Abstract
The immature retinas of preterm neonates are susceptible to insults that disrupt neurovascular growth, leading to retinopathy of prematurity. Suppression of growth factors due to hyperoxia and loss of the maternal–fetal interaction result in an arrest of retinal vascularisation (phase 1). Subsequently, the increasingly metabolically active, yet poorly vascularised, retina becomes hypoxic, stimulating growth factor-induced vasoproliferation (phase 2), which can cause retinal detachment. In very premature infants, controlled oxygen administration reduces but does not eliminate retinopathy of prematurity. Identification and control of factors that contribute to development of retinopathy of prematurity is essential to prevent progression to severe sight-threatening disease and to limit comorbidities with which the disease shares modifiable risk factors. Strategies to prevent retinopathy of prematurity will depend on optimisation of oxygen saturation, nutrition, and normalisation of concentrations of essential factors such as insulin-like growth factor 1 and ω-3 polyunsaturated fatty acids, as well as curbing of the effects of infection and inflammation to promote normal growth and limit suppression of neurovascular development.
Introduction
In the late 1940s, retinopathy of prematurity appeared suddenly in preterm infants. The disorder, initially called retrolental fibroplasia, was characterised by a complete retinal detachment behind the lens. The cause of this first wave of retinopathy of prematurity was the use of supple mental oxygen in closed incubators, which helped to improve the survival of preterm infants,1 but also contributed to blindness.2
Optimum oxygenation to balance risk of retinopathy of prematurity against improved survival is still unknown. Studies3,4 have compared various oxygen saturation targets, but not actual patient oxygen saturation levels. Low oxygenation targets are associated with increased mortality, but the optimum timing and target concentration of oxygen treatment remain unanswered questions. Oxygen administration is better controlled nowadays than in the past in developed countries, but retinopathy of prematurity persists, partly because of the increased survival of infants with extremely low gestational ages and birthweights4 who are at high risk for the disease. In some developing countries un-monitored treatment with 100% oxygen is still used, which can even cause more mature babies to develop severe retinopathy of prematurity.
Where advanced care in neonatal intensive care units is available, most cases of retinopathy of prematurity occur in extremely low-gestational-age neonates (gestational age of less than 28 weeks at birth). The low concentrations of factors important for development that are normally provided in utero prevent the very immature retinas of extremely preterm infants from vascularising normally, which can precipitate the disease,5–11 possibly with different effects during different developmental stages. Identification of postnatal factors that affect the risk for and the course of retinopathy of prematurity might allow neonatologists and ophthalmologists to attempt to prevent the disease and to limit comorbidities with which it shares modifiable risk factors.
Epidemiology
Worldwide about 10% of births occur preterm (before gestational age 37 full weeks).12 Preterm birth is the most common cause of neonatal death,13 and the second most common cause of death in children younger than 5 years.14
Comparisons of the incidence of retinopathy of prematurity from population-based studies is difficult because of substantial variability in study designs, gestational ages of included infants, survival rates, and treatments used. In a prospective study from Sweden15 in infants with a gestational age of less than 27 weeks at birth, retinopathy of prematurity (at any stage) was reported in 73% (368/506) and severe retinopathy of prematurity was reported in 35% (176/506). In a study in Norway16 of infants with a gestational age of less than 28 weeks at birth, retinopathy of prematurity (at any stage) was reported in 33% (95/290). Investigators of a study in Belgium17 in which infants with a gestational age of less than 27 weeks at birth were included reported severe retinopathy of prematurity in 26% (45/175). A study from Australia and New Zealand18 of infants with a gestational age of less than 29 weeks at birth reported severe retinopathy of prematurity in 10% (203/2105). In a study in Austria,19 severe disease was reported in 16% (50/316) of babies with a gestational age of less than 27 weeks at birth. In a Finnish study20 in infants with birthweights of less than 1000 g, severe retinopathy of prematurity was seen in only 5–10% (no numbers reported).
Search strategy and selection criteria.
We searched PubMed using the terms “retinopathy of prematurity”, “retinal vascular development”, “ROP risk factors”, “omega polyunsaturated fatty acids”, “oxygen”, “VEGF”, “erythropoietin”, “IGF-1”, “postnatal growth”, “inflammation”, and “infection”, in various combinations. We mainly selected articles published in the past 5 years, but also included widely referenced and highly regarded older publications. Relevant articles from the reference lists of those identified by this search strategy and additional references suggested by peer reviewers were also included. Review articles and book chapters are cited to direct readers to further information and additional references.
Thus, prevalence estimates from population-based studies vary even among countries with similar neonatal intensive care facilities. This variation might be partly accounted for by differences in the proportions of infants at high risk of retinopathy of prematurity who survive when born at an early gestational age—in Sweden 11·5% of survivors were born in weeks 22–23,15 compared with 0–6% in the other studies.16–22
An alternative to non-uniform and intermittent data collections in many countries or regions would be occasional snapshots of the burden of severe disease in one geographical area with uniform care.23,24 Sweden now has a register (SWEDROP) for all children screened for retinopathy of prematurity, which is used to measure incidence.25 Taken as a whole, the data do not suggest that incidence has changed substantially over time.7,26–30 Perhaps increased survival of very immature infants at high risk for the disease balanced against improved neonatal intensive care can account for this finding. Incidence can also increase when neonatal care is sufficient to save the babies’ lives but insufficient to prevent disease—eg, through use of uncontrolled oxygen delivery.31,32
Pathogenesis
Retinopathy of prematurity can be viewed as an arrest of normal retinal neuronal and vascular development in the preterm infant, with ultimately pathological compensatory mechanisms that result in aberrant vascularisation of the retina. The more profound the immaturity at birth and the persistence of developmental arrest due to exposure of the retina to harmful factors, coupled with deficiencies of factors normally provided in utero, the more aggressive the later pathological response. The disease has two postnatal phases (figure 1),5,6,11 possibly preceded by a prephase10 of antenatal sensitisation via inflammation (figure 2). Understanding these phases and their causes might allow the identification of the optimum postnatal environment for these immature babies.
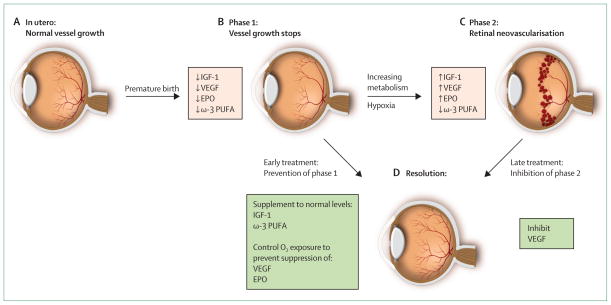
Figure 1. Progression of retinopathy of prematurity.
(A) Oxygen tension is low in utero and vascular growth is normal. (B) Phase 1: after birth until roughly 30 weeks postmenstrual age, retinal vascularisation is inhibited because of hyperoxia and loss of the nutrients and growth factors provided at the maternal–fetal interface. Blood-vessel growth stops and as the retina matures and metabolic demand increases, hypoxia results. (C) Phase 2: the hypoxic retina stimulates expression of the oxygen-regulated factors such as erythropoietin (EPO) and vascular endothelial growth factor (VEGF), which stimulate retinal neovascularisation. Insulin-like growth factor 1 (IGF-1) concentrations increase slowly from low concentrations after preterm birth to concentrations high enough to allow activation of VEGF pathways.
(D) Resolution of retinopathy might be achieved through prevention of phase 1 by increasing IGF-1 to in-utero concentrations and by limiting oxygen to prevent suppression of VEGF; alternatively, VEGF can be suppressed in phase 2 after neovascularisation with laser therapy or an antibody. EPO=erythropoietin. ω-3 PUFA=ω-3 polyunsaturated fatty acids. Adapted from reference 33, by permission of the Association for Research in Vision and Ophthalmology.
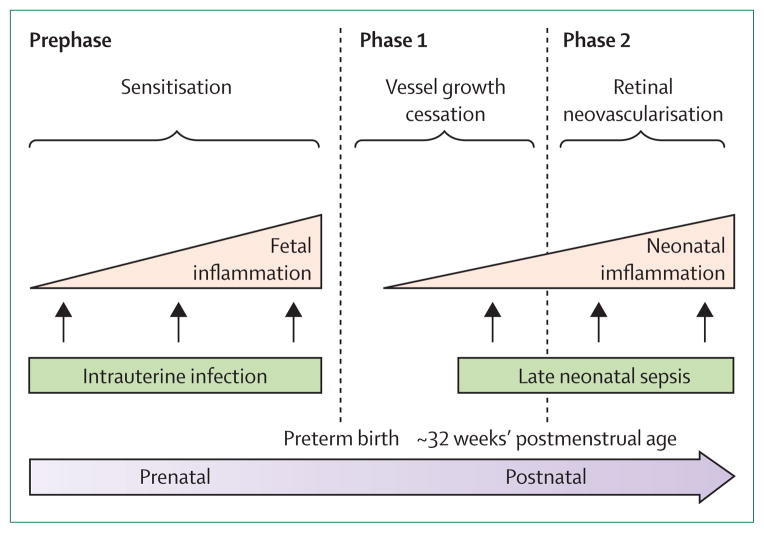
Figure 2. Infection, inflammation, and retinopathy of prematurity.
Exposure to infection and inflammation seems to modify risk of retinopathy of prematurity, especially before (prephase) and a few weeks after birth (phase 2), when oxygen concentrations are relatively low compared with phase 1 (immediately after birth). Whereas prenatal inflammation seems to exert a sensitising effect without directly increasing risk, postnatal infection and inflammation are associated with an increased risk, perhaps most prominently in phase 2. Adapted from reference 10, by permission of Elsevier.
In 1952, Patz and coworkers34 showed in a clinical study the association between administration of very high concentrations of oxygen and retinopathy of prematurity. Ashton35 then established the notion of oxygen toxicity (phase 1) followed by hypoxia-mediated vasoproliferation (phase 2) through work in cats.
In both animal and human studies,11,36,37 hyperoxia is an important driver for the arrest of vascular growth in phase 1. Even room air can lead to hyperoxia compared with the intrauterine environment, where mean oxygen pressure is less than 50 mm Hg during the second half of pregnancy.38 More importantly, supplemental oxygen given to premature infants with respiratory distress can lead to abnormally high oxygen saturation. Hyperoxia leads to suppression of oxygen-regulated angiogenic growth factors, particularly erythropoietin39,40 and vascular endothelial growth factor (VEGF),41 which in turn causes both cessation of retinal vessel growth and loss of some existing retinal vessels42 (a process that has been partly reversed in mice with the replacement of VEGF and erythropoietin).39–42 Some investigators43 speculate that in more mature infants, exposure to high oxygen concentrations causes loss of existing vessels not seen with controlled oxygen delivery, which mainly causes cessation of vessel growth.
