Abstract
Spatial memory facilitates resource acquisition where resources are patchy, but how it influences movement behaviour of wide-ranging species remains to be resolved. We examined African elephant spatial memory reflected in movement decisions regarding access to perennial waterholes. State–space models of movement data revealed a rapid, highly directional movement behaviour almost exclusively associated with visiting perennial water. Behavioural change point (BCP) analyses demonstrated that these goal-oriented movements were initiated on average 4.59 km, and up to 49.97 km, from the visited waterhole, with the closest waterhole accessed 90% of the time. Distances of decision points increased when switching to different waterholes, during the dry season, or for female groups relative to males, while selection of the closest waterhole decreased when switching. Overall, our analyses indicated detailed spatial knowledge over large scales, enabling elephants to minimize travel distance through highly directional movement when accessing water. We discuss the likely cognitive and socioecological mechanisms driving these spatially precise movements that are most consistent with our findings. By applying modern analytic techniques to high-resolution movement data, this study illustrates emerging approaches for studying how cognition structures animal movement behaviour in different ecological and social contexts.
Keywords: cognitive map, hidden Markov model, Loxodonta africana, movement behaviour, movement ecology
1. Introduction
The ability to efficiently access critical resources when they are patchily distributed across large spatial scales is vital for free-ranging animal species. Evidence from conceptual [1], theoretical [2] and experimental [3,4] research demonstrates the importance of spatial memory for foraging animals when faced with the task of accessing patchy resources. Focal follows by humans of habituated animal groups (usually primates) have provided valuable insight into the types of information used by wild animals in situ and the extent and accuracy of their cognitive processes in space and time [5–7].
While direct observation has served as the foundation for cognition-focused animal behavioural research (e.g. [8]), such an approach is applicable to few contexts (i.e. high visibility and easy accessibility by human observers) and at limited spatial and temporal scales. Despite recognition of the importance of spatial cognition for wide-ranging and migratory species [9,10], little is known about the role of spatial memory in large-scale movement behaviour.
Technological advances providing fine-resolution animal movement data are well suited for determining spatial cognitive tasks in wild systems and opening inquiry into the selective history and evolution of these abilities [1,11]. A challenge for in situ studies is extracting useful behavioural information from such remotely collected data and coupling it with contextual information. However, advances in modern statistical approaches allow estimation of behavioural change points (BCPs) [12,13] and latent behavioural modes that can be contextualized relative to spatial habitat features [14], opening new horizons for analyses of decision processes in wide-ranging species.
The movement ecology of the African savannah elephant (Loxodonta africana) provides an exemplary illustration of large-scale, purposeful space use. Individuals in this species have been observed to move over 100 km to distant food and water resources [15–19]. Their well-developed hippocampal structure suggests the ability to perform advanced information processing [20], and experimental evidence suggests they can remember the spatial locations of other (out of site) individuals in relation to themselves [21]. These observations point to the capacity of elephants to use very precise spatial memory and highlight the importance of determining their spatial decision processes in order to fully understand the mechanisms driving their spatial behaviour.
Here, we investigate the scale and accuracy of movement to a collection of perennial waterholes by African savannah elephants in the semi-arid Etosha National Park (ENP), Namibia, using long-term, high-resolution tracking data and modern statistical tools. In ENP, permanent waterholes are widely distributed within a mosaic of variable forage resources, necessitating movements between these two resources [22]. We leverage the occurrence of movement phases [23]—continuous sequences of spatial relocations corresponding to distinct movement behaviours—to investigate whether movement behaviour when accessing water is consistent with what would be expected when cognitive mechanisms are employed to locate resources (i.e. relatively rapid and directed movement to a goal—see [7,24]). Following Byrne et al. [24], we refer to the movement phase change point locations as ‘decision’ points.
After first quantifying movement phases and decision points using hidden Markov models [25], we performed three related analyses on these data to explore the spatial context of movement decisions relative to perennial waterholes and the influence of social and environmental processes on these decisions. First, we compared the properties of movement behaviour in phases associated with accessing widely separated perennial waterholes to movement unrelated to accessing perennial water. Second, we modelled the spatial scales at which the decision points to access water occur as a function of season, gender and whether or not a switch between waterholes was made. Third, we modelled the probability with which individuals moved to the closest waterhole as a function of several covariates, including season, gender, whether or not the individual switched waterholes and the difference in distance between the available waterhole choices from the decision point at which the movement was initiated. We discuss our findings in the context of the most plausible mechanisms structuring elephant spatial memory, how changes in ecological and social constraints influence cognitive driven movement processes and directions for future research.
