Abstract
PURPOSE
To determine the influence of omega-3 supplementation on vitreous vascular endothelial growth factor A (VEGF-A) levels in patients with exudative age-related macular degeneration (wet AMD) receiving intravitreal anti-VEGF treatment.
DESIGN
Prospective, randomized, open-label, single-center, clinical trial, consecutive interventional case series.
METHODS
The study included 3 cohorts with wet AMD and a control group with epiretinal membrane or macular hole. Twenty wet AMD patients being treated with anti-VEGF were randomized to daily supplementation of antioxidants, zinc, and carotenoids with omega-3 fatty acids (docosahexaenoic acid and eicosapentaenoic acid; group 1, n = 10) or without omega-3 fatty acids (group 2, n = 10). They were compared with an anti-VEGF treatment-naïve wet AMD group (group 3, n = 10) and an epiretinal membrane or macular hole group (group 4, n = 10). Primary outcome was vitreal VEGF-A levels (at the time of anti-VEGF injection). Secondary outcomes were plasma VEGF-A and central foveal thickness. Patients with new submacular hemorrhage or any other treatment within 3 months were excluded. Final analyses included 9, 6, 7, and 8 patients in groups 1 through 4, respectively.
RESULTS
Patients receiving omega-3s (group 1) had significantly lower levels of vitreal VEGF-A (141.11 ± 61.89 pg/mL) when compared with group 2 (626.09 ± 279.27 pg/mL; P = .036) and group 3 (735.48 ± 216.43 pg/mL; P = .013), but similar levels to group 4 (235.81 ± 33.99 pg/mL; P=.215). All groups showed similar values for plasma VEGF-A and central foveal thickness measurements.
CONCLUSIONS
This study demonstrated that omega-3 supplementation combined with anti-VEGF treatment is associated with decreased vitreal VEGF-A levels in wet AMD patients.
Age-related macular degeneration (AMD) IS the leading cause of blindness in older individuals in the Western world. The aging of baby boomers is expected to lead to a 2-fold increase in the number of white person 65 years of age or older by 2031.1 Correspondingly, a doubling in the number of North Americans with AMD is expected. The exudative (wet or neovascular) form of AMD is associated most widely with central vision impairment and legal blindness.1 The 15-year cumulative incidence of wet AMD in Americans 75 years of age or older is 4.4%.2 By 2020, in the United States alone, it is estimated that nearly 3 million individuals will be affected by wet AMD.3 The progressive nature of wet AMD, its substantial societal and personal impact, and its high prevalence make it essential to develop clinical strategies to reduce its impact. It represents an important cause of morbidity and presents direct financial burdens of more than $10 billion in direct annual medical costs in the United States and accounts for significant loss of productivity.4 Designing efficient and cost-effective treatment methods therefore is highly desirable.
The management of wet AMD was revolutionized by the introduction of anti–vascular endothelial growth factor (VEGF) therapies.5–7 Regrettably, 5% to 10% of patients proceed to lose 3 lines or more of visual acuity (VA), and most exudative lesions show some sign of activity by the end of follow-up. In addition, increased numbers of thromboembolic events, possible neuronal toxicity, and higher incidence of geographic atrophy in patients with more frequent anti-VEGF injections also may be of concern.8–10 Thus, developing alternative or adjunct therapies to currently available anti-VEGF drugs may increase treatment success, slow AMD progression, and improve VA outcomes.
The abnormal and disproportionate growth of choroidal vessels associated with wet AMD likely stems from a compensatory angiogenic response to overcome an earlier phase of microvessel degeneration and reinstate metabolic equilibrium to the hypoxic macula. A potential strategy to influence and reduce the progression of wet AMD comes from directly modulating the cellular make-up of the retina. In this respect, the outer retina is highly concentrated in diet-derived long-chain polyunsaturated fatty acids (LCPUFAs)11–13 such as docosahexaenoic acid (DHA) of the omega-3 family and arachidonic acid of the omega-6 family. The capacity of lipids to play biological roles beyond energy storage and membrane structure long has been recognized.13,14 Importantly, dysregulation in lipid signaling is a salient feature of conditions associated with chronic inflammation such as metabolic syndrome, atherosclerosis, asthma, allergic response, autoimmunity, hypertension, cancer, and importantly in the context of the current study, ocular vasoproliferative diseases.11,13–18
Because humans are limited in their capacity to biosynthesize omega-3 LCPUFAs de novo, their tissue status is modifiable via diet or supplement intake of DHA and eicosapentaenoic acid (EPA).14 The benefits of omega-3 supplementation on wet AMD consistently have been recognized in multiple observational studies,19–23 and although null results have been reported in a well-nourished nutrient-supplementing cohort with moderate to high risk of AMD progression,24 a clearer understanding of the impact of omega-3 supplementation on wet AMD could prove beneficial for streamlining therapeutic strategies. Furthermore, a number of fundamental studies have demonstrated the beneficial effects of omega-3 metabolites DHA and EPA on pathologic angiogenesis.25–29 Based on the current experimental and epidemiologic data linking omega-3 LCPUFAs and their potential beneficial role in angiogenesis, the purpose of the present pilot trial was to investigate the influence of omega-3 supplementation on VEGF-A levels in the vitreous of patients undergoing anti-VEGF treatment for wet AMD.
