Abstract
The intermediate conductance Ca2+-activated K+ channel, KCa3.1 (IK1/SK4/KCNN4) is widely expressed in the innate and adaptive immune system. KCa3.1 contributes to proliferation of activated T lymphocytes, and in CNS-resident microglia, it contributes to Ca2+ signaling, migration, and production of pro-inflammatory mediators (e.g., reactive oxygen species, ROS). KCa3.1 is under investigation as a therapeutic target for CNS disorders that involve microglial activation and T cells. However, KCa3.1 is post-translationally regulated, and this will determine when and how much it can contribute to cell functions. We previously found that KCa3.1 trafficking and gating require calmodulin (CaM) binding, and this is inhibited by cAMP kinase (PKA) acting at a single phosphorylation site. The same site is potentially phosphorylated by cGMP kinase (PKG), and in some cells, PKG can increase Ca2+, CaM activation, and ROS. Here, we addressed KCa3.1 regulation through PKG-dependent pathways in primary rat microglia and the MLS-9 microglia cell line, using perforated-patch recordings to preserve intracellular signaling. Elevating cGMP increased both the KCa3.1 current and intracellular ROS production, and both were prevented by the selective PKG inhibitor, KT5823. The cGMP/PKG-evoked increase in KCa3.1 current in intact MLS-9 microglia was mediated by ROS, mimicked by applying hydrogen peroxide (H2O2), inhibited by a ROS scavenger (MGP), and prevented by a selective CaMKII inhibitor (mAIP). Similar results were seen in alternative-activated primary rat microglia; their KCa3.1 current required PKG, ROS, and CaMKII, and they had increased ROS production that required KCa3.1 activity. The increase in current apparently did not result from direct effects on the channel open probability (Po) or Ca2+ dependence because, in inside-out patches from transfected HEK293 cells, single-channel activity was not affected by cGMP, PKG, H2O2 at normal or elevated intracellular Ca2+. The regulation pathway we have identified in intact microglia and MLS-9 cells is expected to have broad implications because KCa3.1 plays important roles in numerous cells and tissues.
Keywords: KCa3.1/KCNN4/IK1/SK4 regulation, cGMP-PKG signaling, reactive oxygen species signaling, Ca2+-CaM-CaMKII signaling, patch-clamp electrophysiology, fura-2 calcium measurement, alternative-activated microglia, interleukin-4 stimulation
Introduction
Following the discovery of a Ca2+-dependent K+ efflux (“Gardos” channel) in red blood cells (1), early patch-clamp studies focused on the intermediate-conductance Ca2+-dependent K+ (IK) channel in thymic T cells, B lymphocytes (2), and T cells (3, 4). After the KCNN4 gene was cloned (5–7) and identified as the IK channel (also called KCa3.1, IK1, IKCa1, SK4); its upregulation in activated T lymphocytes and crucial role in their proliferation (8, 9) generated interest in targeting this channel for immunosuppression [reviewed in Ref. (10–12)]. Initially, the KCa3.1 channel was thought to be absent from the CNS, but then KCNN4 transcripts and KCa3.1 protein were detected in microglia (13, 14), astrocytes, and some neurons (15–17). Many CNS disorders involve inflammation and microglial activation, and KCa3.1 blockers or knockdown have improved the outcome in animal models of trauma, spinal cord injury, ischemic stroke, multiple sclerosis, and Alzheimer’s disease [reviewed in Ref. (18, 19)]. Thus, there is increasing interest in roles and regulation of KCa3.1 in the CNS.
KCa3.1 expression, activity, and contributions to T cell functions are governed by the cells’ activation state but until recently, little was known about this aspect for microglia. In rat microglia, we found that KCa3.1 contributes to production of reactive oxygen species (ROS) (14), to p38 MAPK activation, nitric oxide, and peroxynitrite production in classical-activated microglia (“M1,” by analogy with macrophages polarized by T cells), and to their capacity to kill neurons in vitro and in vivo (15). We recently discovered that when rat microglia are skewed to the anti-inflammatory “alternative” (M2) activation state using interleukin-4, both KCa3.1 expression and current are highly upregulated through the type I IL-4 receptor and subsequent signaling through JAK3, Ras/MEK/ERK, and the transcription factor, AP-1 (20). While KCa3.1 is involved in microglial ROS production, it is not known if its activity is regulated by ROS. There is some indirect evidence that this might occur. ROS can evoke Ca2+ release from internal stores in Jurkat T cells (21) and pancreatic β-cells (22). We found that, in microglia, the KCa3.1 current is functionally coupled to Ca2+-release activated Ca2+ (CRAC) channels. When CRAC was activated through P2Y2 metabotropic purinergic receptors, this activated KCa3.1 channels, and they then contributed to the microglia migratory phenotype (23). Migration is also increased in alternative-activated microglia, and this depends on KCa3.1 (20, 24).
KCa3.1 activity is also post-translationally regulated. Most fundamental is its absolute requirement for Ca2+ and calmodulin (CaM) in order for the channels to open (8, 25) and to traffic to the cell surface (26). CaM is bound to the C terminus of the channel but there is evidence that this interaction can be modulated. We found that the KCa3.1 current is inhibited by cAMP kinase (PKA) through a single phosphorylation consensus site (S334 in human; S332 in rodent, which is two amino acids shorter), and a consequent decrease in CaM binding to the channel (27). Many years ago, we observed that the KCa3.1 current in human T lymphoblasts was reduced by the CaM kinase inhibitor, KN-62, but only at 37°C (not room temperature) and not in KCa3.1 heterologously expressed in CHO cells (8). Both observations suggested an indirect modulation by CaM kinase, but this was not investigated further.
More recent studies suggested a possible link between KCa3.1 and CaMKII, ROS and cGMP-protein kinase (PKG). In cardiac and neuronal cells, the PKG pathway can stimulate ROS production and activate CaM/CaMKII signaling (28–30). Numerous stimuli can activate PKG in immune cells, and we noticed that the Ser334/S332 site in KCa3.1 that is regulated by PKA (27) is also a potential consensus site for phosphorylation by PKG. Thus, the present study was designed to test whether KCa3.1 is directly inhibited by cGMP/PKG, and if not, whether it is indirectly regulated and whether this involves crosstalk between Ca2+, ROS, and CaM/CaMKII. First, we analyzed the endogenous KCa3.1 current in a rat microglial cell line because we found that every MLS-9 cell expresses a robust KCa3.1 current that can be easily isolated (23, 31). Then, we corroborated the salient findings on native KCa3.1 channels in primary rat microglia. Importantly, regulation of native channels was studied using the perforated-patch recording configuration to maintain intracellular soluble mediators and biochemical signaling pathways, and to allow cytoplasmic Ca2+ to remain at physiological levels and change with treatments.
Materials and Methods
Cells
Primary cultured rat microglia and the MLS-9 microglia cell line were used to study native KCa3.1 channels, and transfected HEK293 cells were used to facilitate single-channel analysis.
Primary rat microglia
Microglia were isolated from brains of 1–2-day-old Sprague-Dawley rats of either sex (Charles River, St. Constant, QC, Canada) according to our standard protocols (15, 24, 31). The brains were harvested, meninges removed, remaining tissue minced in cold minimum essential medium (MEM; Invitrogen, Burlington, ON, Canada), and then centrifuged (300 g, 10 min) and re-suspended in MEM with 10% fetal bovine serum (FBS; Wisent, St. Bruno, QC, Canada) and 0.05 mg/mL gentamycin (Invitrogen). The culture medium was replaced after 2 days to remove non-adherent cells and debris. After six more days, the mixed cell cultures were shaken on an orbital shaker (65 rpm, 4–5 h, 37°C, 5% CO2), and then the microglia-containing supernatant was centrifuged (300 g, 10 min), and microglial cells were re-suspended in MEM with 2% FBS. Microglia were plated at 7 × 104 cells/coverslip for 24 h, and then exposed to 20 ng/mL rat recombinant interleukin-4 (IL-4; R&D Systems Inc., Minneapolis, MN, USA) for 1 or 2 days (37°C, 5% CO2) before analyzing ROS production, or for 2 days (37°C, 5% CO2) before analyzing KCa3.1 currents. We previously showed that IL-4 shifts them to an alternative-activated state (24, 31) and upregulates KCNN4 mRNA and the KCa3.1 current (20, 27), whereas untreated rat microglia are non-activated (32).
MLS-9 cells
About 20 years ago, we derived the MLS-9 cell line by treating rat microglia cultures for several weeks with M-CSF (colony stimulating factor-1) (33). Although we do not know whether these cells reflect an alternative activation state, M-CSF can shift macrophages to an alternative-activated state (34), and it suppresses the response of microglia to lipopolysaccharide (LPS) (35). We have used MLS-9 cells extensively to study microglial K+, Cl− and TRPM7 channels (23, 31, 36–41). After thawing, the cells were cultured (37°C, 5% CO2) for several days in MEM with 10% FBS and 0.05 mg/mL gentamycin. They were harvested in phosphate buffered saline (PBS) with 0.25% trypsin and 1 mM EDTA, washed with MEM, centrifuged (300 g, 10 min), and re-suspended in MEM. MLS-9 cells were plated on glass coverslips in 12-well plates (4.5 × 104 cells/coverslip) for patch-clamping and Fura-2 analysis, or in 96-well plates (6.0 × 104 cells/well) for measuring intracellular ROS.
Transfection of HEK293 cells
HEK293 cells (embryonic neuronal tumor from a human female kidney) were grown for several days in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) with high glucose, 10% FBS, 100 mg/L penicillin–streptomycin (Invitrogen). They were harvested in PBS with 0.25% trypsin and 1 mM EDTA, washed with MEM, centrifuged (300 g, 10 min), and re-suspended in MEM. The human KCa3.1 gene (wild-type hKCa3.1) was subcloned into the expression vector, pCMV6-XL5 (OriGene, Rockville, MD). The plasmids, pCMV6-XL5-hKCa3.1 and pEF-GFP, were co-transfected using LipofectAMINE (Invitrogen) for 36 h according to the manufacturer’s protocol. HEK293 cells were plated at 5.5 × 104 cells/coverslip for single-channel patch-clamp analysis.
