Abstract
Objectives
To describe and understand varieties and characteristics of sensitization contributing to hyperalgesia for patients with chronic pain conditions.
Methods
Thermal stimulation was delivered to the face, forearm and calf of pain-free subjects and individuals with irritable bowel syndrome (IBS), temporomandibular pain disorder (TMD) and fibromyalgia syndrome (FMS). Three second contacts of a preheated thermode occurred at 30 sec. intervals in ascending and then descending series (0.7°C steps).
Results
Thermal pain ratings during ascending series were greater at each site for individuals diagnosed with chronic pain. Strong pain at the time of testing further enhanced the ratings at all sites, but mild or moderate clinical pain did not have this effect. Thermal pain for all subjects was greater during descending series than during ascending series of arm and leg stimulation. The hypersensitivity during descending series was comparable for pain-free, FMS and TMD subjects but was increased in duration for arm or leg stimulation of FMS subjects.
Discussion
The widespread sensitization for IBS and TMD subjects does not rely on mechanisms of spatial and temporal summation often invoked to explain widespread hyperalgesia associated with chronic pain. Increased sensitivity during descending series during stimulation of an arm or leg but not the face indicates a propensity for sensitization of nociceptive input to the spinal cord. Abnormally prolonged sensitization for FMS patients reveals a unique influence of widespread chronic pain referred to deep somatic tissues.
Keywords: Psychophysics of chronic pain, temporomandibular pain disorder, irritable bowel syndrome, fibromyalgia, sensitization
Introduction
Chronic pain typically is associated with increased sensitivity to somatosensory stimulation. When allodynia and hyperalgesia are present for stimulation of injured tissues, this can be accounted for by a peripheral release of inflammatory mediators, along with central neuronal and glial reactions to partial deafferentation and abnormal input from the injury. In addition, investigations have described hypersensitivity for cutaneous stimulation within the dermatomal distribution of chronic pain localized to deep tissues. Patients with irritable bowel syndrome (IBS; visceral pain) can be hypersensitive to nociceptive stimulation of foot or leg skin 1-6. Similarly, patients with temporomandibular disorder (TMD; facial joint pain) can be hypersensitive to stimulation of cutaneous nociceptors supplying the face 7,8. These effects may result from segmental convergence of input from deep nociceptors and cutaneous nociceptors onto common spinal networks of neurons 6. However, it is difficult to assign mechanisms for hypersensitivity to stimulation outside the dermatomal distribution of regionally referred chronic pain. For example, if convergent interaction underlies central sensitization of cutaneous pain for chronic pain conditions, then sensitization of facial pain should be minimal or absent for IBS subjects, and sensitization of pain during stimulation of a leg would be minimal or absent for TMD subjects. Trigeminothalamic and spinothalamic projection systems are anatomically distinct and are unlikely to mutually interact to the same extent as can occur between segmentally matched inputs to the CNS.
In addition to sensitization of central neurons receiving nociceptive input from injured tissues, pain generates psychological stress, which disrupts autonomic regulation 9,10. Chronic stress in association with chronic pain may constitute a mechanism for extrasegmental sensitization 11,12 that is not limited to caudal spinal dermatomes for IBS or trigeminal areas for TMD. An additional consequence of stress with sympathetic activation is that blood flow to muscles is reduced, which can result in widespread input from nociceptors in ischemic muscles 13-15. Widespread nociceptive input from deep tissues of FMS subjects could result in potent and spatially distributed influences of this condition on cutaneous sensitivity, compared to regionally referred pain conditions (IBS and TMD).
Sensitivity to cutaneous thermal stimulation of the face, arm and leg of FMS, IBS and TMD patients is compared to control subjects in the present study to: 1) describe the spatial extent of sensitization in relation to the regional distribution of chronic pain, 2) determine whether sensitization depends upon the presence or intensity of chronic pain at the time of sensory testing, and 3) evaluate whether cutaneous thermal sensitization differs for the 3 pain conditions.
Materials and Methods
Twenty three healthy subjects (average age: 31; range: 19-66), 12 patients with irritable bowel syndrome (IBS) (average age: 32; range: 18-52), 31 patients with temporomandibular joint disorder (TMD) (average age: 32: range: 20-54) and 9 patients with fibromyalgia syndrome (FMS) (average age: 46; range 23-66) were recruited from clinics at the University of Florida. All participants underwent a screening visit to ensure compliance with all inclusion/exclusion criteria for each disorder. The visit included blood pressure measurement, a health questionnaire, and physical examination. Written informed consent was obtained from all participants once the nature of the study had been thoroughly explained. The procedures were conducted under approval of the University of Florida Institutional Review Board and the Veterans Administration SCI committee.
FMS subjects met the 1990 American College of Rheumatology criteria for FMS which includes widespread pain and the presence of at least 11 tender points (Geel, 1994). Patients of the TMD group met the Research Diagnostic Criteria for an Axis I, Group I disorder (Dworkin et at, 1992). Individuals with TMD were excluded if there was co-morbid fibromyalgia syndrome, irritable bowel syndrome or arthrogenic pain. The criteria for the IBS group required a diagnosis based upon the Rome II criteria 16 and an absence of other diseases (including chronic pain such as FMS). All patients reported the presence of chronic pain for at least 6 months prior to the beginning of the study. All subjects were instructed to take no pain medication for one day prior to each test session. The criteria for members of the control group required no significant spontaneous pain anywhere in the body, no ongoing pharmacotherapy with narcotics or antidepressants, and no disease that might significantly affect pain perception or unduly increase risk of injury (e.g., neurological disorders, serious psychiatric disorders, diabetes, hypertension, serious cardiovascular disorders, and chronic pain diseases such as fibromyalgia syndrome).
Thermal stimuli were administered by brief (3 sec.) contact of a preheated thermode to the face, lateral calf and volar forearm. This method of stimulation is naturalistic, and it avoids ambiguity presented by effects of ramp rate on thermal sensitivity 17. The thermode had a flat square-shaped copper contact surface of 23x23 mm, which was electronically held at the desired temperature by a Peltier thermoelectric device. It was brought from off the skin onto light contact by solenoid activation. The stimulator assembly was mounted on an adjustable arm for positioning to any desired stimulation site.