2. Material and methods
(a). Study area
The collared elephants were located in the eastern area of ENP. A large pan lies in the interior of the park, bordering the area of most visited waterholes to the west, but in general the landscape is topographically featureless with no restrictive mountain ranges, canyons or prominent features by which to orientate. A complex topology of braided wildlife trails emanate out from waterholes, becoming less directed and more transient at distances exceeding approximately 1 km from water. Excluding an isolated waterhole to the west of the pan, the mean nearest neighbour distance among all visited waterholes is 7.54 km (s.d. = 4.85 km). Vegetation is characterized by mopane (Colophospermum mopane) shrubveld and grassy meadows [26] and is generally denuded in the piosphere around perennial waterholes [22]. Annual rainfall from 1 July to the subsequent 30 June averaged 593 mm over the three-year study period (wet seasons occur from approx. November through April).
(b). Tracking data
Global Positioning System (GPS) satellite and Global Systems for Mobile Communications (GSM) collars on five male (GSM, 15 min sampling intervals) and five female (GPS satellite, 30 min sampling intervals) elephants collected location data from October 2009 through June 2012. Tracking data spanned approximately two years per individual with a spatial resolution to about 3 m2; see the electronic supplementary material for individual summaries of data (electronic supplementary material, tables S1 and S2). Fitting and removal of collars were conducted by veterinarians from the Namibian Ministry of Environment and Tourism and in accordance with their best-practice principles. Using the location data for each individual i, time series of net displacements si,t, defined as the distance between consecutive locations at times t and t + 1, are the movement descriptors used for analysis (rationale for these metrics is provided in the electronic supplementary material).
(c). Movement modes
After some preliminary exploratory data analysis (electronic supplementary material, section 3) indicating increased movement speeds as distance to the visited waterhole declined (see also [27]), analysis of individual track log data proceeded according to the following steps (see the electronic supplementary material for details). (i) Subsets of the location data corresponding to movement between two sequential visits to perennial waterholes were identified. To ensure the waterhole visits were distinct, only waterhole visits separated by at least 12 h were retained. (ii) For each movement path k describing a distinct movement track between waterhole visits, the loge(si,t,k) was modelled using hidden Markov models [25] to obtain an estimated latent behavioural state mode bi,t,k at each moment in time based on the speed of the individual (speed was used in modelled state definitions because including turning angle did not improve insight or model diagnostics; see the electronic supplementary material). Based on recognized elephant movement behaviour [19], a total of three distinct latent modes of movement were used to capture different types of incremental movement. These modes are characterized as slow speeds and tortuous directionality (M1—‘resting’), moderate speeds and somewhat persistent movement directions (M2—‘foraging’), fast and directed travel (M3—‘forage as you go’ or ‘goal-oriented walk’). See the electronic supplementary material for an illustration of these movement mode characteristics, evidence for correlation between net displacement and the concentration of the turning angles, and a statistical justification of the choice of three total movement modes. (iii) BCPs were identified, defined as the time t for which bi,t−1,k ≠ bi,t,k and are intuitively thought of as the moments in time when the previous and current movement modes differ. The time intervals with constant bi,t are associated with movement phases (see fig. 1b in [23]); we refer to the BCP locations as ‘decision’ points, as suggested by Byrne et al. [24]. (iv) The BCPs were used to segment each movement path between distinct waterhole visits into three sections: movement from the starting waterhole to the first BCP (movement phase from water), movement from the first BCP to the last BCP (movement phases unrelated to water) and movement from the last BCP to the end waterhole (movement phase to water) (electronic supplementary material, figure S9, provides an illustration).
(d). Behavioural change point analyses
The distances from the locations of the last behavioural change points (LBCPs) prior to visiting water to each of the perennial waterholes were calculated, and were then used to determine if the closest waterhole was accessed from the LBCP location. A mixed model framework [28] was applied to quantify the effects of season, gender, and whether or not the waterhole at the beginning of the movement path was the same as the destination waterhole (revisit choice), on the distance to the accessed waterhole from the LBCP. Generalized linear-mixed models were used to test the importance of these factors in determining the probability of accessing the nearest waterhole, but in addition included as fixed effect predictors the distance to the chosen waterhole from the LBCP and the absolute difference between the distances of the chosen water and the next closest alternative from the LBCP location. See the electronic supplementary material for detailed explication of model construction and inference. Likelihood ratio tests (LRT) were used to test full versus null model significance and individual fixed effect term significance.
3. Results
(a). General movement patterns
A total of 2485 distinct visits to 13 different perennial waterholes were recorded (figure 1; electronic supplementary material, table S2 and figure S1). The most rapid and direct movement phase was associated almost exclusively with movements to/from the perennial waterholes, irrespective of whether the individual was returning to or switching between waterholes (table 1 and figure 2; electronic supplementary material, figure S10). Movement to waterholes was accomplished using the most rapid movement mode for 2422 (97%) of the visits, the intermediate mode for 60 (2%) of the visits, and in the slowest movement mode three times (less than 1%). Waterholes were approached from all directions, with slight dominance by approaches from the North, potentially driven by spatial restrictions related to the dry Etosha pan to the southwest (electronic supplementary material, figures S1 and S11).
Figure 1.