Patch-clamp electrophysiology
Perforated-patch recordings
Endogenous KCa3.1 currents were recorded at room temperature from primary cultured rat microglia and MLS-9 cells. Perforated-patch recordings were obtained by including 200 μM amphotericin B in the pipette (intracellular) solution, which contained (in mM): 100 K aspartate, 40 KCl, 1 MgCl2, 0.5 CaCl2, 1 EGTA, 2 MgATP, 10 HEPES, pH 7.2 (adjusted with KOH), 280 mOsm. Internal free Ca2+ was 120 nM, as calculated by WEB-MAXC Extended software (http://www.stanford.edu/~cpatton/webmaxc/webmaxcE.htm; Stanford University). The extracellular (bath) solution contained (in mM) 125 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 5 glucose, 10 HEPES, pH 7.4 (adjusted with NaOH), and adjusted to ~300 mOsm with sucrose. The bath solution was perfused using a gravity-driven system flowing at 1.5–2 mL/min. Recording pipettes (8–12 MΩ) were pulled from thin-walled borosilicate glass (WPI, Sarasota, FL, USA) using a Narishige puller (Narishige Scientific, Setagaya-Ku, Tokyo, Japan). Recordings were made with an Axopatch 200A amplifier (Molecular Devices, Sunnyvale, CA, USA), digitized with a DigiDATA 1322A board, filtered at 5 kHz and sampled at 10 kHz. Junction potentials were reduced by using agar bridges made with bath solution, and calculated with the pCLAMP utility. After correction, all voltages are ~5 mV more negative than shown in the figures.
Single-channel recordings
Inside-out patches were excised from HEK293 cells that had been transfected with hKCa3.1. Recordings were made at room temperature, sampled at 5 kHz, and low-pass filtered at 1 kHz (−3 dB cut-off frequency). Pipettes were pulled from thin-walled borosilicate glass (6–8 MΩ) and filled with an extracellular solution containing (in mM) 145 KCl, 1 MgCl2, and 1 CaCl2, 5 HEPES; pH 7.4 (adjusted with KOH), adjusted to ~300 mOsm with sucrose. The bath solution contained (in mM) 145 KCl, 1 MgCl2, 1 CaCl2, 0.1 MgATP, 5 HEPES, and 5 glucose and 1.2, 1.5 or 2.8 EGTA, to yield intracellular free Ca2+ concentrations of 1 μM, 500 nM, and 120 nM respectively; pH 7.2 (adjusted with KOH), ~300 mOsm. NPo, the product of the apparent number of active channels in the patch (N) and the channel open probability (Po) was calculated in pClamp by dividing the mean total current (I) by the single-channel current amplitude (i), where NPo = I/i. The single-channel current was determined from the best Gaussian fit to the single-channel event amplitude histogram, and this also indicated the apparent number of active channels in the patch. At the end of each recording, 1 μM TRAM-34 was added to block the channels and identify them as KCa3.1.
Measuring intracellular ROS
Primary rat microglia and MLS-9 cells in 96-well tissue culture plates were incubated with 5 μM of CM-H2DCFDA (5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester) at 37°C for 1 h. After this cell-permeant reagent diffuses into cells, the acetyl group is removed by intracellular esterases, and it can then be oxidized by the intracellular reactive species, hydrogen peroxide (H2O2), hydroxyl radical (OH•), and peroxynitrite (ONOO–) (42). The resulting fluorescent adduct, dichlorofluorescein (DCF), is produced in proportion to the reactive species and remains trapped inside the cell. Before plate-reader analysis, cells were washed with the same extracellular solution used for perforated-patch recordings. Intracellular DCF fluorescence was excited at 480 nm, and measured at 530 nm with a fluorescence plate reader (Victor3 1420 Multilabel Plate Counter; Perkin Elmer Inc, Waltham, MA, USA). For each treatment, the fluorescence was averaged from two separate wells of cells cultured from one animal (for primary microglia) or from one passage (for MLS-9 cells), and then multiple n values were obtained using cultures from different animals or cell passages. Background subtraction was performed using control wells without CM-H2DCFDA. The fluorescence intensity for each treatment group was normalized to the corresponding control value.
Intracellular free Ca2+
The Fura-2 imaging methods were the same as recently described (23, 27, 31). In brief, cells growing on glass coverslips (~7 × 104 cells/15 mm diameter coverslip) were incubated at room temperature with 3.5 μg/mL Fura-2AM (Invitrogen) for 40 min in the dark. For recording, a coverslip was mounted in a 300 μL volume perfusion chamber (Model RC-25, Warner Instruments, Hamden, CT, USA) that contained the same bath solution as for perforated-patch recording. Responses to db-cGMP and H2O2 were assessed on different batches of cells from separate coverslips. Images were acquired at room temperature using a Nikon Diaphot inverted microscope, Retiga-EX camera (Q-Imaging, Burnaby, BC, Canada), and Northern Eclipse image acquisition software (Empix Imaging, Mississauga, ON, Canada). A Lambda DG-4 Ultra High Speed Wavelength Switcher (Sutter Instruments, Novato, CA, USA) was used to alternately acquire images at 340 and 380 nm excitation wavelengths. Images were acquired every 4 s, and the excitation shutter was closed between acquisitions to prevent photobleaching. The intracellular free Ca2+ concentration was calculated from the standard equation (43).
Chemicals
Working solutions were prepared just before use by diluting fresh aliquots of stock solutions of dibutyryl-cGMP (db-cGMP), cGMP, KT5823, N-(2-mercaptopropionyl)glycine (MPG), myristolated autocamtide-2 related inhibitory peptide for CaMKII (mAIP), TRAM-34, apamin, riluzole, and rat recombinant IL-4. Stock solutions were prepared as follows: KT5823, TRAM-34, apamin, and riluzole were prepared in DMSO and stored at –20°C; db-cGMP, cGMP, and mAIP were prepared in distilled H2O and stored at –20°C; MPG was prepared in distilled H2O and stored at 4°C; IL-4 was prepared in sterile PBS containing 0.1% BSA and stored at –20°C. H2O2 was prepared fresh daily from a 30% w/w liquid stock (9.8 M). Aliquots of the original stock of PKG Iα holoenzyme were stored at –80°C, and diluted immediately before use in the bath solution that was used for single-channel recordings. PKG Iα holoenzyme and mAIP were obtained from Calbiochem (EMD Biosciences; San Diego, CA, USA), rat recombinant IL-4 was from R&D Systems Inc. (Minneapolis, MN, USA), and all other reagents were from Sigma Aldrich (Oakville, ON, Canada).
Statistical analysis
Data are expressed as mean ± SEM. For single and multiple comparisons to assess treatment effects on currents, either an unpaired Student’s t-test or 1-way ANOVA with Tukey’s post hoc test was used. Changes in intracellular Ca2+ following treatment with db-cGMP or H2O2 were analyzed using a paired Student’s t-test. For analysis of intracellular ROS production, a 2-way ANOVA followed by Bonferroni’s post hoc test was used, with stimulation (untreated, 24 h IL-4, 48 h IL-4) and inhibitors (untreated, KT5823, TRAM-34) as the two independent variables. Analyses were conducted using GraphPad Prism ver 6.01 (GraphPad Software, San Diego, CA, USA), and statistical significance was taken as p < 0.05.
Results
PKG increases the endogenous KCa3.1 current in microglia
The MLS-9 microglial cell line was used for initial experiments because these cells have a large endogenous KCa3.1 current, and lack Kv1.3, Kv1.5, and Kir2.1 currents that are expressed in primary rat microglia (33, 44, 45). They have a KCa2.3 (SK3) current (31, 41); thus, the bath always contained the blocker, 100 nM apamin. While KCa3.1 channels in many cell types can be activated by sub-micromolar intracellular free Ca2+ (18), we found that the Kd is nearly 8 μM in MLS-9 cells (23), and that 1 μM Ca2+ failed to activate the current in primary rat microglia (20, 27). We do not know why this is the case. However, the microglial current can be activated at lower Ca2+ (e.g., 1 μM) by the activators, riluzole, 1-EBIO, or NS309 (20, 27, 31). These activators act as positive gating modulators that increase the Ca2+ sensitivity of KCa2.x and KCa3.1 channels (18).
To study the native channels in MLS-9 and primary microglial cells, we used riluzole because it reliably activated a KCa3.1 current in perforated-patch recordings. The current was also stable enough to add TRAM-34 to confirm the channel identity and quantify the current density (20, 31). As expected, the KCa3.1 current was not activated at resting levels of intracellular Ca2+ in MLS-9 cells. However, a stable KCa3.1 current was activated by riluzole in all cells tested (Figure 1A). As expected for KCa3.1, current activation was independent of voltage, and it reversed close to the Nernst potential for K+ (–84 mV with the solutions used). The current was entirely KCa3.1 (in the presence of apamin), as demonstrated by full inhibition by the selective KCa3.1 blocker, 1 μM TRAM-34 (Figure 1A). In all subsequent experiments, the KCa3.1 current was quantified as the TRAM-34-sensitive component.
Figure 1.
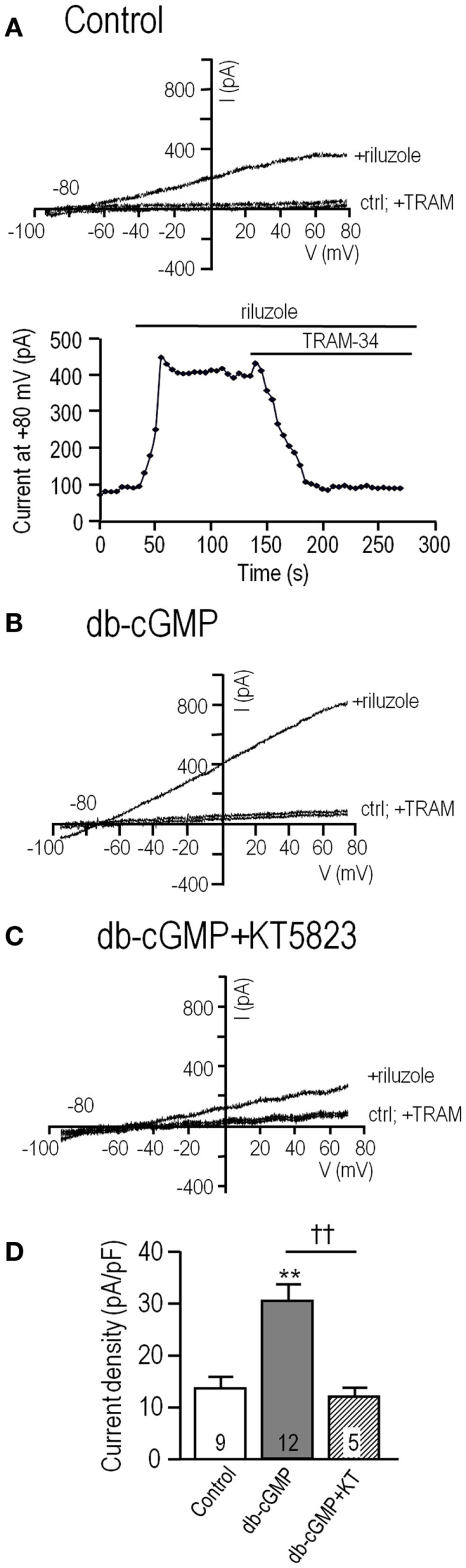
The endogenous KCa3.1 current in MLS-9 microglial cells is increased by cGMP, which requires cGMP-protein kinase. For all traces, the voltage protocol was a holding potential of –70 mV, and repeated ramps from –100 to +80 mV. Recordings were conducted at room temperature in the perforated-patch configuration, and riluzole was used simply to activate the KCa3.1 current at the normal low intracellular Ca2+ concentration. The bath always contained 100 nM apamin, a KCa2.1–2.3 channel blocker. (A) Upper: Representative current traces from a control cell (trace marked “ctrl”), followed by bath addition of 300 μM riluzole, and then 1 μM of the selective KCa3.1 blocker, TRAM-34. Lower: The time course of current activation and block by 1 μM TRAM-34. (B,C) Representative current traces from cells before and after activating the current with riluzole; with or without 1 μM TRAM-34. Cells were pre-treated with the membrane-permeant cGMP analog, db-cGMP (100 μM), for 20 min at room temperature, without (B) or with (C) 1 μM KT5823, a selective inhibitor of cGMP-protein kinase (PKG). (D) Summarized data from a population study using the treatments in panels A–C. For each cell, the KCa3.1 current amplitude was measured at +80 mV, as the component of the riluzole-activated current that was blocked by TRAM-34 (1 μM). The current was always normalized to the cell capacitance (in pF) to account for any differences in cell size and expressed as current density. The TRAM-34-sensitive KCa3.1 current is expressed as mean ± SEM for the number of cells indicated on each bar, and data were compared using one-way ANOVA, with Tukey’s post hoc test. **p < 0.01, indicates a difference from both controls and KT5823-treated cells. ††p < 0.01, for the comparison indicated. There was no difference between the control and KT5823-treated cells.