METHODS
THIS PILOT, PROSPECTIVE, RANDOMIZED, OPEN-LABEL, single-center clinical trial, consecutive, interventional case series was conducted between February and August 2011. The study conformed to the tenets of the Declaration of Helsinki, was approved by the Institutional Review Board of the Maisonneuve-Rosemont Hospital affiliated with the University of Montreal, Quebec, Canada, and is a registered trial (ClinicalTrials.gov identifier, NCT01819415).
PARTICIPANTS
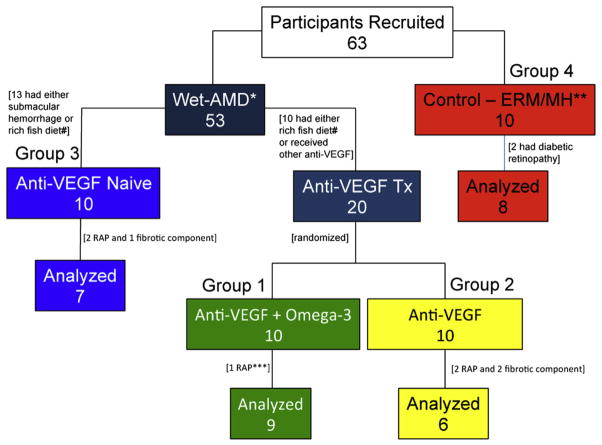
Sixty-three patients were screened for the study. Forty patients were deemed eligible participants and were enrolled at the Department of Ophthalmology Clinic, Maisonneuve-Rosemont Hospital, Montreal, after providing written informed consent (Figure 1). Three cohorts consisted of active wet AMD patients (10 per group) who were eligible for anti-VEGF treatment (bevacizumab 1.25 mg/0.05 mL). They were compared with a non-AMD group with epiretinal membrane (ERM) or macular hole (MH; Figure 1). All participants were nonsmokers with regular consumption less than 1 serving of fish intake per week, according to a food-frequency questionnaire applied during recruitment.30
Figure 1.
Diagram showing study participants, including number of recruited, excluded, and analyzed patients. Three wet age-related macular degeneration (AMD) patient groups, with or without omega-3 supplementation, and a control group are highlighted in distinct colors. *=wet age-related macular degeneration; **=epiretinal membrane and macular hole; ***=retinal angiomatous proliferation; # = 1 or more servings of fish per week. ERM or MH = epiretinal membrane or macular hole; RAP = retinal angiomatous proliferation; Tx = treatment; VEGF = vascular endothelial growth factor.
Patients with wet AMD manifesting new thick submacular hemorrhage and those with treatment other than anti-VEGF or other anti-VEGF drugs within the last 3 months of study entry were ineligible.
STUDY GROUPS AND STUDY SUPPLEMENTS
Twenty patients with active wet AMD who had undergone prior anti-VEGF treatment were divided in 2 groups and were randomized to receive oral supplementation as follows:
Group 1 (n = 10): Vitalux plus Omega-3 (Alcon, Toronto, Ontario, Canada) 4 capsules/day; a formula containing the antioxidants β-carotene (5728 μg), vitamin C (500 mg), vitamin E (400 IU), zinc (25 mg), and copper (1 mg), as well as lutein (10 mg), zeaxanthin (2 mg), and omega 3 (1052 mg fish oil from sardine, mackerel, and anchovy [200 mg of DHA and 400 mg of EPA]).
Group 2 (n = 10): Vitalux AREDS, 2 capsules/day, a formula containing the same concentration of antioxidants and minerals, lutein (10 mg), and zeaxanthin (500 μg; Figure 1).
Group 3 (n = 10): patients with wet AMD starting anti-VEGF treatment (treatment naïve).
Group 4 (n = 10): non-AMD patients with ERM orMH undergoing 25-gauge pars plana vitrectomy.
Patients from groups 3 and 4 were not taking any of the above-mentioned supplements.