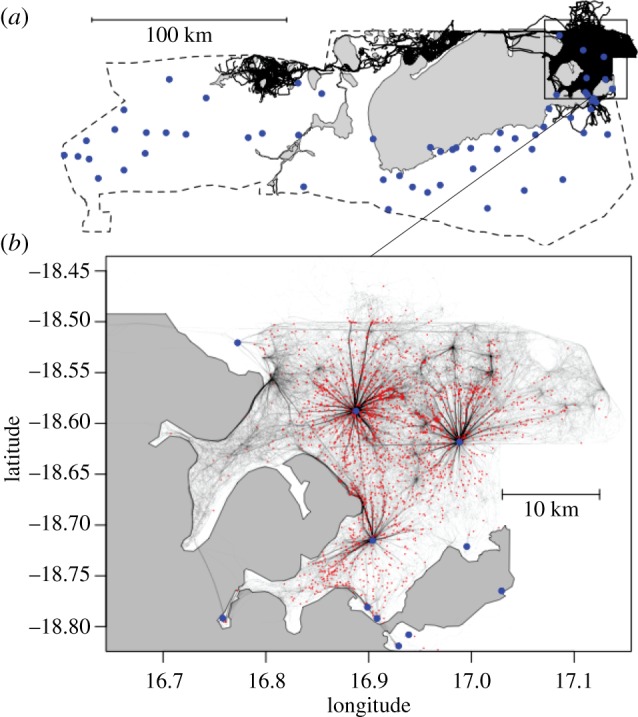
Movement tracks of elephants in ENP. (a) Overview of the park boundaries (dashed lines), salt pans (grey areas), movement trajectories (black lines) and perennial waterholes (circles—blue online). (b) Zoomed-in view of the area where most waterhole visits occur portraying the subsets of movement from the LBCP to the visited waterhole. Small dots (red online) show the LBCP prior to moving to waterholes. Location cluster nodes not associated with perennial waterholes are likely related to ephemeral water located in extended gravel pits not included in the analysis. Larger circles (blue online) show the perennial waterholes. (Online version in colour.)
Table 1.
Number of trips to water by movement mode phase and waterhole classification from the final decision point prior to moving to water.
| phase mode (movement characteristics) | return and closest | switch and closest | return and not closest | switch and not closest |
|---|---|---|---|---|
| mode 1 (slow and tortuous) | 2 | 1 | 0 | 0 |
| mode 2 (moderate and persistent) | 34 | 26 | 0 | 0 |
| mode 3 (rapid and directional) | 1505 | 676 | 96 | 145 |
Figure 2.
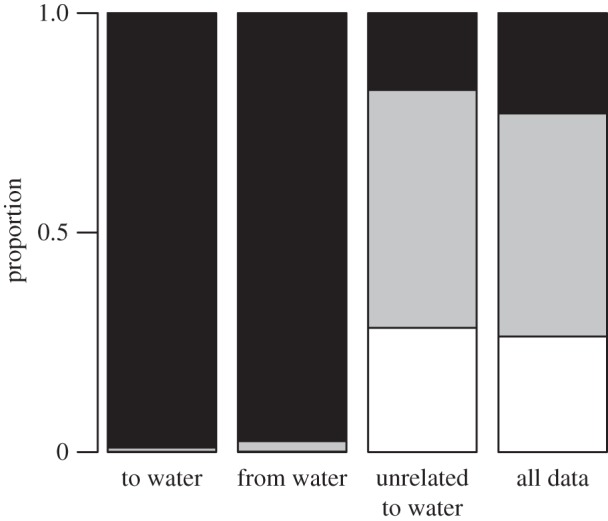
Stacked bar plots showing the proportion of incremental movement (from 521 980 total net displacements across all individuals) in the different behavioural modes by path segment type and for all data. Movement modes are coloured as: white, Mode 1; grey, Mode 2; black, Mode 3. See the electronic supplementary material for corresponding plots for each individual separately.
(b). Distance from decision points to chosen waterholes
The distance from LBCP to the chosen waterhole ranged between 0.1 and 48.97 km (figure 3, mean = 4.59 km, lower quartile = 2.74 km, upper quartile = 5.97 km), with 98 visits where the LBCP was located more than 10 km from the visited waterhole. The full model of the distance from the LBCP was significantly better than a null model that included only random individual and waterhole effects (LRT statistic 14.30, d.f. = 3, p-value < 0.01) and the fixed effect predictor variables included in this model were all significant (table 2 and figure 3). This model showed that the distance from the LBCP to the accessed waterhole decreased in the wet season relative to the dry season and for males relative to females. However, this distance increased when individuals switched to a different waterhole from the waterhole previously visited, averaging 4.91 km when switching and 4.43 km when returning.
Figure 3.
