Abstract
Caffeine is the most widely used psychostimulant in the world, though preclinical studies suggest weaker evidence for abuse-related effects than stimulants with high abuse liability, such as amphetamine or cocaine. Intracranial self-stimulation (ICSS) is one procedure used to assess the abuse liability of drugs, and previous studies have produced mixed results regarding whether caffeine produces an abuse-related facilitation of ICSS. This study assessed both caffeine and its main metabolite in humans, paraxanthine, using a frequency-rate ICSS procedure and compared their effects to those of amphetamine and cocaine. Male Sprague-Dawley rats were implanted with intracranial electrodes targeting the medial forebrain bundle and trained to respond under a fixed-ratio 1 schedule for brain stimulation that varied across a range of frequencies (56–158 Hz in 0.05 log increments). Data analysis focused on three dependent measures: reinforced responding (defined as responses that produced brain stimulation), non-reinforced responding (defined as responses that occurred during each 0.5 sec brain stimulation and that did not produce additional stimulation), and total responding (reinforced + non-reinforced responding). Both amphetamine and cocaine produced robust increases in total, reinforced and non-reinforced responses. Caffeine also increased total, reinforced and non-reinforced responses, but the caffeine dose-effect curve had an inverted-U shape, and peak ICSS facilitation was less than that produced by amphetamine or cocaine. Paraxanthine increased only total responses and non-reinforced responses. These results suggest that paraxanthine has low abuse liability and does not mediate abuse-related effects of caffeine.
Keywords: drug abuse, intracranial self-stimulation, caffeine, paraxanthine, rat
Introduction
Caffeine is a mild psychostimulant and natural constituent of many plant-based beverages, such as coffee and tea. It is also an additive to many soft drinks, energy drinks, and dietary supplements, and it is available in over-the-counter medications and often included as psychoactive filler in illicit drug formulations (Baron, Elie, & Elie, 2011; Gurley, Steelman, & Thomas, 2014; Reissig, Strain, & Griffiths, 2009). As such, caffeine is the most widely used psychostimulant in the world. Despite its wide availability and use, caffeine is not currently treated as a clinically significant drug of abuse. For example, caffeine is not controlled by the Food and Drug Administration in the United States, and caffeine use disorder is not included in the current version of the Diagnostic and Statistical Manual of psychiatric disorders (Hasin et al., 2013). Nonetheless, caffeine does produce detectable though weak effects in preclinical and clinical procedures designed to assess abuse potential (Griffiths & Woodson, 1988; Meredith, Juliano, Hughes, & Griffiths, 2013; Nehlig, 1999). For example, caffeine delivery can serve as a reinforcing stimulus in rodent and human drug self-administration procedures, although it maintains lower and less reliable levels of self-administration than other drugs with high abuse liability, such as amphetamine (Griffiths & Woodson, 1988). Moreover, withdrawal effects are sometimes reported with cessation of caffeine use (Ogawa & Ueki, 2007), and individuals seeking help with caffeine dependence report an average intake of 548 mg of caffeine per day, or ~8 mg/kg in the average 70 kg adult (Juliano, Evatt, Richards, & Griffiths, 2012). Accordingly, some have suggested that caffeine dependence is a clinically meaningful disorder that warrants further study (Meredith, et al., 2013)
In humans, caffeine is primarily metabolized into paraxanthine, which represents approximately 80% of the metabolized products, and appreciable levels (up to 2 mg/L) are found in regular coffee and tea drinkers (Arnaud, 2011; Lelo, Kjellen, Birkett, & Miners, 1989; Lelo, Miners, Robson, & Birkett, 1986). Abuse-related effects of paraxanthine have not been examined in drug self-administration procedures, but in rats, caffeine and paraxanthine are equally potent at increasing locomotor activity, and paraxanthine is more efficacious (Orru et al., 2013). Similarly, both caffeine and paraxanthine can increase striatal dopamine levels, a neurochemical effect also produced by many abused drugs, and again, paraxanthine appears to be more efficacious (Borycz et al., 2007; Okada, Mizuno, & Kaneko, 1996; Orru, et al., 2013; Quarta et al., 2004). These findings suggest that paraxanthine may function as an active metabolite that contributes to abuse-related effects of caffeine.
Intracranial self-administration (ICSS) is another type of procedure, in addition to drug self-administration, that has been used to assess the abuse-related effects of drugs (Carlezon & Chartoff, 2007; Negus & Miller, 2014; Stoker & Markou, 2011; Wise, 1996). In ICSS procedures, operant responding is maintained by delivery of electrical brain stimulation, and drug-induced increases in low rates of responding maintained by low frequencies or amplitudes of brain stimulation are often interpreted as an abuse-related effect. One advantage of ICSS as a tool in abuse potential testing is that it can be used to study effects produced by drugs like paraxanthine that have poor potency and solubility and that are difficult to deliver via intravenous routes common in drug self-administration procedures. Few studies have examined effects of caffeine and paraxanthine in ICSS procedures, and results have been inconsistent. For example, caffeine weakly but significantly increased ICSS in a procedure that evaluated responding maintained by different amplitudes of brain stimulation in rats (Bespalov, Lebedev, Panchenko, & Zvartau, 1999). However, caffeine did not increase break points maintained by delivery of brain stimulation under a progressive-ratio schedule (Bespalov, et al., 1999), and both caffeine and paraxanthine failed to produce abuse-related effects in an autotitration ICSS procedure (Mumford & Holtzman, 1990, 1991; Mumford, Neill, & Holtzman, 1988).