The subjects were asked to rate pain intensity by moving the slider of an electronic visual analog scale (eVAS) from left to right. Instructions regarding the use of the scale and its end-points (“no pain” on the left and “intolerably intense pain” on the right) were given by a standardized video. The slider's position was recorded as a percentage of its total travel (0-100). The eVAS slider was mounted into the surface of a small inclined desk, which was positioned to facilitate precise operation with minimal fatigue. During the experiment, the subject was separated from the investigator by an equipment rack to prevent non-verbal communication and transmission of bias.
Prior to each test session, all subjects rated the intensity of any ongoing pain, using the 0-100 VAS scale, with separate ratings for current pain intensity distributed above and below the umbilicus. Each participant then underwent test sessions on three non-consecutive days. During each session, thermal stimuli were presented to the volar forearm, the lateral calf and the face (over the masseter muscle) in trials separated by 3 min. The order of sites was varied between subjects and sessions, with equal representation of the six sequences. During a trial, three sec. contacts of the Peltier thermode with the skin occurred at intervals of 30 sec. The initial stimulus in a trial was 43°C, and thermode temperature increased by 0.7°C from one stimulus to the next until an eVAS rating of 40 was reached or exceeded. The defined endpoint of ascending series provided stimulus-response functions for low to moderate levels of pain without producing intolerable levels of pain for highly sensitive subjects. A descending temperature series was initiated 30 sec. following the last stimulus in the ascending series, beginning with the temperature of the last stimulus in the ascending series and decreasing in 0.7°C increments to 43°C. The subjects rated pain intensity within 5 seconds of the end of each stimulus pulse. The slider automatically returned to the left endpoint at that time.
The following measures of pain sensitivity were analyzed using 2 way ANOVAs: 1) Ratings of pain during ascending series of stimulus intensities were averaged across three sessions for individual subjects. Statistical comparisons of control subjects wityh each patient group (main effects) utilized eVAS ratings of different temperatures as repeated measures. 2) An influence of the presence or absence of clinical pain on temperatures that elicited pain ratings > eVAS 15 for stimulation of the face, arm or leg was evaluated with t-tests. 3) Temperatures that elicited pain ratings > 15 on the eVAS scale were related to the magnitude of clinical pain on each day of testing, with statistical comparison of clinical pain ranges (main effect) and different sites of stimulation as repeated measures. 4) Ratings of sensation intensity were compared during progressions up to the end of ascending series and down from the beginning of descending series, to describe effects of the ascending series on responses to the same temperatures during the descending series. For each group of subjects and site of stimulation, ratings during ascending series were compared with ratings during descending series (main effect). Intensities relative to the end of ascending series provided repeated measures. 5) Each patient group was compared with control subjects (main effect) in terms of differences between ratings of stimuli in ascending and descending series (repeated measures). Statistical comparisons were conducted with Statistica software (Statsoft, Inc., Tulsa, OK). A probability level 0.05 was corrected to 0.017 to establish statistical significance for results from stimulation of a group of subjects at 3 sites (Bonferroni method).
Results
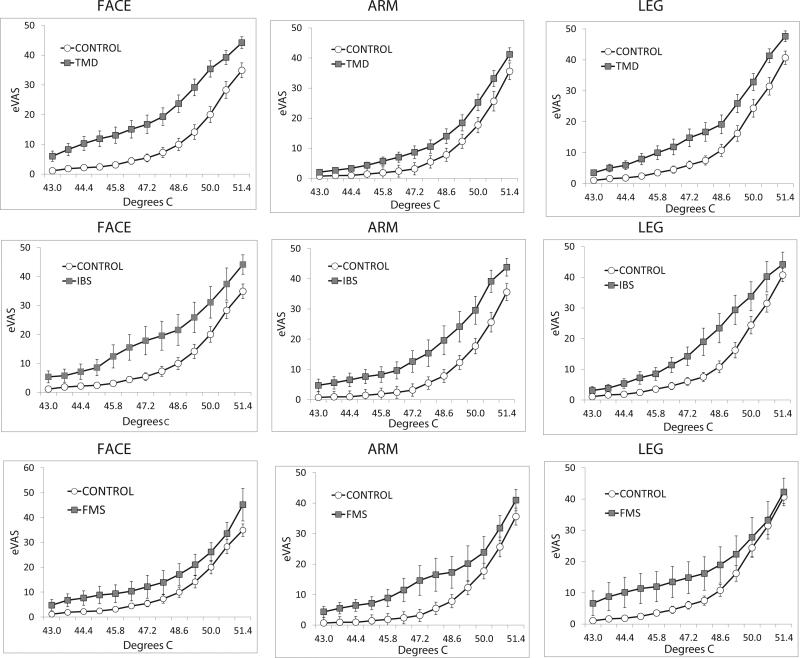
Figure 1 presents eVAS ratings during ascending series of thermal stimulus intensities for the face, arm and leg of TMD, IBS and FMS subjects, compared to control subjects. Statistical analysis utilized stimulus intensities (43°C to 51.4°C) that produced eVAS ratings up to 40 for each group of patients and each stimulation site. For individual subjects with an eVAS rating greater than 40 at a temperature less than 51.4°C, their highest rating was entered for subsequent temperatures up to 51.4°C. This provided a conservative test of differences from the control group. Sensitivity across stimulus intensities was greater for the pain patients at each site of stimulation (Figure 1). Three of these differences were not quite significant at the corrected confidence level of 0.017 (Table 1), but the probability that all 12 comparisons would equal or exceed the 0.02 confidence level is quite low.
Figure 1.
Ratings of pain intensity elicited by ascending series of 3 sec heat stimuli at interstimulus intervals of 30 sec. Each series ended when the eVAS rating equaled or exceeded 40. Stimulation was delivered to the face (left column), arm (middle column) and leg (right column). Pain sensitivity for the ascending progression of stimulus intensities was consistently greater (rating were higher) for subjects with chronic pain (closed squares: TMD, upper row; IBS, middle row; FMS, bottom row) than for pain-free control subjects (open circles). Errors bars depict between subjects’ variability as standard errors of the mean (S.E.M).
