Abstract
Aseptic loosening and osteolysis are the most frequent late complications of total hip arthroplasty (THA) leading to revision of the prosthesis. This review aims to demonstrate how histopathological studies contribute to our understanding of the mechanisms of aseptic loosening/osteolysis development. Only studies analysing periprosthetic tissues retrieved from failed implants in humans were included. Data from 101 studies (5532 patients with failure of THA implants) published in English or German between 1974 and 2013 were included. “Control” samples were reported in 45 of the 101 studies. The most frequently examined tissues were the bone-implant interface membrane and pseudosynovial tissues. Histopathological studies contribute importantly to determination of key cell populations underlying the biological mechanisms of aseptic loosening and osteolysis. The studies demonstrated the key molecules of the host response at the protein level (chemokines, cytokines, nitric oxide metabolites, metalloproteinases). However, these studies also have important limitations. Tissues harvested at revision surgery reflect specifically end-stage failure and may not adequately reveal the evolution of pathophysiological events that lead to prosthetic loosening and osteolysis. One possible solution is to examine tissues harvested from stable total hip arthroplasties that have been revised at various time periods due to dislocation or periprosthetic fracture in multicenter studies.
Keywords: Aseptic loosening, Osteolysis, Total hip, Tissue analysis, Immunostaining
1. Introduction
It is estimated that about two million total hip arthroplasties (THAs) are performed worldwide each year and projections of rising demand are reported at least for the USA [1]. However, some THAs fail during the period of service and require revision surgery, which is more expensive than the primary operation and brings less satisfactory outcomes and increased risk for complications [2,3]. This causes a significant economic impact on the health care system. Therefore, understanding current failure mechanisms of primary THAs and especially the potential for prevention are crucial.
Although instability, infection, pain and periprosthetic bone fractures prevail as reasons for reoperation in the first five years after an index surgery, the most frequent cause of late failure is aseptic loosening accompanied by osteolysis [4]. Since the pioneering work of Willert et al. [5,6], there has been a tendency to associate these late complications with a local tissue response to large numbers of tiny particles generated from bone cement and articulating/non-articulating surfaces of THA. Small particles are phagocytosed by macrophages or stimulate cells in a non-phagocytic manner. These cells then release pro-inflammatory molecules that trigger pathways influencing the osteoclast–osteoblast coupling in bone multicellular units [7,8]. Particle-associated dysregulation of osteoclast–osteoblast coupling in favor of osteoclasts over-weight leads eventually to net bone resorption at the bone–implant interface. In support of this concept, studies have demonstrated inflammatory and osteolytic responses after cell/organ culture stimulation by polymethylmethacrylate, polyethylene and titanium particles [9–15].
Immediately after the surgery, mechanical factors influence the development of the bone–implant interface. These are associated with intermittent loading of the artificial hip during daily living activities, and later with hydrodynamics of the artificial joint fluid creating significant pressures in the adjacent tissues. From some time postoperatively, biological and mechanical pathways interact together, creating conditions appropriate to periprosthetic osteolysis and aseptic loosening. We have previously described these processes in detail [16,17]. Here, we summarize current evidence derived from analyses of tissues retrieved during the reoperation of THA performed due to aseptic loosening and periprosthetic osteolysis.
2. Search strategy and rules for evaluation
We included all research studies that examined human periprosthetic tissues retrieved either during the THA surgery or post-mortem using histopathological examination and immunostaining. Further, we used articles and resources focusing on this issue. One of the authors (J.V.) searched for potentially relevant studies in the PubMed database. Articles published between January 1974 and June 2013 were identified with the keywords and medical subject heading terms “aseptic loosening” and “periprosthetic osteolysis” and “total hip arthroplasty” and “bone loss” or “immunohistochemistry” or “cytokines” or “RANKL” or “hypersensitivity” or “apoptosis” or “interleukin” or “infection”. We reviewed all of the retrieved articles and extracted relevant data, which we incorporated in table in the Excel 2010 software package (Microsoft). Although 223 articles were identified, 82 were excluded (Table 1, Fig. 1) because they lacked data about histopathological examination of retrieved tissues. In agreement with recent requirements for research in biomedicine, data should be considered preliminary until replicated by a different center. Therefore, all molecules, pathways and cell groups reported only in an initial histopathological study were stated as being preliminary, while those also featuring in a replication study were considered as proven.
Table 1.
The sample size of studies.
| No. of patients | No. of studies |
|---|---|
| ≤20 | 55 |
| 21–50 | 20 |
| 51–100 | 9 |
| 101–150 | 5 |
| 151–200 | 5 |
| 201–300 | 2 |
| 301–551 | 3 |
Fig. 1.
Flowchart of the methodology of the study.
3. Results
Data from 101 studies (5532 patients with failure of THA implants) published between 1974 and 2013 were included. “Control” samples were reported in 45 of the 101 studies.