The present study compared effects of caffeine and paraxanthine in a “frequency-rate” ICSS procedure that has been widely used to evaluate other drugs of abuse (Carlezon & Chartoff, 2007; Negus & Miller, 2014; Wise, 1996). We hypothesized that both caffeine and paraxanthine would produce abuse-related increases in ICSS in this procedure with lower efficacy than the other stimulant drugs amphetamine and cocaine. A secondary goal of this study was to compare drug effects on patterns of reinforced and non-reinforced responding. Stimulants such as amphetamine can increase low rates of responding maintained by various contingencies, (Clark & Steele, 1966; Kelleher & Morse, 1968; Sanger & Blackman, 1976). Here we evaluated the degree to which caffeine and paraxanthine might produce dissociable effects on reinforced responding and non-reinforced responding.
Methods
Subjects
A total of 16 adult male Sprague-Dawley rats (Harlan, Frederick Maryland) were used. All rats had ad libitum access to food and water and were housed individually on a 12 h light-dark cycle (6am – 6pm, lights on) in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All rats weighed between 300 and 350g at the time of surgery. All experiments were performed with the approval of the Institutional Animal Care and Use Committee at Virginia Commonwealth University in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals 8th edition.
Assay of Intracranial Self-Stimulation (ICSS)
Surgery
Rats were anesthetized with isoflurane (3% in oxygen; Webster Veterinary, Phoenix, AZ, USA) until unresponsive to toe-pinch prior for implantation of stainless steel electrodes (Plastics One, Roanoke, VA, USA). The cathode, which was 0.25 mm in diameter and covered with polyamide insulation except at the flattened tip, was stereotaxically implanted into the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral to the midsagittal suture, and 8.8 mm ventral to the skull). Three screws were placed in the skull, and the anode (0.125 mm diameter, un-insulated) was wrapped around one of the screws to act as a ground. Dental acrylic was used to secure the electrode to the screws and skull. Ketoprofen (5 mg/kg) was used as a post-operative analgesic immediately and 24 hrs after surgery. Animals were allowed to recover for at least one week before ICSS training.
Apparatus
Operant chambers consisted of sound-attenuating boxes containing modular acrylic and metal test chambers (29.2 cm X 30.5 cm X 24.1 cm) (Med Associates, St. Albans, VT). Each chamber had a response lever (4.5 cm wide, 2.0 cm deep, 3.0 cm above the floor), three stimulus lights (red, yellow and green) centered 7.6 cm above the lever, a 2 W house light, and an ICSS stimulator. Bipolar cables routed through a swivel-commutator (Model SL2C, Plastics One) connected the stimulator to the electrode. MED-PC IV computer software controlled all programming parameters and data collection (Med Associates).
Training
The behavioral procedure was similar to that described previously (Altarifi & Negus, 2011; Bauer, Banks, Blough, & Negus, 2013b; Negus & Miller, 2014). In brief, the house light was illuminated during behavioral sessions, and lever press responding under a fixed-ratio 1 (FR1) schedule produced delivery of a 0.5 s train of square-wave cathodal pulses (0.1 ms per pulse) via the intracranial electrode. During brain stimulation, the stimulus lights over the lever were illuminated, and responding had no scheduled consequences; however, lever presses were recorded during this period. During initial 60 min training sessions, stimulation intensity was set at 150 μA, and stimulation frequency was set at 158 Hz. Stimulation intensity was then individually manipulated in each rat (intensities ranged from 135 μA- 295 μA) to identify an intensity that maintained reinforcement rates >30 stimulations/min. Once an appropriate intensity was identified, changes in frequency were introduced during sessions consisting of three consecutive 10 min components, each of which contained 10 consecutive 60 s trials. The stimulation frequency was 158 Hz for the first trial of each component, and frequency decreased in 0.05 log unit steps during the subsequent nine trials to a final frequency of 56 Hz. Each trial began with a 10 s time-out period, during which responding had no scheduled consequences, and five non-contingent stimulations at the designated frequency were delivered at 1 s intervals during the last 5 s of the time out. During the remaining 50 s of each trial, responding produced both intracranial stimulation at the designated frequency and illumination of the lever lights under an FR1 schedule as described above. ICSS performance was considered to be stable when frequency-rate curves were not statistically different over three consecutive days of training as indicated by lack of a significant effect of ‘day’ in a two-way ANOVA with day and frequency as the main effect variables. All training was completed within six weeks of surgery.
Testing
For dose-effect studies, test sessions consisted of three consecutive ‘baseline’ components followed first by a treatment interval and then by two consecutive ‘test’ components. The following drugs and doses were tested: amphetamine (0.1–1.0 mg/kg), cocaine (10 mg/kg), caffeine (3.2–32 mg/kg), and paraxanthine (3.2–32 mg/kg). Amphetamine and cocaine were assessed following a 10 min post-injection period, and caffeine and paraxanthine were assessed after a 30 min post-injection period. Test sessions were conducted on Tuesdays and Fridays, and three-component training sessions were conducted on other weekdays. The dose order for each drug was varied using a Latin-Square design. Experiments with any one drug were completed before progressing to another, and the order of testing different drugs was irregular across rats. Each drug was tested in groups of six or seven rats. Tests with different drugs with a given rat were separated by at least two weeks, and during this inter-drug interval, a vehicle test session was conducted.
