a 15-year-old tree growing on a site of average quality [24]) of red alder, similar in size across treatment groups (F3,82 = 0.31, p = 0.82). In May 2012, we organized the four treatments into 21 blocks (four trees per block, see the electronic supplementary material, appendix A) with each block at least 100 m apart from each other and each experimental tree within blocks at least 100 m from the next tree to minimize cross contamination of methyl jasmonate, the compound we used to induce plant defences. Jasmonates are volatile chemicals released during herbivory that may be used by undamaged plants to recognize when neighbouring plants have been damaged, and thus constitute an early warning system for plants to manufacture anti-herbivory compounds before they are directly threatened [25]. Prior to experimental treatments, we surveyed each tree for insect damage on four branches (10 leaves per branch). Three-quarters of surveyed leaves showed visible signs of insect damage, but damage levels were consistent across treatment group assignments (F3,83 = 1.56, p = 0.21).
In the herbivory treatment, we wounded every third leaf below 2 m in height by punching two 6.35 mm holes using an office hole punch. We brushed each punched leaf with 50 μl of a 100 mM methyl jasmonate solution in 10% ethanol and 0.125% Triton-X. We repeated this procedure three times over a 6 day period in June, with the same leaves punched and treated each time. Thus, by the end of the treatment, one-third of alder leaves below 2 m had been given six hole punches and brushed with methyl jasmonate three times. We left the remaining leaves untouched for use in leaf pack experiments. This treatment was intended to mimic a sustained threat to the plant, rather than a one-time pulse of damage, which often fails to induce strong defence responses in plants [26]. For the fertilizer treatment, we spread a single addition of 10 g triple superphosphate fertilizer (P2O5) beneath two trees per block on 23 May 2012, which was 10 days or 26 days prior to the start of the methyl jasmonate herbivory treatment depending on the block of trees. We chose to fertilize with phosphorus because it often limits nitrogen-fixers such as red alder, and similar phosphorus additions have increased growth in young of the alder species used here in south-coastal British Columbia [27] as well as speckled alder (Alnus incana) in northeastern North America [28].
We removed fresh green leaves from each of the 84 trees for use in caterpillar feeding trials and in leaf packs deployed in aquatic and terrestrial systems. All leaves collected from trees in the herbivory treated group were adjacent to leaves given the herbivory treatment. No leaves directly receiving the hole punches and methyl jasmonate were used in the experiments to avoid inadvertently applying methyl jasmonate residue to streams. We collected leaves with little or no natural herbivore damage and sealed them in plastic bags.
To test whether experimental treatments affected terrestrial herbivory, we collected leaf-rolling caterpillars (Epinotia albangulana) from inside rolled-up leaves of alder trees. These herbivores were widespread on red alder and often inflicted heavy damage to individual trees. We placed each caterpillar in a separate arena containing two 12 mm diameter leaf cores from two of the four treatments being studied, for example, one leaf core from a leaf was removed from a tree treated with methyl jasmonate versus one leaf core from a control tree from the same block. Caterpillars typically spun webs beneath and ate only one of the two leaf cores offered in the arena, therefore showing an unambiguous measure of preference. Experiments lasted until the caterpillars chose a leaf. We discarded trials if caterpillars did not eat either leaf core after 24 h, and these trials were excluded from the analysis.
At the same time as the caterpillar feeding preference experiment, we used green leaves to make leaf packs. From each tree, we made two packs that contained 12 leaves inside 19 × 15.25 cm bags made of 4.75 mm mesh nylon seine netting. Using 4.75 mm mesh netting allowed colonization by most stream macroinvertebrates in the Pysht River (S Jackrel 2011, unpublished data). We observed that larger caddisflies (Dicosmoecus and Psychoglypha) would feed through the mesh openings but were usually too large to enter the bags. We recorded initial weights of leaf packs and placed the packs in either the aquatic environment or terrestrial environment.
We strung leaf packs designated for the aquatic environment with cable ties onto a steel reinforcing bar, and laid the bar on the stream bottom perpendicular to the flow, pinned at each end by two other bars pounded into the stream bottom at a 45° angle. We deployed leaf packs for 17–21 days at seven locations in the Pysht River. Leaf packs deployed at two locations were not usable, because they were left in the river too long and excessive decomposition made comparisons across packs inaccurate, so we report results only from the remaining five locations. During removal, we placed each leaf pack inside a sealed plastic bag. We removed leaves from mesh bags and gently washed the leaves with tap water to dislodge macroinvertebrates and silt. We blotted the leaves dry with paper towels and weighed leaves to the nearest centigram.
We strung leaf packs designated for the terrestrial environment onto a steel reinforcing bar using cable ties and pounded the bar into the soil. We covered leaf packs with approximately 1.25 cm of debris from the immediate area and deployed packs for either 56 or 67 days at 21 locations (each deployment site was located next to one tree, selected at random, per block of four trees). We periodically watered leaf packs to accelerate decomposition. At the end of the deployment, we removed leaf packs from each mesh bag and gently washed the leaves to dislodge macroinvertebrates and silt. We oven dried and weighed leaves to the nearest centigram.
Either 43 or 56 days after the herbivory treatment, we collected 20 additional leaves per tree in two groups of 10 contiguous leaves starting at the branch tip from two different main branches of the tree. We took leaf cores 12 mm in diameter from each of the 20 leaves per tree, oven dried and weighed each core to the nearest milligram as an indicator of leaf thickness, which often plays an important role in deterring herbivory [29].