TABLE 1.
S-R FUNCTIONS: CONTROLS VS. PATIENTS
| Main Effect | Interaction | ||||||
|---|---|---|---|---|---|---|---|
| F | p | df | F | p | df | ||
| TMD | Face | 15.26 | <0.000* | 1 | 2.66 | 0.001* | 12 |
| Arm | 5.64 | 0.021 | 1 | 1.34 | 0.19 | 12 | |
| Leg | 8.56 | 0.005* | 1 | 1.41 | 0.16 | 12 | |
| IBS | Face | 8.91 | 0.005* | 1 | 1.95 | 0.028 | 12 |
| Arm | 11.34 | 0.002* | 1 | 2.14 | 0.013* | 12 | |
| Leg | 7.11 | 0.012* | 1 | 2.83 | 0.001* | 12 | |
| FMS | Face | 5.73 | 0.023 | 1 | 0.04 | 0.96 | 12 |
| Arm | 7.70 | 0.009* | 1 | 1.06 | 0.39 | 12 | |
| Leg | 5.88 | 0.021 | 1 | 0.30 | 0.99 | 12 | |
Analysis of variance comparisons of stimulus-response functions for ascending series of stimulus intensities presented to control subjects vs. TMD, IBS and FMS subjects. Significant effects indicated by.
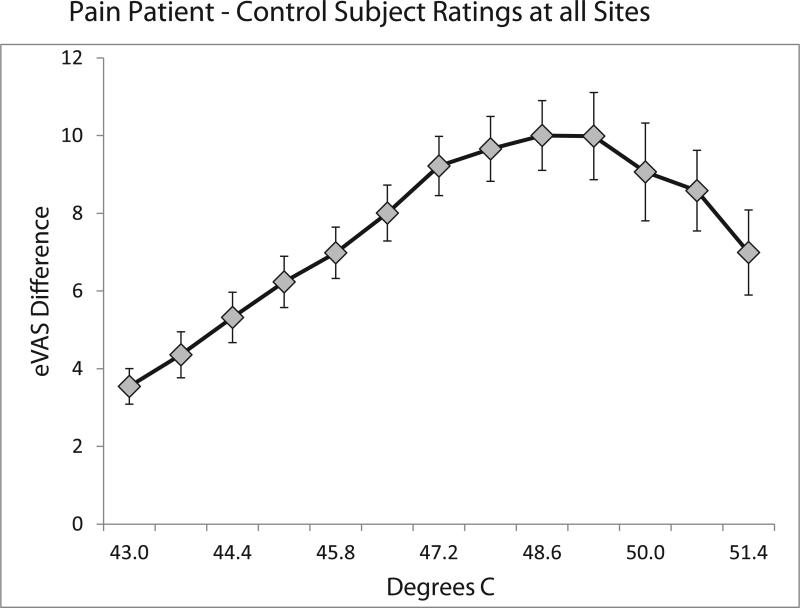
In order to identify temperatures associated with the maximum hypersensitivity for patients, differences between eVAS ratings of control subjects and all pain patients for all sites of stimulation were calculated (Figure 2). The largest differences in sensitivity for patients and controls occurred at temperatures less than 50°C that were associated with eVAS ratings below 20 for control subjects (as shown in Figure 1). Thus, sensitivity to low levels of nociceptive thermal stimulation was especially enhanced for IBS, TMD and FMS subjects. Accordingly, as a basis for evaluation of relationships between cutaneous thermal sensitivity and the presence and intensity of clinical pain, the first temperature in ascending series that produced a rating equal or greater than eVAS 15 was calculated for all subjects.
Figure 2.
The average eVAS ratings of patients with chronic pain (TMD, IBS, FMS) minus the average eVAS ratings of pain-free control subjects for ascending series of stimulation at all sites (with S.E.M. error bars). The differences in ratings peaked for temperatures (48.6°C and 49.3°C) rated below eVAS 20 by control subjects (see Figure 1).
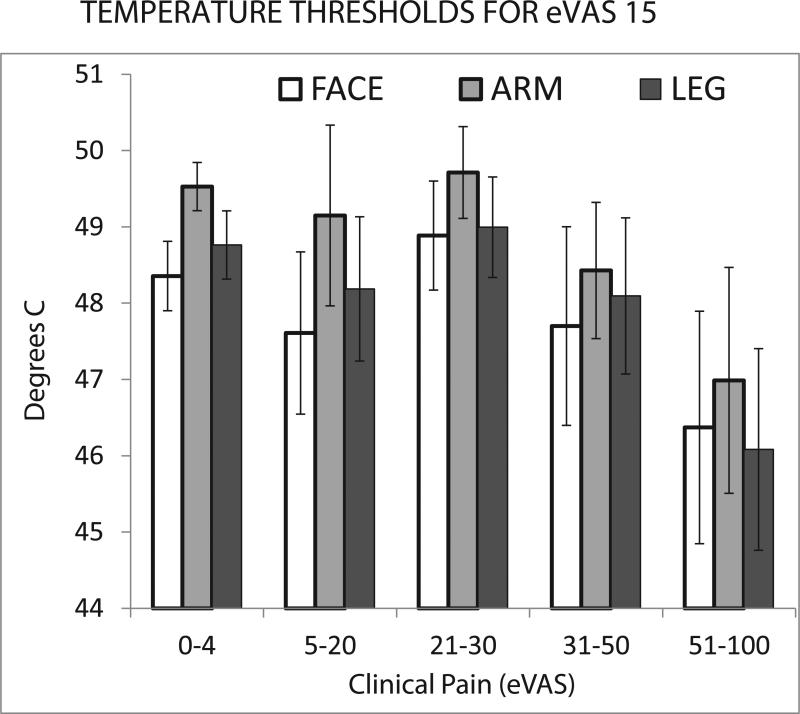
Evidence for an effect of clinical pain magnitude on cutaneous thermal pain sensitivity is provided in Figure 3 where eVAS ranges of maximal clinical pain ratings at the time of testing for all patients are plotted against temperatures sufficient to evoke mild pain (eVAS 15) at the 3 stimulation sites. Statistical evaluation revealed a significant decrease in temperatures that evoked mild thermal pain as a function of 5 ranges of clinical pain intensity (main effect of clinical pain: F=3.23; p=0.014, df=4; differences in sensitivity for stimulation of the face, arm and leg: F=9.60, p<0.000; interaction between stimulus sites and clinical pain: F=0.28, p=0.97; df=8). This effect depended upon the presence of high clinical pain intensity. Post-hoc comparison of temperatures that evoked mild thermal pain in the presence of little or no clinical pain (rated as 0-4) vs. moderate clinical pain (rated as 31-50) was insignificant (F=1.52, p=0.22, df=1; interaction F=0.28, p=0.22, df=2). In contrast, comparison of little or no clinical pain (0-4) vs. strong clinical pain (51-100) was significant (F=10.81, p=0.002, df-1; interaction F=0.55, p=0.58, df=2).