Data Analysis
Three different dependent measures were assessed during each 50 s response period: total responses, reinforced responses, and non-reinforced responses. Total responses represent all lever presses made during the 50 s response period, regardless of reinforcement. Reinforced responses represent lever presses that produced stimulation. Non-reinforced responses represent lever presses that were emitted during stimulation and that did not produce additional stimulation. Therefore, total responses represent the sum of reinforced and non-reinforced responses emitted during each 50 s response period. These variables were averaged for each frequency (responses per trial) across test components for each rat, and then across rats, to produce frequency-response curves for each experimental manipulation. These data were analyzed by two-way ANOVA, with stimulation frequency and drug dose as the two factors. A significant ANOVA was followed by the Holm-Sidak post-hoc test. As a separate measure, responses across all frequencies (responses per component) were totaled and averaged across rats for each dose. These data were analyzed by Student’s t-test (cocaine) or one-way ANOVA followed by the Dunnet’s post-hoc test (all other compounds). For both sets of analyses, the criterion for significance was set at P<0.05.
Opportunities to emit non-reinforced responses were dependent on the occurrence of reinforced responses and the ensuing 0.5 s periods of brain stimulation. Consequently, changes in non-reinforced responding could result from either (a) changes in the number of opportunities to emit non-reinforced responses due to changes in the number of reinforced responses, or (b) changes in the probability (or rate) of responding during each opportunity. To provide a measure of drug effects on the probability of emitting a non-reinforced response after a reinforced response, the number of non-reinforced responses per 100 reinforced responses was calculated as (# Non-Reinforced Responses per Component ÷ # Reinforced Responses per Component) * 100. These values were averaged across rats for each dose of each drug, and dose-effect data for each drug were analyzed by Student’s t-test or one-way ANOVA followed by the Dunnett’s post-hoc test as above.
Drugs
(+)-Amphetamine sulfate and (−)-cocaine HCL were provided by the National Institute on Drug Abuse Supply Program (Bethesda, MD). 1,7-dimethylxanthine (paraxanthine) and 1,3,7- trimethylxanthine (caffeine) were purchased from Sigma Chemical Co. (St. Louis, MO). Paraxanthine was suspended in a solution of 5% dimethyl sulfoxide, 5% Tween80 and 90% sterile water and administered i.p. at 3 ml/kg. All other drugs were dissolved in sterile saline and administered i.p. at 1 ml/kg.
Results
Effect of amphetamine, cocaine, caffeine and paraxanthine on total lever presses
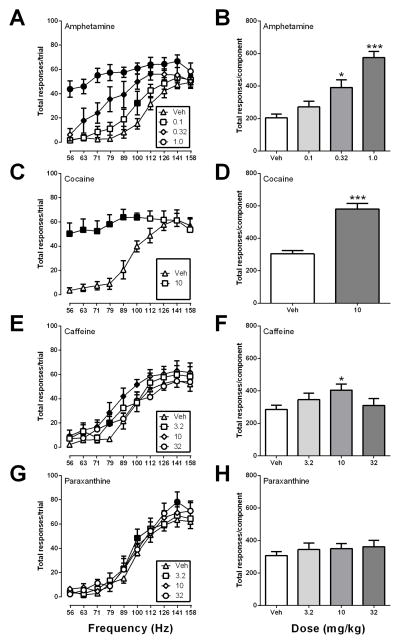
Under baseline conditions, brain stimulation maintained a frequency-dependent increase in responses. There was no significant difference between responding during baseline and vehicle test components for any drug (data not shown), so all comparisons were made to the vehicle test condition. Figure 1 shows effects of all four drugs on total lever presses. Amphetamine produced a robust increase in this measure of ICSS. For analysis of frequency-rate curves, there was a significant main effect of frequency [F(9, 45) = 26.63, p < .0001] and dose [F(3, 15) = 44.64, p < .0001] and a significant frequency x dose interaction [F(27, 135) = 4.06, p < .0001] (Fig. 1A). The number of responses per component also increased in a dose dependent manner [F(3, 5) = 44.64, p < .0001)] (Fig. 1B). Cocaine also increased total responses per trial and per component. For analysis of frequency-rate curves, there was a significant main effect of frequency [F(9, 45) = 7.59, p > .0001] and dose [F(1, 5) = 148.00, p < .0001] and a significant frequency x dose interaction [F(18, 90) = 8.03, p < .0001] (Fig. 1C), and the number of responses per component was also significantly increased [t(5) = 10.43, p < .001] (Fig. 1D). Caffeine and paraxanthine produced significant but smaller increases in ICSS. For caffeine effects on frequency-rate curves, there was a significant main effect of frequency [F(9, 54) = 21.90, p < .0001] and dose [F(3, 18) = 9.92, p < .001] (Fig. 1E). Caffeine also significantly altered total responses per component [F(3, 6) = 9.92, p < .01)], but the dose-effect curve had an inverted-U shape, and ICSS was increased only by the intermediate dose of 10 mg/kg caffeine (Fig. 1F). For paraxanthine effects on frequency-rate curves, there were also significant main effects of frequency [F(9, 45) = 69, p < .0001] and dose [F(3, 15) = 4.826, p < .01] (Fig. 1G). However, ICSS was increased at only a single frequency after paraxanthine doses of 3.2 mg/kg (100 Hz) and 32 mg/kg (141 Hz), and paraxanthine did not significantly alter total responses per component [F(3, 5) = 2.978, p = .09)] (Fig. 1H).
