Abstract
To capture and swallow food on land, a sticky tongue supported by the hyoid and gill arch skeleton has evolved in land vertebrates from aquatic ancestors that used mouth-cavity-expanding actions of the hyoid to suck food into the mouth. However, the evolutionary pathway bridging this drastic shift in feeding mechanism and associated hyoid motions remains unknown. Modern fish that feed on land may help to unravel the physical constraints and biomechanical solutions that led to terrestrialization of fish-feeding systems. Here, we show that the mudskipper emerges onto land with its mouth cavity filled with water, which it uses as a protruding and retracting ‘hydrodynamic tongue’ during the initial capture and subsequent intra-oral transport of food. Our analyses link this hydrodynamic action of the intra-oral water to a sequence of compressive and expansive cranial motions that diverge from the general pattern known for suction feeding in fishes. However, the hyoid motion pattern showed a remarkable resemblance to newts during tongue prehension. Consequently, although alternative scenarios cannot be excluded, hydrodynamic tongue usage may be a transitional step onto which the evolution of adhesive mucosa and intrinsic lingual muscles can be added to gain further independence from water for terrestrial foraging.
Keywords: prey capture, mudskipper, newt, hyoid, tongue, kinematics
1. Background
Identifying the functional modifications enabling transitions from water to land is key to our understanding of how vertebrates managed to invade the terrestrial environment around 400–350 Ma [1,2]. Although studies on skeletal adaptations during the fish-to-tetrapod transition have mainly focused on adaptations of the locomotion system [3–5], modifications to the feeding system are an equally important aspect of terrestrialization [6–9]. Fish rely on generating suction to draw prey and surrounding water into the buccal cavity and to transport it within the mouth towards the oesophagus [10,11]. The hyoid is important for generating suction; it causes buccal volume to increase by depressing the floor of the buccal cavity and laterally abducting the suspensoria [12,13]. On land, however, using flows of air to transport food is virtually impossible [14,15]. Having passed this evolutionary barrier, modern terrestrial tetrapods (e.g. from the groups Lissamphibia, Lepidosauria and Testudines) use a tongue supported by the hyoid skeleton to transport food towards the oesophagus [16–18]. The steps in the transformation of the hyoid (and its associated muscles and ligaments) from a suction-generating structure to supporting and moving the tongue, however, remain unknown.
Examination of extant amphibious fishes may help us better understand the mechanisms that led to the recent evolution of a cranial musculoskeletal system specialized to operate in this novel niche. These model systems provide insight in the basic physical constraints behind this key macro-evolutionary change, and reveal biomechanical solutions for successfully making this drastic shift in feeding environment [6,19]. Probably the most successful group of fishes capable of extended terrestrial foraging excursions are mudskippers (Gobiidae, Oxudercinae). Although the mechanics of the feeding system of mudskippers has been studied previously, these studies focused either exclusively on the functioning of the oral jaws during the initial stages of prey capture [9], or on pharyngeal jaw function during the final processing of prey near the oesophagus entrance [20]. Consequently, it remains to be identified how terrestrial feeding is completed in mudskippers, including the indispensable phase of transporting prey to the posterior end of the buccal cavity. Interestingly, mudskippers come out on land with their opercular and buccal cavities (anatomy shown in figure 1a) filled with water [21]. However, whether this intra-oral water plays a role in feeding remains unknown [22]. The main goal of this study is to unravel how mudskippers capture prey and perform intra-oral transport of prey on land. In addition, these findings will be discussed in the light of the evolution of terrestrial feeding in early tetrapods by means of a comparison of hyoid kinematics between this terrestrially feeding fish (i.e. mudskipper), a typical suction-feeding fish of generalized morphology (in casu sunfish), and a model species for a tongue-using, basal terrestrial tetrapod (in casu newt).
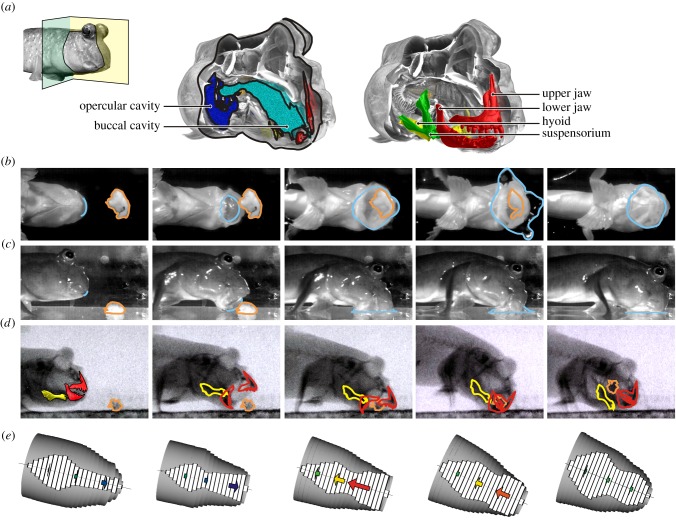
Figure 1.