ANALYTIC SAMPLE
We consecutively recruited 63 patients: 53 with wet AMD and 10 with ERM or MH. Of the wet AMD patients, 23 were excluded because of either higher omega-3 content in their diets, other anti-VEGF treatments, or new submacular hemorrhage. Of the 30 patients recruited with wet AMD, 8 were excluded from statistical analysis (1 from group 1, 4 from group 2, and 3 from group 3) because they either had retinal angiomatous proliferation or a large fibrotic component (more than 50%) of the choroidal neovascularization. Two of 10 patients with ERM or MH from group 4 also were excluded because they were found to have diabetes and mild nonproliferative diabetic retinopathy. A total of 22 patients with wet AMD (9 in group 1, 6 in group 2, and 7 in group 3) and 8 control patients were included for VEGF-A analysis (Figure 1).
END POINTS
The primary outcome was vitreous VEGFA levels, and secondary outcomes were plasma VEGF-A levels and central foveal thickness (CFT) measures. Vitreous and plasma VEGF-A levels were collected at the time of anti-VEGF treatment. At enrollment, we collected data on age, gender, number of previous anti-VEGF injections, time from last anti-VEGF injection, and Snellen visual acuity (converted to logMAR for statistical analysis; Table).
Table.
Characteristics of Patients and Baseline Parameters of Wet Age-Related Macular Degeneration Groups, With or Without Omega-3 Supplementation, and the Control Group
| Parameter | Wet AMD
|
Control (Group 4)a | P Value | ||
|---|---|---|---|---|---|
| Anti-VEGF Plus Omega-3 (Group 1) | Anti-VEGF (Group 2) | Anti-VEGF Naïve (Group 3) | |||
| Age (y) | 79.6 ± 1.81 | 79.00 ± 1.98 | 83.38 ± 2.32 | 68.25 ± 3.56 | .0099b |
| No. female (%) | 5 (56) | 3 (50) | 2 (29) | 7 (86) | .144c |
| Visual acuityd | 0.61 ± 0.14 | 0.84 ± 0.17 | 0.78 ± 0.15 | n.a. | .6606b |
| No. of previous injections | 8 ± 1.19 | 6 ± 1.51 | n.a. | n.a. | .5287b |
| Time from last injection (wks) | 8 ± 0.40 | 8 ± 0.36 | n.a. | n.a. | .9999b |
No. of patients 9 6 7 8 AMD = age-related macular degeneration; n.a. = not applicable; VEGF = vascular endothelial growth factor.
Epiretinal membrane and macular hole patients.
Student t test.
Fisher exact probability test.
Logarithm of the minimal angle of resolution units.
ANTI–VASCULAR ENDOTHELIAL GROWTH FACTOR TREATMENT PROTOCOL
The anti-VEGF treatment regimen consisted of 3 loading doses followed by pro re nata injections based on disease activity measured monthly by spectral-domain optical coherence tomography (Cirrus, Carl Zeiss Meditec, Toronto, Canada). Fluorescein angiography also was performed on all patients with wet AMD on the day of the anti-VEGF injection (when vitreous biopsy and blood samples were collected).
VITREOUS BIOPSY
After the surgical field was sterilized using 5% povidone–iodine, patients were draped in a standard manner with placement of a lid speculum. A 27-gauge self-retaining infusion line (Insight Instruments, Stuart, Florida, USA) of balanced salt solution was placed first, followed by the placement of a 29-gauge trocar with a chandelier light connected to a mercury vapor light source (Synergetics, O’Fallon, Missouri, USA). The surgical view during the procedure was provided through a surgical operative microscope and a Volk contact lens (Volk direct image ×1.5 magnifying disposable vitrectomy lens; Volk Optical, Mentor, Ohio, USA).
The vitreous biopsy was performed using a 23-gauge sutureless Retrector system (Insight Instruments) in all patients. The model used in the study is a portable, battery-powered system with a maximum cut rate of 600 cpm (cuts per minute) and features a retractable sheathed guillotine 25-gauge cutter with an in-built needle (23 gauge). The needle was introduced bevel down through displaced conjunctiva in an oblique 1-plane tunnel into the vitreous cavity 3 to 4 mm from the limbus. At least 0.5 mL of undiluted vitreous fluid was cut and removed from the vitreous right above the macular region (at the premacular bursa) through controlled manual aspiration with the Retrector system. Bevacizumab 2.5 mg/0.1 mL was injected through the 29-gauge trocar after the vitreous biopsy.31
The samples were split in 3 vials: 1 for VEGF-A levels, 1 for lipidomics analysis, and 1 for microbiologic analysis (to verify any contamination during vitreous biopsy). The entire procedure was performed in the minor procedure room within the Department of Ophthalmology Clinic at Maisonneuve Rosemont Hospital, Montreal, Canada.