(c). CNP measurements
From each tree, we collected three to five extra leaves immediately before and after the experiment. We oven dried leaves immediately after collection and then ground them into a fine powder. We packed 3 mg of leaf powder into 3.5 × 5 mm tin capsules prior to analysis and measured per cent nitrogen, per cent carbon, and 15N/14N and 13C/12C ratios using a Costech 4010 Elemental Analyzer combustion system coupled to a Thermo DeltaV Plus IRMS via a Thermo Conflo IV interface at the University of Chicago Stable Isotope Ratio facility. The reproducibility was 0.11‰ for 13C and 0.17‰ for 15N, and we used cocoa powder and glutamic acid as isotopic controls. To measure phosphorus, we modified an ashing, acid-hydrolysis and phosphomolybdate-blue spectrophotometric protocol of [30] (see the electronic supplementary material, appendix B for details of modifications).
(d). Statistical analyses
We completed all statistical analyses using SPSS (IBM Corp. 2013) and R [31]. We report aquatic per cent leaf mass lost in terms of fresh leaf weight, where the final leaf weights were measured as blotted weights and then converted to fresh weight using the regression equation (fresh weight = 0.9412 × blotted weight − 0.0034; R2 = 0.983) generated from a laboratory experiment [14]. Decomposition rate data were arcsine square-root transformed to meet assumptions of normality and controlled statistically by deployment location to standardize results across multiple rounds of experiments.
Analysis of variance was used to test the effects of the fertilizer and herbivory treatments on aquatic and terrestrial decomposition. For the aquatic decomposition results, stepwise multiple regression analysis was used to identify potentially important covariates, some of which varied with experimental treatment, and the analysis of variance was expanded to include these additional covariates. Where treatment effects or covariates were not significant, we removed these terms from the model to maximize statistical power and identify a more parsimonious model.
3. Results
(a). Effect of experimental treatments on leaf traits
The % N and P values at the start of the experimental treatments were high relative to reported literature values (2.78 ± 0.048 s.e. % N versus 1.65–2.45% N; and 0.22 ± 0.0049% P versus 0.13–0.17%) [32]. Natural % N content varied substantially among individuals before the start of the experiment (range: 1.83–4.43% N) and N levels decreased (range: 1.06–3.58%) during the experiment for 80% of trees (n = 80). At the beginning of the experiment, leaves from trees destined to be given the herbivory treatment were similar in % N concentration (%N0 = 2.72 ± 0.056, n = 41) to control trees (%N0 = 2.84 ± 0.079, n = 40) (electronic supplementary material, table C1, p = 0.30), and leaves from trees destined to be given the phosphorus fertilizer were similar in % N concentration (%N0 = 2.85 ± 0.07, n = 41) to control trees (%N0 = 2.71 ± 0.06, n = 40; electronic supplementary material, table C1, p = 0.11).
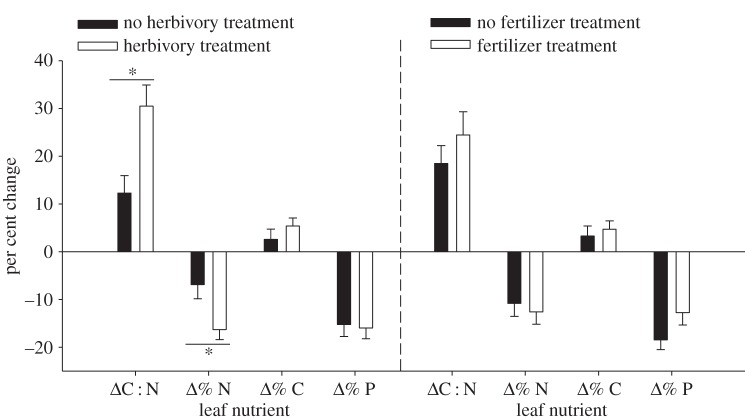
By the completion of the herbivory treatment, leaf composition changed considerably (electronic supplementary material, table C4, p = 0.00235). Leaf C : N content increased 2.5 times faster among trees given the terrestrial herbivory treatment compared to trees in the control group (figure 1, p = 0.0026), leading C : N content of leaves from the herbivory treated group to be 12.7% higher when experimental leaves were deployed (herbivory treated: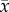 = 21.11 ± 0.45, n = 36; control trees:
= 21.11 ± 0.45, n = 36; control trees: 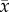 = 18.73 ± 0.74, n = 36; electronic supplementary material, table C4). This C : N pattern was caused by both a twofold greater decline in % N and a tendency for % C to increase among trees given the herbivory treatment (figure 1; electronic supplementary material, table C4, p = 0.0061 and p = 0.40, respectively).
= 18.73 ± 0.74, n = 36; electronic supplementary material, table C4). This C : N pattern was caused by both a twofold greater decline in % N and a tendency for % C to increase among trees given the herbivory treatment (figure 1; electronic supplementary material, table C4, p = 0.0061 and p = 0.40, respectively).
Figure 1.
Change in C : N, % C, % N and % P content of red alder leaves from trees given a herbivory treatment versus control trees (left-hand side), and trees given a fertilizer treatment versus control trees (right-hand side). Significant comparisons (t-tests p < 0.05) between herbivory treated and control trees indicated by a star.