Figure 3.
eVAS ratings of clinical pain immediately prior to psychophysical test sessions are plotted against temperatures sufficient to evoke eVAS ratings of 15 with thermal stimulation of the face, arm or leg. Lower temperatures indicate greater sensitivity to thermal stimulation. Error bars represent S.E.M.
The influence of strong clinical pain at the time of sensory testing questions whether widespread hyperalgesia for patients was present during testing sessions not associated with strong clinical pain. This possibility was evaluated by eliminating data from sessions associated with strong clinical pain for the largest group of pain patients (TMD). Average ratings across the available sessions for each subject at 3 sites and at temperatures from 43°C to 51.4°C were compared for control and TMD subjects, as in Table 1. These stimulus-response functions revealed hyperalgesia for the patients for stimulation of: the face (main effect: F=13.79, p=0.0005, df=1; interaction: F=2.23, p=0.009, df=12), the arm (main effect: F=5.64, p=021, df=1; interaction: F=1.34, p=0.19, df=12) and the leg (main effect: F=8.52, p=0.005, df=1; interaction: F=1.46, p=0.14, df=12).
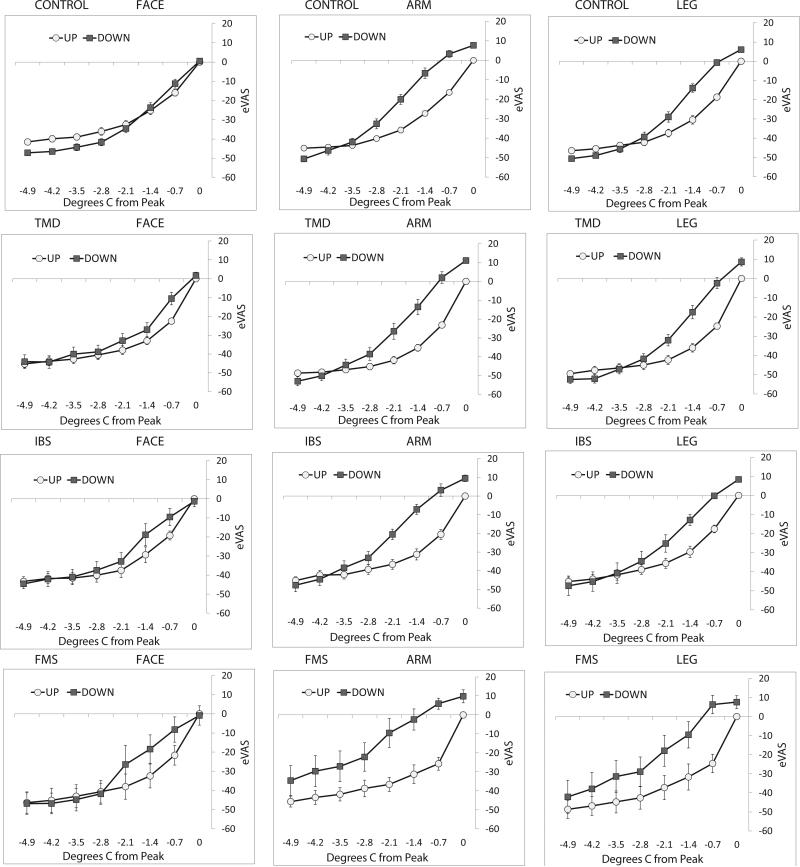
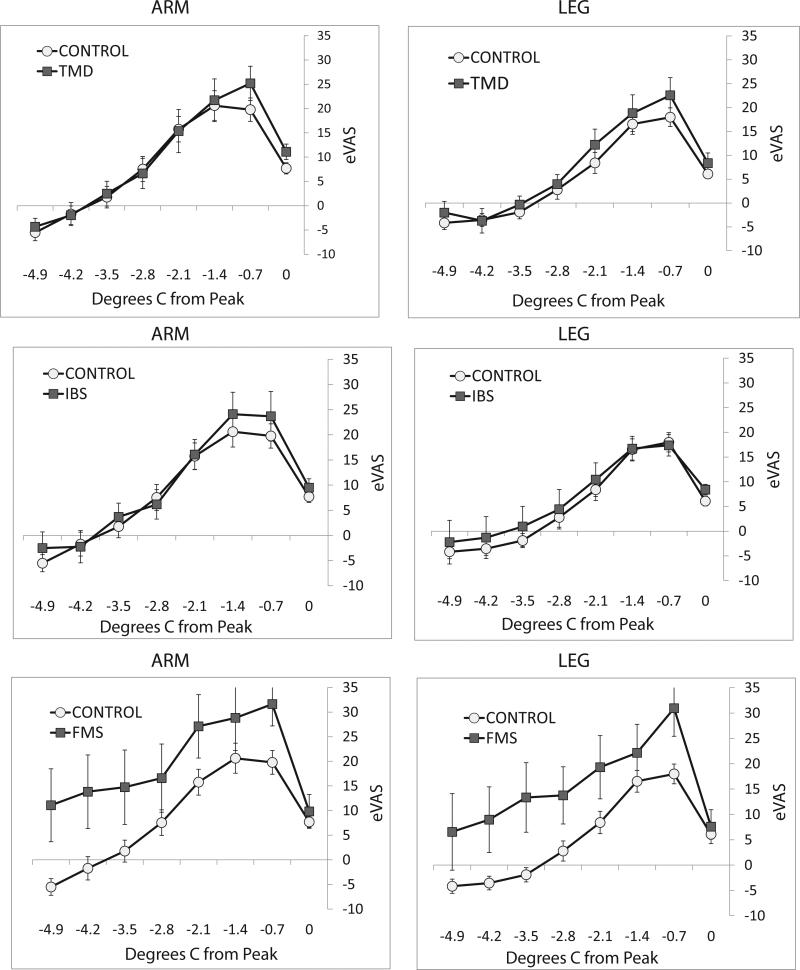
Effects of ascending progressions of nociceptive stimulation on sensitivity to subsequent descending progressions were evaluated by comparing eVAS ratings of temperatures presented in ascending series (up in Figure 4) and the same temperatures in descending series (down in Figure 4). The last stimulus in ascending series elicited an eVAS rating >40, and the first stimulus in descending series was a repeat of the last ascending stimulus intensity. For stimulation of the arm and leg of control, TMD, IBS and FMS subjects, ratings of pain intensity were significantly increased for the first 6 stimuli in descending series of stimulus intensity, compared to the last 6 stimuli in ascending series (Table 2). In contrast, pain ratings were not significantly greater during descending series of facial stimulation for normal, TMD, IBS or FMS subjects. The possibility was considered that the amount of sensitization during descending series was influenced by the temperatures that elicited an eVAS rating >40 in ascending series. However, comparing across all groups of subjects, the average end-temperatures for ascending series were not consistently low for stimulation of the face (Table 2). Furthermore, correlation of the end temperatures during ascending series with the maximum amounts of sensitization (differences between ratings of the second to last stimulus in ascending series and the second stimulus in descending series; see Figure 5) was insignificant (r=−0.026; p=0.68). Thus, for normal subjects and pain patients, a form of sensitization generated by an ascending progression of stimulus intensities (sequence-dependent sensitization) was evident for nociceptive input to central systems of spinal but not trigeminal pain conduction.
Figure 4.
Average eVAS ratings of the last 8 stimuli in ascending series and the first 8 stimuli in descending series, normalized to the last stimulus in ascending series. The range of temperatures spans 4.9°C, progressing in 0.7°C steps of ascending (up) and then descending (down) intensities delivered to the face (left column), forearm (middle column) and calf of control subjects (top row) and TMD (second row), IBS (third row) and FMS (bottom row) patients. Ratings were consistently and significantly higher for descending series of stimulation delivered to the arm and leg of each group of subjects, but ratings did not differ significantly for ascending and descending series of facial stimulation. Errors bars depict between-subjects’ variability (S.E.M.)