Morphology and kinematics of terrestrial feeding in Periophthalmus barbarus. (a) Cut-out section of a scanning 3D-reconstruction illustrates the anatomical elements and their colour codes. Ventral view (b) and lateral view (c) high-speed video frames showing successive stages of feeding, and illustrating the water protruding out of the mouth (light blue contours). X-ray video frames at identical stages are shown in (d), and the outlines of the jaws, hyoid and prey (orange contours) are mapped. (e) Representation of the volumetric ellipse model with the internal volume illustrated by white bars; the flow velocity at 20%, 50% and 80% of the head length are shown by the direction, length and colour of the arrows (see figure 2b for colour legend).
2. Material and methods
(a). Animals
Five adult Atlantic Mudskippers Periophthalmus barbarus (Linnaeus, 1766) (9.9 ± 1.8 cm standard length) originating from Nigeria were obtained commercially. One additional adult individual was sacrificed using an overdose of MS-222 (Sigma Chemical) and used for computed tomography (CT) scanning (scanning protocol was described previously by Michel et al. [9]). The four live animals were housed in individual Plexiglas aquaria (35 × 18 × 30 cm) during testing and recording. The aquaria were equipped with a Plexiglas ramp and a terrestrial excursion area with a transparent floor and sides. A constant temperature of 27°C was maintained, with a 12 L : 12 D cycle. The same set-up was used to house two Italian crested newt Triturus carnifex individuals (75 mm and 77 mm snout-to-vent length) to be used for X-ray video recordings. These animals were collected in Lower Austria, Austria with collection permission RU5-BE-18/022–2011 granted by the local government of Lower Austria. The pumpkinseed sunfish Lepomis gibbosus (74 mm standard length) was wild-caught in Belgium and housed at a room temperature of 20°C. All the specimens used in this study were handled according to University of Antwerp animal care protocols.
(b). Kinematic analysis
Simultaneous high-speed videos were captured from lateral and ventral views of P. barbarus feeding on pieces of brown shrimp presented on the bottom of the terrestrial section of the aquarium using two Redlake cameras (1280 × 1024 pixels; Redlake, San Diego, CA, USA), a Redlake MotionPro HS1000 and a MotionScope M3, at 500 frames per second. Several bright LEDs provided the necessary illumination. The food items provided were approximately 7.5 mm in length, which was somewhat less than 80% of the maximal gape size of the fish. At least five prey-capture videos were recorded for each individual. Two video pairs per individual were selected from these videos based on the quality of the image sharpness and contrast, to be used for further analysis.
In addition to the external video recordings, high-speed X-ray videos were obtained for each of the four individuals. These recordings were made using a Philips Optimus M 200 X-ray generator (Royal Philips Electronics NV, Eindhoven, the Netherlands) coupled to a 14 inch image intensifier set to 6 inch zoom mode and a Redlake Motion Pro 2000 camera (1280 × 1024 pixels; Redlake) recording at 500 frames per second. Prior to the recording sessions, the animals were anaesthetized using MS-222 to insert small lead markers (less than 0.5 mm) subcutaneously in close proximity to the dentary, premaxilla, hyoid and skull roof using hypodermic needles. The same procedure was followed for L. gibbosus. X-ray videos of T. carnifex were recorded at 125 frames per second to improve image contrast. Two-dimensional landmark coordinates were digitized using Didge (Alistair Cullum, Creighton University, USA), and were used to quantify the movement of the gape and hyoid during prey capture. Additional high-speed X-ray video recordings of terrestrial feeding in P. barbarus were scored for successfully transporting prey intra-orally, towards the pharyngeal jaws or oesophagus entrance. In order to capture the intra-oral water during terrestrial feeding, we placed a sturdy, high-performance absorbant beneath prey items. For each of these terrestrial feeding events, prey items were presented on top of 3 cm wide strips, cut from the centre of Always Ultra Normal Plus sanitary pads (Procter & Gamble, Cincinnati, OH, USA).