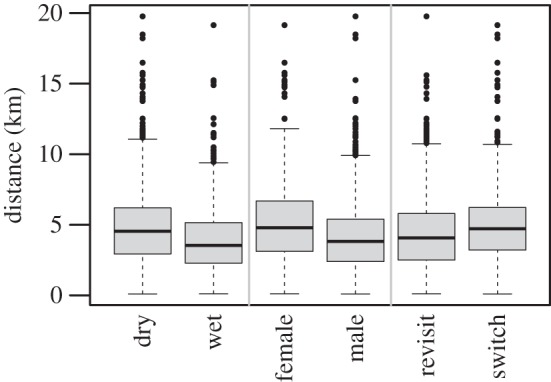
Tukey box plots of the distances from the LBCP to the chosen waterhole, by different categories of the covariates used to model these distances (compare with results shown in table 2). Vertical grey bars delineate covariates. The largest distance recorded is excluded for clarity.
Table 2.
Fixed effect terms and significance in the linear-mixed model describing the distance to the chosen waterhole from the LBCP location. The intercept includes the factor levels dry season, female and return.
| term | estimate | s.e. | t-value | LRT statistic | p-value |
|---|---|---|---|---|---|
| intercept | 4.68 | 0.63 | 7.48 | — | — |
| wet season | −0.85 | 0.24 | −3.50 | 8.11 | <0.01 |
| male | −0.84 | 0.37 | −2.29 | 4.07 | 0.04 |
| switch | 0.55 | 0.22 | 2.53 | 4.80 | 0.03 |
(c). Accuracy from decision points to chosen waterholes
From the LBCP, elephants moved to the closest waterhole 2244 times (table 1) (90% of all distinct visits, individually ranging from 78 to 100% of visits by season—see electronic supplementary material, table S3). The accessed waterhole was closest to the LBCP location 1541 times when returning to a waterhole (94% of all return visits), and 703 times when switching waterholes (83% of all switching visits) (table 1 and figure 4a). The full logistic mixed model of whether or not the closest waterhole was approached was significantly better than a null model that included only random intercept and fixed effect slope (see electronic supplementary material, section 5) effects (LRT statistic 599.33, d.f. = 5, p-value < 0.01). As expected, when the distance from the LBCP location to the chosen water decreased, the probability of moving to the closest waterhole increased (table 3). Notably, even while controlling for the distance from the LBCP to the chosen water, the probability of moving to the closest waterhole decreased when an individual switched to a different waterhole from that previously visited (figure 4a), consistent with the model results of table 2. The effects of season and gender (being male) on the probability of moving to the closest waterhole were positive but non-significant (table 3).
Figure 4.
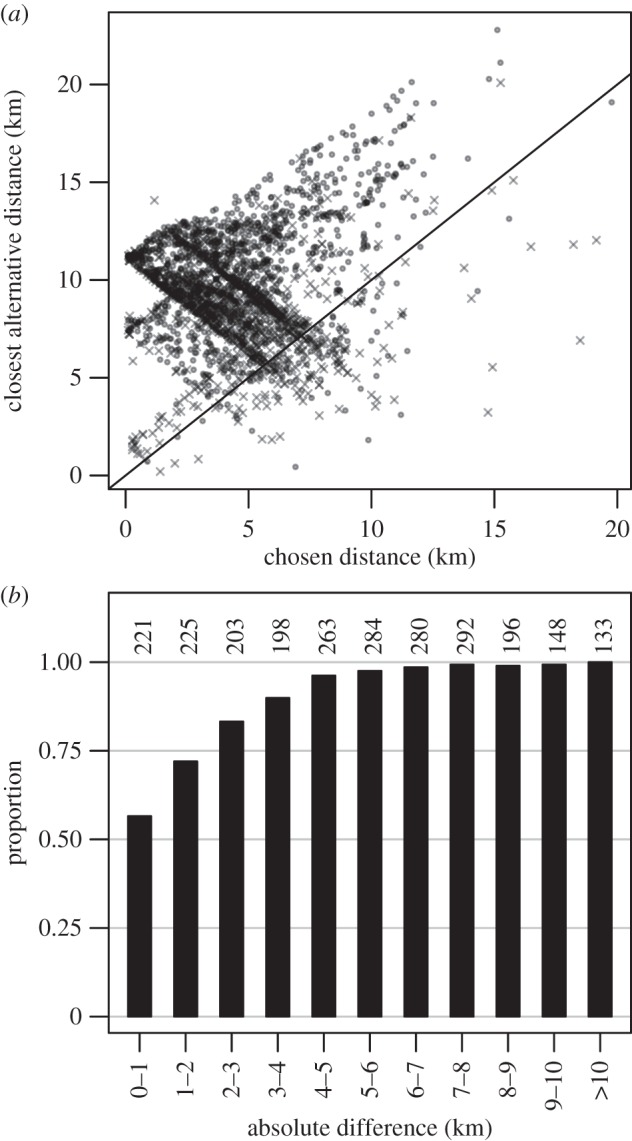
(a) The closest alternative distance to a waterhole versus the distance to the chosen waterhole from the LBCP (grey circles demarcate revisits to the same waterhole and black×symbols switches to a different waterhole). Points below the diagonal are where the chosen waterhole was further from the closest alternative and are dominated by switching events. (b) The proportion of visits that were to the closest waterhole relative to the absolute difference in distances between the chosen and closest alternative waterhole. Distance differences are portrayed in 1 km bins with the exception of the final bin. Numbers above the bars show sample sizes for each bin.