TABLE 2.
UP VS. DOWN eVAS RATINGS
| End Temp. | F | p | df | ||
|---|---|---|---|---|---|
| Control | Face | 51.9 (1.3) | 0.01 | 0.940 | l |
| Arm | 51.8 (0.8) | 48.20 | 0.000* | l | |
| Leg | 51.2 (l.0) | 18.10 | 0.000* | l | |
| TMD | Face | 49.5 (2.6) | 1.81 | 0.180 | l |
| Arm | 50.9 (1.5) | 20.5B | 0.000* | l | |
| Leg | 49.9(1.8) | 15.28 | 0.000* | l | |
| IBS | Face | 49.8 (1.8) | 0.97 | 0.337 | l |
| Arm | 50.1 (1.3) | 21.81 | 0.000* | l | |
| Leg | 49.9 (1.8) | 8.14 | 0.010* | l | |
| FMS | Face | 50.2 (2.1) | 0.71 | 0.410 | l |
| Arm | 50.3 (1.9) | 13.82 | 0.002* | l | |
| Leg | 50.4 (3.0) | 5.7 | 0.030* | l | |
Analysis of variance comparisons of eVAS ratings of stimuli in ascending series and subsequent descending series for stimulation of the face, arm or leg of control, TMD, IBS and FMS subjects. The averages (and standard deviations) of the last temperature in ascending series are given in the column labeled End Temp. Significant effects indicated by.
Figure 5.
Average differences between ratings of stimuli in descending and ascending series of stimulation (down – up) for control subjects vs. each group of pain patients (TMD: top row; IBS: middle row; FMS: bottom row). For TMD and FMS subjects, there was no significant difference in sensitization following an ascending series of stimulation to the forearm (left column) or leg (right column). In contrast, sensitization was significantly greater for FMS subjects, compared to controls, at both sites. Errors bars depict between-subjects’ variability (S.E.M.)
In order to evaluate sequence-dependent sensitization, eVAS ratings of stimuli in ascending series were subtracted from ratings of the corresponding stimulus intensities in descending series. This analysis showed that sequence dependent sensitization reached a maximum difference of up to 25 eVAS units for the second and third stimuli of descending series for stimulation of the arm or leg of control subjects and patients (Figure 5). There were no significant differences in the sensitizing effects of ascending series for IBS or TMD patients, compared to control subjects. However, sensitization during descending series was significantly prolonged for arm or leg stimulation of FMS patients. Sensitization disappeared by the 6th stimulus in descending series for control subjects, but sensitization was apparent through the 8thth stimulus of FMS subjects’ descending series (4 min. from the peak of the ascending series of stimulus intensities). Comparing differences in ratings of ascending and descending stimuli 2.8°C to 4.9°C from the peak of ascending series for control and FMS subjects revealed a near significant effect for arm stimulation (F=6.289, p=0.018, df=1), a significant effect for leg stimulation (F=7.724, p=0.009, df=1) but an insignificant effect for face stimulation (F=1.469, p=0.235, df=1).
As expected, ongoing pain was distributed primarily in the upper region for TMD patients and was more often identified as low by IBS patients (Table 3). For FMS patients, the frequency, intensity and/or widespread distribution of clinical pain may have contributed to the unique sensitization observed for these subjects during descending series. The percentage of patients with ongoing pain and the magnitudes of pain in the upper and lower regions were greatest for FMS patients. Also, clinical pain was equally distributed above and below the umbilicus for FMS patients, increasing the probability of convergent interactions with thermal cutaneous input from the arm and leg.