(c). Volume and flow velocity modelling
The changes in the volume of the head and intra-oral cavities during feeding were calculated using the approach described in a previous study on suction feeding in catfish [8]. This model is based on the ellipse method for calculating the volume of biological objects using lateral and ventral video recordings [23], and previously generated accurate predictions of suction flow velocities in larval carp [23], snake-necked turtles [24] and air-breathing catfish [8]. The model uses the upper and lower contour coordinates of the mudskipper's head excluding the eyes in the lateral and ventral view. It also uses the coordinates of a longitudinal axis connecting the distal tip of the operculum to the upper jaw tip. In the ventral view, this longitudinal axis was set from the central point between the left and right opercula tips to the centre of the snout tip. Next, the contour coordinates were recalculated in the fish frame of reference for every frame of the recording. Interpolation functions were used to extract the four corresponding contour coordinates at 21 equally spaced intervals along the longitudinal axis. With these data, changes in the width and height of the ellipses over time as well as changes in the volume of the 21 elliptical cylinders mimicking the head were calculated. For each elliptical cylinder, the profiles of length and width versus time were filtered with a fourth-order zero-phase-shift low-pass Butterworth filter to reduce landmark coordinate digitization noise (cut-off frequency of 15 Hz).
The internal dimensions of the oral and opercular cavities of the mudskipper at rest were obtained through CT scan and scaled to match the head length of each individual animal. The volume of the oral and opercular cavities was similarly divided into a series of 21 sections along the same longitudinal axis as mentioned above. It was assumed that this situation (i.e. the internal volume of the mouth cavity of the preserved specimen at rest) reflects the moment before start of the prey-capture event. Subsequently, changes in the height and the width of the head over time will cause changes in the width and height of the internal mouth volume ellipses (assuming a constant volume for the head tissues of the mudskipper). The law of continuity requires each volume increase of the internal cavity to be filled with fluid immediately. This allows flow rates to be calculated as long as the mouth and opercular slits are not open at the same time, which does not occur during terrestrial feeding in P. barbarus.
The heights of the ellipses used in the model for P. barbarus were amended to account for the movement of the hyoid. The high-speed X-ray videos showed the hyoid elevating between the suspensoria without causing the outer contours to change from an external view. In order to account for the change of internal volume of the oral cavity owing to the externally invisible hyoid elevation and depression, a spatio-temporal correction factor for hyoid movement was implemented to the ellipse heights. This correction factor was based on the average profile of hyoid movement (three captures × two individuals). The amplitude and timing of the ellipse-height correction factor was scaled to match the size of each individual and the timing of each prey-capture event.
3. Results
Our biomechanical analysis based on dual-view high-speed videos and X-ray videos showed that terrestrial capture and intra-oral food transport in P. barbarus, the Atlantic mudskipper, could best be described as a ‘hydrodynamic tongue’. As soon as the mouth starts opening while pivoting the head about the pectoral fins to approach the prey, a convex meniscus of buccal water was observed at the mouth aperture (figure 1b,c, first column). This water further protruded out of the mouth (figure 1b,c, second column), and just before the jaws were placed around the prey, the water contacted the prey and spread along the surface surrounding the prey (figure 1b,c, third column). While the jaws were closing and the prey was engulfed, part of the expelled water was sucked back into the buccal cavity (figure 1b,c, fourth and fifth columns; see electronic supplementary material, movie S1). Often, a single cycle of the gape and hyoid was sufficient to engulf and transport prey to the pharyngeal jaw region of the buccal cavity (figure 1d; see electronic supplementary material, movie S2). As this ‘protrusion’ and ‘retraction’ of buccal water showed kinematical and functional resemblance to tongue movement during feeding in lower tetrapods, we refer to it as a ‘hydrodynamic tongue’. Note that this term has been previously used to describe the more common, intra-oral, flow-driven transport of prey in aquatic fishes [25]. Since the hydrodynamic tongue of mudskippers also includes an extra-oral component during the initial capture of prey, our definition of a ‘hydrodynamic tongue’ is broader than this original definition.
Mathematical modelling of the volume changes of the head and resulting intra-oral water displacements (figure 1e) confirmed the forward and backward motion of the water observed externally at the mouth region (figure 1b,c). Before the lunge at the prey, the small, valvular slits at the dorso-posterior side of the opercula were closed. Consequently, the connected opercular and buccal volumes could be regarded as a vessel with a small opening at the side of the mouth. While the mudskipper accelerated forwards and pivoted down towards the prey, the left and right gill covers were adducted (on average between 0.1 and 0.025 s before the time of maximal mouth opening; zone z1 in figure 2a), and the hyoid was elevated (between 0.05 and 0.02 s before the time of maximal mouth opening; zone z2 in figure 2a). Because water is incompressible, the decrease in volume resulting from these motions resulted in a flow of water towards the mouth (first and second frame in figure 1e; time −0.10 to −0.02 in figure 2b). As this all happened during forward acceleration of the head (peak accelerations approx. 6 m s−2), the inertia of the water mass cannot cause the anterior flow of water. This means that these compressive motions were powered actively by the mudskipper's cranial muscles.