Table 3.
Fixed effect terms and significance in the generalized linear-mixed model describing whether the closest waterhole was accessed (coded as one for yes, zero for no) relative to distances to other waterholes at the LBCP. Terms are as in table 1 but with the addition of the continuous predictor variables: distance (the distance to the chosen waterhole) and absolute difference (the absolute difference in distances between the chosen and next nearest waterhole locations from the LBCP location).
| term | estimate | s.e. | z-value | LRT statistic | p-value |
|---|---|---|---|---|---|
| intercept | 4.26 | 0.87 | 4.89 | — | — |
| distance (km) | −0.81 | 0.06 | −13.30 | 331.38 | <0.01 |
| absolute difference (km) | 0.65 | 0.06 | 10.39 | 143.03 | <0.01 |
| wet season | 0.43 | 0.49 | 0.88 | 0.83 | 0.36 |
| male | 0.65 | 0.38 | 1.71 | 2.80 | 0.09 |
| switch | −0.79 | 0.30 | −2.67 | 6.07 | 0.01 |
The differences between the distances to the chosen waterhole and the closest alternative from the LBCPs ranged between −11.48 and 12.50 km (mean = 4.99 km, lower quartile = 2.64 km, upper quartile = 5.40 km). As the absolute difference between the closest waterhole and the second closest alternative increased, the probability of moving to the closest waterhole increased (table 3 and figure 4b).
4. Discussion
In semi-arid environments, elephant spatial movement is strongly structured by water resources, with the spatial displacement between water and forage resources shaping individual daily ranging distances [27,29,30]. By identifying movement phases and the change point locations of these phases in relation to perennial waterholes, we were able to inspect movement behaviour related to obtaining a critical resource in the study system.
Elephants demonstrated remarkable spatial acuity when accessing point water sources, initiating highly directional movements to water at considerable distances from the waterhole. The individuals studied overwhelmingly chose the nearest waterhole when moving to it in a directed manner (see also [27]) across a range of scales, suggesting a cognitive-based mechanism for these movements [31]. Presumably, there is considerable heterogeneity in the factors leading to the decision process underlying the patterns revealed here. For example, spatio-temporally varying landscape factors that are both environmental (e.g. distribution of ephemeral waterholes and forage resources of variable quality and quantity) and social (distribution of conspecifics that vary in social rank) in origin (see discussion below) are likely to influence decision processes at both incremental (‘in the moment’) and long-term spatial and temporal scales. Nevertheless, the relatively simplistic models employed here were able to reveal clear structure in movement behaviour related to visiting perennial waterholes.
Our estimates of when an individual made a decision to move to a particular waterhole are conservative as the ultimate decision could have been made well in advance of the final movement phase to water. Indeed, evidence of earlier decisions is suggested by inspection of paths when an individual switches waterholes, whereby the general direction of travel is fairly constant from one waterhole to the next (electronic supplementary material, figure S9). In addition, the preponderance of moving to a further waterhole (i.e. not the closest) when switching (figure 4b) is suspected to reflect purposeful movement to access new foraging areas rather than a failure to minimize Euclidian travel. Pinpointing the causes for elephants revisiting or switching waterholes, these likely lie within the heterogeneity of the decision-making processes and pose exciting challenges for future research.
Several lines of evidence here suggest that spatial memory is the primary mechanism by which elephants access waterholes, resulting in the spatial structuring of the movement behaviour observed here. First, the goal-oriented, rapid movement behaviour was exhibited even when individuals switched waterholes, which involved moving from greater distances than when sequentially revisiting a perennial waterhole and when the chosen waterhole was not the closest. Second, the direction from which the final movement phase was initiated was from all orientations and typically well beyond any visual changes in vegetation related to the piosphere, indicating that proximate sensing (smell or sight) of the water resource was not the mechanism used to navigate to these spatially explicit resources. An alternative olfactory mechanism involves reliance on scent marking of trails by other elephants that includes some capacity to indicate direction, and this remains to be investigated. Third, differences between the wet and dry season probabilities of moving to the closest waterhole were not found (table 3), further suggesting that smell was not important navigationally given the predominance of scattered ephemeral pools in the wet season. The use of auditory cues (e.g. if they hear elephant activity at waterholes) is also unlikely given that discriminatory auditory acuity is thought to be on the order of 1–2 km [32], substantially less than the observed distances at which most directional movements were initiated. Further, it is likely a risky strategy to rely on auditory cues emanating from the location of water, which might not always be available. Our analysis quantitatively supports anecdotes regarding demonstrations of elephant spatial cognitive capacity across large distances in the wild.
