Abstract
High-relaxivity T1-weighted (T1w) MR molecular imaging nanoparticles typically present high surface gadolinium payloads that can elicit significant acute complement activation (CA). The objective of this research was to develop a high T1 contrast nanoparticle, which reduced gadolinium exposure, minimized gadolinium transmetallation risk, and elicited negligible acute complement activation. We report the development, optimization, and characterization of a gadolinium-manganese hybrid nanocolloid (MnOL-Gd NC; 138±10 (Dav)/nm; PDI: 0.06; zeta: −27±2 mV). High r1 particulate relaxivity with minute additions of Gd-DOTA-lipid conjugate to the MnOL nanocolloid surface achieved an unexpected paramagnetic synergism. This hybrid MnOL-Gd NC provided optimal MR TSE signal intensity at 5nM/voxel and lower levels, consistent with the level expression anticipated for sparse biomarkers such as neovascular integrins. MnOL NC produced optimal MR TSE signal intensity at 10nM/voxel concentrations and above. Importantly, MnOL-Gd NC avoided acute CA in vitro and in vivo, while retaining minimal transmetallation risk.
Keywords: MRI, contrast media, manganese, gadolinium, complement activation, nanoparticle
Introduction
T1-weighted (T1w) MR imaging of sparse biomarkers, such as neovascular integrins (e.g. ανβ3 integrin), with lipid-based nanoparticles typically require high surface densities of chelated gadolinium-lipid conjugates. 1–13 Although Gd chelates are safely used as blood pool contrast agents, the recognition of nephrogenic systemic fibrosis (NSF) created significant concerns for gadolinium (Gd3+) including contraindications for its use in patients with renal failure or following liver transplantation. 14–16 Mechanistically, flexible linear acyclic chelates (e.g. DTPA, diethylenetriaminepentaacetic) allow greater Gd3+ transmetallation in comparison to macrocyclics (e.g. DOTA, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid). 17 Coupling Gd-DTPA to lipid anchors for incorporation into phospholipid encapsulated nanoparticles (NP) compromise the stability of the Gd3+ complexation. 18 In contradistinction, Gd3+ chelation to the macrocyclic DOTA chelate is highly stable and functionalizing the complex with lipid anchors imparts negligible increased risk of transmetallation. 19 From a clinical translation perspective, high relaxivity paramagnetic nanoparticle utilizing gadolinium DOTA (Gd-DOTA) is intuitively preferred to minimize NSF risk. Unfortunately, patients administered low dosages of high relaxivity Gd-DOTA perfluorocarbon nanoparticles experienced mild, transient, nonspecific discomfort in an unpublished Phase 1 clinical study, which was subsequently associated with antibody-mediated acute complement activation triggered by membrane inclusion of Gd-DOTA-phosphatidylethanolamine (PE). 20
Development of “soft-particle” preparations with the potential for T1w molecular imaging of biosignatures expressed at low nanomolar/voxel densities have been designed using iron oxide 21 or manganese. 22, 23 Of these, the most attractive translational candidate was a lipid-encapsulated nanocolloid entrapping a high density of manganese oleate (MnOL) in vegetable oil. 23 Manganese (Mn3+/Mn2+) by virtue of its high spin number, labile water exchange, long electronic relaxation time, natural prevalance and known human biochemistry has long been studied as a T1w MR contrast agent. 24 MnOL nanocolloid (NC) had higher relaxivity than its analogue that included equimolar concentrations of metal in the form of manganese oxide (MnOx, 12 nm). 23
We hypothesized that the r1 relaxivity of MnOL NC could be enhanced by inclusion of Gd-DOTA in the lipid membrane. (Figure 1) In contradistinction to the high payloads of gadolinium required with perfluorocarbon (PFC) nanoparticles (NP), for which the fluorine-rich core did not contribute to the NP proton relaxivity, 19, 25 we anticipated that the gadolinium payload required to enhance the relaxivity for the hybrid “mixed-metal” nanocolloid (MnOL-Gd NC) would be reduced given the signal contributions of the Mn-rich core. Further, we hypothesized that a low surface Gd-DOTA presentation density would mitigate against acute compliment activation, lower total gadolinium exposure, and retain minimal transmetallation risk. The overarching objectives of this research were to design, synthesize and characterize the physical, chemical, and magnetic properties of a hybrid MnOL-Gd NC and determine its propensity for complement activation in human sera and mice.
Figure 1.
Illustration of the hypothesis for the enhanced r1 relaxivity appreciated with Gd-DOTA-PE MnOL NC (B) in comparison with (A) MnOL NC alone. The unusual high relaxivity of MnOL is derived from the high core concentration of manganese oleate suspended in polysorbate that was able to permeate the phospholipid layer essentially to the water:particle interface. The addition of Gd-DOTA-PE provides additional relaxivity of the NC by introducing low concentrations of the metal in the water milieu around the periphery. The inherent magnetic dipole of the MnOL particle is synergistically augmented by this dual paramagnetic metal arrangement. The physical basis for the interaction remains unresolved as of this report.