3.1. Methods used for investigation of periprosthetic tissues
The retrieved tissues (or derived cell/organ cultures) were studied histologically and histochemically, especially using immunostaining or molecular biology methods. Another set of methods aimed to detect the prosthetic particles. The aims of all of these investigations were: (i) to distinguish between septic and aseptic THA failures; (ii) to detect prosthetic by-products in periprosthetic tissues; (iii) to analyze cell/tissue structure; and (iv) to detect the signaling proteins and proteolytic enzymes in the periprosthetic tissues.
3.2. Protocols for tissue processing
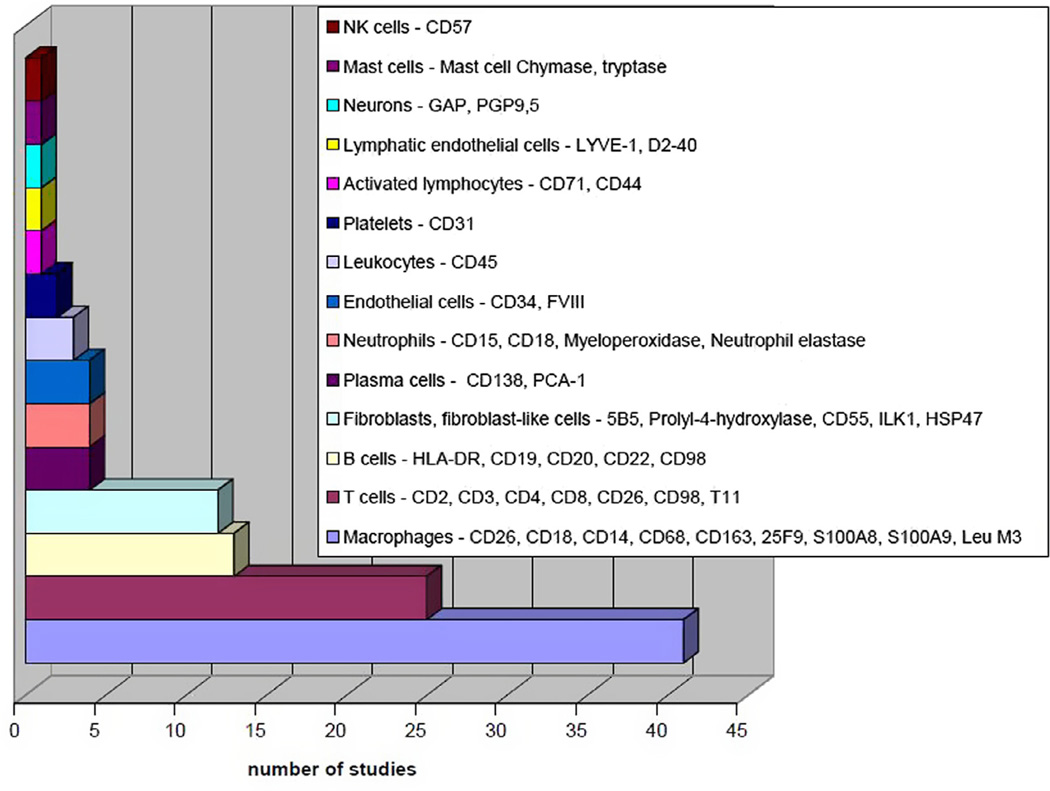
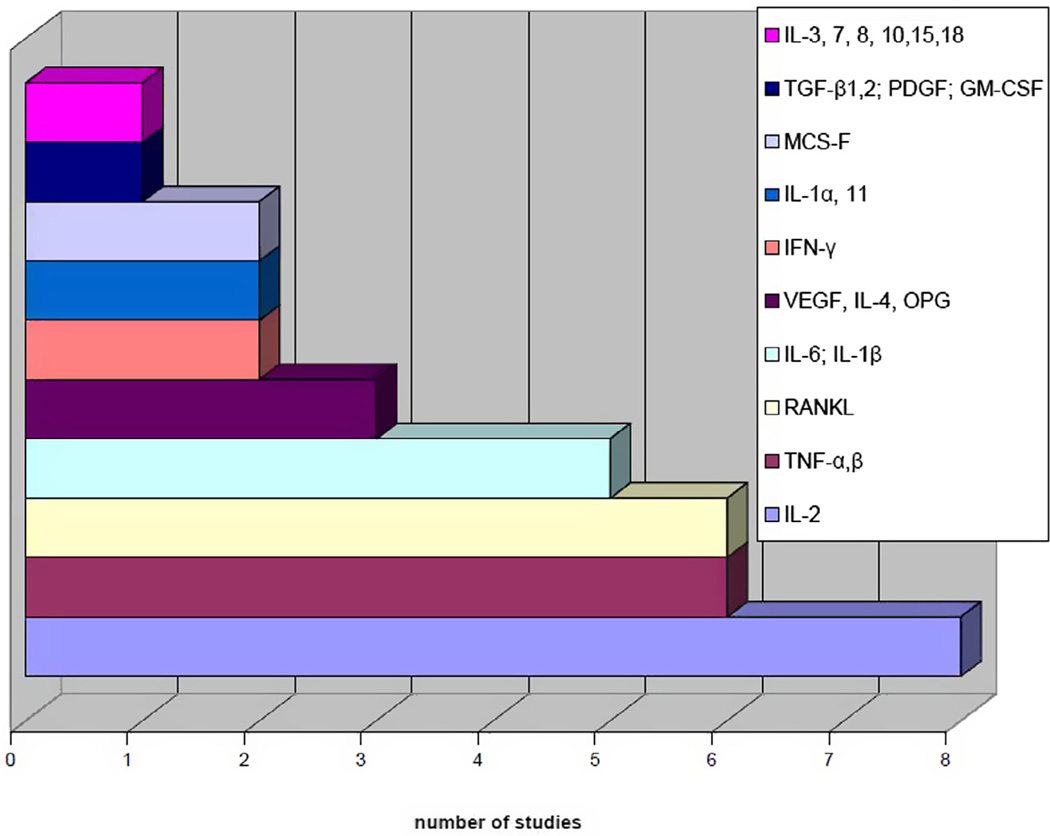
Some authors examined results from cell/organ cultures derived from periprosthetic tissues [18–22], but their results were excluded because we focused on direct histopathologic examinations of the periprosthetic tissues. These were processed post-operatively using approximately 5–6 µm thick frozen tissue sections (41 of 101 studies) or 3–10 µm thick paraffin-embedded tissue sections (45/101), or both methods (10/101). Methylmethacrylate-embedded sections were used in seven studies [5,23–28], and a further two studies did not report the methodology [29,30]. Mostly, the sections were cut from periprosthetic tissues fixed immediately after harvesting in 10% buffered formalin and then embedded in paraffin (formalin fixed, paraffin embedded; FFPE), or were prepared as freshly frozen sections in the cryostat. The sections were stained for comprehensive microscopic evaluation after hematoxylin-eosin staining. For precise demonstration of the specific molecular components within the cells and in the proper tissue context, special histological stains (Table 2) and immunostains were used (Figs. 2 and 3).
Table 2.
Special histological stains.
| Special stain | To demonstrate |
|---|---|
| Fite | microorganisms [30] |
| Gram | microorganisms [74,77–80] |
| Gomori | fungi [30] |
| Giemsa | mast cells, parasites, fungi, wear particles [122,127,128] |
| Grocott | microorganisms [70] |
| Masson’s trichrome | collagen [24] |
| Oil red O | lipid and wear particles [90] |
| PAS reaction | mucin, basement membrane, fungi, inclusions [70,72,92,127,134] |
| Pearl’s reaction | hemosiderin, wear particles [30,92,112,122,127,134] |