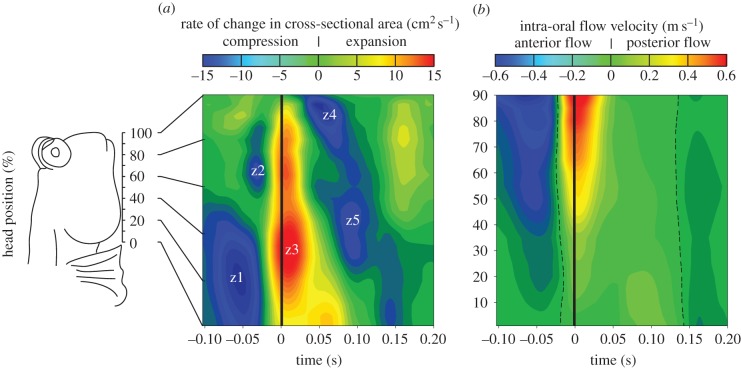
Figure 2.
Intra-oral rate of cross-sectional area change and flow velocity during prey capture in Periophthalmus barbarus. (a) Spatio-temporal-interpolated and averaged (two captures × four individuals) rate of change in the cross-sectional area as a function of the position along the head, showing successive compression and expansion events (z1–z5 delineate spatio-temporal zones of high compression or expansion; time 0, maximal mouth opening). (b) The corresponding intra-oral flow velocities along the anterior-to-posterior axis showing initially forward (blue colouring) and then backward motion (yellow to red colouring) of fluid with respect to the head (dashed line, zero flow velocity).
After the opercular and buccal compression caused protrusion of the hydrodynamic tongue, the head volume increased and suction was generated. This expansion started just before the mouth became maximally opened (time = 0 s), and occurred at the level of the hyoid and suspensorium (zone z3 in figure 2a) and at the gill covers (zone z4 in figure 2a). Fluid flow velocities during suction, which may partly include flows of air, were maximal very close to the time of maximal gape and reach average peak values of 0.6 m s−1 at the mouth entrance (figure 2b). During the final instants of opercular expansion (time = 0.07 s), the opercular slits opened. At the same time, the mouth became fully closed. From this instant on, the opercular and buccal volumes could be treated as a vessel with an opening at the opercular slits. Next, an anterior-to-posterior wave of compression of the head (zone z5 in figure 2a) was formed to cause further posteriorly directed, relatively low-speed fluid flow (figure 2b).
The opercular and buccal cavities were not always filled with water to the same level. Although the volumetric changes described above occurred consistently, water was not always observed protruding out of the mouth during feeding. To test the necessity of the hydrodynamic tongue for terrestrial feeding, additional feeding events were recorded with high-speed X-ray video while a high-performance absorbant was placed beneath the prey items to absorb the water expelled from the buccal cavity. After this decrease of hydrodynamic tongue volume, the mudskipper was still capable of grabbing the prey between the jaws. However, intra-oral transport of prey to the pharyngeal jaws or oesophagus entrance without returning to the water was unsuccessful in 70% of the feeding sequences (n = 18; see electronic supplementary material, movie S3). Without absorbent material, we observed only 36% unsuccessful intra-oral transport cases (n = 24) before moving out of view of the cameras. This shows that the hydrodynamic tongue fails without sufficient intra-oral water.
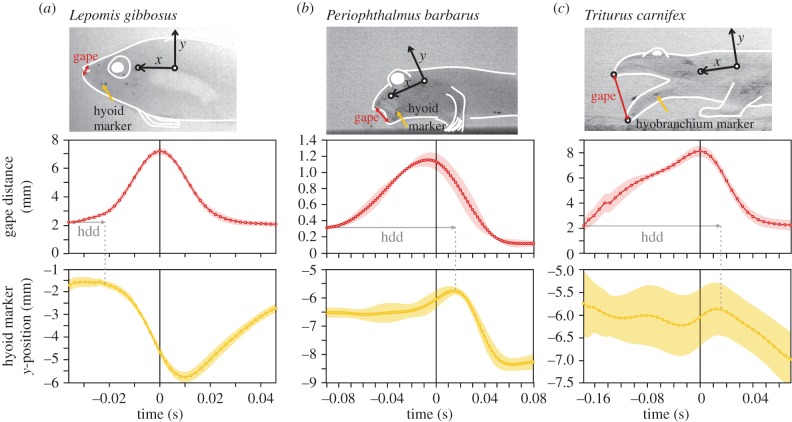
The mudskipper's hyoid motion pattern during terrestrial feeding was different from the general pattern observed for aquatic feeding in fish. During suction feeding of a morphologically generalized perciform fish (e.g. the pumpkinseed sunfish L. gibbosus), depression of the hyoid shortly followed the onset of mouth opening and reached its peak velocity near the instant of maximum mouth opening, as exemplified with marker tracking on X-ray video (figure 3a), which confirms previous kinematical results based on visible light video [26]. In terrestrially fed mudskippers, however, hyoid elevation persisted during the entire mouth opening phase, and depression only started after the onset of mouth closing (figure 3b).