Methods
Materials
Unless otherwise listed, all solvents and reagents were purchased from Aldrich Chemical Co. (St. Louis, MO, USA) and used as received. Anhydrous chloroform was purchased from Aldrich Chemical Co. (St. Louis, MO, USA) and distilled over calcium hydride prior to use. High purity egg yolk phosphatidylcholine was purchased from Avanti Polar Lipids, Inc (Alabaster, AL, USA). Manganese chloride and sorbitan sesquioleate were purchased and used as received from Aldrich Chemical Co. Sodium oleate was purchased and used as received from TCI America. (Portland, OR, USA). Argon and nitrogen (UHP, 99.99%) were used for storage of materials. The Spectra/Por membrane (Cellulose MWCO: 20 000 Da) was used for dialysis (Spectrum Medical Industries, Inc., Laguna Hills, CA, USA).
Synthesis of manganese oleate nanocolloids with titrated gadolinium chelates
Organometallic manganese oleate was synthesized and comixed with almond oil to form the emulsion core, which was encapsulated with phospholipid via microfluidization to produce the base nanocolloid, as previously reported. 23 Briefly, manganese chloride tetrahydrate was reacted with sodium oleate in a mixture of ethanol-water-hexane for 14h maintaining the temperature at 80°C then for 4h at ambient temperature to afford Mn-oleate, which was characterized by FT-IR and TGA. 23 Mn-oleate was suspended with almond oil as an inner matrix then homogenized with the surfactant mixture in a Microfluidics fluidizer (M110s, Westwood, MA) at 14,000 PSI to synthesize ManOL. The surfactant mixtures were comprised of titrated concentrations (mole%) of 0, 0.6, 1.3, 2.5, and 5.0 Gd-DOTA-phospatidylethanolamine19 (Gd-DOTA-PE, a gift of Kereos, Inc, St. Louis, MO, USA) and phosphatidylcholine (PC). The nanocolloids were dialyzed against water using a 20,000 Da MWCO cellulose membrane for prolonged periods of time then passed through a 0.45 μm Acrodisc Syringe filter. The nanocolloids were stored under argon atmosphere at 4°C to reduce oxidation and bacterial growth. Overall, nominal particle sizes and zeta potentials were estimated with dynamic light scattering (DLS, Brookhaven Instrument Corp., Holtsville, NY): DLS (Dav)=134±02 nm; Zeta (ζ)= −25±02 mV; PDI: 0.13±0.03. Manganese and gadolinium concentrations were confirmed by inductively coupled plasma optical emission spectrometry (ICP OES, Perkin Elmer, Waltham, MA). Control nanocolloid (ConNC) was prepared following a similar procedure without incorporation of Mn-oleate; a vegetable oil core (20% v/v) was encapsulated with phosphatidylcholine (100 mole%).
More specifically, the hybrid MnOL-1.25 mol% Gd NC (MnOL-Gd NC) was selected for further detailed experimentation. It had a hydrodynamic particle diameter of 138 ± 10 (Dav) (polydispersity index, PDI: 0.06). The particle stability and successful phospholipid encapsulation were supported by the presence of a negative electrophoretic potential (ζ) value (−27±2 mV). The nanocolloid was comprised of bivalent manganese (Mn2+) oleate and the concentration of manganese was analytically determined as 20.2±0.02 mM Mn2+/ml, equal to ~100,000 Mn2+atoms per nanoparticle. The gadolinium (Gd3+) content was 0.36±0.02 mM Gd3+/ml, equal to 1,700 Gd3+ atoms per particle. Anhydrous state morphology of the particles was observed by atomic force microscopy (AFM), which confirmed the spherical nature of the colloids. (Figure 2)
Figure 2.
A. Schematic representation of 1.25 mol% Gd-DOTA-PE MnOL NC illustrating a phospholipid encapsulation of a high metal density manganese oleate in polysorbate core with surfactant incorporation of the lipophilic chelate. B. Particles size was 138±10 (Dav) (polydispersity indexes, PDI: 0.06) in water with concordant data appreciated by transmission electron microscopy (TEM, C.) and atomic force microscopy (AFM, D.).
Synthesis of Control Paramagnetic and DOTAP Perfluorocarbon Nanoparticles
The synthesis and characterization of control paramagnetic and cationic DOTAP perfluorocarbon nanoparticles for complement activation studies have been previously reported and are provided as Supplemental Data: Methods. 20, 26
MR nanoparticle MR charcterization
All MR imaging characterization experiments were performed with a 3.0T clinical scanner (Achieva; Philips Healthcare, Andover, MA) using a standard birdcage coil (in vitro). 26 The longitudinal relaxation times of the titrated MnOL-Gd NCs were detemined using a Look-Locker inversion-recovery pulse sequence (TE/TR/α: 1.48ms/3000ms/10°, acquisition matrix 272×270, slice thickness 6mm, number of signal averages=4, resolution= 0.4mm × 0.4 mm). After a 180° prepulse, 21 gradient echo images were acquired with a phase interval of 53 ms; all measurements were replicated twice. The transverse relaxation times were measured with a Carr-Purcell-Meiboom-Gill (CPMG) sequence wherein 30 images with equal echo spacing were acquired (TE/TR/α = 40ms/132ms/90°, matrix 204 × 204, slice thickness=6mm, number of signal averages=4, resolution=0.8 × 0.8mm). Ionic and particulate relaxivities (i.e., r1 and r2) were calculated from the slope of the linear least squares regression of relaxation rate versus Mn 2++ Gd3+ or nanocolloid particle concentrations and reported in units of (s*mM)−1 of total metal or nanocolloid, respectively.