Like preterm infants, newborn cats,34 rats,44 and mice36 have incomplete retinal vascularisation at birth and oxygen can be used to induce retinal vessel loss. However, unlike premature infants, the developmental stage at delivery of these animals is appropriate for their species. In human infants born before completion of the third trimester of pregnancy, factors such as insulin-like growth factor 1 (IGF-1),45 normally present at optimum concentrations in utero, are missing, which can also contribute to arrest of vascular growth. IGF-1 is crucial for normal growth and development of many tissues, including brain and blood vessels. Moreover, the loss of maternally-provided ω long-chain polyunsaturated fatty acids seems to have a role in pathogenesis of retinopathy of prematurity (figure 1).46,47
In severe disease, phase 2 begins when the increasingly metabolically active yet poorly vascularised retina (caused by suppression of vessel growth in phase 1) becomes hypoxic. Phase 2 is characterised by proliferation of blood vessels largely in response to hypoxia-driven increases in VEGF and erythropoietin.48,49 The new vessels poorly perfuse the retina and are leaky, which leads to fibrous scar formation and retinal detachment. In most infants retinopathy of prematurity regresses spontaneously and the retina vascularises fairly normally, although neural deficits (loss of photoreceptor function) can remain even in mild cases.50
The transition between phase 1 and 2 seems to depend on the postmenstrual age of the infant rather than the postnatal age. In a study of infants with birthweights of less than 1251 g, disease onset began at roughly 30 weeks’ postmenstrual age and peaked at 36–38 weeks’ post menstrual age, irrespective of gestational age at birth. This important finding suggests that onset of retinopathy of prematurity corresponds more closely with post menstrual age than with postnatal age,51 which points to an association between programmed timing of development and disease pathogenesis. However, this association might not be evident in extreme prematurity. In a study of infants with gestational ages at birth from 22 weeks to 26 weeks and 6 days, the onset of retinal vascular events corresponded more closely with postnatal age (mean range 8·6–9·6 weeks) than with postmenstrual age, which suggests that extreme prematurity confers additional risk for the development of retinopathy of prematurity since vascular events occurred earlier in more immature infants.52
The timing of the phases of retinopathy of prematurity can be also be modified by exposure to very high concentrations of oxygen. In one study,43 even relatively mature preterm infants (gestational age at birth of 31·7 weeks [range 28–35 weeks]) lost retinal vessels (phase 1) and progressed to severe zone-1 neovascularisation (phase 2) when exposed to 100% oxygen after birth.
In some cases factors that cause preterm birth might also affect intrauterine retinal neurovascular development. Antenatal factors such as placental infection and inflammation53 might predispose the fetal retina to severe retinopathy of prematurity, and such a sensitisation effect might constitute a prephase of the disease.10
Risk factors
Oxygen
The question of the correct balance between high oxygen supplementation in the early postnatal period to prevent death and lower oxygen to prevent vessel loss in phase 1 of retinopathy of prematurity remains unsettled, and remains crucially important in neonatology. After the first wave of retinopathy of prematurity, when the use of 100% oxygen made even some mature preterm babies blind, oxygen was restricted to 50% of inspired O2, which resulted in about 16 deaths per case of blindness prevented.54 What constitutes the best oxygen saturation at different gestational ages and in each phase of disease is unknown, although hyperoxia can have different effects during phase 2 (when vascular proliferation is taking place) than it does in phase 1.
Oxygen in phases 1 and 2
Several observational studies55–57 have investigated oxygen saturation (as measured by pulse oximetry, SpO2) during phase 1 of retinopathy of prematurity with respect to progression to severe disease vs morbidity and mortality, although none is definitive with respect to this balance. Tin and colleagues55 reported that in babies with a gestational age at birth of less than 28 weeks, those with an oxygen saturation target of 88–98% for the first 8 weeks of life needed treatment for retinopathy of prematurity four times as often as those with a target of 70–90%. No differences in survival or in incidence of cerebral palsy were seen. In a US national survey,56 infants with a birthweight of less than 1500 g who had a maximum SpO2 greater than 98% in the first 2 postnatal weeks had severe retinopathy in 5·5% of cases, compared with 3·0% in those with a maximum SpO2 of 98% or less (p<0·05). With SpO2 targets after age 2 weeks of greater than 92%, 3·3 % of infants needed treatment for retinopathy of prematurity, compared with 1·3% when the target SpO2 was 92% or less (p< 0·0001). Stage 3 or higher disease was seen in 5·5% of cases from among the infants with the higher saturation target, and in 2·4% of cases from among those with the lower target (p<0·0005). In a study of 1544 infants who weighed less than 1000 g at birth, Sun57 reported that compared with those who had target SpO2 greater than 95%, those with target SpO2 of 95% or less had less stage 3 retinopathy of prematurity (10% vs 29%), less retinal surgery (4% vs 12%), less chronic lung disease (27% vs 53%), and similar mortality (17% vs 24%).
SpO2 target ranges of 85–89% and 91–95% have been compared in two large, multicentre, double-blind, randomised controlled studies. The SUPPORT trial3,4 included 1316 infants born at a gestational age of 24 weeks to 27 weeks and six days. Compared with those in the high-oxygen-target group, infants in the low-oxygen-target group had slightly increased mortality (20% vs 16%, p=0·04) and a significantly smaller proportion had severe retinopathy of prematurity (9% vs 18%, p<0·001). A joint safety analysis4 of survival at 36 weeks’ postmenstrual age of 2315 infants from the BOOST-II trial and the 1316 infants from the SUPPORT trial showed reduced survival with the lower SpO2 target range compared with the higher target range, with mortalities of 17% and 14%, respectively (p=0·015). For the 1055 infants in the UK and Australian components of the BOOST-II trial who were managed after a change of calibration algorithm, mortality differences between the treatment groups were greater (22% vs 13%, p<0·001), which led to recruitment being stopped.4 A collaborative meta-analysis (NeOProM), which includes data for infants from the SUPPORT, BOOST-II Australia, BOOST New Zealand, BOOST-II UK, and COT trials, is due to be reported soon.58
Theoretically, oxygen in phase 2 of retinopathy of prematurity could suppress high concentrations of oxygen-regulated growth factors such as VEGF that cause proliferative disease. Several studies have examined this premise. Investigators of the STOP-ROP study59 reported no change in progression of prethreshold retinopathy of prematurity to proliferative disease after increasing oxygen saturation to 96–99% from the conventional 89–94% for at least 2 weeks. However, increased target oxygen saturation was associated with increased pulmonary complications. The BOOST study60 was a controlled trial in Australia of 358 babies with gestational ages of less than 30 weeks who were dependent on oxygen supplementation at 32 weeks, in which the effect of different oxygen targets during phase 2 of retinopathy of prematurity was investigated. Comparing SpO2 target ranges of 91–94% and 95–98%, the investigators reported no differences in development at 12 months or in numbers of infants with different severities of disease.
Individually, these studies did not conclusively show a benefit from high oxygen saturation in phase 2. In a meta-analysis of ten studies, Chen and colleagues61 showed that the need for oxygen is different at different developmental stages and phases of retinopathy of prematurity; low oxygen saturation (70–96%) in the first few postnatal weeks and high oxygen saturation (94–99%) at postmenstrual ages of 32 weeks or older were both associated with decreased risk for progression to severe retinopathy of prematurity.
Fluctuations in oxygen concentrations during the first few weeks of life are also associated with risk of retinopathy of prematurity.44,62–66 Additionally, high incidence of intermittent hypoxia during the first 8 weeks of life is associated with later severe disease.67,68
Although no individual study has been conclusive as to the best SpO2 target, targets should be different in different stages of development and in the different phases of retinopathy of prematurity. Strict management of oxygen to minimise alternating hypoxia and hyperoxia and avoidance of undesired high oxygen saturations in phase 1 seem to be the most promising strategies to prevent retinopathy of prematurity, although this outcome has to be balanced against the effect on other morbidities such as cerebral palsy and death.69,70
Gestational age and birthweight
Low gestational age and low birthweight for gestational age are major risk factors for retinopathy of prematurity.18 Both factors are related to the extent of immaturity of retinal neural and vascular development at birth, and therefore the retinal vulnerability to insult. Furthermore, the lower the gestational age and birthweight, the more profound the loss of factors normally provided by the intrauterine environment for which the immature fetus is unable to take over production. Additionally, low gestational age increases the duration of an infant’s exposure to adverse postnatal insults, contributing to the risk of retinopathy of prematurity.
When very preterm infants are born with a weight appropriate for gestational age, birthweight is likely to be a proxy for gestational age and not an independent risk factor.71 If growth restriction occurs in utero, a child will be born small for gestational age. Results of several studies have suggested that being small for gestational age at birth is associated with an increased risk for retinopathy of prematurity.18,72–75 Other findings71,76 suggest that being born small for gestational age increases the risk for retinopathy of prematurity only in older infants (ie, older than 29 weeks gestational age at birth),45,51 although one report77 showed an increased risk only among those with a gestational age at birth of less than 30 weeks, and not among those born at 31–32 weeks. Further work is needed to clarify whether growth restriction in utero contributes to risk for retinopathy of prematurity. Further work is needed to clarify whether growth restriction in utero contributes to risk for retinopathy of prematurity.
Gestational age, birthweight, and intrauterine exposure to adverse factors are fixed at birth. Therefore, identification of modifiable postnatal factors that affect retinopathy of prematurity is crucial, not only to assess continuing risk, but also to allow to normalise concentrations of important factors with respect to their concentration in utero, which might allow the immature retina to vascularise. Cessation of normal vascularisation is the precipitating event in retinopathy of prematurity.