Effects of elevating intracellular cGMP were analyzed in MLS-9 cells exposed for 20 min to the membrane-permeant cyclic GMP analog, db-cGMP, before establishing a perforated-patch recording. The KCa3.1 current was much larger after db-cGMP treatment (Figure 1B) than the control current. Two observations provided the initial evidence for an indirect action of cGMP that requires intact cells (perhaps a diffusible mediator is lost when the cell integrity is disrupted). cGMP alone did not activate the channels (riluzole was required), and adding db-cGMP during whole-cell recordings did not increase the current (not shown). Therefore, we focused on perforated-patch recordings, and tested 1 μM KT5823, a membrane-permeant compound that selectively inhibits PKG (cGMP-protein kinase) with no effect on PKA (29). After treatment with db-cGMP in the presence of KT5823, only a small KCa3.1 current was activated (Figure 1C). As summarized in Figure 1D, the current density was 13.9 ± 2.1 pA/pF (n = 9) in control cells, more than twofold larger after db-cGMP (29.0 ± 2.6 pA/pF; n = 12; p < 0.01), and 11.3 ± 1.4 pA/pF (n = 5; not different from control) when the PKG inhibitor was also added. These results indicate that the current enhancement by db-cGMP required PKG.
Lack of direct KCa3.1 activation by cGMP-protein kinase (PKG) in excised patches
The next question, whether PKG directly affects KCa3.1 channel activity, was prompted by our recent study in which KCa3.1 was assessed in excised inside-out patches from transfected HEK293 cells (27). PKA decreased the channel open probability (Po), and this was abolished by mutating the S334 site to S334A, which cannot be phosphorylated (27). Here, the rationale was that S334 is also the only putative PKG phosphorylation site, and the channel could not distinguish which kinase has phosphorylated it. We hypothesized that PKG would decrease Po if it acts directly on the channel. For direct comparison with our earlier PKA study, we used the same experimental system: HEK293 cells transfected with human KCNN4 (hKCa3.1). This system offered several advantages. HEK293 cells lack endogenous KCa3.1 current but after transfection, there were more active channels than in microglia (larger whole-cell currents), which made it easier to find channels in a patch, and the current was readily activated by 1 μM Ca2+ without requiring a gating modifier such as riluzole (27). That is, for human KCNN4 (hKCa3.1), the threshold for current activation is ~100 nM Ca2+, the EC50 is ~270 nM, and the current is essentially fully activated at 1 μM Ca2+ (6, 7).
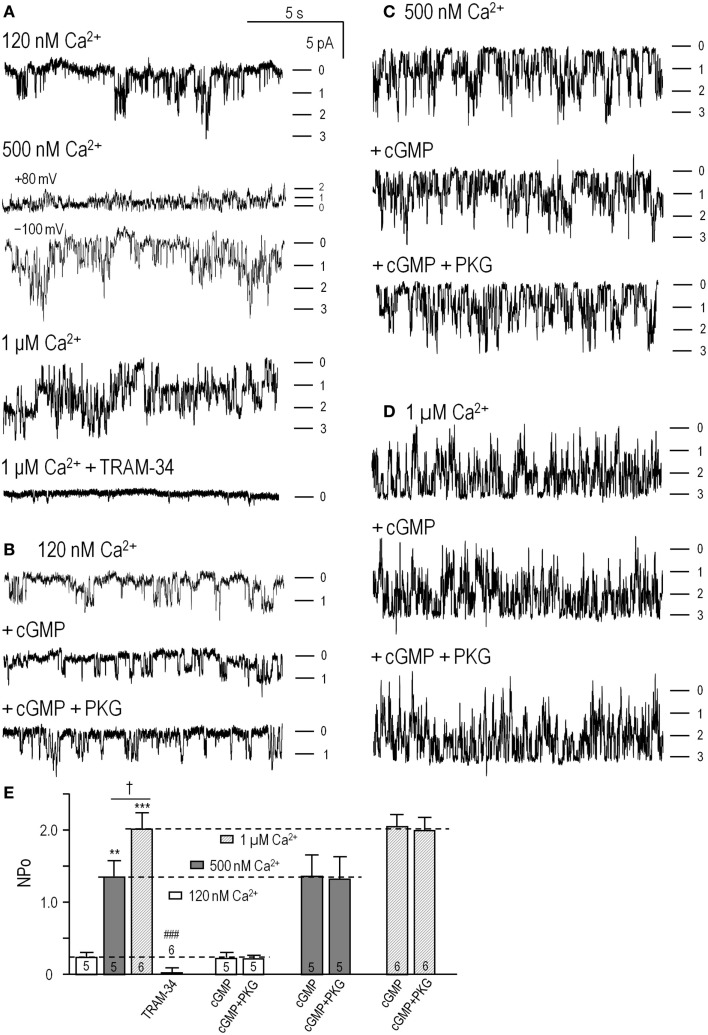
Inside-out patches were excised into a bath (intracellular) solution containing 100 μM ATP and free Ca2+ concentrations of 120 nM, 500 nM, or 1 μM (Figure 2). The intracellular and extracellular solutions contained symmetrical high K+ (140 mM) to set the Nernst potential to 0 mV, increase the unitary inward current amplitude, and expose the innate inward rectification of the single-channel current. One to three channels were usually active in each patch, their activity was stable for several minutes, and channel activity was recorded at –100 mV. Channel activity was quantified as NPo: the number of active channels, N, times the open probability, Po. Using representative 2 min-long segments of each recording, thresholds were set for the closed level and each open level (based on amplitude histograms; see Methods).
Figure 2.
cGMP-protein kinase (PKG) did not directly affect KCa3.1 channel activity. Inside-out patches were excised from HEK293 cells that had been transfected with wild-type human KCNN4 (KCa3.1). The bath and pipette solutions both contained 140 mM potassium, and unless otherwise indicated, inward single-channel currents were recorded at a membrane potential of −100 mV. (A) KCa3.1 channel activity was recorded with intracellular solutions containing ATP and 120 nM, 500 nM, or 1 μM free Ca2+, sequentially perfused into the bath. At the end of the recording, the KCa3.1 selective blocker, 1 μM TRAM-34, was perfused in. Patches usually contained multiple channels, and the dashes indicate the closed level and opening of 1, 2, or 3 channels. (B–D) At each Ca2+ concentration (120 nM, 500 nM, 1 μM), the bath was sequentially perfused with cGMP (100 μM), and cGMP + PKG holoenzyme (1 U/μL). (E) Summarized data show NPo in control bath solution and 4–6 min after adding cGMP or cGMP + PKG. Data are expressed as mean ± SEM for the number of patches indicated on the bars. The dashed lines indicate the NPo value in control bath solution at each Ca2+ concentration. A two-way ANOVA with Tukey’s post hoc test shows that activity increased with intracellular Ca2+ (**p < 0.01 for 500 nM Ca2+, and ***p < 0.001 for 1 μM Ca2+) and was significantly reduced by 1 μM TRAM-34 (only data for 1 μM Ca2+ shown; ###p < 0.001). There were no differences with cGMP or cGMP/PKG treatments.
As expected for KCa3.1, channel activity (NPo) increased with increasing free Ca2+ (Figures 2A–D; summarized in Figure 2E), and the current was fully blocked by 1 μM TRAM-34 (Figures 2A,E). For the 500 nM Ca2+ concentration, both inward (at –100 mV) and outward (at +80 mV) currents are shown to illustrate the inward rectification in symmetrical high K+ solutions (~32 pS at –100 mV, ~12 pS at + 80 mV in our recordings) that is characteristic of this channel (3, 4, 7). Overall, the channels were identified as KCa3.1 from their Ca2+ dependence, voltage-independent activity (–100 to +80 mV tested), inward rectification, reversal at ~0 mV (not shown), and block by 1 μM TRAM-34. Sequential addition of 100 μM cGMP [required for PKG activation (46)] and the PKG holoenzyme (1 U/μL) did not affect the channel activity or Ca2+ dependence (Figures 2B–D). As summarized in Figure 2E, there were no differences in NPo values with or without cGMP or cGMP + PKG at any Ca2+ concentration. At 120 nM Ca2+, NPo was 0.23 ± 0.05 in control solution, 0.22 ± 0.06 after adding cGMP, and 0.21 ± 0.04 after adding PKG (n = 5). At 500 nM Ca2+, NPo was 1.18 ± 0.28 in control solution, 1.20 ± 0.32 after adding cGMP, and 1.17 ± 0.36 after adding PKG (n = 5). At 1 μM Ca2+, NPo was 1.89 ± 0.21 in control solution, 2.01 ± 0.23 after adding cGMP, and 2.00 ± 0.24 after adding PKG (n = 6).
These results on isolated channels show that cGMP and PKG did not change the number or activity of the channels or their Ca2+ dependence, and amplitude histograms (not illustrated) showed that the unitary current amplitude was unaffected. This is in contrast to our recent finding that activated PKA directly reduced Po by ~45%, and required the channel’s PKA phosphorylation site (27). The present results provide strong evidence against direct channel phosphorylation by PKG.