Complement activation
In vitro NP-dependent complement activation/CH50 assay
MnOL-Gd NCs incorporating 0.6, 1.25, and 2.5 mol% Gd-DOTA-PE were evaluated for complement activation using a CH50 hemolysis assay. 20 To quantify the capacity of NPs to activate human complement, we compared the capacity of NP-treated serum and untreated control serum to lyse antibody-sensitized sheep erythrocytes. NPs (10% v/v) were incubated in 10% pooled human serum (CompTech: Tyler, TX) in gelatin veronal buffer supplemented with Mg2+ and Ca2+ (GVB2+) (150 μl total) for 30 min at 37 °C. Reaction mixtures were then chilled to 4 °C and diluted with GVB2+ to a total of 800 μl. Titration curves were constructed from a series of reactions, each composed of 150 μl of variously diluted supernatant plus 5×107 (100 μl) antibody-sensitized sheep erythrocytes (EA) (Supplemental Data: Methods for EA preparation). Reactions were incubated at 37 °C for 1 h with shaking, diluted with 667 μl of GVB2+, and subjected to centrifugation (1000 g for 5 min). Degree of cell lysis was determined by spectroscopy measurement at 414nm. A value for complete cell lysis was provided by a control reaction consisting of EA mixed with water. Residual activity of NP-treated serum was compared with the residual activity of serum incubated with buffer alone. The Z value is the average number of lytic sites per cell (Z =−ln (1−y) where y is the fraction of cells lysed). CH50 is equal to the serum dilution factor that results in 50% cell lysis (when Z=0.69).
In vivo complement activation - C3a ELISA
All animal experiments were performed in compliance with federal laws and in strict accordance with the guidelines established by the Division of Comparative Medicine at Washington University. The animal protocol is subjected to annual review and approval by The Animal Studies Committee of Washington University. Mice (n=33, ≥ 5/treatment group) were injected i.v. with PBS (negative control) or nanoparticles at 5 μl/g of body weight and plasma was obtained at 30 min for C3a ELISA. ELISA plates were coated overnight at 4°C with anti-mouse C3a monoclonal antibody (4 μg/ml; BD Pharmingen). After blocking with 1% BSA, the plates were washed and incubated with samples (100 μl of fresh plasma diluted 1:100 in PBS) for 2 h at room temperature, followed by biotinylated anti-mouse C3a monoclonal antibody (250 ng/ml; BD Pharmingen, San Jose, CA). Following a 20 min incubation with streptavidin-peroxidase (400 ng/ml; Sigma), 100 μl of peroxide-chromogen solution (R&D Systems, Minneapolis, MN) was added to each well, and color development was read at 450 nm with a SpectraMax Plus reader (Molecular Devices, Sunnyvale, CA). Mouse recombinant C3a (BD Pharmingen) was used to establish the standard curve.
Results
Relaxivity of MnOL-Gd Nanocolloids
MnOL-Gd NCs incorporated varying concentrations of lipophilic Gd-DOTA-PE chelate, which positioned the metal beyond the water-particle surface interface for greater 1H relaxivity. 25
As shown in Figure 3 and Supplemental Data: Table 1, the addition of surface gadolinium to the MnOL-Gd NCs enhanced r1 relaxivity over MnOL NC. MnOL-Gd NC achieved the high r1 relaxivity even at the lowest surfactant concentrations evaluated, down to 0.6 mole% with negligible improvement observed with increases of surfactant Gd-DOTA-PE up to 5 mole%. At 5 mole%, r1 relaxivity declined slightly suggesting early T2* dephasing. In the present study, MnOL-Gd NC yielded comparable r1 relaxivity to previously reported Gd-PFC NP with as low as 1/50th of the lanthanide load per NP. 19, 25
Figure 3.

Particulate r1 relaxivity of phospholipid-encapsulated MnOL NC with varying levels of Gd-DOTA-PE included in the surfactant presented as the slope of regression ± standard error of the estimate.
To further elucidate the MR relaxivity (3T @ 25 C) contributions of manganese and gadolinium, four different formulations were characterized on the basis of total metal concentration ([Mn + Gd]) and nanocolloid (NC) concentration ([MnOL-Gd NC]). Specifically, MnOL-Gd NC (1.25% Gd-DOTA-PE), MnOL NC, Gd-vegetable oil (1.25% Gd-DOTA-PE, Gd-only), and 50:50 MnOL:Gd-only NC mixture were compared. (Figure 4A,B; Supplemental Data: Table 2). Gd-DOTA-PE incorporated into the surfactant augmented the r1 of MnOL 25% while no improvement in r1 was appreciated for the 50:50 particle mixture, indicating an unexpected synergistic enhancement of MnOL-Gd NC r1 that was dependent on the magnetic field interactions between the Mn2+ in the core and Gd-DOTA-PE on the surface. The ionic and particulate r2 relaxivities for MnOL-Gd NC, MnOL NC, the 50:50 MnOL: Gd-only NC mixture followed a similar relational pattern. (Figure 4C,D; Supplemental Data: Table 2)
Figure 4.