These analyses suggest that elephants have a keen understanding of the spatial properties of their ecosystems relative to their egocentric location at any given time. Different types of spatial memory have been distinguished in the context of foraging organisms [1,8], with well-developed research on how to measure or detect the types of memory employed. Whether or not the elephant movement behaviour observed here was achieved using a route-based or Euclidean-based memory is beyond the scope of this analysis, i.e. demonstrating novel shortcutting [33] is not possible here. However, given the increasing accuracy (probability of moving to the closest waterhole) as the distance between the elephants' decision location and nearby waterhole choices increased, the range of distances at which directional movements were initiated and the spectrum of routes taken, it is unlikely that elephants rely on simple route-based mapping.
Although dry season conditions typically constrain ranging distances in African savannah elephants [30,34] and decrease the total distance travelled between the visits to perennial water observed here (electronic supplementary material, figure S2), the distance between the final decision point and chosen waterhole increased during the dry season. This suggests that elephants use spatial knowledge to minimize the environmental constraints imposed on them, whereby they access forage further away from waterholes until they make the final decision to return to water. In unreported analyses on the time intervals between visiting waterholes, the distance at which the final movement phase to water was initiated appeared to be partly structured by the time since water was previously accessed. Future work might attempt to more accurately measure water stress in elephants at fine temporal resolutions to understand its influence on decision processes in space as well as time related to accessing water resources.
Males and females demonstrated differences in their movements to waterholes, with females tending to change movement modes and directionally walk to waterholes from greater distances than males. It is likely this difference is related to the higher foraging selectivity of females relative to males [35] and the constraints of dependent young for females. As a result of these two drivers, we hypothesize that females maximize time spent foraging in higher quality patches far from water until rapid movement to water is necessitated by calf needs. Social factors are likely to structure the recorded behaviour in other ways as well. Dominance relations among female elephants tend to be linear and well defined [36], the repercussions of which are manifested in space use [37] and movement properties [34,38]. Male social structure also is complex and likely to influence spatial behaviour and resource access [39,40]. Investigation of the relationship between the accuracy and scale at which point resources are accessed and individual characteristics (age, social status, physiological condition and age of dependent young) would offer a unique approach to understanding the influence of spatial knowledge on population processes and the influence of age (experience) on this interaction [41].
Elucidating the role of cognition, its spatial and temporal extent, and how different social and environmental factors influence cognitive-based behaviour remains a difficult proposition when studying wild animals in situ. Yet, progress can be made by assessing movement in relation to definable goals [31]. When the value of such goals are dynamic, determining cognitive processes can be more challenging, but an opportunity for revealing flexibility in planning and decision processes for achieving future goals by wild animals emerges [42]. Here, we illustrate how social and environmental factors influence goal-based movement in a wide-ranging species but in a relatively simple goal context (i.e. one that is constant in space and time). But our approach is equally applicable to systems with spatio-temporally dynamic goals. A key to realizing the potential of movement ecology for studies of animal cognition by wide-ranging species will be the ability to quantify dynamic constraints and resources.
Supplementary Material
Acknowledgements
We thank the Republic of Namibia's Ministry of Environment and Tourism for facilitating this research. Karline R. L. Janmaat and Henrik B. Rasmussen provided useful discussions. Three anonymous reviewers provided helpful comments on earlier versions of this manuscript.
Data accessibility
Owing to the sensitive conservation status of savannah elephants, access to data may be made by direct request to the Etosha Ecological Institute; contact W.K.
Author contributions
L.P. designed the study, analysed the data and wrote the manuscript. W.K. designed the study, collected data and wrote the manuscript. G.W. designed the study and wrote the manuscript.