Figure 3.
Comparison of gape and hyoid kinematics during prey capture. Data are derived from landmark tracking on high-speed X-ray videos with lead markers inserted subcutaneously (top row; including the reference frames). A strong difference is observed between (a) the hyoid kinematics of a suction-feeding sunfish (eight captures × one individual) and (b) the terrestrial capture of prey by the mudskipper (three captures × two individuals; e.g. in hyoid depression delay, hdd). The latter shows a striking resemblance to (c) terrestrial feeding in newts (four captures × two individuals). Error ribbons, 1 s.e.m.
By contrast, a striking resemblance is observed between the kinematics of the hyoid underlying the hydrodynamic tongue in the mudskipper and that of the hyobranchial elements supporting the true tongue of salamandrids. Position tracking of a radio-opaque marker adhering to the tongue skeleton of the Italian crested newt (T. carnifex) capturing prey on land during X-ray video recordings showed elevation until past the time of maximum gape and a similarly long hyoid depression delay as in mudskippers (figure 3b,c). This hyobranchial elevation helps to protrude the tongue while the subsequent hyobranchial depression causes the tongue to be retracted [17].
4. Discussion and conclusion
The water retained in the buccal and opercular cavity of Atlantic mudskippers has a vital role during terrestrial feeding. As the protrusion and retraction of this water mass was indispensable for intra-oral transport of prey on land, this hydrodynamic tongue empowers mudskippers to capture and swallow several prey sequentially without having to return to the water for swallowing. By contrast, a different fish species previously described to capture prey on land, the eel-catfish (Channallabes apus), does not hold water inside the mouth cavity, and always returns immediately to the water for swallowing after having grabbed the prey between the jaws [15,27]. Other species that only sporadically capture food on the shores, such as aquatic emydid turtles [28] or pipid frogs [29], are also obligatory underwater swallowers. Consequently, the capacity to feed on multiple prey during terrestrial foraging, owing to their hydrodynamic tongue, brings mudskippers up to a higher level of terrestrialization compared with these species.
Our results suggest that fish adapted to use a hydrodynamic tongue for feeding and swallowing on land are likely to evolve a similar hyoid motion pattern as observed in primitive tetrapods using an adhesive tongue to capture prey on land. Although the difference in hyoid kinematics between the mudskipper and the aquatic suction-feeding sunfish is substantial (figure 3), a small amount of buccal compression including hyoid elevation during a short time prior to expansion has also been noted in preparation of aquatic suction feeding in several fish species [30–32]. Yet the amount of hyoid elevation and delay in depression relative to mouth opening in the mudskipper is unprecedented in fish. Nevertheless, it is more likely that the cranial kinematics of terrestrial feeding in mudskippers (figures 2 and 3b) is derived from this preparatory phase of aquatic suction feeding, rather than being the result of a newly gained motor pattern.
The evolution of prey prehension and swallowing by the tongue is considered to be a major step in the terrestrialization of vertebrates [18]. However, due to the scarcity of fossil records of hyobranchial elements of early tetrapods, reconstruction of the skeletal changes associated with the evolution of an adhesive tongue is not possible [33]. Consequently, we are forced to rely almost exclusively on mechanistic scenarios using information from modern systems subjected to similar selection pressures to gain insight on how an adhesive tongue can evolve [1,34]. Although tetrapodomorphs and modern sarcopterygians clearly differ in morphology from mudskippers, the main functional elements of the mudskipper's hydrodynamic tongue are also present in these groups: a hyoid capable of dorsal and ventral rotation, and adductable and abductable gill covers (figure 2) [35,36]. Similar usage of intra-oral water for terrestrial transport of prey at some stage during early evolution of the tetrapod lineage can therefore not be excluded a priori on morphological grounds.
The remarkable similarity in the hyoid's motion pattern between mudskippers and tongue-protruding newts (figure 3b,c) calls for a reconsideration of the current general hypothesis about the evolution of terrestrial feeding behaviour in early tetrapods. This hypothesis states that terrestrial prey transport by the tongue evolved first, while prey capture by a protruding tongue is gained subsequently [37–39]. This hypothesis is based on kinematic similarity between the externally observable hyoid depressions performed by suction-feeding fish (figure 3a) and the depressing hyoid region of terrestrial salamanders during intra-oral transport of prey [37–39]. However, a fundamental gap in this hypothesis is that the tongue-based intra-oral transport by modern terrestrial salamanders moves a prey that is already brought deep into the mouth cavity by the foregoing protrusion and retraction of the tongue: it does not explain how the first land-dwelling tetrapods managed to bring prey inside their mouth cavity. Consequently, this hypothesis presents an incomplete scenario of the evolution of terrestrial feeding.