Ionic and particulate r1 and r2 relaxivities (3T @ 25 °C) of MnOL-Gd NC (1.25% Gd-DOTA-PE), MnOL NC, Gd-vegetable oil (1.25% Gd-DOTA-PE; Gd-only), and a 50:50 MnOL: Gd-only NC mixture (i.e., equimolar metal concentrations) in suspension characterized on the basis of total metal concentration ([Mn + Gd]) (A & C) and nanocolloid (NC) concentration ([MnOL-Gd NC](B & D)).
To assess the potential impact that the addition of 1.25 mole% Gd-MnOL would have on the MR detectability of receptors expressed at a nanomolar/voxel concentration, the relative T1w turbo-spin echo signal intensities (TSE-SI, 3T @25 C) for MnOL-Gd NC and MnOL NC diluted in deionized water were determined. MnOL-Gd NC and MnOL NC peak TSE signal intensity (TSE-SI) in vitro were comparable in magnitude, but peak signal MnOL-Gd NC was measured at 5 nM, and was higher than MnOL NC at 5nM and lower. At higher voxel concentrations, MnOL-Gd NC TSE-SI declined due to T2 (T2*) effects. By comparison, MnOL NCs (i.e., no Gd3+) had an equal magnitued peak TSE-SI, which occured at the voxel concentration of ~10 nM. At this concentration and above, the TSE-SI of MnOL was greater than MnOL-Gd, although it too declined due to T2 (T2*) effects. (Figure 5A) Closer examination of comparative TSE-SI below 5 nM level clearly illustrates the sensitivity advantage of the MnOL-Gd NC for sparser biomarker vascular targets (Figure 5B). Since the MnOL NC TSE-SI peaked at 2-fold higher concentrations than MnOL-Gd NC before declining due to T2(T2*) effects, it may be preferred for imaging higher density epitopes, e.g., fibrin in thrombus; whereas, MnOL-Gd NC would be preferred for less prevalent biosignatures, such as neovascular integrin expression.
Figure 5.
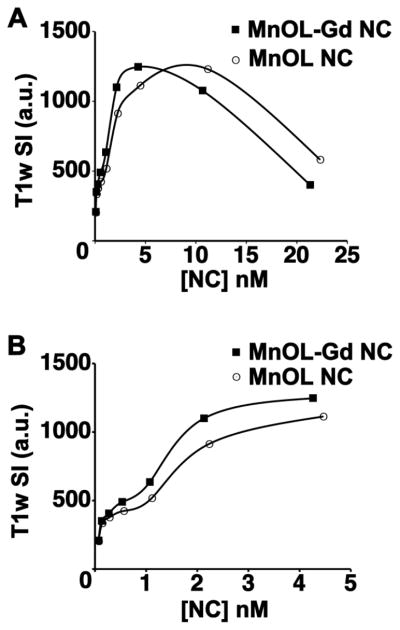
Comparison of T1-weighted (T1w) turbospin echo (TSE) signal intensity for MnOL NC and MnOL-Gd NC as a function of NC concentration. A. Addition of Gd-DOTA-PE to MnOL NC surfactant decreased the voxel concentration required for maximum signal intensity from 10 nM to 5 nM, although the magnitude of the T1w signal was equivalent for the two compositions. B. As NC voxel concentration decreased from 5 nM to 0.5 nM the persistent superiority of the MnOL-Gd NC was appreciated.
Acute Complement Activation
Complement activation (CH50) in human sera was compared between saline-only, MnOL-Gd NCs incorporating 0.0, 0.6, 1.25 or 2.5 mol% Gd-DOTA-PE, and two positive controls, αvβ3-Gd-DOTA-PE (30 mol%) PFC NP and a cationic 50% DOTAP-PE PFC NP. (Figure 6A) Residual complement activity after incubation with MnOL-Gd (0.6, 1.25, or 2.5 mol%) NC did not differ (p>0.05) from the complement activity of normal human sera or human sera incubated with a nonfunctionalized negative control nanoparticle. However, residual complement activity following incubation with the positive controls decreased (p<0.05) moderately with αvβ3-Gd-DOTA-PE (30 mol%) PFC nanoparticles (NP) and markedly with the cationic DOTAP reference NP, which essentially exhausted the complement activity of the sera. These observations suggest that 0.6, 1.25 and 2.5 mol% Gd-DOTA-PE incorporation into the lipid surfactant of MnOL-Gd NC elicited negligible complement activation in human sera, while offering high T1w contrast with substantially reduced Gd-DOTA exposure.
Figure 6.