IGF-1 and postnatal weight gain
In babies born preterm, a strong association exists between early-postnatal low serum IGF-1 concentrations and later retinopathy of prematurity and other prematurity-related morbidities.45 In utero, plasma IGF-1 increases with gestational age, particularly during the third trimester of pregnancy, and decreases after preterm birth78,79 with the loss of the maternal–fetal interaction.80 Most infants born before gestational age 33 weeks have a very slow increase in IGF-1 production after birth until about 44 weeks postmenstrual age, as the preterm infant matures. Full-term infants by comparison have a rapid increase in serum IGF-1 postnatally.80 Postnatal IGF-1 concentrations are nutrition dependent in older preterm infants and are reduced with starvation, infection, and stress.81,82
Low IGF-1 is associated with poor retinal vascular growth in IGF-1-deficient mice, which suggests that low IGF-1 might contribute to suppression of vascular growth in retinopathy of prematurity.83 IGF-1 acts as a permissive factor for VEGF-dependent vascular endothelial cell growth,83–85 and IGFBP3, the major IGF-1-binding protein found in serum, also improves vessel survival in a mouse model of oxygen-induced retinopathy. Importantly, IGFBP3 concentrations are diminished in infants with retinopathy of prematurity.86 In preterm infants, low IGF-1 serum concentrations, as well as directly corresponding with the severity of retinopathy of prematurity, are also associated with poor brain growth as measured by head circumference.87,88
IGF-1 is also strongly associated with postnatal weight gain of preterm children.45 The importance of post-natal weight gain in the development of retinopathy of prematurity was shown in the 1950s in mice89 and was shown in human infants in a clinical study45 in 2003. Low serum IGF-1 after preterm birth associated with poor postnatal growth and retinopathy of prematurity is also affected by immaturity, increased metabolic rate, insufficient nutrition, and concomitant illness, which could result in a vicious circle whereby nutrition is poorly assimilated and both general and vascular growth are impaired during the first few weeks of life.
Hyperglycaemia, insulin, and nutrition
Raised neonatal glucose concentrations also increase the risk for retinopathy of prematurity.90–93 In a study94 of 372 infants born at a gestational age of less than 30 weeks, increased nutrition alone (without IGF-1 supplementation) caused hyperglycaemia, which required increased insulin use. Both hyperglycaemia and insulin use were associated with an increase in both severe (from 4% to 9%) and milder forms of retinopathy of prematurity. These findings emphasise the importance of an integrated approach to prevention.
Increasing nutrition alone does not affect weight gain (normalised for gestational age) or IGF-1 concentrations in extremely low-birthweight infants, who seem unable either to increase IGF-1 concentrations with increased calories or to use calories for growth with low IGF-1 concentrations.95 Exogenous IGF-1 can improve growth in states of undernutrition. In rats fed half of needed calories, exogenous IGF-1 improved weight gain.96 Since postnatal weight gain predicts risk of retinopathy of prematurity,97–99 both increased nutrition and adequate IGF-1 concentrations seem to be necessary for postnatal growth and for a reduction in risk.
Additional attention should also be paid to nutrition components such as adequate protein, appropriate fats, and appropriate use of glucose and other carbohydrates. In animal studies, absence of ω-3 long-chain poly-unsaturated fatty acids increases risk of retinopathy.46 Since total parenteral nutrition rarely contains such fatty acids, provision of this lipid is likely to be beneficial.47 In a study100 of 1706 preterm infants in North America, those with extended use of total parenteral nutrition were at increased risk for the disease, independent of weight gain.
Other risk factors
Neonatal infections, particularly fungal infections,101,102 are also risk factors for retinopathy of prematurity. Late, but not early, neonatal bacteraemia is associated with severe retinopathy of prematurity in extremely low-gestational-age neonates.103 The increased risk associated with infection might be partly due to systemic inflammation,10,104–106 which could act synergistically with hyperoxia107 to mediate the effects of placenta infection.53 Blood transfusions have also been suggested as a possible risk factor for retinopathy of prematurity,108,109 but investigators of the only clinical trial that assessed this question reported no evidence of a link.110
Genetic factors might also affect risk for retinopathy of prematurity. The disease occurs more often in white than in black infants and in boys than in girls.111 Genetic polymorphisms might change gene function, which could affect the disease; however, no genetic factor identified thus far accounts for a substantial number of patients with the disease. Future studies that make use of genomics and proteomics could be helpful in identification of relevant genetic factors.112
Comorbidities
Retinopathy of prematurity could be a window into the state of postnatal development in the premature infant. It often occurs in conjunction with other neonatal morbidities such as neurological dysfunction, poor brain growth, necrotising enterocolitis, intraventricular haemorrhage, and bronchopulmonary dysplasia.113 In extremely preterm infants, severe retinopathy of prematurity predicts risk of death or major disability at age 11 years.114 Therefore addressing poor postnatal growth, hyperoxia, and infection and inflammation to reduce risk of retinopathy of prematurity might also reduce the risk of these comorbidities. Since the retina is part of the CNS, reduction of risk factors that affect postnatal retinal development might also have a positive effect on brain development.
Classification and screening
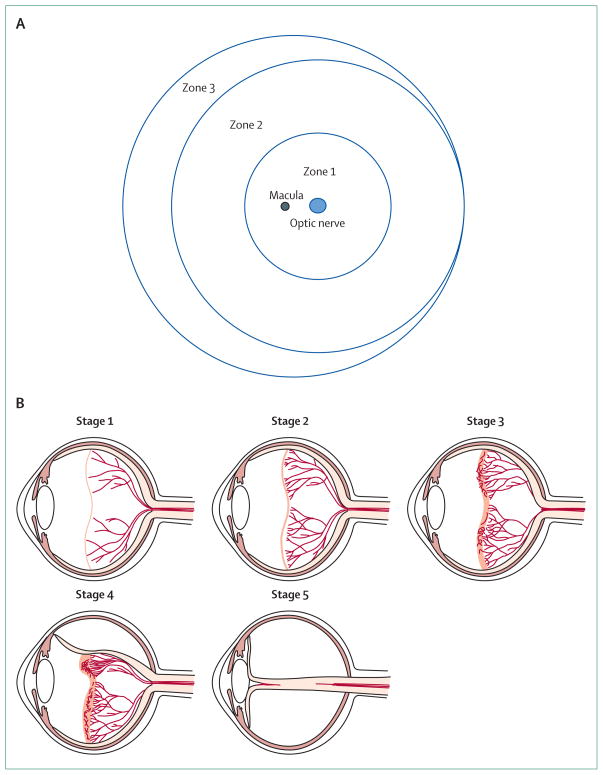
Classification of the stages of retinopathy of prematurity is necessary for the standardisation of treatment practices, and so that interventions can be assessed at a defined stage when progression to blindness is likely. Recommendations are summarised in the International Classification of Retinopathy of Prematurity, first published in 1985113 and revised in 2005.115 The retina is divided into three zones and the extent or severity of disease in these zones is classified as stages (figure 3).116 Stages 1 and 2 are mild and likely to regress spontaneously. In stage 3, extraretinal neovascularisation can become severe enough to cause total retinal detachment (stage 5), which leads to blindness. The presence of increased dilation and tortuosity of posterior vessels (so-called plus disease, figure 4) is an ominous sign of progressive disease. The investigators of the Early Treatment for Retinopathy Of Prematurity (ETROP) study117 reclassified retinopathy of prematurity into type 2 (to be followed up) and type 1 (requires treatment). Type 1 now includes a more virulent form of retinopathy in extremely low-birthweight babies (aggressive posterior retinopathy of prematurity), which involves very central neovascularisation with plus disease.116 Screening guidelines vary with the characteristics of the premature population and neonatal intensive care practices in different settings.
Figure 3. Zones and stages of retinopathy of prematurity.
The retina is divided into three zones (A, diagram shows right eye) and the extent or severity of retinopathy in these zones is classified as stages (B). Stage 1 is characterised by a thin demarcation line between vascularised and non-vascularised retina, stage 2 by a ridge, stage 3 by extraretinal fibrovascular proliferation, stage 4 by part retinal detachment, and stage 5 by total retinal detachment. In stage 3, extraretinal neovascularisation can become severe enough to cause retinal detachment (stages 4–5), which usually leads to blindness. Part B is courtesy of Lisa Hård.
Figure 4. Plus disease.
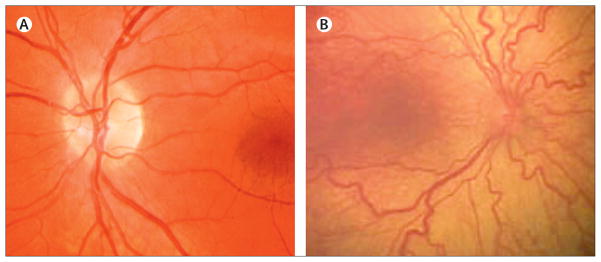
Compared with a normal retina (A), plus disease (B) is characterised by venous dilation and increased arterial tortuosity of posterior vessels. Reproduced from reference 116, by permission of the American Medical Association.
Screening cutoffs range from 30 to 35 weeks’ gestational age at birth and from birthweights of 1500 to 2000 g, and depend on the extent and quality of neonatal intensive care available. Unfortunately, in some parts of the world (eg, some developing countries) screening guidelines do not exist. Guidelines should evolve according to changes in the local preterm population at risk; although screening in the USA and Canada was previously recommended for babies with a gestational age at birth of less than 32 weeks, in a study100 of 2000 preterm infants we noted severe retinopathy of prematurity only in infants born before 29 weeks’ gestational age. US guidelines have been changed and now recommend screening for infants with birthweights of 1500 g or less, or gestational age at birth of 30 weeks or less, as well as more mature infants who had a more unstable clinical course after delivery.118 In Sweden guidelines were adjusted in 2012 to set the screening cutoff for gestational age at birth to 31 weeks instead of 32 weeks.25
To identify all infants who would benefit from treatment, repeated dilated eye examinations are done until the retina is fully vascularised. Eye examination for retinopathy of prematurity can be very painful for preterm infants, even when done by a skilled ophthalmologist.119 In the context of high-quality neonatal intensive care, with existing criteria only about 5–10% of infants120 screened will need treatment. Safely decreasing the number of stressful and costly screening examinations would be beneficial.
To address this issue, Hellström and colleagues121,122 developed an algorithm, WINROP, to identify early after birth infants at high and low risk of development of severe retinopathy of prematurity. Initially, changes in postnatal factors—IGF-1 and weight gain—were used to predict risk for severe retinopathy of prematurity. However, with only serial weight measurements (once per week from birth to 32 weeks postmenstrual age), WINROP also identified early all 35 of the 353 infants in a study98 who later developed proliferative retinopathy of prematurity that required treatment and 76% (268/353) of those who did not develop proliferative disease. A multicentre study100 of about 2000 preterm infants in the USA and Canada substantiated the high sensitivity (98·6%) and negative predictive value (99·7%) of the algorithm, which suggests that the number of screening examinations can be substantially reduced if WINROP is used in combination with traditional screening. That WINROP identified infants at risk for severe retinopathy of prematurity an average of 3 weeks (and as early as 1 week) after preterm birth when early weight gain was poor suggests the importance of early growth in the preterm child.