By contrast, trees given the fertilizer did not have significantly different leaf traits (electronic supplementary material, table C4, p = 0.532). Fertilized trees changed similarly in % N concentration compared to control trees (figure 1, p = 0.64). Fertilized trees retained 44% more phosphorus content (control group declined 18.4%, while fertilized group declined 12.8%, (figure 1; electronic supplementary material, table C2, p = 0.0594), however, this effect of the fertilizer treatment did not lead to a significant difference in % P content of leaves between the treatment groups (control trees: 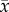 = 0.177 ± 0.0041, n = 42; fertilized trees:
= 0.177 ± 0.0041, n = 42; fertilized trees: 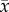 = 0.215 ± 0.0074, n = 42; electronic supplementary material, table C4, p = 0.680). Leaves from fertilized trees were 6.5% thicker (electronic supplementary material, table C4, p = 0.047).
= 0.215 ± 0.0074, n = 42; electronic supplementary material, table C4, p = 0.680). Leaves from fertilized trees were 6.5% thicker (electronic supplementary material, table C4, p = 0.047).
(b). Terrestrial herbivory
Epinotia albangulana leaf-rollers fed less frequently on trees given the herbivory treatment than on untreated control trees (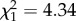 , p = 0.037; figure 2a). The two tree treatment groups (either with or without fertilizer) given the herbivory treatment (eaten in 33.3–37.9% of trials) were eaten half as often as the two treatment groups not given the herbivory treatment (eaten in 63.3–65.5% of trials). Leaf-rollers also fed more frequently on the leaf option lower in C : N content (in 62.2% of trials,
, p = 0.037; figure 2a). The two tree treatment groups (either with or without fertilizer) given the herbivory treatment (eaten in 33.3–37.9% of trials) were eaten half as often as the two treatment groups not given the herbivory treatment (eaten in 63.3–65.5% of trials). Leaf-rollers also fed more frequently on the leaf option lower in C : N content (in 62.2% of trials, 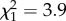 , p = 0.048, n = 74).
, p = 0.048, n = 74).
Figure 2.
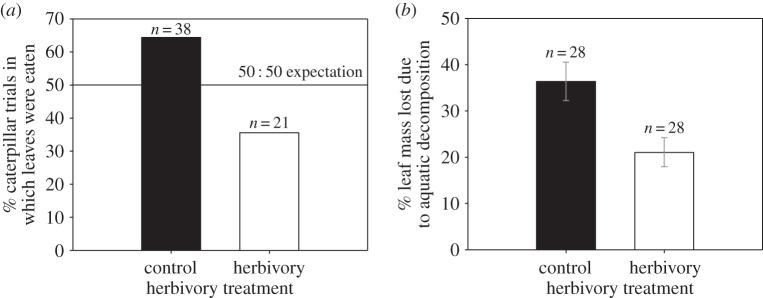
Effects of a jasmonate herbivory treatment applied to foliage of red alder trees on (a) caterpillar feeding trials and (b) average (±s.e.) aquatic decomposition rate of red alder leaf litter. Aquatic results are from analysis of variance after factoring out deployment location.
(c). Aquatic decomposition
Of the leaf packs deployed in the Pysht River, leaves from the control trees decomposed 42% faster than leaves from herbivory treated trees (figure 2b, electronic supplementary material, table D1, p = 0.0056). Leaf decomposition did not vary overall with P addition, but there was a significant interaction (p = 0.028) between herbivory and nutrient treatments on aquatic decomposition: simulated herbivory had a strong inhibitory effect under P addition (60% reduction), but a weak effect without P addition (13% reduction). When evaluating the effect of the P addition independent of the herbivore treatment, among only the subset of trees not given the herbivory treatment, leaf decomposition was 75% greater with the P addition (figure 3, paired t-test t13 = 2.63, p = 0.021).
Figure 3.
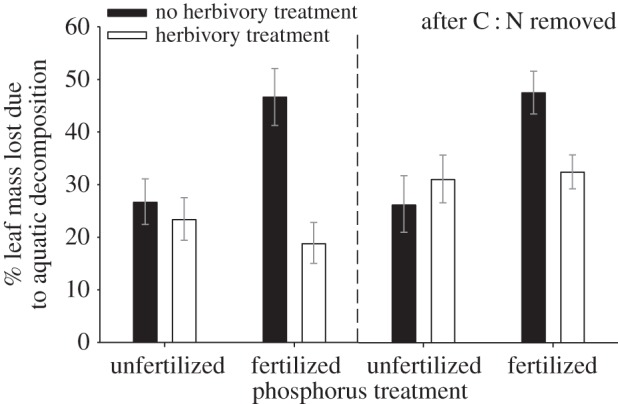
Residual effects (mean ± s.e.) of herbivory and fertilizer treatments on aquatic decomposition of red alder from analysis of variance after factoring out deployment location (electronic supplementary material, table D1) and both deployment location and C : N in leaves at the time of leaf pack assembly, immediately after the herbivory treatment (electronic supplementary material, table D2).