We propose two possible scenarios for the evolution of terrestrial feeding capability in early tetrapods. A first one completes the classical hypothesis described above, while a second one proposes mudskipper-like usage of buccal water to transport and swallow food on land to be an intermediate evolutionary stage:
(1) Kinetic inertial transport of prey (i.e. generating a posterior shift of the prey by forward accelerations of the head) as observed in crocodilians [40] or monitor lizards [41] was evolutionary gained to move prey from being held by the jaws to the level of the hyoid inside the mouth cavity. A tongue evolved to perform salamander-like prey-transport cycles to complete the final stages of intra-oral transport without using water, thereby retaining the ancestral hyoid motion patterns of aquatic suction feeding [34].
(2) A tongue evolved to move prey grabbed between the jaws. In doing so, an elevation followed by a depression of the floor of the mouth by the hyoid skeleton is retained from a behaviour using buccal water for prey transport similar to mudskippers. Independence of water for prey transport is gained by achieving closer contact between the elevated hyoid and prey, coupled with the evolution of adhesive structures. A tongue able to protrude out of the mouth is a logical extension of this behaviour.
It remains an open question which of these two scenarios is the most plausible. The generally large size of early tetrapods, and the presence of a mobile neck in tetrapodomorph fishes [7] and in the earliest known terrestrial tetrapods [42], could be indicative of kinetic inertial transport possibilities by analogy with the feeding style of crocodilians and monitor lizards. However, modern tetrapods show no evidence for an intermediate evolutionary step in combining tongue-retraction transport with foregoing inertial transport: feeding behaviours of reptiles mapped on a phylogenetic tree suggests that their ancestor already used a protruding tongue to capture prey, and so do virtually all extant amphibians that feed on land [43]. The latter may be indicative of a tongue evolving directly to mediate in intra-oral food uptake close to or outside the jaws, which is in line with the second scenario. In that case, kinematic patterns of water-mediated terrestrial feeding similar to the one discovered here for mudskippers may have been important precursor behaviours in the colonization of land. An already established kinematic pattern of the future tongue-bone could then allow a gradual anatomical specialization towards water-independent terrestrial feeding through the increase of the adhesive capacity of the tissues that eventually will form the tongue.
Acknowledgements
We thank Nicolai Konow for help with X-ray video recording and Christian Proy for help in collecting newts. We also thank two anonymous reviewers for their time and effort in reviewing this paper.
Ethics statement
All experiments conducted on the animals for this study were approved by the University of Antwerp ethical animal welfare committee. All of the specimens used in this study were handled according to University of Antwerp animal care protocols.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Author contributions
All authors contributed in collecting data; K.B.M. analysed the data; K.B.M and S.V.W. wrote the manuscript; all authors discussed the results and commented on the manuscript.
Funding statement
This work was funded by the Special Research Fund of the University of Antwerp (NOI-BOF) to S.V.W. and by the Austrian Science Fund (FWF) to E.H.
Conflict of interest
We have no competing interests
References
- 1.Hsieh S-TT. 2010. A locomotor innovation enables water–land transition in a marine fish. PLoS ONE 5, e11197 ( 10.1371/journal.pone.0011197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smithson TR, Wood SP, Marshall JE, Clack JA. 2012. Earliest Carboniferous tetrapod and arthropod faunas from Scotland populate Romer's Gap. Proc. Natl Acad. Sci. USA 109, 4532–4537. ( 10.1073/pnas.1117332109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shubin NH, Daeschler EB, Jenkins FA., Jr 2006. The pectoral fin of Tiktaalik roseae and the origin of the tetrapod limb. Nature 440, 764–771. ( 10.1038/nature04637) [DOI] [PubMed] [Google Scholar]