ASSESSMENT OF VASCULAR ENDOTHELIAL GROWTH FACTOR A LEVELS
Vitreous and plasma samples were frozen on dry ice and immediately were stored at −80 C after biopsy, then centrifuged at 15 000 g for 5 minutes at 4 C before analysis. For plasma analysis, 5 mL venous blood was collected before vitreous biopsy and centrifuged at 3000 g for 15 minutes at 4 C to obtain plasma and was stored at −80 C until assayed. VEGF-A levels were quantified in supernatants using enzyme-linked immunosorbent assays according to manufacturer’s instructions (R&D Systems, Minneapolis, Minnesota, USA).
STATISTICAL ANALYSIS
Statistical analysis was performed using the 2-way analysis of variance nonparametric test, the nonparametric t test (Mann–Whitney U test), parametric Student t test, and the Student t test (GraphPad Prism).We applied the Fisher exact probability test to examine differences in the proportions of women and men in each group. All statistical analysis were performed using the same software (GraphPad Prism, La Jolla, California, USA). Comparisons across all groups yielded an exact P value of .144, suggesting no appreciable differences. Respective P values for comparisons of these proportions across people with wet AMD (groups 1, 2, and 3), between people with wet AMD in the clinical trial (group 1 vs group 2) and all people with AMD vs people with ERM or MH (combined groups 1 through 3 vs group 4) were 0.568, 0.376, and 0.092, respectively. All P values are 2-tailed. P values less than .05 were considered statistically significant. Data are expressed as mean ± standard error of the mean.
RESULTS
BASELINE PARAMETERS WERE SIMILAR FOR EACH GROUP with the exception that patients in group 4 (control) were significantly younger than patients with wet AMD (mean, 68.25 years; standard error of the mean, 3.56, vs 80.66 ± 2.04 years; P = .0099). Patients in groups 1 and 2 had a similar mean (±standard error of the mean) number of anti-VEGF injections of 8 ± 1.19 and 6 ± 1.51, respectively, at the time of their vitreous sampling (P = .5287). They also had similar values for time from last injection (8 ± 0.40 vs 8 ± 0.36; P = .9999; Table). Patients with wet AMD did not show any complications related to the biopsy procedure, and patients in the control group did not have any complications related to the 25-gauge pars plana vitrectomy surgery.
PRIMARY OUTCOME: VITREOUS VASCULAR ENDOTHELIAL GROWTH FACTOR A LEVELS
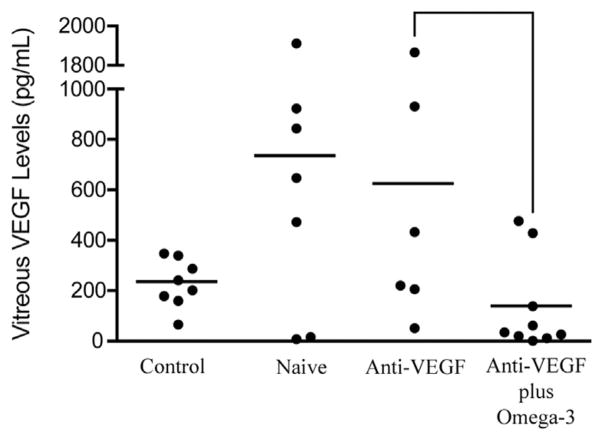
The range of vitreous concentrations of VEGF-A in patients with wet AMD was much wider for groups not receiving the omega-3 LCPUFA supplementation. Group 1 (anti-VEGF injections + Vitalux plus Omega-3) had significantly lower levels of VEGF-A in the vitreous when compared with group 2 (anti-VEGF injections + Vitalux AREDS without omega-3 LCPUFAs; P = .036) and group 3 (treatment-naïve anti-VEGF injections + no planned supplement intervention; P = .014), but not when compared with group 4 (control; P = .215; Figure 2). Both wet AMD groups not taking omega-3 supplementation (groups 2 and 3) had similar levels of vitreous VEGF-A (P = .758). Group 3 (treatment naïve) had significantly higher vitreous levels of VEGF-A when compared with nonvascular ocular pathologic features group 4 (controls; P = .039; Figure 2). Seven of 9 patients in group 1 had concentrations of vitreous VEGF-A lower than all but 1 of the patients in group 2 (Figure 2).
Figure 2.