Figure 1.
Effects of amphetamine, cocaine, caffeine and paraxanthine on total lever presses made during each frequency trial (left panels) and summed across all frequency trials during each component (right panels). Horizontal axes: frequency of electrical stimulation in hertz (A,C,E,G) or dose of drug in mg/kg (B,D,F,H). Vertical axes: total number of lever presses per frequency trial (A,C,E,G) or per component (B,D,F,H). Filled symbols (A,C,E,G) indicate ICSS rates significantly different from vehicle rates as determined by a significant two-way ANOVA followed by the Holm-Sidak post-hoc test (p < .05). Asterisks (B,D,F,H) indicate significantly different from vehicle rates as determined by Student’s t-test (D) or by a significant one-way ANOVA followed by the Dunnet’s post-hoc test (B,F,H) (*p < .05, **p<0.01, ***p < .001). All points show mean ± SEM for 6–7 rats.
Effect of amphetamine, cocaine, caffeine and paraxanthine on reinforced responding
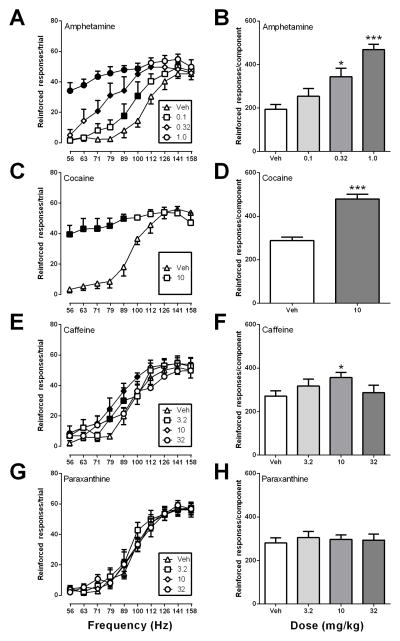
Figure 2 shows effects of all four drugs on reinforced responses. In the case of amphetamine for reinforced responding during each frequency trial, there was a significant main effect of frequency [F(9, 45) = 34.74, p < .0001] and dose [F(3, 15) = 41.79, p < .0001] and a significant interaction [F(27, 135) = 5.348, p < .0001], and amphetamine also increased the number of reinforced responses per component [F(3, 5) = 41.79, p < .0001)] (Fig. 2A,B). For 10 mg/kg cocaine effects on reinforced responses per trial, there was a also significant main effect of frequency [F(9, 45) = 15, p < .0001] and dose [F(1, 5) = 80.97, p < .0001] and a significant frequency x dose interaction [F(9, 45) = 12.04, p < .0001], and cocaine also produced a significant increase [t(5) = 8.99, p < .001] in reinforced responses per component (Fig. 2C,D). For the effects of caffeine on reinforced responses per trial, there was a significant main effect of frequency [F(9, 54) = 30.39, p < .0001] and dose [F(3, 18) = 8.48, p < .01]. Responding was increased at two frequencies for 3.2 mg/kg (79 and 89 Hz), three frequencies for 10 mg/kg (79, 89 and 100 Hz) and one frequency for 32 mg/kg (79 Hz) (Fig. 2E). Caffeine increased the number of reinforced responses per component only at 10 mg/kg [F(3, 6) = 8.480, p < .01] (Fig. 2F). In contrast to the other drugs, paraxanthine produced no changes in reinforced responses at any dose tested. There was only a main effect of frequency [F(9, 45) = 85.10, p < .0001)] following treatment with paraxanthine for reinforced responses per trial and no change in reinforced responding at any frequency for any dose (Fig. 2G). Paraxanthine also did not alter the number of reinforced responses per component (Fig. 2H).
Figure 2.
Effects of amphetamine, cocaine, caffeine and paraxanthine on reinforced responses made during each frequency trial (left panels) and summed across all frequency trials during each component (right panels). All other details as in Figure 1.