PKG increases ROS production, which activates KCa3.1 current through a CaMKII-mediated pathway
The lack of effect of cGMP and PKG on excised patches (Figure 2) suggested that intracellular signaling was required for the current enhancement in MLS-9 cells (Figure 1). Therefore, we used perforated-patch recordings to maintain intracellular signaling when conducting experiments on native channels in MLS-9 cells and primary microglia. We first considered ROS because PKG increases ROS production in cardiac cells and neurons (28–30). Treating MLS-9 cells with db-cGMP increased ROS production by 38 ± 5% (Figure 3A; n = 5; p < 0.001), an effect that was prevented by the PKG inhibitor, KT5823. ROS can activate CaMKII (21, 47) and, as described in the Introduction, CaMK regulates native KCa3.1 channels in T lymphocytes (8). Intriguingly, CaMKII can be activated by ROS without elevated intracellular Ca2+ (21) or in a long-lasting Ca2+-independent manner after a transient Ca2+ elevation (47).
Figure 3.

PKG increases production of reactive oxygen species (ROS), which activates KCa3.1 current through a Ca2+ and CaMKII-mediated pathway. (A) Summarized data showing ROS production by unstimulated MLS-9 microglial cells (control), and after treatment (20 min; 37°C) with db-cGMP (100 μM), with or without the PKG inhibitor, KT5823 (1 μM). Values are expressed as mean ± SEM (n = 5), and compared using a one-way ANOVA with Tukey’s post hoc test. ***p < 0.001 treatment versus control; †††p < 0.001 with and without PKG inhibitor. (B) Acute application of db-cGMP increases intracellular Ca2+ in MLS-9 cells but does not activate KCa3.1 current. Upper panel: Representative Fura-2 recording, in which 100 μM db-cGMP was bath applied during the period marked by the horizontal bar. The inset shows calibrated free intracellular Ca2+ concentration as mean ± SEM, n = 19 cells (***p < 0.001, Student’s t-test). Lower panel: Representative time course of current in a perforated-patch recording (same solutions and voltage protocols as Figure 1) in which db-cGMP (100 μM) was bath applied as indicated by the horizontal bar. (C) KCa3.1 current potentiation by db-cGMP is prevented by the ROS scavenger, MPG, and the CaMKII inhibitor, mAIP. Each set of three traces shows representative currents before and after adding 300 μM riluzole, with or without 1 μM TRAM-34. Upper panel: Representative recording from an MLS-9 cell pre-treated with 100 μM db-cGMP and the ROS scavenger, MPG (500 μM), for 20 min at room temperature. Lower panel: Cells were pre-treated with 100 μM db-cGMP and the CaM kinase II inhibitor, mAIP (1 μM) for 20 min at room temperature. (D) Summarized data from a population study with treatments as in panel C. The TRAM-34-sensitive KCa3.1 current is expressed as mean ± SEM for the number of cells indicated on each bar, and was compared using a one-way ANOVA with Tukey’s post hoc test; ***p < 0.001.
Acute application of db-cGMP to MLS-9 cells evoked a modest rise in intracellular Ca2+, which peaked at ~15 min and began to decline despite the continued presence of db-cGMP (Figure 3B). The calibrated Fura-2 signal indicates that basal Ca2+ was 88 ± 4 nM (n = 37) [consistent with our earlier studies (23, 31)], and the peak after applying db-cGMP was 242 ± 12 nM (n = 19), which was not sufficient to activate the KCa3.1 current (n = 6 cells tested; example in Figure 3B). In a separate experiment (Figure 3C), MLS-9 cells were treated with db-cGMP to increase the KCa3.1 current (activated by riluzole, as in Figure 1), and simultaneously with a membrane-permeant CaMKII inhibitor (1 μM; myristolated autocamtide-2 related inhibitory peptide; mAIP) or a ROS scavenger (500 μM; MPG). MPG is a synthetic oxyradical scavenger that is effective for superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (OH−) (48). Effects of MPG directly implicate ROS, which is important because the probe used to measure ROS production (CM-H2DCFDA) can also detect peroxynitrite (42). As summarized in Figure 1D, the current density was 23.4 ± 0.9 pA/pF (n = 8) after db-cGMP addition alone, and reduced by MPG to 11.1 ± 0.8 pA/pF (n = 4; p < 0.001) and by mAIP to 11.5 ± 2.2 pA/pF (n = 4; p < 0.001). Together, these data provide evidence that the enhancement in KCa3.1 currents by PKG requires both ROS and CaMKII.
Application of H2O2 increases KCa3.1 current through a CaMKII-dependent pathway, not through direct activation of the channel
To further analyze the ROS-mediated increase in KCa3.1 current, we tested acute application of the relatively stable hydrogen peroxide (1 mM H2O2) molecule. Treating MLS-9 cells with H2O2 evoked a moderate, transient rise in intracellular Ca2+ (Figure 4A) that peaked at 456 ± 33 nM (n = 18) by 11.6 ± 0.1 min after treatment. The peak elevation in Ca2+ evoked by H2O2 was higher than for db-cGMP and occurred ~5 min sooner. Again, it was insufficient to activate the KCa3.1 current in MLS-9 cells (n = 6 cells tested; example in Figure 4A), which requires supra-micromolar concentrations (described above). In contrast, in perforated-patch recordings from cells that were pre-incubated with 1 mM H2O2, the KCa3.1 current was more than twofold larger (21.5 ± 1.6 pA/pF; n = 6; p < 0.0001) than in control cells (10.3 ± 0.5 pA/pF; n = 4; Figures 4B,C). The potentiation of the current was prevented if cells were simultaneously pre-treated with the ROS scavenger (500 μM MGP) or the CaMKII inhibitor (1 μM mAIP). The current density remained at 9.5 ± 1.4 pA/pF in MGP-treated cells and 9.2 ± 1.8 pA/pF in mAIP-treated cells. These data show that KCa3.1 function can be enhanced by this identified, stable ROS species through a similar pathway involving CaMKII.
Figure 4.
Direct application of H2O2 increases the KCa3.1 current through a CaMKII-mediated pathway. (A) Hydrogen peroxide elevates intracellular Ca2+ but does not directly activate KCa3.1 current in MLS-9 cells. Upper panel: Representative Fura-2 recording, in which 1 mM H2O2 was bath applied during the period marked by the horizontal bar. The inset shows calibrated free intracellular Ca2+ concentration as mean ± SEM, n = 18 cells (***p < 0.001, Student’s t-test). Lower panel: Representative current in a perforated-patch recording (same solutions and voltage protocols as Figure 1) with 1 mM H2O2 bath applied as indicated. (B) Representative KCa3.1 current traces in perforated-patch recordings, representative currents before and after adding 300 μM riluzole, with or without 1 μM TRAM-34. From top to bottom: control cell, cell pre-treated with 1 mM H2O2 for 10 min at room temperature, cell pre-treated with both 1 mM H2O2 and the ROS scavenger, MPG (500 μM; 10 min, room temperature), cell pre-treated with 1 mM H2O2, and the CaMKII inhibitor, mAIP (1 μM; 10 min, room temperature). (C) Summarized data from a population study with treatments as in panel (B). The TRAM-34-sensitive KCa3.1 current is expressed as mean ± SEM for the number of cells indicated on each bar and was compared using a one-way ANOVA with Tukey’s post hoc test; ****p < 0.0001.
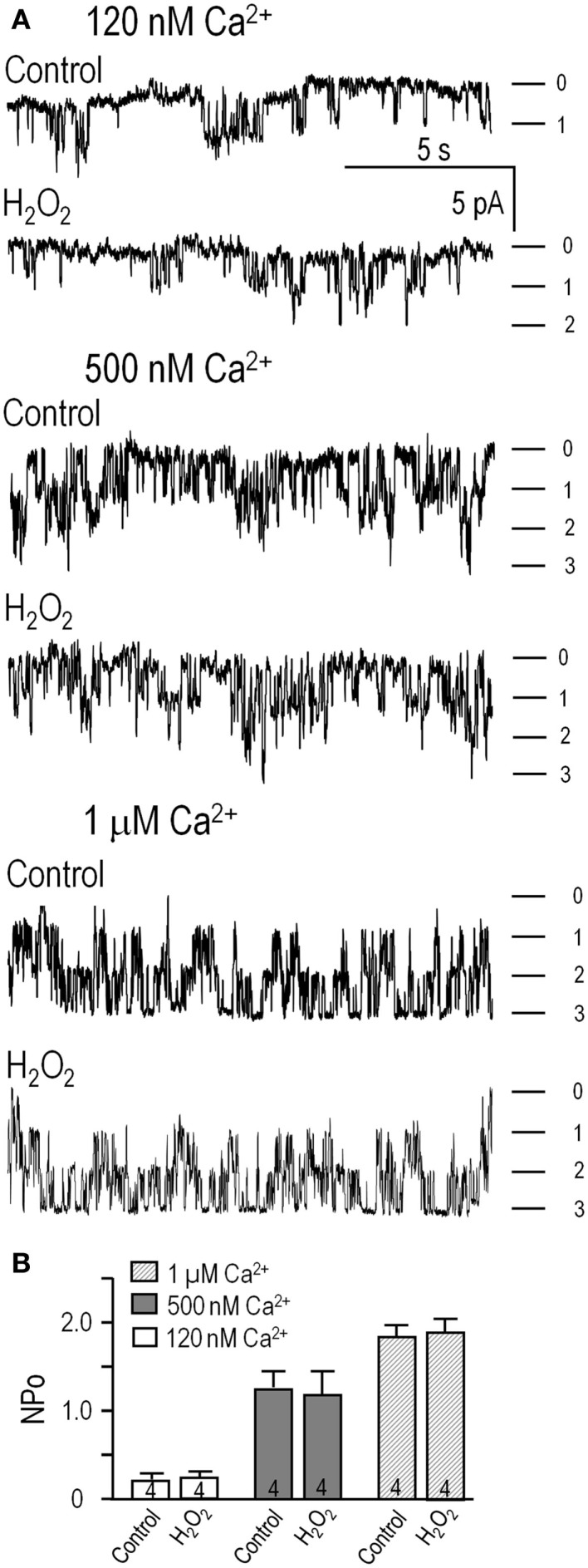
Next, inside-out patches from transfected HEK293 cells were exploited to ask whether H2O2 can directly affect channel activity (NPo; calculated as in Figure 2). There were 1–3 active channels in each patch, channel activity increased with increasing Ca2+ (Figure 5), and the NPo values were the same as in Figure 2. Perfusing 1 mM H2O2 into the bath did not affect channel activity at any of the Ca2+ concentrations (Figure 5A, summarized in Figure 5B). At 120 nM Ca2+, NPo was 0.21 ± 0.05 (n = 4) and 0.23 ± 0.04 after adding H2O2. In 500 nM Ca2+, NPo was 1.17 ± 0.19 (n = 4) and 1.09 ± 0.21 after adding H2O2. At 1 μM Ca2+, NPo was 1.79 ± 0.26 (n = 4) and 1.88 ± 0.31 after adding H2O2. Thus, treatment with this ROS did not directly affect KCa3.1 activity, number of active channels, or their Ca2+ sensitivity; nor was the amplitude of single-channel currents affected (amplitude histograms; not shown). This supports the view that, in order to exert their modulatory effects on KCa3.1 channels, H2O2 and CaMKII require intact cells and possibly another mediator.