A. In vitro CH50 assessment of complement activation (CA) showing that the MnOL-Gd NC do not induce activation in human sera relative to normal human serum (NHS) or ανβ3-targeted phospholipid control nanoparticle (NP). However, the positive controls, i.e., high density ανβ3- targeted 30 mol% Gd-DOTA-PE-PFC NP and 50 mol% DOTAP-PE PFC NPs, elicited significant CA. B. C3a ELISA showed similar results. MnOL-Gd NC induced little CA in vivo relative to a negative mouse control, although a small but significant change was noted at the 1.25 mol% and 2.5 mol% Gd-DOTA-PE surfactant incorporation levels. These responses were minute compared to the positive controls: ανβ3-targeted 20 mol% Gd DOTA-PE PFC and 50 mol% DOTAP-PE PFC NPs. * (p< 0.05)
To confirm and extend the in vitro studies, αvβ3-MnOL-Gd NC incorporating 0.6, 1.25 or 2.5 mol% Gd-DOTA-PE NC were evaluated in mice. Mice were injected intravenously with either MnOL-Gd NC, αvβ3-targeted Gd-DOTA-PE (20 mol%) PFC NP or DOTAP-PE (50 mol%) PFC NP at 5 μl/g of body weight, which exceeded by 2.5-fold the dose of particles typically employed for preclinical studies. Animals were sacrificed 30 min post injection and the extent of complement activation was measured by the generation of C3a using an ELISA-based assay. (Fig 6B) In contrast to the robust complement activation observed following administration of 20 mol% Gd-DOTA-PE and 50 mol% DOTAP-PE PFC NP, 20 mice receiving MnOL-Gd NC had minimal complement activation regardless of Gd-DOTA-PE payload, which was in general agreement with the results obtained with the in vitro analysis using human sera. Administration of MnOL-Gd NC with 0.6 mol% Gd-DOTA-PE elicited minimal C3 cleavage, comparable to control animals injected with PBS. Complement activation induced by MnOL-Gd NC with 1.25, or 2.5 mol% Gd-DOTA-PE was slightly higher but still negligible when compared to animals injected with 20 mol% Gd-DOTA-PE and 50 mol% DOTAP-PE PFC NP. These results reflect the sensitivity of the in vivo assay.
Discussion
In the present study, hybrid “mixed-metal” MnOL-Gd NC were developed as an approach to a high-relaxivity paramagnetic MR molecular imaging agent that would be effective for imaging sparse biomarkers, such as neovascular integrins. The design intent of this agent was to achieve high r1 relaxivity without utilizing a high surface density of Gd-DOTA, which was previously noted to elicit acute complement activation in vitro and in vivo, 20 an effect corroborated in this report. In this study, low surface concentrations of Gd-DOTA-PE incorporated into the outer lipid membrane of MnOL nanocolloid enhanced r1 relaxivity through an unexpected synergism arising between the manganese-rich core and the surface lanthanide. The overall enhanced r1 relaxivity of MnOL-Gd NC did not differ significantly with surface payloads from 0.5 to 5 mole% of the outer membrane lipid complement, although early diminishment in relaxivity was noted at 5 mole% indicative of T2(T2*) effects. The enhanced r1 of MnOL-Gd NC over MnOL NC improved the T1w TSE sensitivity of nanocolloid detection at voxel concentrations of 5nM and below; whereas, the MnOL NC provided better TSE-SI at 10nM/voxel levels and above. Reduction of surfactant Gd-DOTA-PE diminished acute complement activation in vitro and in vivo to negligible levels compared with 30mole% and 20mole% Gd-PFC NP, respectively. In previous studies, MnOL nanocolloids were effective for molecular imaging of fibrin in vitro23, but failed to provide robust imaging of angiogenesis in atherosclerotic rabbits. (Supplemental Data: Figure 1). Collectively, these data suggest that MnOL-Gd NC can provide high T1w MR contrast for targeted imaging of scarce vascular receptors with a lower overall exposure to gadolinium, reduced risk of transmetallation and its sequella, and a highly mitigated potential for acute complement activation.
In contradistinction to small molecules, nanoparticles present unique physical aspects that can trigger the resident immune system, which stands poised to identify and destroy “foreign” intruders. Lipid-encapsulated particles, such as liposomes, micelles, and emulsions, represent classes of nanoparticles of varying sizes that have been introduced into the clinical setting. While simple phospholipid membrane exteriors of these agents bear a striking resemblance to biological cell structures, functionalization of these particles with metals for imaging, complexation with polyethylene glycol (PEG) for “stealthiness”, or other unusual surface chemistry modifications can incite immune response 27, 28. Such blood contact issues are well documented for Doxil™, a PEG-coated liposomal form of doxorubicin, that has elicited moderate to severe hypersensitivity reactions, often correlated to complement activation, in up to 45% of patients in some series 27. Similar reactions in liposome-treated pigs were diminished by pre-treatment with complement inhibitors. 29 While simple lipid-encapsulated NPs can activate the complement system, the fundamental question is to what degree?