Currently, WINROP has been used for more than 10 000 babies in neonatal intensive care units in Sweden, the USA, Canada, Brazil, Switzerland, and Mexico. Its variable specificity and poor positive predictive value in studies (table)98,100,121–126 suggest that the algorithm should be used in addition to conventional screening guidelines. WINROP does not currently include infants with a gestational age at birth of more than 32 weeks. This limitation excludes more mature babies who are at risk for retinopathy of prematurity in developing countries, where a reduction in the conventional screening burden would be beneficial. Modification of WINROP to include these more mature infants would be valuable. Other groups have developed similar prediction methods based on postnatal weight gain.127–129 Telemedicine might further improve screening for retinopathy of prematurity by reducing the need for skilled ophthalmologists in every neonatal intensive care unit, through the centralisation of readings.130
Table 1.
Assessments of WINROP algorithm for prediction of severe retinopathy of prematurity
| n | Number of centres | NICU level* | Years | Cohort definition | Alarm definition | Definition of severe ROP | Prevalence of severe ROP | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hellström et al,98 2009 | 353 | 1 | 3 | 2004–07 | GA <32 weeks | High risk or low risk at <32 weeks’ PMA | Stage 3 | 10% | 100 | 84 | 41 | 100 |
| Flückiger et al,123 2011 | 376 | 1 | 3 | 2003–08 | GA <32 weeks or birthweight <1500 g | High risk or low risk at <32 weeks’ PMA | Stage 3 or threshold | 3% | 90 | 63 | 6 | 99 |
| Zepeda-Romero et al,124 2012 | 192 | 1 | 1 | 2005–10 | GA <32 weeks | High-risk or low risk at <33 weeks’ PMA | Type 1 | 51% | 85 | 27 | 54 | 53 |
| Löfqvist et al,121 2006 | 79 | 2 | 3 | 1999–2002 | GA <32 weeks | ·· | Stage 3 or treated ROP | 16% | 100 | 84 | ·· | ·· |
| Löfqvist et al,125 2009 | 50 | 1 | 3 | 2005–07 | GA <31 weeks | High risk at <32 weeks’ PMA | Type 1 | 18% | 100 | 54 | 41 | ·· |
| Hård et al,126 2010 | 366 | 1 | 3 | 2002–08 | GA <32 weeks | High risk or low risk at <32 weeks’ PMA | Stage 3 | 6% | 91 | 55 | 11 | 99 |
| Wu et al,122 2010 | 318 | 1 | 3 | 2005–08 | GA <32 weeks | High risk | Prethreshold or threshold and stage 3 | 9% | 100 | 82 | 35 | 100 |
| Wu et al,100 2012 | 1706 | 10 | 3 | 2006–09 | GA <32 weeks | High risk or low risk at <32 weeks’ PMA | Type 1 | 9% | 99 | 39 | 13 | 100 |
NICU=neonatal intensive care unit. ROP=retinopathy of prematurity. PPV=positive predictive value. NPV=negative predictive value. GA=gestational age. PMA=post-menstrual age.
NICU levels are 1 (special care), 2 (high-dependency care), and 3 (intensive care).
Treatment
Cryotherapy emerged in the 1980s as the method used on the first widely studied intervention—ablation of non-vascularised retina—that reduced structural and functional disease associated with retinopathy of prematurity.131 In the CRYO-ROP study,132 preterm infants were treated at the point of progression of retinopathy of prematurity (a subcategory of stage 3), at which time retinal neovascularisation was equally likely to progress to retinal detachment (high risk for blindness) or to regress. This point in disease progression was defined as the threshold. One eye was selected at random to be treated with cryotherapy, “while the other eye would run its natural course and serve as a control”.132 Treatment reduced blindness by 17% at age 10 years (70/227 in the treated group were blind, compared with 106/222 in the non-treated group).133
After CRYO-ROP, many ophthalmologists believed that earlier treatment (before threshold) might benefit some extremely premature infants with severe retinopathy of prematurity. A computer-based algorithm, RM-ROP,134 which included more detailed risk criteria than were used in CRYO-ROP, was used to estimate new criteria for earlier treatment. The results of the sub sequent ETROP study135 to test this hypothesis showed that blindness could be further reduced with earlier treatment in some patients, particularly those with aggressive posterior retinopathy of prematurity. The CRYO-ROP treatment criteria have now been replaced with the ETROP designations of type 1 retinopathy of prematurity (requires treatment) and type 2 (to be followed up). Treatment of type 1 disease with retinal ablation is intended to minimise unnecessary retinal destruction in cases that would regress spontaneously and to maximise the number of cases in which treatment prevents progression to retinal detachment.136 The designation of type 1 disease relies on assessment of plus disease. Despite widely accepted guidelines and diagnostic criteria for identification of plus disease, large interobserver differences exist,137 although this situation might be improved with computer-based image analysis of the retina.130,138–141 However, vessel calibre might be changed by non-adverted compression by the camera lens.142
Transpupillary laser treatment to ablate non-vascularised retina117 has effectively replaced cryotherapy, because of better visual outcomes and fewer adverse effects such as systemic complications. Treatment of retinopathy of prematurity can also include other modalities. Some reports143 suggest that with early retinal detachment (stage 4), lens-sparing vitrectomy might help to preserve vision.
Long-term outcomes
Much of our knowledge about outcomes in children with retinopathy of prematurity comes from the CRYO-ROP132,133,144–146 and ETROP117,135,147,148 studies. Severe retinopathy of prematurity often leads to long-term visual loss, with blindness in the most severe cases.149 Without treatment, most non-proliferative retinopathy of prematurity regresses, but even non-proliferative disease is associated with visual deficits,150 since preterm birth itself has lasting effects on the developing visual system. Retinopathy of prematurity is also associated with other eye problems. Infants treated with transpupillary laser for severe retinopathy of prematurity have an increased risk of myopia (up to 70% of such infants are affected).151 Preterm birth is a risk factor for hyperopia and astigmatism.152 80% of children with a history of severe retinopathy of prematurity develop strabismus during the first 6 years of life.153
At age 8 years, a third of children with threshold retinopathy of prematurity from the CRYO-ROP study needed special education and almost half had lower-than-grade-level academic performance.145 Some of the academic problems might be due to neurological deficits, which are strongly associated with retinopathy of prematurity.154 At age 10 years, the proportion of eyes with good acuity (20/40 or better) was similar in the treated and untreated groups (roughly 25%), but fewer treated eyes were blind compared with untreated controls (33% vs 50%).133
For children from the ETROP study, at age 6 years poor visual acuity was equally likely in treated and untreated eyes. A subgroup analysis suggested a benefit from earlier (before threshold) compared with later (at threshold) treatment in more severe cases (type 1 disease; 16% vs 25%; p=0·004), but not in milder cases (type 2 disease; 21% vs 16%; p=0·29).147
Candidate interventions for prevention and treatment
Ablative treatment of non-vascularised retina when the risk of retinal detachment is substantial helps to prevent blindness, but does not address the underlying cause of retinopathy of prematurity or other comorbidities, which is the failure of normal neural and vascular growth. Furthermore, peripheral retina is destroyed to save central vision. Addressing the postnatal risk factors for retinopathy of prematurity might help to normalise postnatal growth and reduce risk.
Increasing nutrition alone seems to be insufficient to increase IGF-1 and promote postnatal weight gain in the early postnatal period in the most immature babies,95 and insufficient to decrease risk of retinopathy of prematurity.155 Instead, hyperglycaemia and insulin requirement are raised, both of which are associated with an increased risk.94 Since persistently low serum IGF-1 concentrations in preterm infants are associated with retinopathy of prematurity and other morbidities,45,156,157 and since supplemental IGF-1 seems to improve food utilisation in undernutrition,96 supplementation with exogenous IGF-1 might improve early postnatal growth and outcome. A pharmacokinetic and dosing study158 of intravenous IGF-1–IGFBP3 complex in preterm infants showed no adverse effects and an increase in serum IGF-1 to in-utero concentrations. An IGF-1–IGFBP3 replacement trial (NCT01096784) is now underway, with reduction in the severity of retinopathy of prematurity as the primary endpoint and brain growth and other complications of premature birth as secondary endpoints.
During the third trimester of pregnancy, a massive transfer of essential fatty acids (ω-3 and ω-6 long-chain polyunsaturated fatty acids) from the mother to the fetus takes place; these essential fatty acids (especially ω-3 fatty acids) are often not provided after preterm birth.159 In a study in mice, adequate ω-3 long-chain polyunsaturated fatty acids reduced retinopathy by 50%,46 which suggests that replacement of these fatty acids in infants would reduce risk of retinopathy of prematurity.
Suppression of proliferative retinopathy (phase 2) with injection of anti-VEGF antibody has been reported in many small case-series—eg, by Harder and colleagues.160 In a trial161 of 150 infants randomly assigned to receive laser or intravitreal bevacizumab treatment, recurrence was slightly less likely in the bevacizumab group than in laser-treated group at 54 weeks’ postmenstrual age. A significant treatment effect with bevacizumab was seen for retinopathy of prematurity in zone 1, but not zone 2.161 However, visual outcomes and adverse systemic effects were not reported. Bevacizumab injected into the eye leaks into systemic circulation and reduces systemic VEGF concentrations,162 and might suppress systemic vascular growth or have other as-yet-unknown negative effects.163 Additional studies to assess the best choice of anti-VEGF drug, the optimum dose, the pharmacokinetics, and short-term and long-term safety are warranted.164,165
The β blocker propranolol has been proposed as a potential treatment to reduce retinal neovascularisation166 and clinical studies are underway in Israel (NCT01238471) and Italy (NCT01079715). However, investigators of a 2012 study167 report that propranolol does not reduce retinopathy in a mouse model of retinopathy of prematurity. Results of a meta-analysis suggest that the carbohydrate inositol can reduce the number of stage 3 or higher cases of retinopathy of prematurity (two trials, n=262, typical relative risk 0·09, 95% CI 0·01–0·67).168 Further studies into inositol are underway (NCT00349726, NCT01030575). Penicillamine has been assessed for the prevention of retinopathy of prematurity, but further clinical studies would be necessary to establish efficacy and safety.169 Antioxidants have also been investigated for the prevention of retinopathy of prematurity; in a study of extremely low-gestational-age neonates treated with recombinant human Cu/Zn super-oxide dismutase (rhSOD), no significant difference was seen between the rhSOD group and the placebo group. However, subgroup analysis suggested that the most immature infants (ie, those with a gestational age of less than 26 weeks at birth) might have benefited from the treatment.170 Finally, for many decades the potential role of vitamin E in retinopathy of prematurity has been the focus of some attention; however, so far no conclusive positive treatment effect has been shown.171
Conclusions
Retinopathy of prematurity continues to be a challenge in neonatology. International standards are needed for postnatal care to minimise risk of the disease, which differs substantially between countries. Although ablation of the non-vascularised retina according to ETROP criteria reduces blindness, many treated patients do not achieve good visual acuity. Prevention by reduction of risk factors that disrupt normal retinal vascularisation is likely to be more effective than late treatment of neovascularisation, not only with respect to vision, but also other comorbidities of premature birth. Careful control of oxygen saturation, normalisation of serum IGF-1 concentrations, provision of adequate nutrition, minimisation of hyperglycaemia and insulin use, normalisation of ω-3 polyunsaturated fatty acid concentrations, and curbing the negative effects of infection and inflammation could promote adequate postnatal growth and improve neural and vascular development of the retina. The coming decade will hopefully see the development of these and other new treatment approaches to prevent the disease and to reduce associated complications of preterm birth.