TABLE 3.
PERCENTAGES OF CLINICAL PAIN
| eVAS Range | Low Clinical Pain Location | Upper Clinical Pain Location | ||||
|---|---|---|---|---|---|---|
| TMD | FMS | IBS | TMD | FMS | IBS | |
| 0-4 | 89.8% | 26.1% | 28.1% | 51.1% | 26.1% | 71.9% |
| 5-50 | 7.9% | 47.8% | 59.4% | 45.5% | 52.2% | 25.0% |
| 51-100 | 2.3% | 26.1% | 12.5% | 3.4% | 21.7% | 3.1% |
Percentages of testing sessions with prior eVAS ratings within different ranges of clinical pain intensity at the time of testing, separately tabulated for pain above the umbilicus (upper clinical pain) and below the umbilicus (lower clinical pain).
Discussion
Brief thermal stimulation at a deliberate pace revealed widespread sensitization for TMD, IBS and FMS patients. Widespread sensitization is generally acknowledged to accompany chronic pain and often has been attributed to central mechanisms of synaptic enhancement by tonic nociceptive input to the spinal cord 18,19. For example, temporal summation (windup) of second pain from repetitive thermal stimulation is affected by the presence of FMS 20,21, IBS 4 and TMD pain 8,22-24. However, the influences of chronic pain on thermal sensitivity may not be related to windup enhancement. Relatively low intensity thermal stimulation generates temporal summation for pain patients but not controls 4, indicating that the threshold for elicited pain has been lowered by chronic pain. Accordingly, the rate (slope) of temporal summation of thermal pain usually has not been shown to be enhanced for individuals with IBS, TMD or FMS. In these studies, more pain has been elicited throughout a series of stimuli for pain patients, without a difference in the rate (slope) of temporal summation compared to normal subjects 8,18,19,25. Similarly, the rate of temporal summation has not differed between groups when the temperature is adjusted to accommodate different sensitivities of individual subjects 19,26,27. Thus, excessive central amplification as a result of stimulus repetition has been difficult to demonstrate for clinical pain conditions. Also, the NMDA receptor antagonist dextromethorphan does not differentially affect temporal summation of second pain for FMS subjects relative to controls 28. These findings indicate that increased sensitivity to repetitive thermal stimulation of FMS subjects represents an increased sensitivity to C nociceptive input, not an increase in the rate of windup as a measure of central sensitization.
Consistent with the above interpretation, the present study demonstrates an increase in sensitivity to thermal stimulation without a dependency on the rate of repetition. A preheated thermode contacted the skin for 3 sec, with an interstimulus interval of 30 sec. Sensitivity to thermal stimulation was enhanced for all sites and all patient groups. Of particular importance, sensitivity to stimulation of the face was enhanced for IBS subjects, and sensitivity to stimulation of the leg was enhanced for TMD subjects. Also, hypersensitivity has been demonstrated for cutaneous stimulation of the arm or hand of IBS patients 1,3,5,6,29 and TMD patients 7,8,22-24,26,30. Demonstrations of remote (extrasegmental) hypersensitivity open the possibility that widespread cutaneous sensitization can result from mechanisms other than convergent central input from the viscera or deep somatic tissues and input from the skin 31. The first synaptic targets for chronic input from the gut (IBS) or face (TMD) and cutaneous input from the face or the leg, respectively, are maximally separated along the neuraxis. Spinal and trigeminal pathways subserving pain from the leg and the face originate in the caudal spinal cord or brain stem, relay in separate thalamic nuclei and terminate at opposite ends of the primary somatosensory cortex.
Irrespective of the relative locations of referred clinical pain and thermal stimulation, high levels of clinical pain (rated >eVAS 50) on the day of psychophysical testing were associated with increased sensitivity to nociceptive thermal stimulation, in contrast to levels of clinical pain from mild to moderate (rated from 5 to 50 on the eVAS scale). This result is consistent with other findings that high levels of clinical pain have sensitizing effects that differ from those observed in the presence of low levels of clinical pain 32,33. However, widespread cutaneous hyperalgesia did not depend upon the presence of strong clinical pain on the day of testing. Thermal pain sensitivity for the cohort of TMD patients was significantly elevated, relative to control subjects, for stimulation of the face or the leg on test days without strong clinical pain. Thus, a mechanism other than convergent sensitization from ongoing clinical pain must be identified as responsible for the widespread cutaneous hyperalgesia associated with many pain conditions. Hyperalgesia can be associated with psychological stress11,34, with mood changes including anxiety, depression and a perceptual bias toward catastrophizing. Psychological stress is enhanced by pain 11,35,36, setting up a vicious cycle.
Following ascending series of stimulus intensities with descending progressions from a defined eVAS rating revealed a phenomenon of sequence-dependent sensitization. The sensitization was not influenced by the temperatures which produced an eVAS rating >40 prior to the beginning of descending series. Peripheral sensitization is unlikely to have accounted for hyperalgesia following ascending series because of the slow pace of stimulation and the presentation only of stimulus intensities that produced mild to moderate levels of pain. In fact, habituation, rather than sensitization might have been expected with repeated stimulation of the same peripheral site 37. The sensitization effect is similar to a previous demonstration with continuous stimulation and much shorter intervals (1.7 sec.) between small changes in temperature that maintained an eVAS setpoint (e.g., 40) 38. In this study, sensitization was observed following ascending progressions of stimulus intensity, and desensitization was observed descending progressions of intensity. Aternations of sensitization and desensitization established the importance of intensity sequences, as opposed to mechanisms for these phonomena that depend upon stimulus intensity or duration or repetition rate.