We determined whether other traits not intentionally directly manipulated in our experiment were associated with aquatic decomposition. In particular, aquatic decomposition declined significantly as C : N content increased, either when considering all trees (R2 = 0.232, p < 0.001; figure 4a), which included trees that shifted C : N in response to simulated herbivory (figure 1), or when considering only control trees that naturally varied in C : N (R2 = 0.542, p < 0.003). When including all measured leaf traits in a stepwise multiple regression, the best model (indicated by the lowest AIC score) of aquatic decomposition included C : N and 15N/14N (R2 = 0.31, p < 0.001; electronic supplementary material, table D3; figure D1). To test the degree to which these covariates could explain observed treatment differences, we conducted a follow-up ANCOVA, including a leaf C : N term in addition to both treatment effects. Leaf C : N exhibited a negative effect on aquatic decomposition in the analysis (p < 0.001), but the N-isotope term was not significant and did not vary by experimental treatments (electronic supplementary material, table C4), so was removed. After factoring out variation in C : N, the residual effect of simulated herbivory was much weaker (13% decline) and not statistically significant (p = 0.27, electronic supplementary material, table D2), but a positive effect of adding P on average was revealed (39% increase, p = 0.044, electronic supplementary material, table D2). The interactive effect of simulated herbivory and P addition remained (p = 0.06; electronic supplementary material, table D2, figure 3): herbivory reduced aquatic decomposition by 32% under P addition, but tended to elevate aquatic decomposition by 19% without P addition. These results suggest that the fertilizer treatment had a qualitative effect on leaf traits that we could not detect from our measurements of quantitative nutrient levels.
Figure 4.
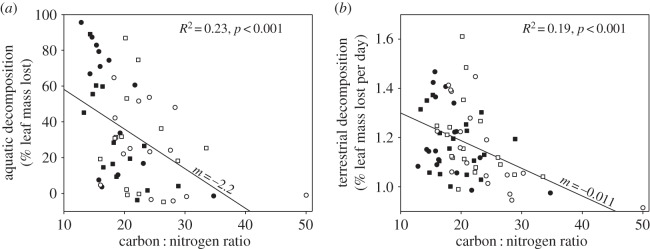
Effects of innate and experimentally induced variation in C : N of red alder leaves on the leaf decomposition rates in streams (a) and forest soil (b). Carbon : nitrogen ratios of leaves at the time of leaf pack deployment after the implementation of a herbivory treatment (hollow) versus control (filled) and a phosphorus fertilizer treatment (circles) versus control (squares). Coefficients of determination and two-tailed p-values are reported for the entire dataset.
(d). Terrestrial decomposers
Leaf packs from the herbivory treated trees decomposed at a similar rate (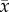 = 1.188 ± 0.025 s.e. % leaf mass lost per day, n = 42) to leaves from control trees (
= 1.188 ± 0.025 s.e. % leaf mass lost per day, n = 42) to leaves from control trees (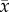 = 1.194 ± 0.020% leaf mass lost per day, n = 41) (electronic supplementary material, table E1). However, decomposition increased by 1% for every 5% percentage decrease in leaf C : N content (figure 4b). For terrestrial soil decomposition, the best model (indicated by the lowest AIC score) included leaf thickness, C : N, tree diameter, N : P and 13C/12C (R2 = 0.424) (electronic supplementary material, figure D1 and table E2). Interestingly, decomposers showed a local preference for leaves from the nearest tree. Each deployment location was next to one of the four trees per block, and at 52% of these sites, highest decomposition occurred for leaf packs from the tree growing immediately above (electronic supplementary material, table E1, p = 0.009). Repeating analyses without these home leaf packs did not change conclusions for the herbivory or fertilizer treatments.
= 1.194 ± 0.020% leaf mass lost per day, n = 41) (electronic supplementary material, table E1). However, decomposition increased by 1% for every 5% percentage decrease in leaf C : N content (figure 4b). For terrestrial soil decomposition, the best model (indicated by the lowest AIC score) included leaf thickness, C : N, tree diameter, N : P and 13C/12C (R2 = 0.424) (electronic supplementary material, figure D1 and table E2). Interestingly, decomposers showed a local preference for leaves from the nearest tree. Each deployment location was next to one of the four trees per block, and at 52% of these sites, highest decomposition occurred for leaf packs from the tree growing immediately above (electronic supplementary material, table E1, p = 0.009). Repeating analyses without these home leaf packs did not change conclusions for the herbivory or fertilizer treatments.
4. Discussion
The aquatic decomposition rate of red alder leaves varies substantially across individual source trees [14]. Two mechanisms that may contribute to these individual tree differences include varying leaf nutrient quality and leaf defence traits [33,34]. Here we have shown that a terrestrial herbivory treatment that induced a systemic defence response in red alder trees decreased terrestrial insect feeding as expected and had a cascading effect into aquatic ecosystems by significantly inhibiting aquatic decomposition. Leaf C : N content varied widely among our study trees and we found that terrestrial herbivores, aquatic decomposers and terrestrial decomposers all strongly preferred leaves with low C : N levels. Our analysis of leaf traits indicated that the herbivory treatment caused a decrease in nitrogen levels and this resulting change in C : N played a large role in why the terrestrial herbivory treatment negatively affected aquatic decomposition.