Complement reactions to high payload Gd-PFC-NP have been reported and well characterized through a series in vitro and in vivo studies. 20 Paramagnetic PFC NP presenting high surface densities of chelated gadolinium in two different forms yielded markedly different complement activation responses. 20 One metal complex was Gd-DTPA-BOA, which incorporates into the outer lipid membrane through insertion of oleic acid anchors into the hydrophobic region of the mono-layered phospholipid membrane. Although the molecular interactions of Gd-DTPA-BOA with the membrane have not been mathematically modelled in detail, the high stablity of these formulations suggest that the oleic acid groups are deeply invested into the hydrophobic acyl region of the membrane. This positions the Gd-DTPA complex proximate to or intermingled among the phospholipid head groups at the PFC particle surface. Interesting, Gd-DTPA-BOA incorporated into PFC NP in high density did not elicit significant complement activation in vitro or in vivo. 20 In contradistinction, a robust complement activation response was measured in vitro and in vivo for PFC NPs incorporating equimolar levels of Gd-DOTA-PE. 20 This effect was again corroborated in the present study. Gd-DOTA-PE positions the metal complex well beyond phospholipid headgroups, which markedly enhances particle relaxivity by increasing the interactions of the metal with surrounding water protons. 19 The more closely membrane-associated Gd-DTPA-BOA chelate has much lower r1 for the same lanthanide content. 19 Gd-DOTA-PFC NP activated complement through the antibody-mediated classical pathway, the effects of which can be further amplified by the alternative pathway. 30 In contradistinction, the location of Gd-DTPA-BOA just at the NP surface potentially reduced antibody recognition and complement activation. 20, 25 However, the supposition that Gd-DTPA when positioned further from the nanoparticle membrane surface for higher relaxivity 19 would not activate complement cannot be assumed. Gd-DTPA-BOA enhanced the r1 relaxivity of MnOL nanoparticles similar to that observed with Gd-DOTA-PE if added to the surfactant at 10mol%. However, the lower metal complexation stability of the linear lipid-chelate complex decreases its safety for clinical translation from a transmetallation perspective. 17–19 (Supplemental Data: Figure 2)
In the present study, reducing the surface density of Gd-DOTA-PE presented on MnOL reduced in vitro and in vivo complement activation to negligible levels when referenced to Gd-DOTA-PFC NP or the highly cationic, positive control, DOTAP-PFC NP. In vitro, the extent of complement activation with MnOL-Gd NCs was essentially nondetectable. However, these in vitro studies utilized pooled human sera and individual variation in complement responses exists among patients, which could result in detectable in vitro responses, albeit at a very low frequency. 30
In mice, the dosage of nanocolloids given was 5-fold greater than the highest preclinical dosages typically studied. To some extent, this high dose compensates for the early, rapid biliary elimination of nanoparticles unique to rodent models. 31 However, acute complement activation begins essentially immediately with particle-blood contact and blood samples were acquired at 30 min and processed immediately. Yet, even under this toxicological dosage regimen, the complement activation of MnOL-Gd at metal payloads ranging from 0.6mole% to 2.5mole% was minimal in magnitude, particularly when referenced to the levels of activation elicited by the positive controls. Collectively, these human sera CH50 and mouse model data suggest that the overall complement response expected by the hybrid ultralow gadolinium-manganese oleate nanocolloids is minimized.
The particulate relaxivity of the 50:50 combination of MnOL + Gd-only NCs was 607,504 ± 13,578 (s•mmol [MnOL+Gd-only NC])−1), very similar to the expected sum of the individual MnOL and Gd-only NC r1s, yet this r1 was ~25% lower than the particulate r1 for the hybrid NC, 748,199 ± 30,464 (s•mmol [ManOL-Gd NC])−1. The contrast improvement of MnOl-Gd NC over MnOL NC increased detection sensitivity at nanoparticle/voxel concentrations of 5nM and below, which we anticipate will be required for in vivo molecular imaging of sparse receptors, such as neovascular integrins. The higher relaxivity of the hybrid NC versus the 50:50 mixture revealed an unexpected magnetic synergism between surfactant coupled Gd3+ and the Mn2+ rich core, which was influenced by placement of the Gd3+ relative to the particle surface. Positioning of Gd3+ slightly beyond the particle surface with Gd-DOTA-PE improved lanthanide paramagnetic interaction with the magnetic dipole field generated by the MnOL core. Positioning the Gd3+ near the particle-water interface offered similar enhancement at larger surface concentrations of the metal, which is consistent with the lower r1 relaxivity previously reported for this agent. 25 The ionic and particulate r2 relaxivities for MnOL-Gd-NC, MnOL NC, 50:50 MnOL: Gd-only NC mixture followed similar relational patterns with r1. The underlying physical mechanism that underpins the unexpected synergism between the rich manganese core and surface gadolinium is undefined and will require further study.
The present report focused on the physical, magnetic and biological characterization of a hybrid “mixed metal” nanocolloid. These MRI contrast agents clearly ameliorated the issue of complement activation. Characterization of the pharmacokinetics, biodistribution and efficacy of MnOL-Gd in a large animal model as well as large animal in vivo demonstration that these improvements in relaxivity and detection sensitivity translate into efficacy for molecular imaging of the neovasculature are addressed in the companion paper. The continuation of this extensive report focuses on the in vivo pharmacology of the hybrid “mixed metal” nanocolloid and its utility for imaging atherosclerotic neovasculature in hyperlipidemic rabbits.
In summary, the development and characterization of a hybrid MnOL-Gd NC is reported. The relaxivity of paramagnetic manganese, the predominant contrast metal, entrapped within the core of a “soft” vascular-constrained nanocolloid was augmented by low levels of surface gadolinium in an unanticipated magnetic synergy that resulted in high MR T1w contrast. The hybrid nanocolloids substantially reduced the gadolinium payload, reduced the risk of lanthanide transmetallation, and mitigated acute complement activation, which has plagued paramagnetic particles bearing high surface gadolinium payloads. With improved safety, high r1, and MR sensitivity to low nanomolar contrast signal, this new molecular imaging agent may provide a translatable tool for evaluating sparse biomarker expression associated with the neovasculature of atherosclerotic plaques and cancers.