Sequence-dependent sensitization during descending series was comparable for control subjects and patients with regionally referred pain. For IBS and TMD patients and control subjects, substantial and comparable increases in eVAS ratings were observed for the first two stimuli in descending series relative to the last two stimuli in ascending series, and then ratings in descending and ascending series were equal by the 5th stimulus from the peak (3.5°C from the highest temperature). In contrast, differences in ratings of ascending and descending temperature progressions were significantly greater for FMS subjects relative to controls. Sensation intensity decayed very little for FMS subjects as temperatures were reduced in descending series from the peak of ascending series. For these subjects, sensitization remained elevated through the 8th stimulus of descending series (see Figure 5). Similarly, prolongation of painful after-sensations is characteristic of FMS 21. When prolonged sensitization has been observed following stimulation with a windup paradigm, an implicit assumption has been that central NMDA channels have not reset normally 39. However, prolonged sensitization of FMS subjects during descending series was observed in the present study for interstimulus intervals of 30 sec. -- well beyond those which support windup of thermal second pain 40.
Enhanced sequence-dependent sensitization for FMS subjects reinforces a need to discriminate between different forms of sensitization and reveal whether and how they are associated with categories of chronic pain 41. For example, NMDA sensitive windup during repetitive cutaneous thermal stimulation requires activation of C nociceptors at interstimulus intervals up to 3 sec 40. In contrast, repetitive compression of muscles produces substantial summation of pain at interstimulus intervals well beyond 3 sec 42. Consistent with this psychophysical result, stimulation of nociceptors in deep tissues generates a more prolonged response than stimulation of cutaneous nociceptors 43. Thus, enhanced pain sensations and after-sensations from repetitive palpation of FMS patients’ muscles 44 can be attributed to exaggerated and prolonged discharge from muscle nociceptors rather than windup. Similarly, the sequence-dependent sensitization following ascending series for FMS subjects might have been increased in duration by widespread input from nociceptors in muscles rendered ischemic from chronic peripheral vasoconstriction 11,45. The clinical pain reported by FMS patients was greater in magnitude, more prevalent at the time of testing and more widespread than the pain reported by IBS and TMD patients (Table 3).
Summary
Psychophysical testing of pain sensitivity for the face, arm and leg of chronic pain patients provided evidence for 4 varieties of sensitization: 1) Near (segmental) and remote (extra-segmental) sensitization of cutaneous thermal sensitivity was observed for stimulation of the face, arm and leg of TMD and IBS subjects. These widespread effects were not consistent with frequently invoked mechanisms such as temporal summation of convergent inputs from deep structures and the skin onto a common set of central neurons. 2) Sensitization was observed for subjects not experiencing strong pain on the day of psychophysical testing, but a more substantial sensitization was observed in the presence of strong clinical pain. 3) Evidence for differences between spinal and trigeminal processing of pain was provided by sequence-dependent sensitization of cutaneous thermal pain, which was observed for stimulation of an arm or leg but not the face of all subjects. 4) For FMS subjects, the sequence-dependent sensitization for cutaneous thermal stimulation of an arm or leg was considerably prolonged, but FMS did not influence ratings of facial stimulation during descending series.
Deficiencies for the present study have to do with numbers of subjects with each clinical condition and numbers of psychophysical test sessions associated with descriptions of the locations and intensities of clinical pain. Surprisingly, chronic pain was not present or was rated as weak on the majority of test days, limiting evaluation of relationships between the intensity of chronic pain and thermal pain sensitivity. Ideally, this analysis would include both within- and between-subject comparisons, permitting a determination of whether the effect of strong clinical pain is related to individual differences or variations in pain intensity over time (or both). Also, determining relationships between the location of clinical pain and thermal sensitivity at different sites requires a large number of subjects and testing sessions. For example, correlations between the intensity of clinical pain for all patients and thermal sensitivity at each site indicated that lower body pain is more sensitizing than upper body pain (data not shown). However, the number of subjects and clinical pain reports were insufficient to verify this effect with direct statistical comparisons of lower and upper pain effects for each group of patients.
Acknowledgments
Supported by grant #AG039659 from the National Institute of Aging.
Footnotes
Disclosures
There are no conflicts of interest of the authors with respect to this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhou Q, Fillingim RB, Riley JL, III, Verne GN. Thermal hypersensitivity in a subset of irritable bowel syndrome patients. World J Gastroenterol. 2009;15:3254–60. doi: 10.3748/wjg.15.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moshiree B, Price DD, Robinson ME, Gaible R, Verne GN. Thermal and visceral hypersensitivity in irritable bowel syndrome patients with and without fibromyalgia. Clin J Pain. 2007;23:323–30. doi: 10.1097/AJP.0b013e318032e496. [DOI] [PubMed] [Google Scholar]
- 3.Piche M, Arsenault M, Poitras P, Rainville P, Bouin M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain. 2010;148:49–58. doi: 10.1016/j.pain.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Q, Price DD, Callam CS, Woodruff MA, Verne GN. Effects of the N-methyl-D-aspartate receptor on temporal summation of second pain (wind-up) in irritable bowel syndrome. J Pain. 2011;12:297–303. doi: 10.1016/j.jpain.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues AC, Nicholas VG, Schmidt S, Mauderli AP. Hypersensitivity to cutaneous thermal nociceptive stimuli in irritable bowel syndrome. Pain. 2005;115:5–11. doi: 10.1016/j.pain.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Q, Fillingim RB, Riley JL, III, Malarkey WB, Verne GN. Central and peripheral hypersensitivity in the irritable bowel syndrome. Pain. 2010;148:454–61. doi: 10.1016/j.pain.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayesh EE, Jensen TS, Svensson P. Hypersensitivity to mechanical and intra-articular electrical stimuli in persons with painful temporomandibular joints. J Dent Res. 2007;86:1187–92. doi: 10.1177/154405910708601209. [DOI] [PubMed] [Google Scholar]