Effect of amphetamine, cocaine, caffeine and paraxanthine on non-reinforced responding
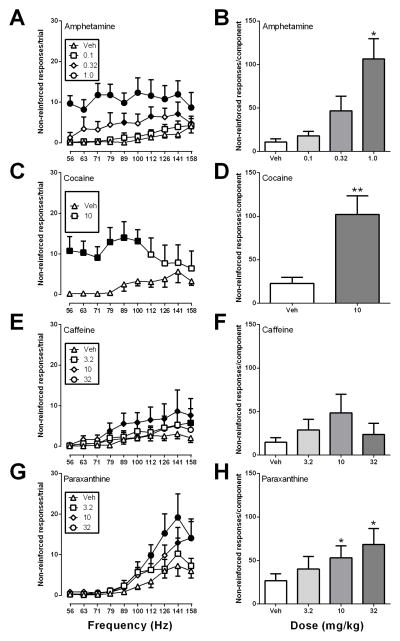
Figure 3 shows effects of all four drugs on non-reinforced responding. For amphetamine, there was a significant main effect of dose [F(3, 15) = 13.24, p < .001] on non-reinforced responses per trial, and amphetamine also dose-dependently increased the number of non-reinforced responses per component [F(3, 5) = 13.24, p < .01] (Fig. 3A,B). Similarly, for 10 mg/kg cocaine, there was a significant main effect of dose [F(1, 5) = 24.66, p < .01] on non-reinforced responses per trial, and cocaine also increased non-reinforced responses per component [t(5) = 4.96, p < .01] (Fig. 3C,D). For caffeine, there was a significant main effect of dose [F(3, 18) = 4.01, p < .05] on non-reinforced responses per trial. Non-reinforced responding per trial was significantly increased at one frequency for 3.2 mg/kg (79 Hz), seven frequencies for 10 mg/kg, and no frequencies at 32 mg/kg compared to vehicle (Fig. 3E). However, there was not a significant effect of caffeine on the number of non-reinforced responses per component. For paraxanthine effects on non-reinforced responding per trial, there was a significant main effect of frequency [F(9, 45) = 9.701, p < .0001] and dose [F(3, 15) = 7.573, p < .01] and a significant frequency x dose interaction [F(27, 135) = 3.468, p < .0001]. Non-reinforced responding per trial was increased at two frequencies at 10 mg/kg (141 and 158 Hz) and four frequencies at 32 mg/kg (112–158 Hz) (Fig. 2G). Paraxanthine also significantly increased the number of non-reinforced responses per component [F(3, 5) = 7.57, p < .01] (Fig. 2H).
Figure 3.
Effects of amphetamine, cocaine, caffeine and paraxanthine on non-reinforced responses made during each frequency trial (left panels) and summed across all frequency trials during each component (right panels). All other details as in Figure 1
Table 1 shows the probability of non-reinforced response given the occurrence of a reinforced response [expresses as (# Non-Reinforced Responses per Component ÷ # Reinforced Responses per Component) * 100]. This measure of the probability of non-reinforced response given a reinforced response was increased by amphetamine [F(3, 5) = 8.870, p < .01], cocaine [t(5) = 3.891, p< .05] and paraxanthine [F(3, 5) = 10.76, p < .01], but not by caffeine.
Table 1.
Drug effects on the probability that a reinforced response would be followed by a non-reinforced response (expressed as mean±SEM #Non-Reinforced Responses per 100 Reinforced Responses).
| Treatment | # Non-Reinforced Responses per 100 Reinforced Responses |
|---|---|
| Amphetamine | Mean ± SEM |
| Vehicle | 6.1 ± 1.9 |
| 0.1 mg/kg | 7.8 ± 2.5 |
| 0.32 mg/kg | 13.1 ± 3.9 |
| 1.0 mg/kg | 22.7 ± 4.6 ** |
| Cocaine | |
| Vehicle | 7.9 ± 2.9 |
| 10 mg/kg | 21.3 ± 4.7* |
| Caffeine | |
| Vehicle | 5.4 ± 1.9 |
| 3.2 mg/kg | 8.2 ± 3.5 |
| 10 mg/kg | 13.2 ± 5.7 |
| 32 mg/kg | 7.4 ± 3.7 |
| Paraxanthine | |
| Vehicle | 9.8 ± 2.9 |
| 3.2 mg/kg | 12.3 ± 3.3 |
| 10 mg/kg | 17.3 ± 3.9* |
| 32 mg/kg | 23.2 ± 5.1* |
p < .01,
p < .05 compared to vehicle using Student’s t-test or one-way ANOVA and Dunnett’s post-hoc test.
Discussion
This study used a frequency-rate ICSS procedure to compare abuse-related effects of caffeine and its primary metabolite paraxathine with effects of the other abused psychostimulants amphetamine and cocaine. There were three main findings. First, as reported previously, both amphetamine and cocaine produced robust increases in ICSS responding consistent with their status as drugs with high abuse potential, and both drugs increased both reinforced and non-reinforced responses. Second, caffeine and paraxanthine produced significant but smaller increases in ICSS relative to amphetamine and cocaine, and these two drugs also differed in their effects on reinforced vs. non-reinforced responding. Specifically, caffeine increased both reinforced and non-reinforced responding, whereas paraxanthine increased only non-reinforced responding. Finally, these results suggest that measures of reinforced and non-reinforced responding may contribute to characterization of drug effects on ICSS.
The facilitation of ICSS by amphetamine and cocaine in this study is similar to previous results from our laboratory (Bauer, Banks, Blough, & Negus, 2013a; Bauer, Banks, & Negus, 2014), and from several other laboratories using various ICSS procedures (for review see (Negus & Miller, 2014)). Our results for the effect of caffeine agree with previous studies that examined caffeine effects in procedures in which various rates of ICSS were maintained by various frequencies or amplitudes of brain stimulation (Bespalov, et al., 1999; Burov Iu & Borisenko, 1976). Moreover, the present results are consistent with previous findings that caffeine produces an inverted U-shaped dose-effect curve, and has weaker abuse-related effects than either amphetamine or cocaine, in ICSS procedures (Bespalov, et al., 1999; Valdes, McGuire, & Annau, 1982), place conditioning procedures (Brockwell, Eikelboom, & Beninger, 1991; Patkina & Zvartau, 1998) and drug self-administration procedures (Griffiths & Woodson, 1988). In contrast to the present study, caffeine did not facilitate ICSS in studies that used a different type of ICSS procedure (an autotitration procedure; (Mumford & Holtzman, 1990, 1991; Mumford, et al., 1988). The limited range of conditions across which caffeine produces an abuse-related facilitation of ICSS resembles the limited range of conditions across which caffeine maintains self-administration (Griffiths and Woodson, 1988) and provides additional evidence for the weak abuse-related effects of caffeine relative to some other drugs, such as amphetamine or cocaine.