Graph showing omega-3 supplementation and vitreous vascular endothelial growth factor (VEGF) A concentrations in wet age-related macular degeneration and control patients. Concentrations of vitreous VEGF-A, demonstrating that group 1 (anti-VEGF plus omega-3; n=9) had significantly lower levels than group 2 (anti-VEGF alone; n=6; P=.0360) and group 3 (treatment naïve, starting on anti-VEGF; n = 7; P = .0139). Data also demonstrate that group 1 (anti-VEGF plus omega-3) and group 4 (control; n=8) had similar vitreous VEGF levels (P = .2153) and that group 3 (treatment naïve) had significantly higher vitreous VEGF levels than group 4 (control; P = .0387). Group 2 (anti-VEGF alone) and group 3 (treatment naïve), both not taking omega-3 supplementation, had similar vitreous VEGF levels (P = .7582, t test).
SECONDARY OUTCOMES
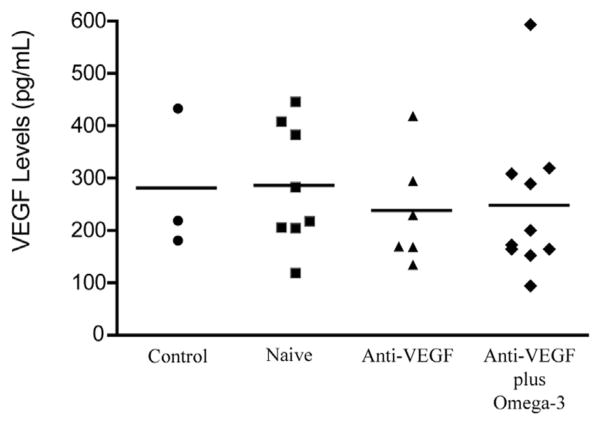
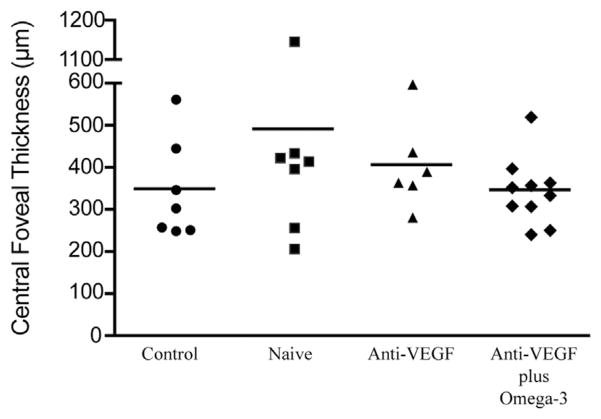
Analysis of plasma levels of VEGF-A revealed no significant change between groups (P = .736; Figure 3). Similarly, although values for CFT tended toward improvement, no significant benefit was noted with omega-3 supplementation in the sample population investigated in this pilot study (P = .211; Figure 4).
Figure 3.
Graph showing omega-3 supplementation and systemic vascular endothelial growth factor (VEGF) A concentrations in wet age-related macular degeneration and control patients. Plasma VEGF levels demonstrating no significant difference between group 4 (control; n = 8) and group 1 (anti-VEGF plus omega-3; n = 9; P = .7361), group 2 (anti-VEGF alone; n=6; P=.6194), and group 3 (treatment naïve, starting on anti-VEGF; n = 7; P = .9474, t test).
Figure 4.
Graph showing omega-3 supplementation and central foveal thickness in wet age-related macular degeneration and control patients. Assesment of central foveal thickness showed no significant difference between group 1 (anti–vascular endothelial growth factor [VEGF] plus omega-3) and group 2 (anti-VEGF alone; P=.2108), group 3 (treatment naïve, starting on anti-VEGF; P = .1511), and group 4 (control; P = .9579, t test).
DISCUSSION
IN THIS PILOT CLINICAL TRIAL, WE INVESTIGATED THE INfluence of omega-3 supplementation on VEGF-A levels in the vitreous of patients undergoing anti-VEGF treatment for wet AMD and noted a significant decrease of VEGF-A in patients receiving omega-3. Dietary intake of omega-3 LCPUFAs and its influence on processes implicated in pathologic retinal angiogenesis has been proposed.18 We previously reported on the pronounced anti-angiogenic effects of certain omega-3 LCPUFA metabolites such as 4-hydroxy-docosahexaenoic acid (a metabolite produced via the 5-lipoxygenase pathway and acting through the peroxisome proliferator-activated receptor). We also demonstrated that increased omega-3 LCPUFA dietary intake reduces pathologic angiogenesis in experimental animal models of neovascular retinopathies.27,29,32 Our previous genetic work in humans extended these findings to support the influence of omega-3 activated pathway on angiogenesis in wet AMD patients via complement and VEGF signaling systems.33 In the time frame of the current human study, the effects of omega-3 supplementation were exclusive to modulating vitreous levels of VEGF-A in proximity of the site of neovascularization, but not on systemic levels as determined by analysis of plasma.