- 4.Ijspeert AJ, Crespi A, Ryczko D, Cabelguen JM. 2007. From swimming to walking with a salamander robot driven by a spinal cord model. Science 315, 1416–1420. ( 10.1126/science.1138353) [DOI] [PubMed] [Google Scholar]
- 5.Pierce SE, Clack JA, Hutchinson JR. 2012. Three-dimensional limb joint mobility in the early tetrapod Ichthyostega. Nature 486, 523–526. ( 10.1038/nature11124) [DOI] [PubMed] [Google Scholar]
- 6.Ashley-Ross MA, Hsieh ST, Gibb AC, Blob RW. 2013. Vertebrate land invasions-past, present, and future: an introduction to the symposium. Integr. Comp. Biol. 53, 192–196. ( 10.1093/icb/ict048) [DOI] [PubMed] [Google Scholar]
- 7.Daeschler EB, Shubin NH, Jenkins FA., Jr 2006. A Devonian tetrapod-like fish and the evolution of the tetrapod body plan. Nature 440, 757–763. ( 10.1038/nature04639) [DOI] [PubMed] [Google Scholar]
- 8.Van Wassenbergh S, Aerts P, Herrel A. 2006. Scaling of suction feeding performance in the catfish clarias gariepinus. Physiol. Biochem. Zool. 79, 43–56. ( 10.1086/498188) [DOI] [PubMed] [Google Scholar]
- 9.Michel KB, Adriaens D, Aerts P, Dierick M, Wassenbergh SV. 2014. Functional anatomy and kinematics of the oral jaw system during terrestrial feeding in Periophthalmus barbarus. J. Morphol. 275, 1145–1160. ( 10.1002/jmor.20291) [DOI] [PubMed] [Google Scholar]
- 10.Alexander R. 1970. Mechanics of the feeding action of various teleost fishes. J. Zool. 162, 145–156. ( 10.1111/j.1469-7998.1970.tb01261.x) [DOI] [Google Scholar]
- 11.Muller M, Osse J. 1984. Hydrodynamics of suction feeding in fish. Trans. Zool. Soc. Lond. 37, 51–135. ( 10.1111/j.1096-3642.1984.tb00068.x) [DOI] [Google Scholar]
- 12.Muller M. 1989. A quantitative theory of expected volume changes of the mouth during feeding in teleost fishes. J. Zool. 217, 639–661. ( 10.1111/j.1469-7998.1989.tb02515.x) [DOI] [Google Scholar]
- 13.Aerts P. 1991. Hyoid morphology and movements relative to abducting forces during feeding in Astatotilapia elegans (Teleostei, Cichlidae). J. Morphol. 208, 323–345. ( 10.1002/jmor.1052080308) [DOI] [PubMed] [Google Scholar]
- 14.Herrel A, Van Wassenbergh S, Aerts P. 2012. Biomechanical studies of food and diet selection. eLS. ( 10.1002/9780470015902.a0003213.pub2) [DOI] [Google Scholar]
- 15.Van Wassenbergh S. 2013. Kinematics of terrestrial capture of prey by the eel-catfish Channallabes apus. Integr. Comp. Biol. 53, 258–268. ( 10.1093/icb/ict036) [DOI] [PubMed] [Google Scholar]
- 16.Bramble DM, Wake D. 1985. Feeding mechanisms of lower tetrapods. In Functional vertebrate morphology (eds Hildebrand DMBM, Liem KF, Wake DB.), pp. 230–261. Cambridge, MA: Harvard University Press. [Google Scholar]
- 17.Miller BT, Larsen JH. 1990. Comparative kinematics of terrestrial prey capture in salamanders and newts (Amphibia, Urodela, Salamandridae). J. Exp. Zool. 256, 135–153. ( 10.1002/jez.1402560204) [DOI] [Google Scholar]
- 18.Reilly SM, Lauder GV. 1989. Kinetics of tongue projection in Ambystoma tigrinum—quantitative kinematics, muscle function, and evolutionary hypotheses. J. Morphol. 199, 223–243. ( 10.1002/jmor.1051990208) [DOI] [PubMed] [Google Scholar]
- 19.Markey MJ, Marshall CR. 2007. Terrestrial-style feeding in a very early aquatic tetrapod is supported by evidence from experimental analysis of suture morphology. Proc. Natl Acad. Sci. USA 104, 7134–7138. ( 10.1073/pnas.0701706104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sponder DL, Lauder GV. 1981. Terrestrial feeding in the mudskipper Periophthalmus (Pisces, teleostei)—a cineradiographic analysis. J. Zool. 193, 517–530. ( 10.1111/j.1469-7998.1981.tb01501.x) [DOI] [Google Scholar]
- 21.Stebbins RC, Kalk M. 1961. Observations on the natural history of the mud-skipper, Periophthalmus sobrinus. Copeia 1961, 18–27. ( 10.2307/1440166) [DOI] [Google Scholar]
- 22.Clayton David A. 1993. Mudskippers. In Oceanogr. Mar. Biol. Annu. Rev. 31, 507–577. [Google Scholar]
- 23.Drost MR, Vandenboogaart JGM. 1986. A simple method for measuring the changing volume of small biological objects, illustrated by studies of suction feeding by fish larvae and of shrinkage due to histological fixation. J. Zool. 209, 239–249. ( 10.1111/j.1469-7998.1986.tb03579.x) [DOI] [Google Scholar]