Induced defences are costly to plants [35] and we aimed to contrast plant defence to herbivory both in isolation and in combination with a nutrient-rich environment. The effect of the herbivory treatment on aquatic decomposition was strongly context dependent on nutrient availability. Phosphorus fertilizer treatment on red alder has reportedly caused extensive foliar changes including increased foliar N, P, Ca, S, Mg and Fe and decreased K, Cu, Zn [36]. In contrast to other studies [27,28], our fertilization treatment failed to show quantitative changes in leaf traits (including % N, C, P, 15N/14N and 13C/12C). This discrepancy may be due to the duration between fertilization and sampling. Our fertilization treatment occurred in May (similar to cited studies fertilizing between May and July) and our leaf sampling was within six to eight weeks of fertilization, which was similar to a seedling study (eight weeks [36]) but far shorter than other studies on trees (15 months [28] and 20 months [17]). Fertilized trees retained slightly more of their starting P content than control trees, but due to variation in initial P levels, there was no significant difference in P levels between treatment groups. However, the significant interaction between the fertilizer and herbivory treatments suggests that this retention of P among the fertilized trees (or some other leaf trait change) when paired with the herbivory treatment did have a qualitative effect, perhaps by spurring a different physiological response that resulted in qualitative trait shifts and a stronger defence.
We found that low C : N, which was largely determined by variation in N content, was an important diet consideration for two communities that are not typically regarded as N-limited. Freshwater ecosystems are typically limited by P, and there is evidence that P is more limiting than N in many Olympic Peninsula rivers [23,37]. Although terrestrial ecosystems are most often limited by N, the forests in our study area are largely dominated by A. rubra, an N-fixer, and thus are typically less limited by N [38]. Finding a strong C : N effect is not surprising, however, given recent meta-analyses that have shown many terrestrial and freshwater ecosystems are co-limited by N and P rather than by a single limiting resource [39].
The physiological mechanism that caused the elevated C : N among our herbivory treatments is not known. There are many scenarios that could explain the decrease in N, some of which directly involve plant defence, while others are more indirect consequences of herbivory stress. Several possible explanations include: alders released N-containing volatile emissions in response to herbivory (such as methyl anthranilate and indole) [40], methyl jasmonate or the herbivory stress as a whole interfered with or deprioritized nitrogen fixation [41], or N was shunted out of the leaves into other parts of the plant (such as the roots) as a means of resource protection and/or to dissuade further herbivory [42]. The physiological responses resulting in the increased C : N among the herbivory treated trees are quite possibly different among the fertilized versus unfertilized trees (plants growing on N-poor soils often use C-based defences and vice versa) [43]. A study simulating herbivory only using mechanical damage in Alnus incana and A. glutinosa found no change in % N content [44]. This may indicate this response is unique to A. rubra or that by using methyl jasmonate, our study invoked a more complex defence response. Reduced N foliage in response to mechanical defoliation was documented in black oak (Quercus velutina) but not gray birch (Betula populifolia) [45]. Research evaluating the physiology behind this response would be an interesting future direction.
Determining underlying physiology may explain why the herbivory treatment impeded consumption by terrestrial herbivores and aquatic decomposers, but not terrestrial decomposers. Plants benefit from the action of decomposers breaking down organic material on the forest floor [46]. These decomposers can be negatively affected by the plant defensive properties produced to deter herbivores [9,46,47], thus impeding nutrient recycling, and presenting a trade-off for the host tree that produced those defences and now relies on nutrient recycling for future growth. A host tree would presumably benefit if terrestrial decomposers were insensitive to plant defence responses aimed to deter terrestrial herbivores. In the light of these results, further experiments testing the effects of herbivore defence on terrestrial decomposers are warranted.
For our herbivory treatment, we aimed to mimic a natural herbivory threat by using both mechanical and chemical stressors. Mechanical defoliation has been shown to trigger production of anti-herbivory compounds in a European species of alder [48], but mechanical damage alone probably only triggers a partial defensive response [49]. In order to further induce plant defences, we applied jasmonates because a chemical treatment is a more controlled and reliable method than introducing herbivores. There have been extensive laboratory studies on the role that jasmonates play in inducing plant defences [50,51], but there are far fewer studies that have successfully used jasmonates to induce defence production in the foliage of trees in nature [52–54].
Our herbivory treatment provided a very generalized form of terrestrial herbivory. Different species of terrestrial herbivores may induce separate defence pathways that in turn differentially affect aquatic decomposers. Plants often defend themselves differently depending on how damage is inflicted and sometimes by the composition of insect saliva [55]. Natural complexes of herbivores that feed on a single tree may produce varied defence responses that could affect aquatic ecosystems in complex ways and warrants further study.
Complex responses to herbivore stress may cause red alder tree individuals to have very different leaf traits across space. Herbivore loads and nutrient availability are patchily distributed across landscapes, and varying genetic makeup can also affect how an individual tree responds to herbivore stresses and nutrient availability. Spatial variation in leaf traits could explain the pattern of local adaptation in rivers to different alder populations that we have previously documented in this ecosystem [14]. These aquatic decomposer communities are well matched to the specific red alder trees growing in the immediate area that drop their leaves regularly into that local stretch of river. Here we show that a heavy herbivory treatment lowered aquatic decomposition rates, which supports the idea that spatially variable leaf traits such as leaf defence traits could be driving the previously documented pattern of local adaptation in aquatic ecosystems to their local leaf subsidies. We did not aim to explicitly test local adaptation among terrestrial decomposition, but our results suggest soil communities show similar local preferences. A reciprocal transplant design, as in Jackrel & Wootton [14], would be necessary to conclusively demonstrate local preference by soil organisms. Hence these results warrant further study of how leaf defence traits influence aquatic and terrestrial ecosystems.