Interestingly, despite the significantly lower levels of VEGF-A in the vitreous of group 1, CFT values were similar to those of group 2 (after an average of 7 prior anti-VEGF injections) and of group 3 (Figure 3 and Table). In accordance with recent work in diabetic macular edema by Sonoda and associates, our findings also demonstrated a lack of correlation between CFT values and vitreous levels of VEGF in patients with active wet AMD (data not shown).34 These data agree with the notion that other factors besides VEGF-A may contribute to disease activity in wet AMD and that combination therapy with other agents is likely necessary in many patients to completely stall CNV activity and promote regression. In addition, the bevacizumab pro re nata treatment protocol used for groups 1 and 2 may have contributed to the absence of significant variations in plasma levels of VEGF-A when compared with group 3 (treatment naïve). Lesser influence of bevacizumab treatment on systemic levels of VEGF also has been found in patients in the discontinuous treatment arm of the Inhibit VEGF in Age-related choroidal Neovascularization (IVAN) trial.35
The biopsy technique applied was performed specifically to collect vitreous samples as close as possible to the macula, under microscope visualization, to obtain a representative vitreal sample in close proximity to neovascular membranes.31 This accurate sampling by vitreous biopsy directly adjacent to the macula also may explain in part the higher levels of VEGF-A detected in our patients with wet AMD when compared with previous reports.36,37 Despite high levels of LCPUFA metabolites in retinal tissue,29 lipidomic analysis of the undiluted vitreous in wet AMD did not yield consistent results, and we were not able to detect consistent levels of omega-3 and omega-6 metabolites (data not shown).
Epidemiologic studies consistently have shown protective relationships of increased omega-3 LCPUFA-rich food intake with advanced AMD.19–23 The Age-Related Eye Disease Study 2 did not report a protective effect of 350 mg/day of DHA plus 650 mg/day of EPA supplementation for progression to wet AMD in their phase 3 clinical trial.24 The lack of positive results in this trial could be because it was performed on a very well-nourished study population, in which 11% of the placebo group were taking omega-3 LCPUFAs outside the study regimen, or that a higher supplemental dose or higher composition of DHA plus EPA was needed for efficacy.24
The Nutritional AMD Treatment 2 study research team randomly assigned high-risk AMD patients to 840 mg/day DHA plus 270 mg/day EPA or a placebo for 3 years. Time to occurrence of CNV did not differ between omega-3 vs placebo groups; however, patients in the group receiving omega-3 LCPUFAs were in the higher tertile of the area under the receiver operating characteristic curve for serum and red blood cell membrane levels of DHA plus EPA and had nearly a 70% lower risk of developing CNV when compared with the lower tertile.38
The limitations of the current pilot study include its small sample size, the inability to detect vitreal lipid profiles, lack of DHA serum levels measurements, and perhaps low doses of omega-3 LCPUFAs in supplements.
In summary, we demonstrated that daily omega-3 fatty acid supplementation as part of a formulation also containing antioxidants, zinc, lutein, and zeaxanthin in patients with wet AMD and being treated with anti-VEGF injections (group 1) was associated with significantly lower vitreous levels of VEGF-A than those observed in patients treated with bevacizumab plus daily omega-3-free supplements (group 2). Anti-VEGF–naïve eyes without any nutrient supplement and an omega-3 LCPUFA poor diet (group 3) also were associated with higher VEGF-A levels than those detected in the omega-3 supplemented group (group 1). Furthermore, we showed that omega-3 supplementation specifically lowers vitreous levels of VEGF-A without influencing plasma levels of VEGF-A in patients with wet AMD who were receiving a bevacizumab pro re nata regimen. This is likely because AMD provokes a local rise in VEGF-A, and hence only vitreous, but not systemic, levels increase. The average time from last injection in both groups being treated with bevacizumab was 8 weeks, without any significant difference between groups 1 and 2 (Table). Although recent studies have demonstrated decreased systemic VEGF levels up to 4 weeks after intravitreal bevacizumab injection, our study did not show any significant difference between groups 1 and 2 (treated with bevacizumab) and group 3 (treatment naïve) at 8 weeks after their last anti-VEGF injection.39,40 Therefore, our data suggest that omega-3 supplementation selectively lowers pathologic ocular VEGF-A in the retina, but not physiologic systemic VEGF-A. Long-term studies will be required to determine if the observed reduction in VEGF-A by omega-3- supplementation combined with anti-VEGF translates into lesser CNV progression or activity.