References
- 1.Fagan WF, et al. 2013. Spatial memory and animal movement. Ecol. Lett. 16, 1316–1329. ( 10.1111/ele.12165) [DOI] [PubMed] [Google Scholar]
- 2.Mueller T, Fagan WF, Grimm V. 2011. Integrating individual search and navigation behaviors in mechanistic movement models. Theor. Ecol. 4, 341–355. ( 10.1007/s12080-010-0081-1) [DOI] [Google Scholar]
- 3.Cole S, Hainsworth FR, Kamil AC, Mercier T, Wolf LL. 1982. Spatial learning as an adaptation in hummingbirds. Science 217, 655–657. ( 10.1126/science.217.4560.655) [DOI] [PubMed] [Google Scholar]
- 4.Bailey DW, Gross JE, Laca EA, Rittenhouse LR, Coughenour MB, Swift DM, Sims PL. 1996. Mechanisms that result in large herbivore grazing distribution patterns. J. Range Manag. 49, 386–400. ( 10.2307/4002919) [DOI] [Google Scholar]
- 5.Noser R, Byrne RW. 2007. Travel routes and planning of visits to out-of-sight resources in wild chacma baboons, Papio ursinus. Anim. Behav. 73, 257–266. ( 10.1016/j.anbehav.2006.04.012) [DOI] [Google Scholar]
- 6.Ban SD, Boesch C, Janmaat KRL. 2014. Taï chimpanzees anticipate revisiting high-valued fruit trees from further distances. Anim. Cogn. 17, 1353–1364. ( 10.1007/s10071-014-0771-y) [DOI] [PubMed] [Google Scholar]
- 7.Janmaat KRL, Ban SD, Boesch C. 2013. Chimpanzees use long-term spatial memory to monitor large fruit trees and remember feeding experiences across seasons. Anim. Behav. 86, 1183–1205. ( 10.1016/j.anbehav.2013.09.021) [DOI] [Google Scholar]
- 8.Boinski S, Garber PA. 2000. On the move: how and why animals travel in groups. Chicago, IL: Chicago University Press. [Google Scholar]
- 9.Brooks CJ, Harris S. 2008. Directed movement and orientation across a large natural landscape by zebras, Equus burchelli antiquorum. Anim. Behav. 76, 277–285. ( 10.1016/j.anbehav.2008.02.005) [DOI] [Google Scholar]
- 10.Papastamatiou YP, Cartamil DP, Lowe CG, Meyer CG, Wetherbee BM, Holland KN. 2011. Scales of orientation, directed walks and movement path structure in sharks. J. Anim. Ecol. 80, 864–874. ( 10.1111/j.1365-2656.2011.01815.x) [DOI] [PubMed] [Google Scholar]
- 11.Shettleworth SJ. 2001. Animal cognition and animal behaviour. Anim. Behav. 61, 277–286. ( 10.1006/anbe.2000.1606) [DOI] [Google Scholar]
- 12.Patterson TA, Thomas L, Wilcox C, Ovaskainen O, Matthiopoulos J. 2008. State–space models of individual animal movement. Trends Ecol. Evol. 23, 87–94. ( 10.1016/j.tree.2007.10.009) [DOI] [PubMed] [Google Scholar]
- 13.Gurarie E, Andrews RD, Laidre KL. 2009. A novel method for identifying behavioral changes in animal movement data. Ecol. Lett. 12, 395–408. ( 10.1111/j.1461-0248.2009.01293.x) [DOI] [PubMed] [Google Scholar]
- 14.McClintock BT, King R, Thomas L, Matthiopoulos J, McConnell BJ, Morales JM. 2012. A general discrete-time modeling framework for animal movement using multistate random walks. Ecol. Monogr. 82, 335–349. ( 10.1890/11-0326.1) [DOI] [Google Scholar]
- 15.Leggett KEA. 2006. Home range and seasonal movement of elephants in the Kunene Region, northwestern Namibia. Afr. Zool. 41, 17–36. ( 10.3377/1562-7020(2006)41[17:HRASMO]2.0.CO;2) [DOI] [Google Scholar]
- 16.Thouless CR. 1995. Long distance movements of elephants in northern Kenya. Afr. J. Ecol. 33, 321–334. ( 10.1111/j.1365-2028.1995.tb01042.x) [DOI] [Google Scholar]
- 17.Verlinden A, Gavor IKN. 1998. Satellite tracking of elephants in northern Botswana. Afr. J. Ecol. 36, 105–116. [Google Scholar]
- 18.Viljoen PJ. 1989. Spatial distribution and movements of elephants (Loxodonta africana) in the northern Namib Desert region of the Kaokoveld, South West Africa/Namibia. J. Zool. 219, 1–19. ( 10.1111/j.1469-7998.1989.tb02561.x) [DOI] [Google Scholar]
- 19.Wall J, Wittemyer G, Klinkenberg B, LeMay V, Douglas-Hamilton I. 2013. Characterizing properties and drivers of long distance movements by elephants (Loxodonta africana) in the Gourma, Mali. Biol. Conserv. 157, 60–68. ( 10.1016/j.biocon.2012.07.019) [DOI] [Google Scholar]
- 20.Hart BL, Hart LA, Pinter-Wollman N. 2008. Large brains and cognition: where do elephants fit in? Neurosci. Biobehav. Rev. 32, 86–98. ( 10.1016/j.neubiorev.2007.05.012) [DOI] [PubMed] [Google Scholar]
- 21.Bates LA, Sayialel KN, Njiraini NW, Poole JH, Moss CJ, Byrne RW. 2008. African elephants have expectations about the locations of out-of-sight family members. Biol. Lett. 4, 34–36. ( 10.1098/rsbl.2007.0529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franz M, Kramer-Schadt S, Kilian W, Wissel C, Groeneveld J. 2010. Understanding the effects of rainfall on elephant–vegetation interactions around waterholes. Ecol. Model. 221, 2909–2917. ( 10.1016/j.ecolmodel.2010.09.003) [DOI] [Google Scholar]