Figure 5.
H2O2 did not directly affect the KCa3.1 channels. (A) HEK293 cells transfected with hKCa3.1 were used to assess channel activity in inside-out patches in intracellular (bath) solutions containing 120 nM, 500 nM, or 1 μM free Ca2+ (as in Figure 2), with or without perfusing in 1 mM H2O2. Inward single-channel currents were recorded at −100 mV. (B) Summarized data show NPo in control bath solution, and 4–6 min after adding H2O2. Data are expressed as mean ± SEM from four patches per Ca2+ concentration, and a one-way ANOVA with Tukey’s post hoc test showed no differences following treatments.
In alternative-activated rat microglia, increased ROS production potentiates the KCa3.1 current through a PKG- and CaMKII-dependent pathway
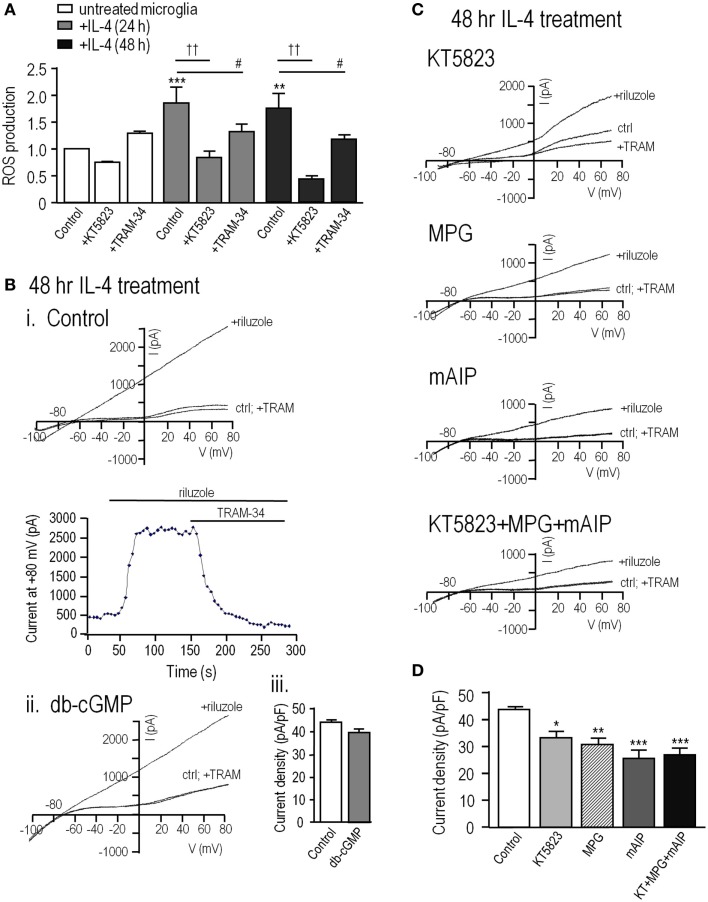
The above results on MLS-9 cells show that ROS and cGMP increase KCa3.1 current through a pathway requiring PKG and CaMKII. It was important to determine whether the same pathway regulates KCa3.1 in primary cultured microglia. For these experiments, we used alternative-activated (IL-4 treated) rat microglia, which have upregulated KCNN4 expression and a much larger KCa3.1 current than resting microglia (20), and in which KCa3.1 contributes to Ca2+ signaling (27) and migration (20). Here, we found that at 24 and 48 h after IL-4 treatment, ROS production was increased by 93 ± 31% (p < 0.01) and 78 ± 21% (p < 0.01), respectively (Figure 6A). The PKG inhibitor, KT5823, decreased this induced ROS production by 53% at 24 h and 73% at 48 h after IL-4 treatment, but did not significantly affect resting microglia. The enhanced ROS production in alternative-activated microglia was moderately dependent on KCa3.1 channels, as TRAM-34 reduced it by 29% at 24 h and 31% at 48 h after IL-4 treatment.
Figure 6.
In alternative-activated rat microglia, ROS production requires and potentiates KCa3.1 channel activity through the ROS-PKG-CaMKII pathway. To evoke alternative activation, primary rat microglia were treated with rat recombinant interleukin-4 (IL-4); 20 ng/mL for 24 or 48 h. (A) Summarized data show ROS production, detected by CM-H2DCFDA (see Methods) in untreated versus IL-4 treated microglia. Under each condition, separate batches of microglia were exposed to the PKG inhibitor (1 μM KT5823) or the KCa3.1 blocker (1 μM TRAM-34) for 24 or 48 h at 37°C. Values are expressed as mean ± SEM (n = 6 replicates experiments each), and compared using a two-way ANOVA with Tukey’s post hoc test. **p < 0.01 and ***p < 0.001 for non-activated versus IL-4 treated cells; #p < 0.05 and ††p < 0.01, for drug treatments, as indicated. (B) KCa3.1 currents were recorded in alternative-activated microglia 48 h after IL-4 treatment, using the perforated-patch configuration and the same solutions and voltage protocols as in Figure 1. (i) Representative currents from a cell before and after adding the KCa3.1 activator, 300 μM riluzole, and the KCa3.1 blocker, 1 μM TRAM-34. (ii) A cell pre-treated with 100 μM db-cGMP for 20 min at room temperature. (iii) Summarized data from a population study, in which the TRAM-34-sensitive KCa3.1 current amplitude is expressed as mean ± SEM (n = 4 cells each). The difference was non-significant based on Student’s t-test. (C). KCa3.1 currents were recorded from alternative-activated microglia, with and without the activator, riluzole, as in panel B. All drug pre-treatments were for 1 h at 37°C. From top to bottom, different microglia were treated with 1 μM KT5823; the ROS scavenger, 500 μM MPG; the CaMKII inhibitor, 1 μM mAIP; KT5823, MPG and mAIP. (D). Summarized data from a population study of experiments as in panel (C). The TRAM-34-sensitive KCa3.1 current is expressed as the mean ± SEM (n = 5 cells each), and was compared using a one-way ANOVA with Tukey’s post hoc test; *p < 0.05, **p < 0.01, ***p < 0.001.
Perforated-patch recordings from alternative-activated rat microglia were used to assess KCa3.1 regulation. For all recordings, the bath contained 100 nM apamin to block the KCa2.3 channels (41), KCa3.1 was activated by riluzole, and 1 μM TRAM-34 was added at the end of each recording to quantify the TRAM-34-sensitive KCa3.1 component. This subtraction procedure was used because primary rat microglia have a voltage-dependent Kv1.3 current, which can be seen in control traces before riluzole was added to activate KCa3.1. Note, however, that riluzole reduces the microglial Kv1.3 current (20). As for MLS-9 cells, riluzole-activated a KCa3.1 current in every microglia cell that was examined. Again, the current activation was not voltage-dependent; it reversed near the K+ Nernst potential, and was fully blocked by 1 μM TRAM-34 (Figure 6B). The only differences from MLS-9 cells were that the current was several-fold larger in IL-4-treated primary microglia (compare with Figure 1) and pre-treatment with db-cGMP did not further increase it. The current density was 43.7 ± 1.4 pA/pF in control microglia and 39.9 ± 1.9 pA/pF in cells pre-treated with db-cGMP, which raises the possibility that the current is already maximal in alternative-activated microglia. The key finding was that the KCa3.1 current in primary microglia was also regulated by ROS, PKG and CaMKII, as it was in MLS-9 cells. The current density was reduced from 43.7 ± 1.4 pA/pF in control microglia to 29.8 ± 2.1 pA/pF by the ROS scavenger (MPG), to 32.6 ± 2.2 pA/pF by the PKG inhibitor (KT5823), and to 23.8 ± 2.7 pA/pF by the CaMKII inhibitor (mAIP) (Figures 6C,D). Evidence for a common regulatory pathway was that combining all three inhibitors did not further reduce the current, as it would have for separate, additive pathways. Together, these results suggest that in alternative-activated microglia, the KCa3.1 current is maximally activated through a regulation pathway involving endogenous PKG, ROS, and CaMKII.
Discussion
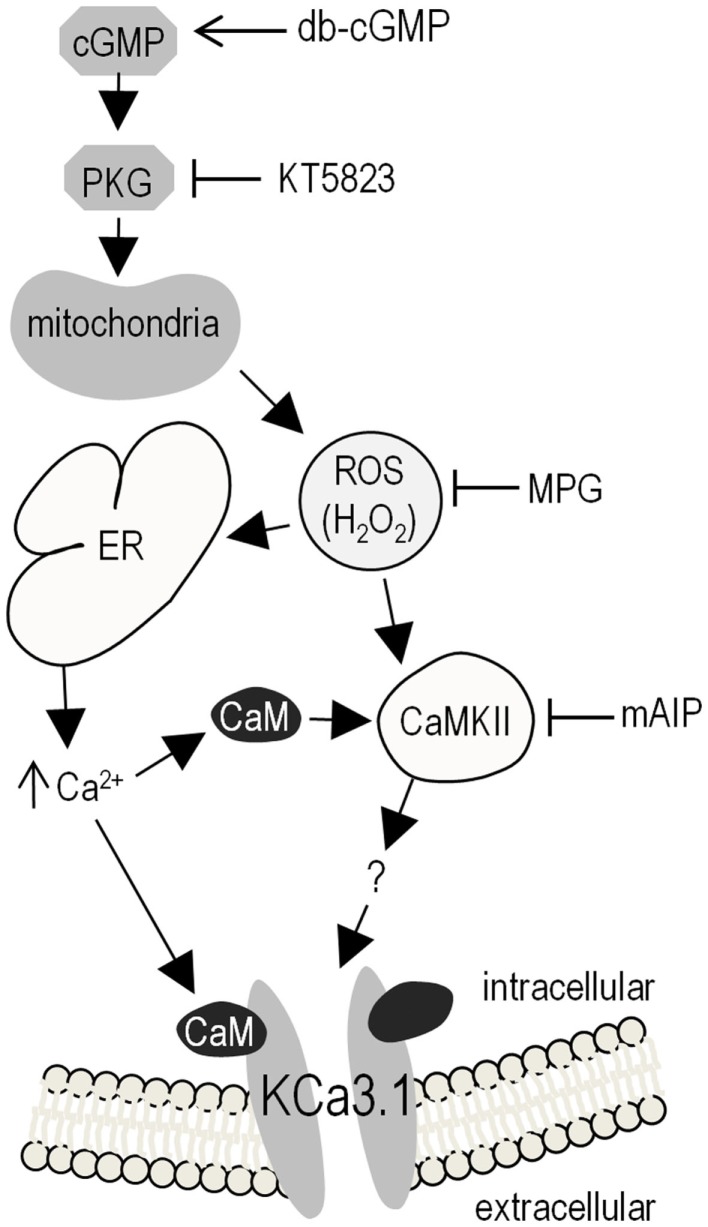
Figure 7 summarizes our results and presents a model of KCa3.1 post-translational regulation based on our observations and the literature. Elevating cGMP activates PKG, which increases ROS production, evokes Ca2+ release from intracellular stores, which binds to CaM, and opens the KCa3.1 channel. CaM also activates CaMKII, which enhances the KCa3.1 current through an unknown mechanism. This is the first report of KCa3.1 regulation by cGMP/PKG and ROS. In comparing the present results with the literature, it is important to note several experimental considerations.