Supplementary Material
Acknowledgments
Funding: The financial support from the NIH in part under grant numbers CA154737 (GML), CA136398 (GML), NS073457 (DH/CP), HL112518 (GML), HL113392 (GML), CA100623 (GML), SBIR (GML), and the Barnes-Jewish Hospital Charitable Foundation is greatly appreciated. This research was also supported in part by the National Natural Science Foundation of China (81130028,31210103913) (BS), the Key Grant Project of Heilongjiang Province (GA12C302) (BS), the Ph.D. Programs Foundation of Ministry of Education of China (201123071100203) (BS), and the Key Laboratory of Molecular Imaging Foundation (College of Heilongjiang Province) (BS), the National Natural Science Foundation for Young Scholars of China (81101086) (KW), China Postdoctoral Science Foundation (20100471020) (KW), China Postdoctoral Special Science Foundation (2012T50375) (KW) and Scientific Research Grant of Heilongjiang Province Natural Science Foundation for Returned Chinese Scholars (LC201436) (KW).
Footnotes
Conflicts of Interest: Intellectual property surrounding the hybrid MnOL NC technology has been licensed nonexclusively by Washington University School of Medicine to Ocean NanoTech, LLC, San Diego, CA. Further, the gadolinium perfluorocarbon nanoparticles referenced in this manuscript were licensed from Barnes-Jewish Hospital Research Foundation to Kereos, Inc, St. Louis, MO, for which SAW and GL were scientific co-founders.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumor angiogenesis in vivo by alpha v beta3-targeted magnetic resonance imaging. Nat Med. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 2.Lanza G, Lorenz C, Fischer S, Scott M, Cacheris W, Kaufman R, et al. Enhanced detection of thrombi with a novel fibrin-targeted magnetic resonance imaging agent. Acad Radiol. 1998;5(suppl 1):s173–s176. doi: 10.1016/s1076-6332(98)80097-4. [DOI] [PubMed] [Google Scholar]
- 3.Anderson SA, Rader RK, Westlin WF, Null C, Jackson D, Lanza GM, et al. Magnetic resonance contrast enhancement of neovasculature with alpha v beta 3-targeted nanoparticles. Magn Reson Med. 2000;44:433–439. doi: 10.1002/1522-2594(200009)44:3<433::aid-mrm14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Flacke S, Fischer S, Scott M, Fuhrhop R, Allen J, Mc Lean M, et al. A novel MRI contrast agent for molecular imaging of fibrin:Implications for detecting vulnerable plaques. Circulation. 2001;104:1280–1285. doi: 10.1161/hc3601.094303. [DOI] [PubMed] [Google Scholar]
- 5.Winter PM, Caruthers SD, Kassner A, Harris TD, Chinen LK, Allen JS, et al. Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel alpha v beta 3-targeted nanoparticle and 1.5 Tesla magnetic resonance imaging. Cancer Res. 2003;63:5838–5843. [PubMed] [Google Scholar]
- 6.Schmieder A, Winter P, Caruthers S, Harris T, Williams T, Allen J, et al. Mr molecular imaging of melanoma angiogenesis with αvβ3-targeted paramagnetic nanoparticles. Magn Reson Med. 2005;53:621–627. doi: 10.1002/mrm.20391. [DOI] [PubMed] [Google Scholar]
- 7.Strijkers GJ, Mulder WJ, van Heeswijk RB, Frederik PM, Bomans P, Magusin PC, et al. Relaxivity of liposomal paramagnetic MRI contrast agents. Magma. 2005;18:186–192. doi: 10.1007/s10334-005-0111-y. [DOI] [PubMed] [Google Scholar]
- 8.Mulder WJ, Strijkers GJ, van Tilborg GA, Griffioen AW, Nicolay K. Lipid-based nanoparticles for contrast-enhanced mri and molecular imaging. NMR Biomed. 2006;19:142–164. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 9.Mulder WJ, van der Schaft DW, Hautvast PA, Strijkers GJ, Koning GA, Storm G, et al. Early in vivo assessment of angiostatic therapy efficacy by molecular MRI. FASEB J. 2007;21:378–383. doi: 10.1096/fj.06-6791com. [DOI] [PubMed] [Google Scholar]
- 10.Schmieder AH, Caruthers SD, Zhang H, Williams TA, Robertson JD, Wickline SA, et al. Three-dimensional MR mapping of angiogenesis with alpha 5 beta 1(alpha v beta 3)-targeted theranostic nanoparticles in the MDA-MB-435 xenograft mouse model. FASEB J. 2008;22:4179–4189. doi: 10.1096/fj.08-112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winter PM, Schmieder AH, Caruthers SD, Keene JL, Zhang H, Wickline SA, et al. Minute dosages of alpha v beta 3-targeted fumagillin nanoparticles impair Vx-2 tumor angiogenesis and development in rabbits. FASEB J. 2008;22:2758–2767. doi: 10.1096/fj.07-103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanza GM, Winter PM, Caruthers SD, Hughes MS, Hu G, Schmieder AH, et al. Theragnostics for tumor and plaque angiogenesis with perfluorocarbon nanoemulsions. Angiogenesis. 2010;13:189–202. doi: 10.1007/s10456-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmieder AH, Winter PM, Williams TA, Allen JS, Hu G, Zhang H, et al. Molecular mr imaging of neovascular progression in the Vx2 tumor with alpha v beta 3-targeted paramagnetic nanoparticles. Radiology. 2013;268:470–480. doi: 10.1148/radiol.13120789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 15.Ersoy H, Rybicki FJ. Biochemical safety profiles of gadolinium-based extracellular contrast agents and nephrogenic systemic fibrosis. Journal of Magnetic Resonance Imaging. 2007;26:1190–1197. doi: 10.1002/jmri.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perkins JD. Warning regarding gadolinium and nephrogenic systemic fibrosis. Liver Transpl. 2007;13:1752–1753. doi: 10.1002/lt.21296. [DOI] [PubMed] [Google Scholar]