Paraxanthine effects on ICSS have been examined in only two previous studies, which used an autotitration ICSS procedure (Mumford & Holtzman, 1990, 1991). In these studies, paraxanthine, like caffeine, failed to facilitate ICSS. The present study extends on these previous results by evaluating paraxanthine effects in a procedure in which caffeine did produce an abuse-related facilitation of ICSS. In this procedure, paraxanthine also significantly increased responding for brain stimulation; however, these effects were smaller than those of caffeine, and as will be discussed further below, paraxanthine effects were also qualitatively different from caffeine effects. Accordingly, these results suggest that paraxanthine has little or no abuse potential, and these results also do not support the hypothesis that abuse-related effects of caffeine are mediated by its metabolism to paraxanthine. These findings in an ICSS procedure also differ from previous findings that paraxanthine was more efficacious than caffeine in rats to stimulate both locomotor activity and dopamine release in dorsolateral striatum (Orru, et al., 2013). An implication of this discrepancy is that mechanisms underlying striatal dopamine release and locomotor stimulant effects of paraxanthine are distinct from mechanisms that mediate facilitation of ICSS.
A secondary goal if this study was to compare drug effects on reinforced responding and non-reinforced responding during behavioral sessions. Reinforced responding was defined as responses that produced brain stimulation, whereas non-reinforced responding was defined as responses that occurred during the 0.5 sec periods of brain stimulation delivery and did not produce further brain stimulation. Under vehicle treatment conditions, reinforced responding was approximately 10–20 fold higher than non-reinforced responding, and amphetamine and cocaine increased both reinforced and non-reinforced responding. Opportunities to emit non-reinforced responses were dependent on the occurrence of a reinforced response, and as a result, drug-induced increases in non-reinforced responding could have merely reflected the increased opportunities afforded by increases in reinforced responding. To address this issue, we calculated the probability of a non-reinforced response given the occurrence of a reinforced response, and both amphetamine and cocaine increased this measure. This finding suggests that amphetamine and cocaine effects on non-reinforced responding were due not only to an increased number opportunities for non-reinforced responding, but also to increased probability of responding during those opportunities. Taken together, these findings agree with previous studies reporting that amphetamine and cocaine can increase low rates of responding maintained by other contingencies (Clark & Steele, 1966; Kelleher & Morse, 1968; Lobarinas & Falk, 1999; Lobarinas, Lau, & Falk, 1999; Sanger & Blackman, 1976).
Caffeine also increased both reinforced responding and non-reinforced responding, and this agrees with evidence for caffeine-induced stimulation of low response rates under other circumstances, such as the early segments of intervals from fixed-interval schedules of food-maintained responding (McMillan, 1979; Michaelis, Holloway, Bird, & Huerta, 1987; Randall et al., 2011; Spealman, 1988; Valdes, et al., 1982). However, in contrast to the effects of amphetamine, the response increasing effects of caffeine on both reinforced and non-reinforced responding were characterized by an inverted-U shaped dose-effect curve, and peak increases in reinforced and non-reinforced responding were smaller than those produced by amphetamine or cocaine. Moreover, unlike amphetamine and cocaine, caffeine did not produce a significant increase in the probability of a non-reinforced response given a reinforced response. Thus, the caffeine-induced increases in non-reinforced responding can be attributed primarily to the increased number of opportunities for non-reinforced responding.
In contrast to the other drugs tested here, paraxanthine failed to increase reinforced responding, and instead increased only non-reinforced responding and thus the probability of non-reinforced responding after a reinforced response. The lack of paraxanthine effects on reinforced responding cannot be attributed to inadequate dosing. In addition to producing significant increases in non-reinforced responding in the present study, paraxanthine was tested here at doses that also stimulated both locomotor activation and striatal dopamine release in previous studies (Orru, et al., 2013). The narrow range of conditions across which paraxanthine increased responding, and especially the lack of paraxanthine effects on reinforced responding, provides further evidence to suggest that paraxanthine does not produce stimulant-like abuse-related effects in this ICSS procedure.
It is unknown if different neurobiological mechanisms mediate drug effects on reinforced vs. non-reinforced responding in this ICSS procedure; however, the present results do provide evidence to suggest that these effects can be dissociated. For example, the low dose of 0.1 mg/kg amphetamine significantly increased reinforced responding maintained by intermediate frequencies of brain stimulation (89 and 100 Hz, see Figure 2A), but this dose did not increase non-reinforced responding at any frequency of brain stimulation. Conversely, as noted above, paraxanthine increased non-reinforced responding but not reinforced responding. Finally, as implied by Table 1, drug effects on non-reinforced responding could be attributed not only to an increased number of opportunities due to increased reinforced responding, but also to increases in the probability of non-reinforced response give the occurrence of a reinforced response.