Figure 7.
Summary of results and proposed model of KCa3.1 regulation in microglia. Elevating intracellular cGMP activates PKG, which can then phosphorylate numerous downstream cellular targets, one of which triggers mitochondrial production of ROS (proposed to be via the “5-hydroxydecanoate-sensitive factor” that is likely the mitoKATP channel). Intracellular ROS can then contributes to KCa3.1 regulation through its role as a signaling intermediate; e.g., by evoking Ca2+ release from intracellular stores on the ER, leading to CaM-dependent activation of CaMKII, which then increases KCa3.1 activity (by an unknown mechanism). Activator used: 100 μM db-cGMP (membrane-permeant cGMP analog) to activate PKG. Inhibitors used: 1 μM KT5823 for PKG; 500 μM MPG as a general ROS scavenger (including O2–, H2O2, OH∙); 1 μM mAIP for CaMKII. Acronyms: CaMKII, Ca2+/CaM-dependent protein kinase II; cGMP, cyclic guanosine monophosphate; db-cGMP, dibutyryl-cyclic guanosine monophosphate; ER, endoplasmic reticulum; H2O2, hydrogen peroxide; mAIP, myristolated autocamtide-2 related inhibitory peptide; mitoKATP, mitochondrial ATP-sensitive potassium channel; MPG, N-(2-mercaptopropionyl)glycine; O2–, superoxide; OH∙, hydroxyl radical; PKG, cGMP-dependent protein kinase; ROS, reactive oxygen species.
One major consideration is the patch-clamp configuration. When studying signaling pathways that regulate ion channel function, diffusible regulators can be lost or compromised in whole-cell recordings or excised patches. Therefore, we performed all recordings of native channels in the perforated-patch configuration to maintain the cytoplasmic integrity, allow intracellular Ca2+ to “free-run,” and prevent loss of soluble mediators. We applied all modulators by pre-incubation (10–60 min) before recordings were established; i.e., db-cGMP with or without KT5823 (PKG inhibitor), MPG (ROS scavenger), or mAIP (CaMKII inhibitor); H2O2 with or without MPG or mAIP; or in alternative-activated (IL-4 treated) primary microglia with or without KT5823, MPG or mAIP. Results can differ in excised patches (e.g., our observed lack of effect of PKG in inside-out patches) or in whole-cell recordings. For instance, we showed that cAMP/PKA increased activity of Kv1.3 channels in intact human T lymphocytes but this regulation was lost in whole-cell recordings (49). In a pilot study using whole-cell recordings from MLS-9 cells, acute application of db-cGMP (data not shown) did not increase the KCa3.1 current, and this provided the first evidence for loss of a soluble mediator.
Another concern is whether the channels have been convincingly identified as KCa3.1. Following cloning of KCNN4 [the gene coding for KCa3.1 (5–7)], KCa3.1 currents were identified by their requirement for Ca2+ (EC50 200–700 nM); voltage-independent gating; reversal near the Nernst potential for K+ (–85 mV with physiological internal and external K+), an inward-rectifying single-channel conductance in symmetrical high K+ solutions (~10 pS at positive potentials and 25–35 pS at very negative potentials); block by TRAM-34 (selective at ≤1 μM) and the less selective blockers, charybdotoxin (ChTx; IC50 ~5 nM), and clotrimazole (IC50 < 70 nM). Here, and in several recent papers on microglia, MLS-9 cells (20, 27, 31), we identified the KCa3.1 current by several of these criteria: (i) voltage-independent gating and thus, current seen at all voltages tested; (ii) a need for elevated intracellular Ca2+ (although unusually high); (iii) current enhancement by positive gating modulators (riluzole, 1-EBIO, NS309); and (iv) essentially full block by 1 μM TRAM-34.
Four papers have implicated cGMP and/or PKG in activating Ca2+-dependent K+ channels; two published before the channel was cloned and two afterward. In the early study of vascular smooth muscle cells (50), the type of K+ channel activated by cGMP was not identified, but is unlikely to be KCa3.1. That is, the whole-cell current was depolarization-activated and blocked by 10 mM tetraethylammonium (TEA) as well as 200 nM ChTx, which is more consistent with large-conductance Ca2+-dependent K+ (BK) channels. It is now well known that BK channels are activated by the NO-cGMP-PKG pathway [reviewed in Ref. (51–53)]. In the study on rat cortical collecting duct epithelial cells, inside-out patches showed that two channel types (28 and 85 pS) were activated by cGMP and this was prevented by the PKG inhibitor, KT5823 (54). However, these recordings were made in the absence of intracellular Ca2+ or a gating modifier, and thus neither is likely to be KCa3.1. The two later papers reported that cGMP regulates a clotrimazole-sensitive channel, and one concluded that it was KCa3.1. We reported that the IC50 for KCa3.1 block by clotrimazole was 40 and 56 nM for native channels in human T lymphoblasts and hKCa3.1 expressed in CHO cells, respectively (8) and we subsequently used 200 nM in functional studies (14). However, clotrimazole also inhibits Ca2+ channels (55), and at micromolar concentrations, it inhibits NMDA channels (56), TRPM2 channels (57), and several K+ channels; i.e., transient outward, ultra-rapid delayed-rectifier, hERG and KCNQ1/KCNE1 channels (58). The third paper on potential KCa channel regulation by PKG showed that a K+ current in interstitial cells of Cajal was activated by Ca2+ and by the nitric oxide donor, sodium nitroprusside (SNP), blocked by 1 μM clotrimazole, and had a single-channel conductance of ~38 pS (59), which are consistent with KCa3.1. However, it exhibited a steeply voltage-dependent activation at –40 mV, which is not consistent with KCa3.1. Finally, a study of single-channel activity in human dermal fibroblasts showed that activity was increased by the NO donor, S-nitroso-N-acetylpenicillamine (SNAP) in cell-attached patches, and by applying cGMP + PKG to the cytoplasmic face of inside-out patches (60). The channel was blocked by 10 μM clotrimazole, was depolarization activated at about –40 mV, and had a large single-channel conductance (116 pS reported, >150 pS in some recordings); properties that are inconsistent with KCa3.1.
We began this study by noting that KCa3.1 has a single consensus motif for phosphorylation by PKG (331RKES334 in the human gene), but our results rule out this mechanism. First, applying PKG (with cGMP and ATP) to the cytoplasmic membrane face had no effect on channel activity and second, when S334 is phosphorylated by PKA the current is decreased, not increased (27). Failure of a kinase to phosphorylate a potential consensus motif is not unusual. For instance, PKG might have a low affinity due to conformational considerations; activated PKG (2 PKG monomers + 4 cGMP molecules) is ~148 kDa compared with the catalytic subunit of PKA (43.5 kDa). Our results instead support a linear mechanism for enhancing the native KCa3.1 current: elevating cGMP (by adding membrane-permeant db-cGMP) activates PKG (inhibited by KT5823), evokes ROS production (mimicked by H2O2 and inhibited by MPG), elevates intracellular Ca2+ (but not high enough to directly activate the channel), and activates CaMKII (inhibited by mAIP). Activation of KCa3.1 by CaMKII is consistent with our earlier study in which this current in human T lymphoblasts was inhibited by the CaMK antagonist, KN-62 (8).
Several aspects would be worth considering in future studies. (i) We do not know the mechanism by which CaMKII increases the KCa3.1 current. One possibility is that it promotes channel trafficking/insertion into the surface membrane or, conversely, reduces endocytosis. CaM is involved in assembly and trafficking of KCa3.1 (26) but there is no evidence that CaMKII is involved. In expression systems, KCa3.1 turns over rapidly through clathrin-mediated endocytosis and is then targeting for lysosomal degradation (61). (ii) CaMK-dependent changes in gene transcription can produce long-lasting cellular outcomes, such as learning and memory [reviewed in Ref. (62, 63)] but we think altered gene expression is unlikely. For instance, the CaMKII inhibitor, mAIP, was only applied for 1 h to alternative-activated microglia. (iii) In alternative-activated microglia, ROS production was increased, and dependent on both PKG signaling and KCa3.1 channels. We previously found that KCa3.1 was necessary for efficient ROS production in classical-activated microglia. Because ROS increased the KCa3.1 current this might be a positive feedback mechanism to increase KCa3.1 contributions to microglia functions under conditions of oxidative stress; e.g., after acute injuries, such as stroke. (iv) When exposed to classical-activation stimuli (e.g., LPS) or in conjunction with phagocytosis, microglia produce ROS through an NADPH oxidase (NOX)-mediated respiratory burst (64, 65). While ROS play anti-microbial roles, endogenously generated ROS can potentially feed back onto physiological functions of microglia; e.g., activating NFκB and synthesis of TNFα (66). In contrast, ROS can reduce classical activation of peritoneal macrophages (67), suggesting complex roles in regulating immune cell functions. One question is whether the source matters; i.e., ROS produced by NOX versus mitochondria. We think the long-lasting ROS production in IL-4-treated microglia (elevated for at least 2 days) is likely mediated by mitochondria, because PKG is known to stimulate mitochondrial ROS production in cardiomyocytes and a neuroblastoma cell line (28–30). However, the IL-4 signaling pathway increased ROS production through PI3K-dependent activation of NOX enzymes in an epithelial cell line (68); thus, we cannot rule out NOX contributions.