- 17.Laurent S, Elst LV, Copoix F, Muller RN. Stability of mri paramagnetic contrast media: A proton relaxometric protocol for transmetallation assessment. Invest Radiol. 2001;36:115–122. doi: 10.1097/00004424-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Sherry A, Cacheris W, Kuan K. Stability constants for Gd3+ binding to model DTPA-conjugates and DTPA-proteins: Implications for their use as magnetic resonance contrast agents. Magn Reson Med. 1988;8:180–190. doi: 10.1002/mrm.1910080208. [DOI] [PubMed] [Google Scholar]
- 19.Winter P, Athey P, Kiefer G, Gulyas G, Frank K, Fuhrhop R, et al. Improved paramagnetic chelate for molecular imaging with MRI. J Magn Magn Mater. 2005;293:540–545. [Google Scholar]
- 20.Pham CT, Mitchell LM, Huang JL, Lubniewski CM, Schall OF, Killgore JK, et al. Variable antibody-dependent activation of complement by functionalized phospholipid nanoparticle surfaces. J Biol Chem. 2011;286:123–130. doi: 10.1074/jbc.M110.180760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senpan A, Caruthers SD, Rhee I, Mauro NA, Pan D, Hu G, et al. Conquering the dark side: Colloidal iron oxide nanoparticles. ACS Nano. 2009;3:3917–3926. doi: 10.1021/nn900819y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan D, Caruthers SD, Hu G, Senpan A, Scott MJ, Gaffney PJ, et al. Ligand-directed nanobialys as theranostic agent for drug delivery and manganese-based magnetic resonance imaging of vascular targets. J Am Chem Soc. 2008;130:9186–9187. doi: 10.1021/ja801482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan D, Senpan A, Caruthers SD, Williams TA, Scott MJ, Gaffney PJ, et al. Sensitive and efficient detection of thrombus with fibrin-specific manganese nanocolloids. Chem Commun (Camb) 2009;22:3234–3236. doi: 10.1039/b902875g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan D, Caruthers SD, Senpan A, Schmieder AH, Wickline SA, Lanza GM. Revisiting an old friend: Manganese-based mri contrast agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:162–173. doi: 10.1002/wnan.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winter P, Caruthers S, Yu X, Song S, Fuhrhop R, Chen J, et al. Improved molecular imaging contrast agent for detection of human thrombus. Mag Reson Med. 2003;50:411–416. doi: 10.1002/mrm.10532. [DOI] [PubMed] [Google Scholar]
- 26.Hockett F, Wallace K, Schmieder A, Caruthers S, Pham C, Wickline S, et al. Simultaneous dual frequency 1H and 19F open coil imaging of arthritic rabbit knee at 3T. IEEE Trans Med Imaging. 2011;30:22–27. doi: 10.1109/TMI.2010.2056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szebeni J. Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology. 2005;216:106–121. doi: 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson B, Korsgren O, Lambris JD, Ekdahl KN. Can cells and biomaterials in therapeutic medicine be shielded from innate immune recognition? Trends in immunology. 2010;31:32–38. doi: 10.1016/j.it.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szebeni J, Fontana JL, Wassef NM, Mongan PD, Morse DS, Dobbins DE, et al. Hemodynamic changes induced by liposomes and liposome-encapsulated hemoglobin in pigs: A model for pseudoallergic cardiopulmonary reactions to liposomes. Role of complement and inhibition by soluble cr1 and anti-C5a antibody. Circulation. 1999;99:2302–2309. doi: 10.1161/01.cir.99.17.2302. [DOI] [PubMed] [Google Scholar]
- 30.Pham CT, Thomas DG, Beiser J, Mitchell LM, Huang JL, Senpan A, et al. Application of a hemolysis assay for analysis of complement activation by perfluorocarbon nanoparticles. Nanomedicine. 2014;10:651–660. doi: 10.1016/j.nano.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulte JW, Schmieder AH, Keupp J, Caruthers SD, Wickline SA, Lanza GM. Mr cholangiography demonstrates unsuspected rapid biliary clearance of nanoparticles in rodents: Implications for clinical translation. Nanomedicine. 2014 doi: 10.1016/j.nano.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meoli DF, Sadeghi MM, Krassilnikova S, Bourke BN, Giordano FJ, Dione DP, et al. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest. 2004;113:1684–1691. doi: 10.1172/JCI20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan D, Pramanik M, Senpan A, Allen JS, Zhang H, Wickline SA, et al. Molecular photoacoustic imaging of angiogenesis with integrin-targeted gold nanobeacons. FASEB J. 2011;25:875–882. doi: 10.1096/fj.10-171728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan N, Arnaoutakis GJ, George TJ, Allen JG, Ju DG, Schaffer JM, et al. The spectrum of complications following left ventricular assist device placement. J Card Surg. 2012;27:630–638. doi: 10.1111/j.1540-8191.2012.01504.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