The basis for distinct profiles of effects for caffeine and paraxanthine on ICSS is also not currently known. However, previous studies have identified differences in the pharmacology of caffeine and paraxanthine in regards to their effects on locomotor activity and extracellular dopamine leves in dorsal striatum. For instance, paraxanthine is able to reverse locomotor-suppressing effects of both the adenosine type A2 (A2A) receptor agonist CGS 21680 and adenosine type A1 (A1) receptor agonist N6-cyclopentyladenosine (CPA), whereas caffeine is only able to reverse the latter (Orru, et al., 2013). Moreover, paraxanthine-induced increases in locomotor activity and dopamine levels in dorsolateral striatum appear to be partly mediated by increases in cGMP levels, whereas cGMP does not appear to mediate locomotor stimulation by caffeine, and caffeine does not increase dopamine levels in dorsolateral striatum (Orru, et al., 2013). These results together with results from the present study suggest that paraxanthine might be more likely than caffeine to produce motor activating effects mediated by A2A receptor antagonism, cGMP accumulation, and enhanced DA signaling in dorsolateral striatum but less likely than caffeine to produce rewarding effects mediated by DA signaling in ventral striatum. These differences may suggest a role of adenosine receptors and cGMP signaling in mediating the increases in non-reinforced vs reinforced responding as they pertain to the actions of psychostimulants.
Abbreviations
- ICSS
intracranial self-stimulation
- paraxanthine
1,7-dimethylxanthine
- caffeine
1,3,7 trimethylxanthine
- CGS 21860
-[4-[2-[[6-amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxy-oxolan-2-yl]purin-2-yl]amino]ethyl]phenyl]propanoic acid
- CPA
N6-cyclopentyladenosine
- ANOVA
analysis of variance
- i.p
intraperitoneal
- A2A
adenosine type A2A
- A1
adenosine type A1
- FR
fixed ratio
- Veh
vehicle
References
- Altarifi AA, Negus SS. Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behavioural Pharmacology. 2011;22:663–673. doi: 10.1097/FBP.0b013e32834aff54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud MJ. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handbook of Experimental Pharmacology. 2011:33–91. doi: 10.1007/978-3-642-13443-2_3. [DOI] [PubMed] [Google Scholar]
- Baron M, Elie M, Elie L. An analysis of legal highs: do they contain what it says on the tin? Drug Testing and Analysis. 2011;3:576–581. doi: 10.1002/dta.274. [DOI] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Rate-dependent effects of monoamine releasers on intracranial self-stimulation in rats: implications for abuse liability assessment. Behavioural Pharmacology. 2013a;24:448–458. doi: 10.1097/FBP.0b013e328363d1a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. British Journal of Pharmacology. 2013b;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Negus SS. The effect of chronic amphetamine treatment on cocaine-induced facilitation of intracranial self-stimulation in rats. Psychopharmacology. 2014;231:2461–2470. doi: 10.1007/s00213-013-3405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov A, Lebedev A, Panchenko G, Zvartau E. Effects of abused drugs on thresholds and breaking points of intracranial self-stimulation in rats. European Neuropsychopharmacology. 1999;9:377–383. doi: 10.1016/s0924-977x(99)00008-5. [DOI] [PubMed] [Google Scholar]
- Borycz J, Pereira MF, Melani A, Rodrigues RJ, Kofalvi A, Panlilio L, Ferre S. Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. Journal of Neurochemistry. 2007;101:355–363. doi: 10.1111/j.1471-4159.2006.04386.x. [DOI] [PubMed] [Google Scholar]
- Brockwell NT, Eikelboom R, Beninger RJ. Caffeine-induced place and taste conditioning: production of dose-dependent preference and aversion. Pharmacology, Biochemistry, and Behavior. 1991;38:513–517. doi: 10.1016/0091-3057(91)90006-n. [DOI] [PubMed] [Google Scholar]
- Burov Iu V, Borisenko SA. The effect of neurotropic substances on the self-stimulation reaction at the thalamic level. Biulleten’ Eksperimental’noi Biologii i Meditsiny. 1976;81:43–45. [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nature Protocols. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Clark FC, Steele BJ. Effects of D-amphetamine on performance under a multiple schedule in the rat. Psychopharmacologia. 1966;9:157–169. doi: 10.1007/BF00404720. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Woodson PP. Reinforcing properties of caffeine: studies in humans and laboratory animals. Pharmacology, Biochemistry, and Behavior. 1988;29:419–427. doi: 10.1016/0091-3057(88)90180-3. [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Steelman SC, Thomas SL. Multi-ingredient, Caffeine-Containing Dietary Supplements: History, Safety, and Efficacy. Clinical Therapeutics. 2014 doi: 10.1016/j.clinthera.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Grant BF. DSM-5 criteria for substance use disorders: recommendations and rationale. The American Journal of Psychiatry. 2013;170:834–851. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano LM, Evatt DP, Richards BD, Griffiths RR. Characterization of individuals seeking treatment for caffeine dependence. Psychology of Addictive Behaviors. 2012;26:948–954. doi: 10.1037/a0027246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. Determinants of the specificity of behavioral effects of drugs. Ergebnisse der Physiologie, Biologischen Chemie und Experimentellen Pharmakologie. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- Lelo A, Kjellen G, Birkett DJ, Miners JO. Paraxanthine metabolism in humans: determination of metabolic partial clearances and effects of allopurinol and cimetidine. The Journal of Pharmacology and Experimental Therapeutics. 1989;248:315–319. [PubMed] [Google Scholar]