Broader implications
KCa3.1 is expressed in numerous cell types, including red blood cells, some immune cells [see Introduction, and reviewed in Ref. (69)], neurons (15–17), epithelia (70), vascular smooth muscle and endothelial cells (71, 72), fibroblasts (73, 74), stem cells (75, 76), and cancer cells (77, 78). Because KCa3.1 plays diverse roles in these cells (e.g., proliferation, volume regulation, migration, cytokine production, and others) its post-translational regulation by cGMP/PKG, ROS, and Ca2+/CaM/CaMKII is likely to have broad consequences. There are many opportunities for KCa3.1 to be regulated by cGMP/PKG signaling because this pathway can be activated through NO produced by nNOS (neurons), eNOS (endothelial cells) and iNOS (innate immune cells), and acting on soluble guanylate cyclase. In microglia, iNOS is upregulated and NO is produced in the classical-activated state. Pro-inflammatory mediators are induced by LPS, and there is evidence that atrial natriuretic peptide (ANP)-induced cGMP/PKG signaling can reduce this (79). In microglia, the roles of PKG are not well known but we found that PKG is involved in ROS production in alternative-activated microglia. Further evidence for involvement of PKG and ROS in alternative activation is that PKG increased IL-4 production by Th2 lymphocytes (80), and ROS increased IL-4 release by macrophages (81, 82). The widespread coincidence of this channel and signaling pathway is reinforces the importance of future studies addressing its regulation in other cell types.
Author Contributions
LS, RF, and RW contributed to the conception, design of this study, and wrote the manuscript. RF and RW performed the experiments. LS, RF, and RW agree to be accountable for all aspects of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by an operating grant to LS from the Heart & Stroke Foundation, Ontario chapter (HSFO #00493), an Ontario Graduate Scholarship (RF), and donations to the Toronto General & Western Hospital Foundation. We thank Tamjeed Siddiqui for help measuring intracellular ROS, and Xiaoping Zhu for help preparing microglial cells each week.
References
- 1.Gardos G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta (1958) 30:653–4 10.1016/0006-3002(58)90124-0 [DOI] [PubMed] [Google Scholar]
- 2.Mahaut-Smith MP, Schlichter LC. Ca2+-activated K+ channels in human B lymphocytes and rat thymocytes. J Physiol (1989) 415:69–83. 10.1113/jphysiol.1989.sp017712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grissmer S, Nguyen AN, Cahalan MD. Calcium-activated potassium channels in resting and activated human T lymphocytes. Expression levels, calcium dependence, ion selectivity, and pharmacology. J Gen Physiol (1993) 102:601–30. 10.1085/jgp.102.4.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlichter LC, Pahapill PA, Schumacher PA. Reciprocal regulation of K+ channels by Ca2+ in intact human T lymphocytes. Receptors Channels (1993) 1:201–15. [PubMed] [Google Scholar]
- 5.Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci U S A (1997) 94:11651–6. 10.1073/pnas.94.21.11651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joiner WJ, Wang LY, Tang MD, Kaczmarek LK. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc Natl Acad Sci U S A (1997) 94:11013–8. 10.1073/pnas.94.20.11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logsdon NJ, Kang J, Togo JA, Christian EP, Aiyar J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J Biol Chem (1997) 272:32723–6. 10.1074/jbc.272.52.32723 [DOI] [PubMed] [Google Scholar]
- 8.Khanna R, Chang MC, Joiner WJ, Kaczmarek LK, Schlichter LC. hSK4/hIK1, a calmodulin-binding KCa channel in human T lymphocytes. Roles in proliferation and volume regulation. J Biol Chem (1999) 274:14838–49. 10.1074/jbc.274.21.14838 [DOI] [PubMed] [Google Scholar]
- 9.Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, Gutman GA, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem (2000) 275:37137–49. 10.1074/jbc.M003941200 [DOI] [PubMed] [Google Scholar]
- 10.Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci (2004) 25:280–9. 10.1016/j.tips.2004.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev (2009) 231:59–87. 10.1111/j.1600-065X.2009.00816.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam J, Wulff H. The lymphocyte potassium channels Kv1.3 and KCa3.1 as targets for immunosuppression. Drug Dev Res (2011) 72:573–84. 10.1002/ddr.20467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eder C, Klee R, Heinemann U. Pharmacological properties of Ca2+-activated K+ currents of ramified murine brain macrophages. Naunyn Schmiedebergs Arch Pharmacol (1997) 356:233–9. 10.1007/PL00005046 [DOI] [PubMed] [Google Scholar]
- 14.Khanna R, Roy L, Zhu X, Schlichter LC. K+ channels and the microglial respiratory burst. Am J Physiol Cell Physiol (2001) 280:C796–806. [DOI] [PubMed] [Google Scholar]
- 15.Kaushal V, Koeberle PD, Wang Y, Schlichter LC. The Ca2+-activated K+ channel KCNN4/KCa3.1 contributes to microglia activation and nitric oxide-dependent neurodegeneration. J Neurosci (2007) 27:234–44. 10.1523/JNEUROSCI.3593-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouhy D, Ghasemlou N, Lively S, Redensek A, Rathore KI, Schlichter LC, et al. Inhibition of the Ca2+-dependent K+ channel, KCNN4/KCa3.1, improves tissue protection and locomotor recovery after spinal cord injury. J Neurosci (2011) 31:16298–308. 10.1523/JNEUROSCI.0047-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engbers JD, Anderson D, Asmara H, Rehak R, Mehaffey WH, Hameed S, et al. Intermediate conductance calcium-activated potassium channels modulate summation of parallel fiber input in cerebellar Purkinje cells. Proc Natl Acad Sci U S A (2012) 109:2601–6. 10.1073/pnas.1115024109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wulff H, Zhorov BS. K+ channel modulators for the treatment of neurological disorders and autoimmune diseases. Chem Rev (2008) 108:1744–73 10.1021/cr078234p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maezawa I, Jenkins DP, Jin BE, Wulff H. Microglial KCa3.1 channels as a potential therapeutic target for Alzheimer’s disease. Int J Alzheimers Dis (2012) 2012:868972. 10.1155/2012/868972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira R, Lively S, Schlichter LC. IL-4 type 1 receptor signaling up-regulates KCNN4 expression, and increases the KCa3.1 current and its contribution to migration of alternative-activated microglia. Front Cell Neurosci (2014) 8:183. 10.3389/fncel.2014.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howe CJ, Lahair MM, McCubrey JA, Franklin RA. Redox regulation of the calcium/calmodulin-dependent protein kinases. J Biol Chem (2004) 279:44573–81 10.1074/jbc.M404175200 [DOI] [PubMed] [Google Scholar]
- 22.Nakazaki M, Kakei M, Yaekura K, Koriyama N, Morimitsu S, Ichinari K, et al. Diverse effects of hydrogen peroxide on cytosolic Ca2+ homeostasis in rat pancreatic β-cells. Cell Struct Funct (2000) 25:187–93. 10.1247/csf.25.187 [DOI] [PubMed] [Google Scholar]
- 23.Ferreira R, Schlichter LC. Selective activation of KCa3.1 and CRAC channels by P2Y2 receptors promotes Ca2+ signaling, store refilling and migration of rat microglial cells. PLoS One (2013) 8:e62345. 10.1371/journal.pone.0062345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lively S, Schlichter LC. The microglial activation state regulates migration and roles of matrix-dissolving enzymes for invasion. J Neuroinflammation (2013) 10:75. 10.1186/1742-2094-10-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanger CM, Ghanshani S, Logsdon NJ, Rauer H, Kalman K, Zhou J, et al. Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J Biol Chem (1999) 274:5746–54. 10.1074/jbc.274.9.5746 [DOI] [PubMed] [Google Scholar]
- 26.Joiner WJ, Khanna R, Schlichter LC, Kaczmarek LK. Calmodulin regulates assembly and trafficking of SK4/IK1 Ca2+-activated K+ channels. J Biol Chem (2001) 276:37980–5. 10.1074/jbc.M104965200 [DOI] [PubMed] [Google Scholar]
- 27.Wong R, Schlichter LC. PKA reduces the rat and human KCa3.1 current, CaM binding, and Ca2+ signaling, which requires Ser332/334 in the CaM-binding C terminus. J Neurosci (2014) 34:13371–83. 10.1523/JNEUROSCI.1008-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z, Ji X, Boysen PG. Exogenous nitric oxide generates ROS and induces cardioprotection: involvement of PKG, mitochondrial KATP channels, and ERK. Am J Physiol Heart Circ Physiol (2004) 286:H1433–40. 10.1152/ajpheart.00882.2003 [DOI] [PubMed] [Google Scholar]
- 29.Chai Y, Lin YF. Stimulation of neuronal KATP channels by cGMP-dependent protein kinase: involvement of ROS and 5-hydroxydecanoate-sensitive factors in signal transduction. Am J Physiol Cell Physiol (2010) 298:C875–92. 10.1152/ajpcell.00196.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai Y, Zhang DM, Lin YF. Activation of cGMP-dependent protein kinase stimulates cardiac ATP-sensitive potassium channels via a ROS/calmodulin/CaMKII signaling cascade. PLoS One (2011) 6:e18191. 10.1371/journal.pone.0018191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu BS, Ferreira R, Lively S, Schlichter LC. Microglial SK3 and SK4 currents and activation state are modulated by the neuroprotective drug, riluzole. J Neuroimmune Pharmacol (2013) 8:227–37. 10.1007/s11481-012-9365-0 [DOI] [PubMed] [Google Scholar]
- 32.Sivagnanam V, Zhu X, Schlichter LC. Dominance of E. coli phagocytosis over LPS in the inflammatory response of microglia. J Neuroimmunol (2010) 227:111–9. 10.1016/j.jneuroim.2010.06.021 [DOI] [PubMed] [Google Scholar]
- 33.Schlichter LC, Sakellaropoulos G, Ballyk B, Pennefather PS, Phipps DJ. Properties of K+ and Cl- channels and their involvement in proliferation of rat microglial cells. Glia (1996) 17:225–36. [DOI] [PubMed] [Google Scholar]
- 34.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep (2014) 6:13. 10.12703/P6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodge PA, Sriram S. Regulation of microglial activation by TGF-β, IL-10, and CSF-1. J Leukoc Biol (1996) 60:502–8. [DOI] [PubMed] [Google Scholar]
- 36.Cayabyab FS, Khanna R, Jones OT, Schlichter LC. Suppression of the rat microglia Kv1.3 current by src-family tyrosine kinases and oxygen/glucose deprivation. Eur J Neurosci (2000) 12:1949–60. 10.1046/j.1460-9568.2000.00083.x [DOI] [PubMed] [Google Scholar]