Acknowledgments
AH received support from the Swedish Medical Research Council (2011-2432), a Swedish Government grant (ALFGB-137491), and VINNOVA (2009-00221). LEHS received support from a Research to Prevent Blindness Senior Investigator Award, US National Institutes of Health grants (NEI EY017017, NEI EY022275, NIH P01 HD18655), and from the Lowy Medical Foundation. OD was supported by a grant from the US National Eye Institute (EY021820), a cooperative agreement with the US National Institute of Neurological Disorders and Stroke (ELGAN, NS040069), and by grants from the European Union (NEUROBID 241778, NEO-CIRC 282533). The authors thank Anna-Lena Hård for her contribution to researching the clinical data for the Seminar.
Footnotes
Contributors
OD wrote the early drafts of the Seminar, cowrote the major revision, and provided figures. AH and LEHS made substantial contributions to the early drafts, cowrote the major revision, and provided figures. All authors approved the final version.
Conflicts of interest
AH previously owned shares in PremaCure Holding, which controlled PremaCure (Uppsala, Sweden), a company that had rights to the WINROP system and held patents and patent applications that covered prevention of retinopathy of prematurity with insulin-like growth factor 1. LEHS and OD declare that they have no conflicts of interest.
References
- 1.Silverman WA. Retrolental fibroplasia: a modern parable. New York: Grune & Stratton; 1980. [Google Scholar]
- 2.Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasias: a clinical approach. Med J Aust. 1951;2:48–50. [PubMed] [Google Scholar]
- 3.Carlo WA, Finer NN, Walsh MC, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–69. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenson B, Brocklehurst P, Tarnow-Mordi W. Increased 36-week survival with high oxygen saturation target in extremely preterm infants. N Engl J Med. 2011;364:1680–82. doi: 10.1056/NEJMc1101319. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–40. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 6.Heidary G, Vanderveen D, Smith LE. Retinopathy of prematurity: current concepts in molecular pathogenesis. Semin Ophthalmol. 2009;24:77–81. doi: 10.1080/08820530902800314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera JC, Sapieha P, Joyal JS, et al. Understanding retinopathy of prematurity: update on pathogenesis. Neonatology. 2011;100:343–53. doi: 10.1159/000330174. [DOI] [PubMed] [Google Scholar]
- 8.Raghuveer TS, Bloom BT. A paradigm shift in the prevention of retinopathy of prematurity. Neonatology. 2011;100:116–29. doi: 10.1159/000322848. [DOI] [PubMed] [Google Scholar]
- 9.Mataftsi A, Dimitrakos SA, Adams GG. Mediators involved in retinopathy of prematurity and emerging therapeutic targets. Early Hum Dev. 2011;87:683–90. doi: 10.1016/j.earlhumdev.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Dammann O. Perinatal infection, inflammation, and retinopathy of prematurity. Semin Fetal Neonatal Med. 2012;17:26–29. doi: 10.1016/j.siny.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367:2515–26. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C GAPPS Review Group. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth. 2010;10 (suppl 1):S1. doi: 10.1186/1471-2393-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Johnson HL, Cousens S, et al. for the Child Health Epidemiology Reference Group of WHO, UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 15.Austeng D, Källen KB, Ewald UW, Jakobsson PG, Holmström GE. Incidence of retinopathy of prematurity in infants born before 27 weeks gestation in Sweden. Arch Ophthalmol. 2009;127:1315–19. doi: 10.1001/archophthalmol.2009.244. [DOI] [PubMed] [Google Scholar]
- 16.Markestad T, Kaaresen PI, Rønnestad A, et al. Early death, morbidity, and need of treatment among extremely premature infants. Pediatrics. 2005;115:1289–98. doi: 10.1542/peds.2004-1482. [DOI] [PubMed] [Google Scholar]
- 17.Allegaert K, de Coen K, Devlieger H for the EpiBel Study group. Threshold retinopathy at threshold of viability: the EpiBel study. Br J Ophthalmol. 2004;88:239–42. doi: 10.1136/bjo.2003.027474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darlow BA, Hutchinson JL, Henderson-Smart DJ, Donoghue DA, Simpson JM, Evans NJ for the Australian and New Zealand Neonatal Network. Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand Neonatal Network. Pediatrics. 2005;115:990–96. doi: 10.1542/peds.2004-1309. [DOI] [PubMed] [Google Scholar]
- 19.Weber C, Weninger M, Klebermass K, et al. Mortality and morbidity in extremely preterm infants (22 to 26 weeks of gestation): Austria 1999–2001. Wien Klin Wochenschr. 2005;117:740–46. doi: 10.1007/s00508-005-0468-y. [DOI] [PubMed] [Google Scholar]
- 20.Tommiska V, Heinonen K, Lehtonen L, et al. No improvement in outcome of nationwide extremely low birth weight infant populations between 1996–1997 and 1999–2000. Pediatrics. 2007;119:29–36. doi: 10.1542/peds.2006-1472. [DOI] [PubMed] [Google Scholar]
- 21.Fledelius HC, Dahl H. Retinopathy of prematurity, a decrease in frequency and severity. Trends over 16 years in a Danish county. Acta Ophthalmol Scand. 2000;78:359–61. doi: 10.1034/j.1600-0420.2000.078003359.x. [DOI] [PubMed] [Google Scholar]
- 22.Lundqvist P, Källen K, Hallström I, Westas LH. Trends in outcomes for very preterm infants in the southern region of Sweden over a 10-year period. Acta Paediatr. 2009;98:648–53. doi: 10.1111/j.1651-2227.2008.01155.x. [DOI] [PubMed] [Google Scholar]
- 23.Haines L, Fielder AR, Scrivener R, Wilkinson AR, Pollock JI for the Royal College of Paediatrics and Child Health, the Royal College of Ophthalmologists and British Association of Perinatal Medicine. Retinopathy of prematurity in the UK I: the organisation of services for screening and treatment. Eye (Lond) 2002;16:33–38. doi: 10.1038/sj.eye.6700030. [DOI] [PubMed] [Google Scholar]
- 24.Haines L, Fielder AR, Baker H, Wilkinson AR. UK population based study of severe retinopathy of prematurity: screening, treatment, and outcome. Arch Dis Child Fetal Neonatal Ed. 2005;90:F240–44. doi: 10.1136/adc.2004.057570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmström GE, Hellström A, Jakobsson PG, Lundgren P, Tornqvist K, Wallin A. Swedish national register for retinopathy of prematurity (SWEDROP) and the evaluation of screening in Sweden. Arch Ophthalmol. 2012;130:1418–24. doi: 10.1001/archophthalmol.2012.2357. [DOI] [PubMed] [Google Scholar]
- 26.Tan SZ, Dhaliwal C, Becher JC, Fleck B. Trends in the incidence of retinopathy of prematurity in Lothian, south-east Scotland, from 1990 to 2009. Arch Dis Child Fetal Neonatal Ed. 2012;97:F310–11. doi: 10.1136/fetalneonatal-2011-301464. [DOI] [PubMed] [Google Scholar]
- 27.Hameed B, Shyamanur K, Kotecha S, et al. Trends in the incidence of severe retinopathy of prematurity in a geographically defined population over a 10-year period. Pediatrics. 2004;113:1653–57. doi: 10.1542/peds.113.6.1653. [DOI] [PubMed] [Google Scholar]
- 28.Lad EM, Nguyen TC, Morton JM, Moshfeghi DM. Retinopathy of prematurity in the United States. Br J Ophthalmol. 2008;92:320–25. doi: 10.1136/bjo.2007.126201. [DOI] [PubMed] [Google Scholar]
- 29.Lad EM, Hernandez-Boussard T, Morton JM, Moshfeghi DM. Incidence of retinopathy of prematurity in the United States: 1997 through 2005. Am J Ophthalmol. 2009;148:451–58. doi: 10.1016/j.ajo.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Gunn DJ, Cartwright DW, Gole GA. Incidence of retinopathy of prematurity in extremely premature infants over an 18-year period. Clin Experiment Ophthalmol. 2012;40:93–99. doi: 10.1111/j.1442-9071.2011.02724.x. [DOI] [PubMed] [Google Scholar]
- 31.Zin AA, Moreira ME, Bunce C, Darlow BA, Gilbert CE. Retinopathy of prematurity in 7 neonatal units in Rio de Janeiro: screening criteria and workload implications. Pediatrics. 2010;126:e410–17. doi: 10.1542/peds.2010-0090. [DOI] [PubMed] [Google Scholar]
- 32.Darlow BA, Zin AA, Beecroft G, Moreira ME, Gilbert CE. Capacity building of nurses providing neonatal care in Rio de Janeiro, Brazil: methods for the POINTS of care project to enhance nursing education and reduce adverse neonatal outcomes. BMC Nurs. 2012;11:3. doi: 10.1186/1472-6955-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith LE. Through the eyes of a child: understanding retinopathy through ROP the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2008;49:5177–82. doi: 10.1167/iovs.08-2584. [DOI] [PubMed] [Google Scholar]
- 34.Patz A, Hoeck LE, De la Cruz E. Studies on the effect of high oxygen administration in retrolental fibroplasia, I: nursery observations. Am J Ophthalmol. 1952;35:1248–53. doi: 10.1016/0002-9394(52)91140-9. [DOI] [PubMed] [Google Scholar]
- 35.Ashton N. Pathological basis of retrolental fibroplasia. Br J Ophthalmol. 1954;38:385–96. doi: 10.1136/bjo.38.7.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–11. [PubMed] [Google Scholar]
- 37.Connor KM, Krah NM, Dennison RJ, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–73. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolaides KH, Economides DL, Soothill PW. Blood gases, pH, and lactate in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol. 1989;161:996–1001. doi: 10.1016/0002-9378(89)90770-9. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Connor KM, Aderman CM, Smith LE. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008;118:526–33. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, Smith LE. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2009;50:1329–35. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA. 1995;92:905–09. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114:1219–28. doi: 10.1001/archopht.1996.01100140419009. [DOI] [PubMed] [Google Scholar]
- 43.Shah PK, Narendran V, Kalpana N. Aggressive posterior retinopathy of prematurity in large preterm babies in South India. Arch Dis Child Fetal Neonatal Ed. 2012;97:F371–75. doi: 10.1136/fetalneonatal-2011-301121. [DOI] [PubMed] [Google Scholar]
- 44.Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Invest Ophthalmol Vis Sci. 1993;34:576–85. [PubMed] [Google Scholar]
- 45.Hellström A, Engström E, Hård AL, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112:1016–20. doi: 10.1542/peds.112.5.1016. [DOI] [PubMed] [Google Scholar]
- 46.Connor KM, Sangiovanni JP, Löfqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pawlik D, Lauterbach R, Turyk E. Fish-oil fat emulsion supplementation may reduce the risk of severe retinopathy in VLBW infants. Pediatrics. 2011;127:223–28. doi: 10.1542/peds.2010-2427. [DOI] [PubMed] [Google Scholar]