- 24.Aerts P, Van Damme J, Herrel A. 2001. Intrinsic mechanics and control of fast cranio-cervical movements in aquatic feeding turtles. Am. Zool. 41, 1299–1310. ( 10.1668/0003-1569(2001)041[1299:IMACOF]2.0.CO;2) [DOI] [Google Scholar]
- 25.Liem KF. 1991. Functional morphology—hydrodynamic tongue. In Cichlid fishes: behaviour ecology and evolution (ed. Keenleyside MHA.), pp. 143–144. New York, NY: Chapman & Hall. [Google Scholar]
- 26.Lauder GV. 1980. Evolution of the feeding mechanism in primitive actionopterygian fishes: a functional anatomical analysis of Polypterus, Lepisosteus, and Amia. J. Morphol. 163, 283–317. ( 10.1002/jmor.1051630305) [DOI] [PubMed] [Google Scholar]
- 27.Van Wassenbergh S, Herrel A, Adriaens D, Huysentruyt F, Devaere S, Aerts P. 2006. Evolution: a catfish that can strike its prey on land. Nature 440, 881 ( 10.1038/440881a) [DOI] [PubMed] [Google Scholar]
- 28.Stayton CT. 2011. Terrestrial feeding in aquatic turtles: environment-dependent feeding behavior modulation and the evolution of terrestrial feeding in Emydidae. J. Exp. Biol. 214, 4083–4091. ( 10.1242/jeb.060574) [DOI] [PubMed] [Google Scholar]
- 29.Measey GJ. 1998. Terrestrial prey capture in Xenopus laevis. Copeia 1998, 787–791. ( 10.2307/1447816) [DOI] [Google Scholar]
- 30.Lauder G. 1985. Aquatic feeding in lower vertebrates. In Functional vertebrate morphology (eds Hildebrand DMBM, Liem KF, Wake D.), pp. 210–229. Cambridge, MA: Harvard University Press. [Google Scholar]
- 31.Hernandez LP, Barresi MJ, Devoto SH. 2002. Functional morphology and developmental biology of zebrafish: reciprocal illumination from an unlikely couple. Integr. Comp. Biol. 42, 222–231. ( 10.1093/icb/42.2.222) [DOI] [PubMed] [Google Scholar]
- 32.Sanford CPJ, Wainwright PC. 2002. Use of sonomicrometry demonstrates the link between prey capture kinematics and suction pressure in largemouth bass. J. Exp. Biol. 205, 3445–3457. [DOI] [PubMed] [Google Scholar]
- 33.Schoch RR. 2001. Can metamorphosis be recognised in Palaeozoic amphibians? Neues Jahrb. Geol. Palaontol. Abh. 220, 335–367. [Google Scholar]
- 34.Reilly S. 1996. The metamorphosis of feeding kinematics in Salamandra salamandra and the evolution of terrestrial feeding behavior. J. Exp. Biol. 199, 1219–1227. [DOI] [PubMed] [Google Scholar]
- 35.Downs JP, Daeschler EB, Jenkins FA, Jr, Shubin NH. 2008. The cranial endoskeleton of Tiktaalik roseae. Nature 455, 925–929. ( 10.1038/nature07189) [DOI] [PubMed] [Google Scholar]
- 36.Bemis WE, Lauder GV. 1986. Morphology and function of the feeding apparatus of the lungfish, Lepidosiren paradoxa (Dipnoi). J. Morphol. 187, 81–108. ( 10.1002/jmor.1051870108) [DOI] [PubMed] [Google Scholar]
- 37.Reilly SM, Lauder GV. 1990. The evolution of tetrapod feeding-behavior—kinematic homologies in prey transport. Evolution 44, 1542–1557. ( 10.2307/2409336) [DOI] [PubMed] [Google Scholar]
- 38.Gillis G, Lauder G. 1995. Kinematics of feeding in bluegill sunfish: is there a general distinction between aquatic capture and transport behaviors? J. Exp. Biol. 198, 709–720. [DOI] [PubMed] [Google Scholar]
- 39.Reilly S. 1995. The ontogeny of aquatic feeding behavior in Salamandra salamandra: stereotypy and isometry in feeding kinematics. J. Exp. Biol. 198, 701–708. [DOI] [PubMed] [Google Scholar]
- 40.Cleuren J, Devree F. 1992. Kinematics of the jaw and hyolingual apparatus during feeding in Caiman crocodilus. J. Morphol. 212, 141–154. ( 10.1002/jmor.1052120205) [DOI] [PubMed] [Google Scholar]
- 41.Elias JA, McBrayer LD, Reilly SM. 2000. Prey transport kinematics in Tupinambis teguixin and Varanus exanthematicus: conservation of feeding behavior in ‘chemosensory-tongued’ lizards. J. Exp. Biol. 203, 791–801. [DOI] [PubMed] [Google Scholar]
- 42.Ahlberg PE, Clack JA, Blom H. 2005. The axial skeleton of the Devonian tetrapod Ichthyostega. Nature 437, 137–140. ( 10.1038/nature03893) [DOI] [PubMed] [Google Scholar]
- 43.Nishikawa K, Schwenk K. 2001. Ingestion in reptiles and amphibians. eLS. ( 10.1038/npg.els.0001835) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.