- 8.Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 9.Wong F, Vierck CJ, Riley JL, III, King C, Mauderli AP. A new thermal stimulation method for human psychophysical studies: pain intensity clamping. J Neurosci Methods. 2010;188:83–8. doi: 10.1016/j.jneumeth.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Lavin M. Fibromyalgia: When distress becomes (un)sympathetic pain. Pain Res and Treatment. 2011;2012:6. doi: 10.1155/2012/981565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vierck CJ., Jr. Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia). Pain. 2006;124:242–63. doi: 10.1016/j.pain.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Vierck C. A mechanism-based aproach to prevention of and therapy for fibromyalgia. Pain Res and Treatment. 2011;2012:12. doi: 10.1155/2012/951354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spieker LE, Hurlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, et al. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 2002;105:2817–20. doi: 10.1161/01.cir.0000021598.15895.34. [DOI] [PubMed] [Google Scholar]
- 14.Light KC, Bragdon EE, Grewen KM, Brownley KA, Girdler SS, Maixner W. Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder. J Pain. 2009;10:542–52. doi: 10.1016/j.jpain.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein JC, Crandall CG, Brothers RM, Carter JR. Combined heat and mental stress alters neurovascular control in humans. J Appl Physiol. 2010;109:1880–6. doi: 10.1152/japplphysiol.00779.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiller R, Camilleri M, Longstreth GF. Do the symptom-based, Rome criteria of irritable bowel syndrome lead to better diagnosis and treatment outcomes? Clin Gastroenterol Hepatol. 2010;8:125–9. doi: 10.1016/j.cgh.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Pertovaara A. The influence of stimulus temperature rise rate, adapting temperature, and stimulus duration on suprathreshold responses evoked by noxious heat in the glabrous skin of the limb. Comparison of neuronal discharge in the rat spinal dorsal horn with human sensations. Exp Brain Res. 1999;126:482–94. doi: 10.1007/s002210050756. [DOI] [PubMed] [Google Scholar]
- 18.Price DD, Staud R. Neurobiology of fibromyalgia syndrome. J Rheumatol Suppl. 2005;75:22–8. [PubMed] [Google Scholar]
- 19.Staud R, Bovee CE, Robinson ME, Price DD. Cutaneous C-fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain. 2008;139:315–23. doi: 10.1016/j.pain.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 21.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–75. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 22.Sarlani E, Garrett PH, Grace EG, Greenspan JD. Temporal summation of pain characterizes women but not men with temporomandibular disorders. J Orofac Pain. 2007;21:309–17. [PMC free article] [PubMed] [Google Scholar]
- 23.Sarlani E, Greenspan JD. Why look in the brain for answers to temporomandibular disorder pain? Cells Tissues Organs. 2005;180:69–75. doi: 10.1159/000086200. [DOI] [PubMed] [Google Scholar]
- 24.Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Evidence for up-regulated central nociceptive processing in patients with masticatory myofascial pain. J Orofac Pain. 2004;18:41–55. [PubMed] [Google Scholar]
- 25.Staud R, Robinson ME, Vierck C, Jr., Price DD. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101(1-2):167–174. doi: 10.1016/s0304-3959(02)00325-1. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- 26.Raphael KG, Janal MN, Anathan S, Cook DB, Staud R. Temporal summation of heat pain in temporomandibular disorder patients. J Orofac Pain. 2009;23:54–64. [PMC free article] [PubMed] [Google Scholar]
- 27.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8:893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staud R, Vierck CJ, Robinson ME, Price DD. Effects of the N-methyl-D-aspartate receptor antagonist dextromethorphan on temporal summation of pain are similar in fibromyalgia patients and normal control subjects. J Pain. 2005;6:323–32. doi: 10.1016/j.jpain.2005.01.357. [DOI] [PubMed] [Google Scholar]
- 29.King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL., III. Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain. 2009;143:172–8. doi: 10.1016/j.pain.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63:341–51. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 31.Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain. 2006;7:529–35. doi: 10.1016/j.jpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Coombes BK, Bisset L, Vicenzino B. Thermal Hyperalgesia Distinguishes Those With Severe Pain and Disability in Unilateral Lateral Epicondylalgia. Clin J Pain. 2012 doi: 10.1097/AJP.0b013e31823dd333. [DOI] [PubMed] [Google Scholar]
- 33.Wong F, Rodrigues A, Schmidt S, Vierck CJ, Jr., Mauderli AP. Extreme thermal sensitivity and pain-induced sensitization in a fibromyalgia patient. Pain Res Treat. 2010;2010:912513. doi: 10.1155/2010/912513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong F, Rodrigues AC, King CD, Riley JL, III, Schmidt S, Vierck CJ, et al. Relationships between Irritable Bowel Syndrome Pain, Skin Temperature Indices of Autonomic Dysregulation, and Sensitivity to Thermal Cutaneous Stimulation. Pain Res Treat. 2010;2010:949027. doi: 10.1155/2010/949027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis MC, Zautra AJ, Reich JW. Vulnerability to stress among women in chronic pain from fibromyalgia and osteoarthritis. Ann Behav Med. 2001;23:215–26. doi: 10.1207/S15324796ABM2303_9. [DOI] [PubMed] [Google Scholar]
- 36.Vierck CJ, Green M, Yezierski RP. Pain as a stressor: effects of prior nociceptive stimulation on escape responding of rats to thermal stimulation. Eur J Pain. 2010;14:11–6. doi: 10.1016/j.ejpain.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greffrath W, Baumgartner U, Treede RD. Peripheral and central components of habituation of heat pain perception and evoked potentials in humans. Pain. 2007;132:301–11. doi: 10.1016/j.pain.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 38.Vierck CJ, Riley JL, III, Wong F, King CD, Mauderli AP. Psychophysical demonstration of bidirectional pain modulation (sensitization and desensitization) by ascending or descending progressions of thermal stimulus intensity. Brain Res. 2010;1347:58–64. doi: 10.1016/j.brainres.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Staud R, Price DD, Robinson ME, Mauderli AP, Vierck CJ. Maintenance of windup of second pain requires less frequent stimulation in fibromyalgia patients compared to normal controls. Pain. 2004;110:689–96. doi: 10.1016/j.pain.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Vierck CJ, Jr., Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 41.Woolf CJ. Windup and central sensitization are not equivalent. Pain. 1996;66:105–8. [PubMed] [Google Scholar]
- 42.Nie H, Arendt-Nielsen L, Madeleine P, Graven-Nielsen T. Enhanced temporal summation of pressure pain in the trapezius muscle after delayed onset muscle soreness. Exp Brain Res. 2006;170:182–90. doi: 10.1007/s00221-005-0196-6. [DOI] [PubMed] [Google Scholar]
- 43.Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol. 1984;356:443–58. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ., Jr. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 45.Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, et al. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Appl Physiol. 2001;91:2611–8. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]