- 48.Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA. 1995;92:10457–61. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe D, Suzuma K, Matsui S, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:782–92. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 50.Fulton AB, Hansen RM, Moskowitz A, Akula JD. The neurovascular retina in retinopathy of prematurity. Prog Retin Eye Res. 2009;28:452–82. doi: 10.1016/j.preteyeres.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1991;98:1628–40. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 52.Austeng D, Källen KB, Hellström A, et al. Screening for retinopathy of prematurity in infants born before 27 weeks’ gestation in Sweden. Arch Ophthalmol. 2011;129:167–72. doi: 10.1001/archophthalmol.2010.346. [DOI] [PubMed] [Google Scholar]
- 53.Chen ML, Allred EN, Hecht JL, et al. Placenta microbiology and histology and the risk for severe retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2011;52:7052–58. doi: 10.1167/iovs.11-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bolton DPG, Cross KW. Further observations on cost of preventing retrolental fibroplasia. Lancet. 1974;303:445–48. doi: 10.1016/s0140-6736(74)92395-2. [DOI] [PubMed] [Google Scholar]
- 55.Tin W, Milligan DW, Pennefather P, Hey E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonatal Ed. 2001;84:F106–10. doi: 10.1136/fn.84.2.F106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson CG, Benitz WE, Madan A. Retinopathy of prematurity and pulse oximetry: a national survey of recent practices. J Perinatol. 2004;24:164–68. doi: 10.1038/sj.jp.7211067. [DOI] [PubMed] [Google Scholar]
- 57.Sun SC. Relation of target SpO2 levels and clinical outcome in ELBW infants on supplemental oxygen. Pediatr Res. 2002;51:350A. [Google Scholar]
- 58.Askie LM, Brocklehurst P, Darlow BA, Finer N, Schmidt B, Tarnow-Mordi W. NeOProM: neonatal oxygenation prospective meta-analysis collaboration study protocol. BMC Pediatr. 2011;11:6. doi: 10.1186/1471-2431-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.The STOP-ROP Multicenter Study Group. Supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial I: primary outcomes. Pediatrics. 2000;105:295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- 60.Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM. Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med. 2003;349:959–67. doi: 10.1056/NEJMoa023080. [DOI] [PubMed] [Google Scholar]
- 61.Chen ML, Guo L, Smith LE, Dammann CE, Dammann O. High or low oxygen saturation and severe retinopathy of prematurity: a meta-analysis. Pediatrics. 2010;125:e1483–92. doi: 10.1542/peds.2009-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saito Y, Omoto T, Cho Y, Hatsukawa Y, Fujimura M, Takeuchi T. The progression of retinopathy of prematurity and fluctuation in blood gas tension. Graefes Arch Clin Exp Ophthalmol. 1993;231:151–56. doi: 10.1007/BF00920938. [DOI] [PubMed] [Google Scholar]
- 63.Cunningham S, Fleck BW, Elton RA, McIntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–65. doi: 10.1016/s0140-6736(95)92475-2. [DOI] [PubMed] [Google Scholar]
- 64.York JR, Landers S, Kirby RS, Arbogast PG, Penn JS. Arterial oxygen fluctuation and retinopathy of prematurity in very-low-birth-weight infants. J Perinatol. 2004;24:82–87. doi: 10.1038/sj.jp.7211040. [DOI] [PubMed] [Google Scholar]
- 65.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–31. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 66.Penn JS, Henry MM, Wall PT, Tolman BL. The range of PaO2 variation determines the severity of oxygen-induced retinopathy in newborn rats. Invest Ophthalmol Vis Sci. 1995;36:2063–70. [PubMed] [Google Scholar]
- 67.Di Fiore JM, Bloom JN, Orge F, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. 2010;157:69–73. doi: 10.1016/j.jpeds.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coleman RJ, Beharry KD, Brock RS, Abad-Santos P, Abad-Santos M, Modanlou HD. Effects of brief, clustered versus dispersed hypoxic episodes on systemic and ocular growth factors in a rat model of oxygen-induced retinopathy. Pediatr Res. 2008;64:50–55. doi: 10.1203/PDR.0b013e31817307ac. [DOI] [PubMed] [Google Scholar]
- 69.Chow LC, Wright KW, Sola A. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111:339–45. doi: 10.1542/peds.111.2.339. [DOI] [PubMed] [Google Scholar]
- 70.Castillo A, Deulofeut R, Critz A, Sola A. Prevention of retinopathy of prematurity in preterm infants through changes in clinical practice and SpO2 technology. Acta Paediatr. 2011;100:188–92. doi: 10.1111/j.1651-2227.2010.02001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.The EXPRESS Group. Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS) Acta Paediatr. 2010;99:978–92. doi: 10.1111/j.1651-2227.2010.01846.x. [DOI] [PubMed] [Google Scholar]
- 72.Bardin C, Zelkowitz P, Papageorgiou A. Outcome of small-for-gestational age and appropriate-for-gestational age infants born before 27 weeks of gestation. Pediatrics. 1997;100:E4. doi: 10.1542/peds.100.2.e4. [DOI] [PubMed] [Google Scholar]
- 73.Regev RH, Lusky A, Dolfin T, Litmanovitz I, Arnon S, Reichman B. Excess mortality and morbidity among small-for-gestational-age premature infants: a population-based study. J Pediatr. 2003;143:186–91. doi: 10.1067/S0022-3476(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 74.Allegaert K, Vanhole C, Casteels I, et al. Perinatal growth characteristics and associated risk of developing threshold retinopathy of prematurity. J AAPOS. 2003;7:34–37. doi: 10.1067/mpa.2003.S1091853102420150. [DOI] [PubMed] [Google Scholar]
- 75.Dhaliwal CA, Fleck BW, Wright E, Graham C, McIntosh N. Retinopathy of prematurity in small-for-gestational age infants compared with those of appropriate size for gestational age. Arch Dis Child Fetal Neonatal Ed. 2009;94:F193–95. doi: 10.1136/adc.2008.143552. [DOI] [PubMed] [Google Scholar]
- 76.Fortes Filho JB, Valiatti FB, Eckert GU, Costa MC, Silveira RC, Procianoy RS. Is being small for gestational age a risk factor for retinopathy of prematurity? A study with 345 very low birth weight preterm infants. J Pediatr (Rio J) 2009;85:48–54. doi: 10.2223/JPED.1870. [DOI] [PubMed] [Google Scholar]
- 77.Qiu X, Lodha A, Shah PS, et al. Neonatal outcomes of small for gestational age preterm infants in Canada. Am J Perinatol. 2012;29:87–94. doi: 10.1055/s-0031-1295647. [DOI] [PubMed] [Google Scholar]
- 78.Lassarre C, Hardouin S, Daffos F, Forestier F, Frankenne F, Binoux M. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr Res. 1991;29:219–25. doi: 10.1203/00006450-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 79.Reece EA, Wiznitzer A, Le E, Homko CJ, Behrman H, Spencer EM. The relation between human fetal growth and fetal blood levels of insulin-like growth factors I and II, their binding proteins, and receptors. Obstet Gynecol. 1994;84:88–95. [PubMed] [Google Scholar]
- 80.Lineham JD, Smith RM, Dahlenburg GW, et al. Circulating insulin-like growth factor I levels in newborn premature and full-term infants followed longitudinally. Early Hum Dev. 1986;13:37–46. doi: 10.1016/0378-3782(86)90096-4. [DOI] [PubMed] [Google Scholar]
- 81.Larnkjær A, Mølgaard C, Michaelsen KF. Early nutrition impact on the insulin-like growth factor axis and later health consequences. Curr Opin Clin Nutr Metab Care. 2012;15:285–92. doi: 10.1097/MCO.0b013e328351c472. [DOI] [PubMed] [Google Scholar]
- 82.Demendi C, Börzsönyi B, Nagy ZB, Rigó J, Jr, Pajor A, Joó JG. Gene expression patterns of insulin-like growth factor 1, 2 (IGF-1, IGF-2) and insulin-like growth factor binding protein 3 (IGFBP-3) in human placenta from preterm deliveries: influence of additional factors. Eur J Obstet Gynecol Reprod Biol. 2012;160:40–44. doi: 10.1016/j.ejogrb.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 83.Hellström A, Perruzzi C, Ju M, et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci USA. 2001;98:5804–08. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith LE, Kopchick JJ, Chen W, et al. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science. 1997;276:1706–09. doi: 10.1126/science.276.5319.1706. [DOI] [PubMed] [Google Scholar]
- 85.Smith LE, Shen W, Perruzzi C, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5:1390–95. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- 86.Löfqvist C, Chen J, Connor KM, et al. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci USA. 2007;104:10589–94. doi: 10.1073/pnas.0702031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Löfqvist C, Engström E, Sigurdsson J, et al. Postnatal head growth deficit among premature infants parallels retinopathy of prematurity and insulin-like growth factor-1 deficit. Pediatrics. 2006;117:1930–38. doi: 10.1542/peds.2005-1926. [DOI] [PubMed] [Google Scholar]
- 88.Hansen-Pupp I, Hövel H, Hellström A, et al. Postnatal decrease in circulating insulin-like growth factor-I and low brain volumes in very preterm infants. J Clin Endocrinol Metab. 2011;96:1129–35. doi: 10.1210/jc.2010-2440. [DOI] [PubMed] [Google Scholar]
- 89.Gyllensten LJ, Hellström BE. Experimental approach to the pathogenesis of retrolental fibroplasia III: changes in the eye induced by exposure of newborn mice to general hypoxia. Br J Ophthalmol. 1955;39:409–15. doi: 10.1136/bjo.39.7.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garg R, Agthe AG, Donohue PK, Lehmann CU. Hyperglycemia and retinopathy of prematurity in very low birth weight infants. J Perinatol. 2003;23:186–94. doi: 10.1038/sj.jp.7210879. [DOI] [PubMed] [Google Scholar]
- 91.Ertl T, Gyarmati J, Gaal V, Szabo I. Relationship between hyperglycemia and retinopathy of prematurity in very low birth weight infants. Biol Neonate. 2006;89:56–59. doi: 10.1159/000088199. [DOI] [PubMed] [Google Scholar]
- 92.Blanco CL, Baillargeon JG, Morrison RL, Gong AK. Hyperglycemia in extremely low birth weight infants in a predominantly Hispanic population and related morbidities. J Perinatol. 2006;26:737–41. doi: 10.1038/sj.jp.7211594. [DOI] [PubMed] [Google Scholar]
- 93.Wilson AG, di Giovine FS, Blakemore AI, Duff GW. Single base polymorphism in the human tumour necrosis factor alpha (TNFα) gene detectable by NcoI restriction of PCR product. Hum Mol Genet. 1992;1:353. doi: 10.1093/hmg/1.5.353. [DOI] [PubMed] [Google Scholar]
- 94.Kaempf JW, Kaempf AJ, Wu Y, Stawarz M, Niemeyer J, Grunkemeier G. Hyperglycemia, insulin and slower growth velocity may increase the risk of retinopathy of prematurity. J Perinatol. 2011;31:251–57. doi: 10.1038/jp.2010.152. [DOI] [PubMed] [Google Scholar]
- 95.Hansen-Pupp I, Löfqvist C, Polberger S, et al. Influence of insulin-like growth factor I and nutrition during phases of postnatal growth in very preterm infants. Pediatr Res. 2011;69:448–53. doi: 10.1203/PDR.0b013e3182115000. [DOI] [PubMed] [Google Scholar]
- 96.Fryklund L, Gluckman P, Skottner A. Treatment of catabolic states using authentic IGF-I and hypocaloric amount of nutrients. 6034059. . [accessed April 3, 2013];US Patent #. http://patents.com/us-6034059.html.