Acknowledgments
Supported by the Department of Ophthalmology, University of Montreal; Department of Ophthalmology, Maisonneuve-Rosemont Hospital; Fond de Recherche en Ophtalmologie, University of Montreal; Foundation Fighting Blindness Canada; Grant 324573 from the Canadian Institutes of Health Research; Retina Foundation of Canada; Insight Instruments, Stuart, Florida, USA; Synergetics, Inc., O’Fallon, Missouri, USA; Novartis Canada, Montreal, Quebec, Canada; Grants EY022275, EY017017, and P01 HD18655 from the National Institutes of Health, Bethesda, Maryland; a Senior Investigator Award from Research to Prevent Blindness, New York, New York, USA; the Lowy Medical Foundation; and FP7 project 305485 of the European Commission (LEHS). The sponsors or funding organizations had no role in the design or conduct of this research. Involved in Design and conduct of study (F.A.R., P.S.); Collection of data (F.A.R., E.L., C.X.Q.); Management of data (F.A.R., E.L., P.S.); Analysis and interpretation of data (F.A.R., E.L., L.S., J.P.S., P.S.); Preparation of manuscript (F.A.R., E.L., P.S.); and Review and approval of manuscript (F.A.R., L.S., J.P.S., P.S.). The authors thank Karsten Gronert, School of Optometry, University of California, Berkeley, California, USA, for carrying out lipidomic assay on patient vitreous (data was not included).
Biography

Dr Flavio A. Rezende is an Associate Professor of Ophthalmology and Director of the Retina Section, Department of Ophthalmology, University of Montreal, Quebec, Canada. He is in charge of the vitreoretinal surgical fellowship program of the same institution. He is concurrently an Associate Professor, Department of Ophthalmology, Pontifícia Universidade Católica, Rio de Janeiro, Brazil. His primary research interests include surgical and medical diseases of the retina as well as translational research in this field.
Footnotes
ALL AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST and the following were reported. Dr Rezende has received consultation fees from Novartis, Lachine, Quebec, Canada, Alcon Canada, Bausch & Lomb, Montreal, Quebec, Canada, Allergan, Markham, Ontario, Canada, and Bayer, Toronto, Ontario, Canada, none of which are related to the current study. Przemyslaw Sapieha holds a Canada Research Chair and has received consultation fees from Gerson Lehman Group not related to the current research.
References
- 1.Cruess A, Zlateva G, Xu X, Rochon S. Burden of illness of neovascular age-related macular degeneration in Canada. Can J Ophthalmol. 2007;42(6):836–843. doi: 10.3129/i07-153. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114(2):253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DS, O’Colmain BJ, Muñoz B, et al. Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 4.Rein DB, Zhang P, Wirth KE, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124(12):1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 6.Heier JS, Brown DM, Chong V, et al. VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Martin DF, Maguire MG, Fine SL, et al. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart MW. The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clin Proc. 2012;87(1):77–88. doi: 10.1016/j.mayocp.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson GS, Ju M, Shih SC, et al. Nonvascular role for VEGF: VEGFR-1, 2 activity is critical for neural retinal development. FASEB J. 2001;15(7):1215–1217. doi: 10.1096/fj.00-0598fje. [DOI] [PubMed] [Google Scholar]
- 10.Grunwald JE, Daniel E, Huang J, et al. CATT Research Group. Risk of geographic atrophy in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2014;121(1):150–161. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Euler US. On the specific vaso-dilating and plain muscle stimulating substances from accessory genital glands in man and certain animals (prostaglandin and vesiglandin) J Physiol. 1936;88(2):213–234. doi: 10.1113/jphysiol.1936.sp003433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldberg W, Holden HF, Kellaway CH. The formation of lysocithin and of a muscle-stimulating substance by snake venoms. J Physiol. 1938;94(2):232–248. doi: 10.1113/jphysiol.1938.sp003676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9(2):162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 14.Fetterman JW, Jr, Zdanowicz MM. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm. 2009;66(13):1169–1179. doi: 10.2146/ajhp080411. [DOI] [PubMed] [Google Scholar]
- 15.Fortin PR, Lew RA, Liang MH, Wright EA, Beckett LA, Chalmers TC. Validation of a meta-analysis: the effects of fish oil in rheumatoid arthritis. J Clin Epidemiol. 1995;48(11):1379–1390. doi: 10.1016/0895-4356(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 16.Holub BJ. Docosahexaenoic acid (DHA) and cardiovascular disease risk factors. Prostaglandins Leukot Essent Fatty Acids. 2009;81(2–3):199–204. doi: 10.1016/j.plefa.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Calder PC, Yaqoob P. Omega-3 polyunsaturated fatty acids and human health outcomes. Biofactors. 2009;35(3):266–272. doi: 10.1002/biof.42. [DOI] [PubMed] [Google Scholar]