Our findings have broad conservation implications for the functioning of these ecosystems. Both nutrient availability and terrestrial herbivory pressures are affected by anthropogenic activities. Logging practices frequently include fertilization of trees to boost growth rates, locally elevating N. Further, global N availability has been increasing due to fossil fuel combustion and synthetic nitrogen fixation [56]. The intensity of terrestrial insect herbivory would be predicted to increase due in part to this addition of nitrogen, as well as an increase in global temperature [57]. Density patterns of herbivores may also change over time due to the individual or interactive effects of climate change and nitrogen deposition. Our findings suggest that the consequences of these environmental changes may have far-reaching implications. Increased terrestrial herbivory has the potential to affect a key ecosystem process that may decrease the abundance of insect decomposers and may lead to a food shortage at higher trophic levels.
Supplementary Material
Acknowledgements
We thank C. Pfister, T. Price, J. Bergelson and G. Dwyer for constructive comments and discussion on this work and L. Harris for assistance in the field. We thank Merrill & Ring Inc. and J. Murray for facilitating research on their lands, A. Colman, A. Mine, G. Olack and the Colman Isotope Lab at University of Chicago for nitrogen, carbon and phosphorus analyses, and D. Hurd and M. Hurd for providing work facilities.
Author contributions
S.L.J. implemented the experiment and collected field and laboratory data; S.L.J. and J.T.W. designed experiment, analysed data and wrote the paper.
Funding statement
This work was supported by a NSF GRFP fellowship, DOE GAANN training grant, University of Chicago Hinds Fund grant and NSF DDIG DEB-1311293 to S.L.J., an Olympic National Resources Grant and NSF grant DEB 09-19420 to J.T.W., and NSF OCE-0928232 to C.A. Pfister.
Competing interests
We have no competing interests.
References
- 1.Breedlove DE, Ehrlich PR. 1968. Plant–herbivore coevolution: lupines and lycaenids. Science 162, 671–672. ( 10.1126/science.162.3854.671) [DOI] [PubMed] [Google Scholar]
- 2.Agrawal AA. 2007. Macroevolution of plant defense strategies. Trends Ecol. Evol. 22, 103–109. ( 10.1016/j.tree.2006.10.012) [DOI] [PubMed] [Google Scholar]
- 3.Agrawal AA. 1998. Induced responses to herbivory and increased plant performance. Science 279, 1201–1202. ( 10.1126/science.279.5354.1201) [DOI] [PubMed] [Google Scholar]
- 4.Allmann S, Baldwin IT. 2010. Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science 329, 1075–1078. ( 10.1126/science.1191634) [DOI] [PubMed] [Google Scholar]
- 5.Choudhury D. 1988. Herbivore induced changes in leaf-litter resource quality: a neglected aspect of herbivory in ecosystem nutrient dynamics. Oikos 51, 389–393. ( 10.2307/3565324) [DOI] [Google Scholar]
- 6.Kotilainen T, Haimi J, Tegelberg R, Julkunen-Tiitto R, Vapaavuori E, Aphalo PJ. 2009. Solar ultraviolet radiation alters alder and birch litter chemistry that in turn affects decomposers and soil respiration. Oecologia 161, 719–728. ( 10.1007/s00442-009-1413-y) [DOI] [PubMed] [Google Scholar]
- 7.Cyr H, Face MLF. 1993. Magnitude and patterns of herbivory in aquatic and terrestrial ecosystems. Nature 361, 148–150. ( 10.1038/361148a0) [DOI] [Google Scholar]
- 8.Cebrian J. 1999. Patterns in the fate of production in plant communities. Am. Nat. 154, 449–468. ( 10.1086/303244) [DOI] [PubMed] [Google Scholar]
- 9.Hättenschwiler S, Vitousek PM. 2000. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 15, 238–243. ( 10.1016/S0169-5347(00)01861-9) [DOI] [PubMed] [Google Scholar]
- 10.Schindler DW. 1997. Evolution of phosphorus limitation in lakes. Science 195, 260–262. ( 10.1126/science.195.4275.260) [DOI] [PubMed] [Google Scholar]
- 11.Vitousek PM, Howarth RW. 1991. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13, 87–115. ( 10.1007/BF00002772) [DOI] [Google Scholar]
- 12.Motomori K, Mitsuhashi H, Nakano S. 2001. Influence of leaf litter quality on the colonization and consumption of stream invertebrate shredders. Ecol. Res. 16, 173–182. ( 10.1046/j.1440-1703.2001.00384.x) [DOI] [Google Scholar]
- 13.Lecerf A, Dobson M, Dang C, Chauvet E. 2005. Riparian plant species loss alters trophic dynamics in detritus-based stream ecosystems. Oecologia 146, 432–442. ( 10.1007/s00442-005-0212-3) [DOI] [PubMed] [Google Scholar]
- 14.Jackrel SL, Wootton JT. 2014. Local adaptation of stream communities to intraspecific variation in a terrestrial ecosystem subsidy. Ecology 95, 37–43. ( 10.1890/13-0804.1) [DOI] [PubMed] [Google Scholar]