- 97.Wallace DK, Kylstra JA, Phillips SJ, Hall JG. Poor postnatal weight gain: a risk factor for severe retinopathy of prematurity. J AAPOS. 2000;4:343–47. doi: 10.1067/mpa.2000.110342. [DOI] [PubMed] [Google Scholar]
- 98.Hellström A, Hård AL, Engström E, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009;123:e638–45. doi: 10.1542/peds.2008-2697. [DOI] [PubMed] [Google Scholar]
- 99.Fortes Filho JB, Bonomo PP, Maia M, Procianoy RS. Weight gain measured at 6 weeks after birth as a predictor for severe retinopathy of prematurity: study with 317 very low birth weight preterm babies. Graefes Arch Clin Exp Ophthalmol. 2009;247:831–36. doi: 10.1007/s00417-008-1012-3. [DOI] [PubMed] [Google Scholar]
- 100.Wu C, Löfqvist C, Smith LE, VanderVeen DK, Hellström A for the WINROP Consortium. Importance of early postnatal weight gain for normal retinal angiogenesis in very preterm infants: a multicenter study analyzing weight velocity deviations for the prediction of retinopathy of prematurity. Arch Ophthalmol. 2012;130:992–99. doi: 10.1001/archophthalmol.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mittal M, Dhanireddy R, Higgins RD. Candida sepsis and association with retinopathy of prematurity. Pediatrics. 1998;101:654–57. doi: 10.1542/peds.101.4.654. [DOI] [PubMed] [Google Scholar]
- 102.Manzoni P, Maestri A, Leonessa M, Mostert M, Farina D, Gomirato G. Fungal and bacterial sepsis and threshold ROP in preterm very low birth weight neonates. J Perinatol. 2006;26:23–30. doi: 10.1038/sj.jp.7211420. [DOI] [PubMed] [Google Scholar]
- 103.Tolsma KW, Allred EN, Chen ML, Duker J, Leviton A, Dammann O. Neonatal bacteremia and retinopathy of prematurity: the ELGAN study. Arch Ophthalmol. 2011;129:1555–63. doi: 10.1001/archophthalmol.2011.319. [DOI] [PubMed] [Google Scholar]
- 104.Dammann O, Brinkhaus MJ, Bartels DB, et al. Immaturity, perinatal inflammation, and retinopathy of prematurity: a multi-hit hypothesis. Early Hum Dev. 2009;85:325–29. doi: 10.1016/j.earlhumdev.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 105.Dammann O. Inflammation and retinopathy of prematurity. Acta Paediatr. 2010;99:975–77. doi: 10.1111/j.1651-2227.2010.01836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sood BG, Madan A, Saha S, et al. for the NICHD neonatal research network. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res. 2010;67:394–400. doi: 10.1203/PDR.0b013e3181d01a36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen M, Citil A, McCabe F, et al. Infection, oxygen, and immaturity: interacting risk factors for retinopathy of prematurity. Neonatology. 2011;99:125–32. doi: 10.1159/000312821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cooke RW, Clark D, Hickey-Dwyer M, Weindling AM. The apparent role of blood transfusions in the development of retinopathy of prematurity. Eur J Pediatr. 1993;152:833–36. doi: 10.1007/BF02073381. [DOI] [PubMed] [Google Scholar]
- 109.Giannantonio C, Papacci P, Cota F, et al. Analysis of risk factors for progression to treatment-requiring ROP in a single neonatal intensive care unit: is the exposure time relevant? J Matern Fetal Neonatal Med. 2012;25:471–77. doi: 10.3109/14767058.2011.587056. [DOI] [PubMed] [Google Scholar]
- 110.Brooks SE, Marcus DM, Gillis D, Pirie E, Johnson MH, Bhatia J. The effect of blood transfusion protocol on retinopathy of prematurity: a prospective, randomized study. Pediatrics. 1999;104:514–18. doi: 10.1542/peds.104.3.514. [DOI] [PubMed] [Google Scholar]
- 111.Relationships between maternal ethnicity, gestational age, birth weight, weight gain, and severe retinopathy of prematurity. J Pediatr. 2013 doi: 10.1016/j.jpeds.2012.12.038.. published online Jan 23. [DOI] [PubMed] [Google Scholar]
- 112.Shastry BS. Genetic susceptibility to advanced retinopathy of prematurity (ROP) J Biomed Sci. 2010;17:69. doi: 10.1186/1423-0127-17-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leviton A, Dammann O, Engelke S, et al. The clustering of disorders in infants born before the 28th week of gestation. Acta Paediatr. 2010;99:1795–800. doi: 10.1111/j.1651-2227.2010.01973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Farooqi A, Hagglof B, Sedin G, Serenius F. Impact at age 11 years of major neonatal morbidities in children born extremely preterm. Pediatrics. 2011;127:e1247–57. doi: 10.1542/peds.2010-0806. [DOI] [PubMed] [Google Scholar]
- 115.An international classification of retinopathy of prematurity. Pediatrics. 1984;74:127–33. [PubMed] [Google Scholar]
- 116.International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–99. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 117.Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 118.American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus American Association of Certified Orthoptists. . Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131:189–95. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 119.Kleberg A, Warren I, Norman E, et al. Lower stress responses after Newborn Individualized Developmental Care and Assessment Program care during eye screening examinations for retinopathy of prematurity: a randomized study. Pediatrics. 2008;121:e1267–78. doi: 10.1542/peds.2006-2510. [DOI] [PubMed] [Google Scholar]
- 120.Section on Ophthalmology American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117:572–76. doi: 10.1542/peds.2005-2749. [DOI] [PubMed] [Google Scholar]
- 121.Löfqvist C, Andersson E, Sigurdsson J, et al. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol. 2006;124:1711–18. doi: 10.1001/archopht.124.12.1711. [DOI] [PubMed] [Google Scholar]
- 122.Wu C, Vanderveen DK, Hellström A, Löfqvist C, Smith LE. Longitudinal postnatal weight measurements for the prediction of retinopathy of prematurity. Arch Ophthalmol. 2010;128:443–47. doi: 10.1001/archophthalmol.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Flückiger S, Bucher HU, Hellström A, Lövqist C, Sturm V, Arri SJ. Der frühe postnatale Gewichtsverlauf als Prädiktor einer Frühgeborenenretinopathie. Klin Monbl Augenheilkd. 2011;228:306–10. doi: 10.1055/s-0031-1273217. [DOI] [PubMed] [Google Scholar]
- 124.Zepeda-Romero LC, Hard AL, Gomez-Ruiz LM, et al. Prediction of retinopathy of prematurity using the screening algorithm WINROP in a Mexican population of preterm infants. Arch Ophthalmol. 2012;130:720–23. doi: 10.1001/archophthalmol.2012.215. [DOI] [PubMed] [Google Scholar]
- 125.Löfqvist C, Hansen-Pupp I, Andersson E, et al. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulinlike growth factor I. Arch Ophthalmol. 2009;127:622–27. doi: 10.1001/archophthalmol.2009.69. [DOI] [PubMed] [Google Scholar]
- 126.Hård AL, Löfqvist C, Fortes Filho JB, Procianoy RS, Smith L, Hellström A. Predicting proliferative retinopathy in a Brazilian population of preterm infants with the screening algorithm WINROP. Arch Ophthalmol. 2010;128:1432–36. doi: 10.1001/archophthalmol.2010.255. [DOI] [PubMed] [Google Scholar]
- 127.Binenbaum G, Ying GS, Quinn GE, et al. for the Premature Infants in Need of Transfusion Study Group. A clinical prediction model to stratify retinopathy of prematurity risk using postnatal weight gain. Pediatrics. 2011;127:e607–14. doi: 10.1542/peds.2010-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aydemir O, Sarikabadayi YU, Aydemir C, et al. Adjusted poor weight gain for birth weight and gestational age as a predictor of severe ROP in VLBW infants. Eye (Lond) 2011;25:725–29. doi: 10.1038/eye.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eckert GU, Fortes Filho JB, Maia M, Procianoy RS. A predictive score for retinopathy of prematurity in very low birth weight preterm infants. Eye (Lond) 2012;26:400–06. doi: 10.1038/eye.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Salcone EM, Johnston S, VanderVeen D. Review of the use of digital imaging in retinopathy of prematurity screening. Semin Ophthalmol. 2010;25:214–17. doi: 10.3109/08820538.2010.523671. [DOI] [PubMed] [Google Scholar]
- 131.Palmer EA, Hardy RJ, Dobson V, et al. 15-year outcomes following threshold retinopathy of prematurity: final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol. 2005;123:311–18. doi: 10.1001/archopht.123.3.311. [DOI] [PubMed] [Google Scholar]
- 132.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity—preliminary results. Arch Ophthalmol. 1988;106:471–79. doi: 10.1001/archopht.1988.01060130517027. [DOI] [PubMed] [Google Scholar]
- 133.Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: ophthalmological outcomes at 10 years. Arch Ophthalmol. 2001;119:1110–18. doi: 10.1001/archopht.119.8.1110. [DOI] [PubMed] [Google Scholar]
- 134.Hardy RJ, Palmer EA, Dobson V, et al. Risk analysis of prethreshold retinopathy of prematurity. Arch Ophthalmol. 2003;121:1697–701. doi: 10.1001/archopht.121.12.1697. [DOI] [PubMed] [Google Scholar]
- 135.Good WV, Hardy RJ ETROP Multicenter Study Group. The multicenter study of early treatment for retinopathy of prematurity (ETROP) Ophthalmology. 2001;108:1013–14. doi: 10.1016/s0161-6420(01)00540-1. [DOI] [PubMed] [Google Scholar]