- 18.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24(1):87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Seddon JM, Rosner B, Sperduto RD, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119(8):1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- 20.SanGiovanni JP, Chew E, Clemons TE, et al. Age-Related Eye Disease Study Research Group. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS report no. 20. Arch Ophthalmol. 2007;125(5):671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- 21.Augood C, Chakravarthy U, Young I, et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am J Clin Nutr. 2008;88(2):398–406. doi: 10.1093/ajcn/88.2.398. [DOI] [PubMed] [Google Scholar]
- 22.Chong EW, Kreis AJ, Wong TY, Simpson JA, Guymer RH. Dietary omega-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: a systematic review and meta-analysis. Arch Ophthalmol. 2008;126(6):826–833. doi: 10.1001/archopht.126.6.826. [DOI] [PubMed] [Google Scholar]
- 23.SanGiovanni JP, Agrón E, Meleth AD, et al. AREDS Research Group. ω-3 long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2009;90(6):1601–1607. doi: 10.3945/ajcn.2009.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 25.Zhang YW, Morita I, Yao XS, Murota S. Pretreatment with eicosapentaenoic acid prevented hypoxia/reoxygenation-induced abnormality in endothelial gap junctional intercellular communication through inhibiting the tyrosine kinase activity. Prostaglandins Leukot Essent Fatty Acids. 1999;61(1):33–40. doi: 10.1054/plef.1999.0070. [DOI] [PubMed] [Google Scholar]
- 26.Zhang YW, Yao XS, Murota S, Morita I. Inhibitory effects of eicosapentaenoic acid (EPA) on the hypoxia/reoxygenation-induced tyrosine kinase activation in cultured human umbilical vein endothelial cells. Prostaglandins Leukot Essent Fatty Acids. 2002;67(4):253–261. doi: 10.1054/plef.2002.0427. [DOI] [PubMed] [Google Scholar]
- 27.Stahl A, Sapieha P, Connor KM, et al. PPARγ mediates a direct antiangiogenic effect of ω 3-PUFAs in proliferative retinopathy. Circ Res. 2010;107(4):495–500. doi: 10.1161/CIRCRESAHA.110.221317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang G, Panigrahy D, Mahakian LM, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci U S A. 2013;110(16):6530–6535. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sapieha P, Stahl A, Chen J, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of ω-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3(69):69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 31.Rezende FA, Qian CX, Sapieha P. Evaluation of the vitreous microbial rate in office-based three-port microincision vitrectomy surgery using Retrector technology. BMC Ophthalmol. 2014;14(1):58. doi: 10.1186/1471-2415-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13(7):868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SanGiovanni JP, Chen J, Sapieha P, et al. DNA sequence variants in PPARGC1A, a gene encoding a coactivator of the ω-3 LCPUFA sensing PPAR-RXR transcription complex, are associated with NV AMD and AMD-associated loci in genes of complement and VEGF signaling pathways. PLoS One. 2013;8(1):e53155. doi: 10.1371/journal.pone.0053155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Otsuka H, Sonoda Y. Retinal morphologic changes and concentrations of cytokines in eyes with diabetic macular edema. Retina. 2014;34(4):741–748. doi: 10.1097/IAE.0b013e3182a48917. [DOI] [PubMed] [Google Scholar]
- 35.Chakravarthy U, Harding SP, Rogers CA, et al. IVAN Study Investigators. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Duh EJ, Yang HS, Haller JA, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor: implications for ocular angiogenesis. Am J Ophthalmol. 2004;137(4):668–674. doi: 10.1016/j.ajo.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Q, Ziemssen F, Henke-Fahle S, et al. Tübingen Bevacizumab Study Group. Vitreous levels of bevacizumab and vascular endothelial growth factor-Ain patients with choroidal neovascularization. Ophthalmology. 2008;115(10):1750–1755. doi: 10.1016/j.ophtha.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Souied EH, Delcourt C, Querques G, et al. Nutritional AMD Treatment 2 Study Group. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: the Nutritional AMD Treatment 2 study. Ophthalmology. 2013;120(8):1619–1631. doi: 10.1016/j.ophtha.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Carneiro AM, Costa R, Falcão MS, et al. Vascular endothelial growth factor plasma levels before and after treatment of neovascular age-related macular degeneration with bevacizumab or ranibizumab. Acta Ophthalmol. 2012;90(1):e25–e30. doi: 10.1111/j.1755-3768.2011.02240.x. [DOI] [PubMed] [Google Scholar]
- 40.Zehetner C, Kirchmair R, Huber S, Kralinger MT, Kieselbach GF. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br J Ophthalmol. 2013;97(4):454–459. doi: 10.1136/bjophthalmol-2012-302451. [DOI] [PubMed] [Google Scholar]