- Lelo A, Miners JO, Robson R, Birkett DJ. Assessment of caffeine exposure: caffeine content of beverages, caffeine intake, and plasma concentrations of methylxanthines. Clinical Pharmacology and Therapeutics. 1986;39:54–59. doi: 10.1038/clpt.1986.10. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Falk JL. Dose-dependent effects but not sensitization of DRL 45-s performance by oral d-amphetamine with cumulative- and repeated-dosing regimens. Behavioural Pharmacology. 1999;10:739–746. doi: 10.1097/00008877-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Lau CE, Falk JL. Sensitization of operant behavior to oral cocaine with increasing- and repetitive-dose regimens. Behavioural Pharmacology. 1999;10:15–26. doi: 10.1097/00008877-199902000-00002. [DOI] [PubMed] [Google Scholar]
- McMillan DE. Effects of d-amphetamine and caffeine on schedule-controlled and schedule-induced responding. Journal of the Experimental Analysis of Behavior. 1979;32:445–456. doi: 10.1901/jeab.1979.32-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith SE, Juliano LM, Hughes JR, Griffiths RR. Caffeine Use Disorder: A Comprehensive Review and Research Agenda. Journal of Caffeine Research. 2013;3:114–130. doi: 10.1089/jcr.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis RC, Holloway FA, Bird DC, Huerta PL. Interactions between stimulants: effects on DRL performance and lethality in rats. Pharmacology, Biochemistry, and Behavior. 1987;27:299–306. doi: 10.1016/0091-3057(87)90573-9. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Holtzman SG. Methylxanthines elevate reinforcement threshold for electrical brain stimulation: role of adenosine receptors and phosphodiesterase inhibition. Brain Research. 1990;528:32–38. doi: 10.1016/0006-8993(90)90191-d. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Holtzman SG. Do adenosinergic substrates mediate methylxanthine effects upon reinforcement thresholds for electrical brain stimulation in the rat? Brain Research. 1991;550:172–178. doi: 10.1016/0006-8993(91)90425-u. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Neill DB, Holtzman SG. Caffeine elevates reinforcement threshold for electrical brain stimulation: tolerance and withdrawal changes. Brain Research. 1988;459:163–167. doi: 10.1016/0006-8993(88)90298-3. [DOI] [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacological Reviews. 2014;66:869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neuroscience and Biobehavioral Reviews. 1999;23:563–576. doi: 10.1016/s0149-7634(98)00050-5. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Ueki H. Clinical importance of caffeine dependence and abuse. Psychiatry and Clinical Neurosciences. 2007;61:263–268. doi: 10.1111/j.1440-1819.2007.01652.x. [DOI] [PubMed] [Google Scholar]
- Okada M, Mizuno K, Kaneko S. Adenosine A1 and A2 receptors modulate extracellular dopamine levels in rat striatum. Neuroscience Letters. 1996;212:53–56. doi: 10.1016/0304-3940(96)12780-4. [DOI] [PubMed] [Google Scholar]
- Orru M, Guitart X, Karcz-Kubicha M, Solinas M, Justinova Z, Barodia SK, Ferre S. Psychostimulant pharmacological profile of paraxanthine, the main metabolite of caffeine in humans. Neuropharmacology. 2013;67:476–484. doi: 10.1016/j.neuropharm.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkina NA, Zvartau EE. Caffeine place conditioning in rats: comparison with cocaine and ethanol. European Neuropsychopharmacology. 1998;8:287–291. doi: 10.1016/s0924-977x(97)00086-2. [DOI] [PubMed] [Google Scholar]
- Quarta D, Ferre S, Solinas M, You ZB, Hockemeyer J, Popoli P, Goldberg SR. Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. Journal of Neurochemistry. 2004;88:1151–1158. doi: 10.1046/j.1471-4159.2003.02245.x. [DOI] [PubMed] [Google Scholar]
- Randall PA, Nunes EJ, Janniere SL, Stopper CM, Farrar AM, Sager TN, Salamone JD. Stimulant effects of adenosine antagonists on operant behavior: differential actions of selective A2A and A1 antagonists. Psychopharmacology. 2011;216:173–186. doi: 10.1007/s00213-011-2198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks--a growing problem. Drug and Alcohol Dependence. 2009;99:1–10. doi: 10.1016/j.drugalcdep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger DJ, Blackman DE. Rate-dependent effects of drugs: a review of the literature. Pharmacology, Biochemistry, and Behavior. 1976;4:73–83. doi: 10.1016/0091-3057(76)90178-7. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Psychomotor stimulant effects of methylxanthines in squirrel monkeys: relation to adenosine antagonism. Psychopharmacology. 1988;95:19–24. doi: 10.1007/BF00212759. [DOI] [PubMed] [Google Scholar]
- Stoker AK, Markou A. The intracranial self-stimulation procedure provides quantitative measures of brain reward function. In: Gould TD, editor. Mood and anxiety related phenotypes in mice: characterization using behavioral tests, Volume II. Vol. 63. Totowa: Humana Press; 2011. pp. 307–331. [Google Scholar]
- Valdes JJ, McGuire PS, Annau Z. Xanthines alter behavior maintained by intracranial electrical stimulation and an operant schedule. Psychopharmacology. 1982;76:325–328. doi: 10.1007/BF00449119. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annual Review of Neuroscience. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]