- 15.Kominoski JS, Marczak LB, Richardson JS. 2011. Riparian forest composition affects stream litter decomposition despite similar microbial and invertebrate communities. Ecology 92, 151–159. ( 10.1890/10-0028.1) [DOI] [PubMed] [Google Scholar]
- 16.Bryant JP, Clausen TP, Reichardt PB, McCarthy MC, Werner RA. 1987. Effect of nitrogen fertilization upon the secondary chemistry and nutritional value of quaking aspen (Populus tremuloides Michx.) leaves for the large aspen tortrix (Choristoneura conflictana (Walker)). Oecologia 73, 513–517. ( 10.1007/BF00379408) [DOI] [PubMed] [Google Scholar]
- 17.Bryant JP, Chapin FS, III, Reichardt PB, Clausen TP. 1987. Response of winter chemical defense in Alaska paper birch and green alder to manipulation of plant carbon/nutrient balance. Oecologia 72, 510–514. ( 10.1007/BF00378975) [DOI] [PubMed] [Google Scholar]
- 18.Mattson WJ. 1980. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Syst. 11, 119–161. ( 10.1146/annurev.es.11.110180.001003) [DOI] [Google Scholar]
- 19.Behmer ST, Joern A. 1993. Diet choice by a grass-feeding grasshopper based on the need for a limiting nutrient. Funct. Ecol. 7, 522–527. ( 10.2307/2390127) [DOI] [Google Scholar]
- 20.Hunter MD, Schultz JC. 1995. Fertilization mitigates chemical induction and herbivore responses within damaged oak trees. Ecology 76, 1226–1232. ( 10.2307/1940929) [DOI] [Google Scholar]
- 21.Cipollini D, Bergelson J. 2001. Plant density and nutrient availability constrain constitutive and wound-induced expression of trypsin inhibitors in Brassica napus. J. Chem. Ecol. 27, 593–610. ( 10.1023/A:1010384805014) [DOI] [PubMed] [Google Scholar]
- 22.Luken JO, Fonda RW. 1983. Nitrogen accumulation in a chronosequence of red alder communities along the Hoh River, Olympic National Park, Washington. Can. J. Forest Res. 13, 1228–1237. ( 10.1139/x83-161) [DOI] [Google Scholar]
- 23.Wootton JT. 2012. Effects of timber harvest on river food webs: physical, chemical and biological responses. PLoS ONE 7, e43561 ( 10.1371/journal.pone.0043561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worthington NP, Johnson FA, Staebler GR, Lloyd WJ. 1960. Normal yield tables for red alder. Pacific Northwest Forest and Range Experimental Station. U.S. Dept. of Agriculture Forest Service. [Google Scholar]
- 25.Bruin J, Sabelis MW, Dicke M. 1995. Do plants tap SOS signals from their infested neighbours? Trends Ecol. Evol. 10, 167–170. ( 10.1016/S0169-5347(00)89033-3) [DOI] [PubMed] [Google Scholar]
- 26.Mithöfer A, Wanner G, Boland W. 2005. Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol. 137, 1160–1168. ( 10.1104/pp.104.054460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown KR, Courtin PJ, Negrave RW. 2011. Growth, foliar nutrition and δ13C responses of red alder (Alnus rubra) to phosphorus additions soon after planting on moist sites. Forest Ecol. Manag. 262, 791–802. ( 10.1016/j.foreco.2011.05.013) [DOI] [Google Scholar]
- 28.Gökkaya K, Hurd TM, Raynal DJ. 2006. Symbiont nitrogenase, alder growth, and soil nitrate response to phosphorus addition in alder (Alnus incana ssp. rugosa) wetlands of the Adirondack Mountains, New York State, USA. Environ. Exp. Bot. 55, 97–109. ( 10.1016/j.envexpbot.2004.10.004) [DOI] [Google Scholar]
- 29.Coley PD. 1983. Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol. Monogr. 53, 209–234. ( 10.2307/1942495) [DOI] [Google Scholar]
- 30.Monaghan EJ, Ruttenberg KC. 1999. Dissolved organic phosphorus in the coastal ocean: reassessment of available methods and seasonal phosphorus profiles from the Eel River Shelf. Limnol. Oceanogr. 44, 1702–1714. ( 10.4319/lo.1999.44.7.1702) [DOI] [Google Scholar]
- 31.R Development Core Team. 2013. R version 3. Vienna, Austria: R Project for Statistical Computing; See http://www.r-project.org. [Google Scholar]
- 32.DeBell DS, Radwan MA. 1984. Foliar chemical concentrations in red alder stands of various ages. Plant Soil 77, 391–394. ( 10.1007/BF02182943) [DOI] [Google Scholar]
- 33.Driebe EM, Whitham TG. 2000. Cottonwood hybridization affects tannin and nitrogen content of leaf litter and alters decomposition. Oecologia 123, 99–107. ( 10.1007/s004420050994) [DOI] [PubMed] [Google Scholar]
- 34.LeRoy CJ, Whitham TG, Wooley SC, Marks JC. 2007. Within-species variation in foliar chemistry influences leaf-litter decomposition in a Utah river. J. North Am. Benthol. Soc. 26, 426–438. ( 10.1899/06-113.1) [DOI] [Google Scholar]
- 35.Baldwin IT. 1998. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc. Natl Acad. Sci. USA 95, 8113–8118. ( 10.1073/pnas.95.14.8113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown KR, Courtin PJ. 2003. Effects of phosphorus fertilization and liming on growth, mineral nutrition, and gas exchange of Alnus rubra seedlings grown in soils from mature alluvial Alnus stands. Can. J. Forest Res. 33, 2089–2096. ( 10.1139/x03-125) [DOI] [Google Scholar]