- 37.Cayabyab FS, Schlichter LC. Regulation of an ERG K+ current by Src tyrosine kinase. J Biol Chem (2002) 277:13673–81. 10.1074/jbc.M108211200 [DOI] [PubMed] [Google Scholar]
- 38.Cayabyab FS, Tsui FW, Schlichter LC. Modulation of the ERG K+ current by the tyrosine phosphatase, SHP-1. J Biol Chem (2002) 277:48130–8. 10.1074/jbc.M208448200 [DOI] [PubMed] [Google Scholar]
- 39.Ducharme G, Newell EW, Pinto C, Schlichter LC. Small-conductance Cl- channels contribute to volume regulation and phagocytosis in microglia. Eur J Neurosci (2007) 26:2119–30. 10.1111/j.1460-9568.2007.05802.x [DOI] [PubMed] [Google Scholar]
- 40.Schlichter LC, Kaushal V, Moxon-Emre I, Sivagnanam V, Vincent C. The Ca2+ activated SK3 channel is expressed in microglia in the rat striatum and contributes to microglia-mediated neurotoxicity in vitro. J Neuroinflammation (2010) 7:1–15. 10.1186/1742-2094-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siddiqui T, Lively S, Ferreira R, Wong R, Schlichter LC. Expression and contributions of TRPM7 and KCa2.3/SK3 channels to the increased migration and invasion of microglia in anti-inflammatory activation states. PLoS One (2014) 9:e106087. 10.1371/journal.pone.0106087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kristiansen KA, Jensen PE, Moller IM, Schulz A. Monitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM-H2DCFDA and confocal laser microscopy. Physiol Plant (2009) 136:369–83. 10.1111/j.1399-3054.2009.01243.x [DOI] [PubMed] [Google Scholar]
- 43.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem (1985) 260:3440–50. [PubMed] [Google Scholar]
- 44.Kotecha SA, Schlichter LC. A Kv1.5 to Kv1.3 switch in endogenous hippocampal microglia and a role in proliferation. J Neurosci (1999) 19:10680–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newell EW, Schlichter LC. Integration of K+ and Cl- currents regulate steady-state and dynamic membrane potentials in cultured rat microglia. J Physiol (2005) 567:869–90. 10.1113/jphysiol.2005.092056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott JD. Cyclic nucleotide-dependent protein kinases. Pharmacol Ther (1991) 50:123–45. 10.1016/0163-7258(91)90075-W [DOI] [PubMed] [Google Scholar]
- 47.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell (2008) 133:462–74. 10.1016/j.cell.2008.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fantinelli JC, Gonzalez Arbelaez LF, Perez Nunez IA, Mosca SM. Protective effects of N-(2-mercaptopropionyl)-glycine against ischemia-reperfusion injury in hypertrophied hearts. Exp Mol Pathol (2013) 94:277–84. 10.1016/j.yexmp.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 49.Pahapill PA, Schlichter LC. Modulation of potassium channels in intact human T lymphocytes. J Physiol (1992) 445:407–30. 10.1113/jphysiol.1992.sp018931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K+ channel by cGMP-dependent protein kinase. Proc Natl Acad Sci U S A (1994) 91:7583–7. 10.1073/pnas.91.16.7583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol (1985) (2001) 91:1421–30. [DOI] [PubMed] [Google Scholar]
- 52.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev (2010) 62:525–63. 10.1124/pr.110.002907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bice JS, Burley DS, Baxter GF. Novel approaches and opportunities for cardioprotective signaling through 3’,5’-cyclic guanosine monophosphate manipulation. J Cardiovasc Pharmacol Ther (2014) 19:269–82. 10.1177/1074248413518971 [DOI] [PubMed] [Google Scholar]
- 54.Hirsch J, Schlatter E. K+ channels in the basolateral membrane of rat cortical collecting duct are regulated by a cGMP-dependent protein kinase. Pflugers Arch (1995) 429:338–44 10.1007/BF00374148 [DOI] [PubMed] [Google Scholar]
- 55.Fearon IM, Ball SG, Peers C. Clotrimazole inhibits the recombinant human cardiac L-type Ca2+ channel α 1C subunit. Br J Pharmacol (2000) 129:547–54. 10.1038/sj.bjp.0703106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isaev NK, Stelmashook EV, Dirnagl U, Andreeva NA, Manuhova L, Vorobjev VS, et al. Neuroprotective effects of the antifungal drug clotrimazole. Neuroscience (2002) 113:47–53 10.1016/S0306-4522(02)00164-1 [DOI] [PubMed] [Google Scholar]
- 57.Hill K, McNulty S, Randall AD. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn Schmiedebergs Arch Pharmacol (2004) 370:227–37. 10.1007/s00210-004-0981-y [DOI] [PubMed] [Google Scholar]
- 58.Tian M, Dong MQ, Chiu SW, Lau CP, Li GR. Effects of the antifungal antibiotic clotrimazole on human cardiac repolarization potassium currents. Br J Pharmacol (2006) 147:289–97. 10.1038/sj.bjp.0706590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu Y, Ye J, Huizinga JD. Clotrimazole-sensitive K+ currents regulate pacemaker activity in interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol (2007) 292:G1715–25. 10.1152/ajpgi.00524.2006 [DOI] [PubMed] [Google Scholar]
- 60.Bae H, Lee HJ, Kim K, Kim JH, Kim T, Ko JH, et al. The stimulating effects of nitric oxide on intermediate conductance Ca2+-activated K+ channels in human dermal fibroblasts through PKG pathways but not the PKA pathways. Chin J Physiol (2014) 57:137–51. 10.4077/CJP.2014.BAB171 [DOI] [PubMed] [Google Scholar]
- 61.Balut CM, Hamilton KL, Devor DC. Trafficking of intermediate KCa3.1 and small KCa2.x conductance, Ca2+-activated K+ channels: a novel target for medicinal chemistry efforts? ChemMedChem (2012) 7:1741–55. 10.1002/cmdc.201200226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bading H. Nuclear calcium signalling in the regulation of brain function. Nat Rev Neurosci (2013) 14:593–608. 10.1038/nrn3531 [DOI] [PubMed] [Google Scholar]
- 63.Giese KP, Mizuno K. The roles of protein kinases in learning and memory. Learn Mem (2013) 20:540–52. 10.1101/lm.028449.112 [DOI] [PubMed] [Google Scholar]
- 64.Colton CA, Gilbert DL. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett (1987) 223:284–8. 10.1016/0014-5793(87)80305-8 [DOI] [PubMed] [Google Scholar]
- 65.Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch Biochem Biophys (1998) 353:312–21. 10.1006/abbi.1998.0658 [DOI] [PubMed] [Google Scholar]
- 66.Kaul N, Forman HJ. Activation of NFκB by the respiratory burst of macrophages. Free Radic Biol Med (1996) 21:401–5. 10.1016/0891-5849(96)00178-5 [DOI] [PubMed] [Google Scholar]
- 67.Marikovsky M, Ziv V, Nevo N, Harris-Cerruti C, Mahler O. Cu/Zn superoxide dismutase plays important role in immune response. J Immunol (2003) 170:2993–3001. 10.4049/jimmunol.170.6.2993 [DOI] [PubMed] [Google Scholar]
- 68.Sharma P, Chakraborty R, Wang L, Min B, Tremblay ML, Kawahara T, et al. Redox regulation of interleukin-4 signaling. Immunity (2008) 29:551–64 10.1016/j.immuni.2008.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wulff H, Castle NA. Therapeutic potential of KCa3.1 blockers: recent advances and promising trends. Expert Rev Clin Pharmacol (2010) 3:385–96. 10.1586/ecp.10.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol (2000) 279:C461–79. [DOI] [PubMed] [Google Scholar]
- 71.Grgic I, Eichler I, Heinau P, Si H, Brakemeier S, Hoyer J, et al. Selective blockade of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler Thromb Vasc Biol (2005) 25:704–9. 10.1161/01.ATV.0000156399.12787.5c [DOI] [PubMed] [Google Scholar]
- 72.Su XL, Wang Y, Zhang W, Zhao LM, Li GR, Deng XL. Insulin-mediated upregulation of KCa3.1 channels promotes cell migration and proliferation in rat vascular smooth muscle. J Mol Cell Cardiol (2011) 51:51–7. 10.1016/j.yjmcc.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 73.Grgic I, Kiss E, Kaistha BP, Busch C, Kloss M, Sautter J, et al. Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels. Proc Natl Acad Sci U S A (2009) 106:14518–23. 10.1073/pnas.0903458106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang C, Shen S, Ma Q, Gill A, Pollock CA, Chen XM. KCa3.1 mediates activation of fibroblasts in diabetic renal interstitial fibrosis. Nephrol Dial Transplant (2013) 29:313–24. 10.1093/ndt/gft431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deng XL, Lau CP, Lai K, Cheung KF, Lau GK, Li GR. Cell cycle-dependent expression of potassium channels and cell proliferation in rat mesenchymal stem cells from bone marrow. Cell Prolif (2007) 40:656–70. 10.1111/j.1365-2184.2007.00458.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tao R, Lau CP, Tse HF, Li GR. Regulation of cell proliferation by intermediate-conductance Ca2+-activated potassium and volume-sensitive chloride channels in mouse mesenchymal stem cells. Am J Physiol Cell Physiol (2008) 295:C1409–16. 10.1152/ajpcell.00268.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jager H, Dreker T, Buck A, Giehl K, Gress T, Grissmer S. Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreaticn cancer cell growth in vitro. Mol Pharmacol (2004) 65:630–8. 10.1124/mol.65.3.630 [DOI] [PubMed] [Google Scholar]
- 78.Fioretti B, Castigli E, Micheli MR, Bova R, Sciaccaluga M, Harper A, et al. Expression and modulation of the intermediate- conductance Ca2+-activated K+ channel in glioblastoma GL-15 cells. Cell Physiol Biochem (2006) 18:47–56. 10.1159/000095135 [DOI] [PubMed] [Google Scholar]
- 79.Boran MS, Baltrons MA, Garcia A. The ANP-cGMP-protein kinase G pathway induces a phagocytic phenotype but decreases inflammatory gene expression in microglial cells. Glia (2008) 56:394–411 10.1002/glia.20618 [DOI] [PubMed] [Google Scholar]
- 80.Gomes B, Savignac M, Cabral MD, Paulet P, Moreau M, Leclerc C, et al. The cGMP/protein kinase G pathway contributes to dihydropyridine-sensitive calcium response and cytokine production in TH2 lymphocytes. J Biol Chem (2006) 281:12421–7. 10.1074/jbc.M510653200 [DOI] [PubMed] [Google Scholar]
- 81.Dobashi K, Aihara M, Araki T, Shimizu Y, Utsugi M, Iizuka K, et al. Regulation of LPS induced IL-12 production by IFN-gamma and IL-4 through intracellular glutathione status in human alveolar macrophages. Clin Exp Immunol (2001) 124:290–6. 10.1046/j.1365-2249.2001.01535.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murata Y, Shimamura T, Hamuro J. The polarization of Th1/Th2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int Immunol (2002) 14:201–12. 10.1093/intimm/14.2.201 [DOI] [PubMed] [Google Scholar]