- 136.Fielder AR. Premature for life. Arch Ophthalmol. 2005;123:392–94. doi: 10.1001/archopht.123.3.392. [DOI] [PubMed] [Google Scholar]
- 137.Slidsborg C, Forman JL, Fielder AR, et al. Experts do not agree when to treat retinopathy of prematurity based on plus disease. Br J Ophthalmol. 2012;96:549–53. doi: 10.1136/bjophthalmol-2011-300573. [DOI] [PubMed] [Google Scholar]
- 138.Shah DN, Wilson CM, Ying GS, et al. Comparison of expert graders to computer-assisted image analysis of the retina in retinopathy of prematurity. Br J Ophthalmol. 2011;95:1442–45. doi: 10.1136/bjo.2010.185363. [DOI] [PubMed] [Google Scholar]
- 139.Wittenberg LA, Jonsson NJ, Paul Chan RV, Chiang MF. Computer-based image analysis for plus disease diagnosis in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2012;49:11–19. doi: 10.3928/01913913-20110222-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Silva RA, Murakami Y, Lad EM, Moshfeghi DM. Stanford University network for diagnosis of retinopathy of prematurity (SUNDROP): 36-month experience with telemedicine screening. Ophthalmic Surg Lasers Imaging. 2011;42:12–19. doi: 10.3928/15428877-20100929-08. [DOI] [PubMed] [Google Scholar]
- 141.Murakami Y, Silva RA, Jain A, Lad EM, Gandhi J, Moshfeghi DM. Stanford University network for diagnosis of retinopathy of prematurity (SUNDROP): 24-month experience with telemedicine screening. Acta Ophthalmol. 2010;88:317–22. doi: 10.1111/j.1755-3768.2009.01715.x. [DOI] [PubMed] [Google Scholar]
- 142.Zepeda-Romero LC, Martinez-Perez ME, Ruiz-Velasco S, Ramirez-Ortiz MA, Gutierrez-Padilla JA. Temporary morphological changes in plus disease induced during contact digital imaging. Eye (Lond) 2011;25:1337–40. doi: 10.1038/eye.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Singh R, Reddy DM, Barkmeier AJ, Holz ER, Ram R, Carvounis PE. Long-term visual outcomes following lens-sparing vitrectomy for retinopathy of prematurity. Br J Ophthalmol. 2012;96:1395–98. doi: 10.1136/bjophthalmol-2011-301353. [DOI] [PubMed] [Google Scholar]
- 144.Multicenter trial of cryotherapy for retinopathy of prematurity. Snellen visual acuity and structural outcome at 5 1/2 years after randomization. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1996;114:417–24. doi: 10.1001/archopht.1996.01100130413008. [DOI] [PubMed] [Google Scholar]
- 145.Msall ME, Phelps DL, Hardy RJ, et al. for the Cryotherapy for Retinopathy of Prematurity Cooperative Group. Educational and social competencies at 8 years in children with threshold retinopathy of prematurity in the CRYO-ROP multicenter study. Pediatrics. 2004;113:790–99. doi: 10.1542/peds.113.4.790. [DOI] [PubMed] [Google Scholar]
- 146.Quinn GE, Dobson V, Saigal S, et al. Health-related quality of life at age 10 years in very low-birth-weight children with and without threshold retinopathy of prematurity. Arch Ophthalmol. 2004;122:1659–66. doi: 10.1001/archopht.122.11.1659. [DOI] [PubMed] [Google Scholar]
- 147.Early Treatment for Retinopathy of Prematurity Cooperative Group. Grating visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2011;129:840–46. doi: 10.1001/archophthalmol.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Repka MX, Tung B, Good WV, Capone A, Jr, Shapiro MJ. Outcome of eyes developing retinal detachment during the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2011;129:1175–79. doi: 10.1001/archophthalmol.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Repka MX. Ophthalmological problems of the premature infant. Ment Retard Dev Disabil Res Rev. 2002;8:249–57. doi: 10.1002/mrdd.10045. [DOI] [PubMed] [Google Scholar]
- 150.Hansen RM, Harris ME, Moskowitz A, Fulton AB. Deactivation of the rod response in retinopathy of prematurity. Doc Ophthalmol. 2010;121:29–35. doi: 10.1007/s10633-010-9228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang CS, Wang AG, Sung CS, Hsu WM, Lee FL, Lee SM. Long-term visual outcomes of laser-treated threshold retinopathy of prematurity: a study of refractive status at 7 years. Eye (Lond) 2010;24:14–20. doi: 10.1038/eye.2009.63. [DOI] [PubMed] [Google Scholar]
- 152.Chen TC, Tsai TH, Shih YF, et al. Long-term evaluation of refractive status and optical components in eyes of children born prematurely. Invest Ophthalmol Vis Sci. 2010;51:6140–48. doi: 10.1167/iovs.10-5234. [DOI] [PubMed] [Google Scholar]
- 153.VanderVeen DK, Bremer DL, Fellows RR, et al. Prevalence and course of strabismus through age 6 years in participants of the early treatment for retinopathy of prematurity randomized trial. J AAPOS. 2011;15:536–40. doi: 10.1016/j.jaapos.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Slidsborg C, Bangsgaard R, Fledelius HC, Jensen H, Greisen G, la Cour M. Cerebral damage may be the primary risk factor for visual impairment in preschool children born extremely premature. Arch Ophthalmol. 2012;130:1410–17. doi: 10.1001/archophthalmol.2012.1393. [DOI] [PubMed] [Google Scholar]
- 155.Wilson DC, Cairns P, Halliday HL, Reid M, McClure G, Dodge JA. Randomised controlled trial of an aggressive nutritional regimen in sick very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1997;77:F4–11. doi: 10.1136/fn.77.1.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hellström A, Carlsson B, Niklasson A, et al. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab. 2002;87:3413–16. doi: 10.1210/jcem.87.7.8629. [DOI] [PubMed] [Google Scholar]
- 157.Perez-Munuzuri A, Fernandez-Lorenzo JR, Couce-Pico ML, Blanco-Teijeiro MJ, Fraga-Bermudez JM. Serum levels of IGF1 are a useful predictor of retinopathy of prematurity. Acta Paediatr. 2010;99:519–25. doi: 10.1111/j.1651-2227.2009.01677.x. [DOI] [PubMed] [Google Scholar]
- 158.Löfqvist C, Niklasson A, Engström E, et al. A pharmacokinetic and dosing study of intravenous insulin-like growth factor-I and IGF-binding protein-3 complex to preterm infants. Pediatr Res. 2009;65:574–79. doi: 10.1203/PDR.0b013e31819d9e8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Crawford MA, Golfetto I, Ghebremeskel K, et al. The potential role for arachidonic and docosahexaenoic acids in protection against some central nervous system injuries in preterm infants. Lipids. 2003;38:303–15. doi: 10.1007/s11745-003-1065-1. [DOI] [PubMed] [Google Scholar]
- 160.Harder BC, von Baltz S, Jonas JB, Schlichtenbrede FC. Intravitreal bevacizumab for retinopathy of prematurity. J Ocul Pharmacol Ther. 2011;27:623–27. doi: 10.1089/jop.2011.0060. [DOI] [PubMed] [Google Scholar]
- 161.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–15. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sato T, Wada K, Arahori H, et al. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153:327–33. e1. doi: 10.1016/j.ajo.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 163.Hård AL, Hellström A. On safety, pharmacokinetics and dosage of bevacizumab in ROP treatment—a review. Acta Paediatr. 2011;100:1523–27. doi: 10.1111/j.1651-2227.2011.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Darlow BA, Ells AL, Gilbert CE, Gole GA, Quinn GE. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed. 2013;98:F170–74. doi: 10.1136/archdischild-2011-301148. [DOI] [PubMed] [Google Scholar]
- 165.Hård AL, Hellström A. On the use of antiangiogenetic medications for retinopathy of prematurity. Acta Paediatr. 2011;100:1063–65. doi: 10.1111/j.1651-2227.2011.02330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Filippi L, Cavallaro G, Fiorini P, et al. Study protocol: safety and efficacy of propranolol in newborns with retinopathy of prematurity (PROP-ROP): ISRCTN18523491. BMC Pediatr. 2010;10:83. doi: 10.1186/1471-2431-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen J, Joyal JS, Hatton CJ, et al. Propranolol inhibition of β-adrenergicreceptor does not suppress pathologic neovascularization in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2012;53:2968–77. doi: 10.1167/iovs.12-9691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Howlett A, Ohlsson A, Plakkal N. Inositol for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. 2012;3:CD000366. doi: 10.1002/14651858.CD000366.pub2. [DOI] [PubMed] [Google Scholar]
- 169.Phelps DL, Lakatos L, Watts JL. D-Penicillamine for preventing retinopathy of prematurity in preterm infants. Cochrane Database Syst Rev. 2001;1:CD001073. doi: 10.1002/14651858.CD001073. [DOI] [PubMed] [Google Scholar]
- 170.Parad RB, Allred EN, Rosenfeld WN, Davis JM. Reduction of retinopathy of prematurity in extremely low gestational age newborns treated with recombinant human Cu/Zn superoxide dismutase. Neonatology. 2012;102:139–44. doi: 10.1159/000336639. [DOI] [PubMed] [Google Scholar]
- 171.Raju TN, Langenberg P, Bhutani V, Quinn GE. Vitamin E prophylaxis to reduce retinopathy of prematurity: a reappraisal of published trials. J Pediatr. 1997;131:844–50. doi: 10.1016/s0022-3476(97)70031-3. [DOI] [PubMed] [Google Scholar]