- 37.Wootton JT. 2012. River food web response to large-scale riparian zone manipulations. PLoS ONE 7, e51839 ( 10.1371/journal.pone.0051839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bormann BT, DeBell DS. 1981. Nitrogen content and other soil properties related to age of red alder stands. Soil Sci. Soc. Am. J. 45, 428–432. ( 10.2136/sssaj1981.03615995004500020038x) [DOI] [Google Scholar]
- 39.Elser JJ, et al. 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142. ( 10.1111/j.1461-0248.2007.01113.x) [DOI] [PubMed] [Google Scholar]
- 40.Tscharntke T, Thiessen S, Dolch R, Boland W. 2001. Herbivory, induced resistance, and interplant signal transfer in Alnus glutinosa. Biochem. Syst. Ecol. 29, 1025–1047. ( 10.1016/S0305-1978(01)00048-5) [DOI] [Google Scholar]
- 41.Sun J, Cardoza V, Mitchell DM, Bright L, Oldroyd G, Harris JM. 2006. Crosstalk between jasmonic acid, ethylene and Nod factor signaling allows integration of diverse inputs for regulation of nodulation. Plant J. 46, 961–970. ( 10.1111/j.1365-313X.2006.02751.x) [DOI] [PubMed] [Google Scholar]
- 42.Gómez S, Ferrieri RA, Schueller M, Orians CM. 2010. Methyl jasmonate elicits rapid changes in carbon and nitrogen dynamics in tomato. New Phytol. 188, 835–844. ( 10.1111/j.1469-8137.2010.03414.x) [DOI] [PubMed] [Google Scholar]
- 43.Bryant JP, Chapin FS, III, Klein DR. 1983. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40, 357–468. ( 10.2307/3544308) [DOI] [Google Scholar]
- 44.Oleksyn J, Karolewski P, Giertych MJ, Zytkowiak R, Reich PB, Tjoelker MG. 1988. Primary and secondary host plants differ in leaf-level photosynthetic response to herbivory: evidence from Alnus and Betula grazed by the alder beetle, Agelastica alni. New Phytol. 140, 239–249. ( 10.1046/j.1469-8137.1998.00270.x) [DOI] [PubMed] [Google Scholar]
- 45.Valentine H, Wallner W, Wargo P. 1983. Nutritional changes in host foliage during and after defoliation, and their relation to the weight of gypsy moth pupae. Oecologia 57, 298–302. ( 10.1007/BF00377171) [DOI] [PubMed] [Google Scholar]
- 46.Horner JD, Gosz JR, Cates RG. 1988. The role of carbon-based plant secondary metabolites in decomposition in terrestrial ecosystems. Am. Nat. 132, 869–883. ( 10.1086/284894) [DOI] [Google Scholar]
- 47.Kuiters AT. 1990. Role of phenolic substances from decomposing forest litter in plant–soil interactions. Acta Botanica Neerlandica 39, 329–348. [Google Scholar]
- 48.Dolch R, Tscharntke T. 2000. Defoliation of alders (Alnus glutinosa) affects herbivory by leaf beetles on undamaged neighbours. Oecologia 125, 504–511. ( 10.1007/s004420000482) [DOI] [PubMed] [Google Scholar]
- 49.Reymond P, Weber H, Damond M, Farmer EE. 2000. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12, 707–720. ( 10.1105/tpc.12.5.707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farmer EE, Ryan CA. 1990. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl Acad. Sci. USA 87, 7713–7716. ( 10.1073/pnas.87.19.7713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gundlach H, Müller MJ, Kutchan TM, Zenk MH. 1992. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc. Natl Acad. Sci. USA 89, 2389–2393. ( 10.1073/pnas.89.6.2389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin DM, Gershenzon J, Bohlmann J. 2003. Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol. 132, 1586–1599. ( 10.1104/pp.103.021196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeneli G, Krokene P, Christiansen E, Krekling T, Gershenzon J. 2006. Methyl jasmonate treatment of mature Norway spruce (Picea abies) trees increases the accumulation of terpenoid resin components and protects against infection by Ceratocystis polonica, a bark beetle-associated fungus. Tree Physiol. 26, 977–988. ( 10.1093/treephys/26.8.977) [DOI] [PubMed] [Google Scholar]
- 54.Elderd BD, Rehill BJ, Haynes KJ, Dwyer G. 2013. Induced plant defenses, host–pathogen interactions, and forest insect outbreaks. Proc. Natl Acad. Sci. USA 110, 14 978–14 983. ( 10.1073/pnas.1300759110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howe GA, Jander G. 2008. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 59, 41–66. ( 10.1146/annurev.arplant.59.032607.092825) [DOI] [PubMed] [Google Scholar]
- 56.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. 1997. Human domination of Earth's ecosystems. Science 277, 494–499. ( 10.1126/science.277.5325.494) [DOI] [Google Scholar]
- 57.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363. ( 10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.