- 23.Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. 2008. A movement ecology paradigm for unifying organismal research. Proc. Natl Acad. Sci. USA 105, 19 052–19 059. ( 10.1073/pnas.0800375105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrne RW, Noser R, Bates LA, Jupp PE. 2009. How did they get here from there? Detecting changes of direction in terrestrial ranging. Anim. Behav. 77, 619–631. ( 10.1016/j.anbehav.2008.11.014) [DOI] [Google Scholar]
- 25.Rabiner LR. 1989. A tutorial on hidden Markov models and selected applications in speech recognition. Proc. IEEE 77, 257–286. ( 10.1109/5.18626) [DOI] [Google Scholar]
- 26.Le Roux CJG, Grunow JO, Morris JW, Bredenkamp GJ, Scheepers JC. 1988. A classification of the vegetation of the Etosha National Park. South Afr. J. Bot. 54, 1–10. [Google Scholar]
- 27.Chamaillé-Jammes S, Mtare G, Makuwe E, Fritz H. 2013. African elephants adjust speed in response to surface-water constraint on foraging during the dry-season. PLoS ONE 8, e59164 ( 10.1371/journal.pone.0059164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. ( 10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 29.Loarie SR, Van Aarde RJ, Pimm SL. 2009. Fences and artificial water affect African savannah elephant movement patterns. Biol. Conserv. 142, 3086–3098. ( 10.1016/j.biocon.2009.08.008) [DOI] [Google Scholar]
- 30.Bohrer G, Beck PS, Ngene SM, Skidmore AK, Douglas-Hamilton I. 2014. Elephant movement closely tracks precipitation-driven vegetation dynamics in a Kenyan forest–savanna landscape. Mov. Ecol. 2, 2 ( 10.1186/2051-3933-2-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janson CH, Byrne R. 2007. What wild primates know about resources: opening up the black box. Anim. Cogn. 10, 357–367. ( 10.1007/s10071-007-0080-9) [DOI] [PubMed] [Google Scholar]
- 32.McComb K, Reby D, Baker L, Moss C, Sayialel S. 2003. Long-distance communication of acoustic cues to social identity in African elephants. Anim. Behav. 65, 317–329. ( 10.1006/anbe.2003.2047) [DOI] [Google Scholar]
- 33.Bennett AT. 1996. Do animals have cognitive maps? J. Exp. Biol. 199, 219–224. [DOI] [PubMed] [Google Scholar]
- 34.Polansky L, Douglas-Hamilton I, Wittemyer G. 2013. Using diel movement behavior to infer foraging strategies related to ecological and social factors in elephants. Mov. Ecol. 1, 1–11. ( 10.1186/2051-3933-1-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owen-Smith RN. 1992. Megaherbivores: the influence of very large body size on ecology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 36.Wittemyer G, Getz WM. 2007. Hierarchical dominance structure and social organization in African elephants. Anim. Behav. 73, 671–681. ( 10.1016/j.anbehav.2006.10.008) [DOI] [Google Scholar]
- 37.Wittemyer G, Getz WM, Vollrath F, Douglas-Hamilton I. 2007. Social dominance, seasonal movements, and spatial segregation in African elephants: a contribution to conservation behavior. Behav. Ecol. Sociobiol. 61, 1919–1931. ( 10.1007/s00265-007-0432-0) [DOI] [Google Scholar]
- 38.Wittemyer G, Polansky L, Douglas-Hamilton I, Getz WM. 2008. Disentangling the effects of forage, social rank, and risk on movement autocorrelation of elephants using Fourier and wavelet analyses. Proc. Natl Acad. Sci. USA 105, 19 108–19 113. ( 10.1073/pnas.0801744105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiyo PI, Moss CJ, Alberts SC. 2012. The influence of life history milestones and association networks on crop-raiding behavior in male African elephants. PLoS ONE 7, e31382 ( 10.1371/journal.pone.0031382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldenberg SZ, de Silva S, Rasmussen HB, Douglas-Hamilton I, Wittemyer G. 2014. Controlling for behavioural state reveals social dynamics among male African elephants, Loxodonta africana. Anim. Behav. 95, 111–119. ( 10.1016/j.anbehav.2014.07.002) [DOI] [Google Scholar]
- 41.McComb K, Shannon G, Durant SM, Sayialel K, Slotow R, Poole J, Moss C. 2011. Leadership in elephants: the adaptive value of age. Proc. R. Soc. B 278, 3270–3276. ( 10.1098/rspb.2011.0168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janmaat KRL, Polansky L, Ban SD, Boesch C. 2014. Wild chimpanzees plan their breakfast time, type, and location. Proc. Natl Acad. Sci. USA 111, 16 343–16 348. ( 10.1073/pnas.1407524111) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Owing to the sensitive conservation status of savannah elephants, access to data may be made by direct request to the Etosha Ecological Institute; contact W.K.