| Sudan III | lipid and wear particles [5,90] |
| Toluidin blue | mast cells [74,90] |
| Van Gieson | elastic fibers and collagen [5,92,113,122,127] |
| Ziehl–Neelsen | mycobacteria [70] |
Fig. 2.
Cell types identified by immunostains.
Fig. 3.
Studies focused on cytokines produced by harvested tissues.
Different staining methods and molecular and cellular biology techniques were used. The most frequently used methods capable of identifying specific RNA or DNA molecules are polymerase chain reaction (PCR) and in situ hybridization (ISH). Some authors also used PCR to detect bacterial DNA, or inflammatory cytokines and other mediators. In most cases, the results from the molecular biology methods were compared to immunostaining of the same tissue samples [31,32].
Immunostaining can specifically identify categories of cell lineage and their regulatory molecules (proteins), and detect the presence of specific antigens in cells with high sensitivity. The immunostains used were based on the reaction between antigen and primary and secondary antibodies, with one of them being labeled with an enzyme (horseradish peroxidase, alkaline phosphatase, biotin), the fluorophore fluorescein isothiocyanate [33,34] or tetramethylrhodamine isothiocyanate [34].
Immunoenzyme protocols with many different principles were applied for antibody-aided detection, including: (i) the avidin-biotin complex method [19,25,35–51]; (ii) the labeled streptavidin-biotin method [32,33,52–59]; and (iii) the polymer-based detection method [60–67]. The presence of antigen was most often visualized by chromogen 3,3′-diaminobenzidine tetrachloride, which produces a brown reaction that can be seen with a light microscope. Occasionally, a fast red TR salt [35], aminoethylcarbazole [38] or fuchsine [39,52], giving a red stain, or chromogen, with the blue-colored precipitate nitro-blue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate [41], was used. Finally, sections were counterstained with hematoxylin prior to mounting.
In some cases, double immunostains were used, which can identify two sets of antigens in the same section when the antibodies are applied in sequence or at the same time. Some authors followed an immunofluorescence staining protocol [38,40,50,61,68], while others preferred antigens labeled by enzymes [38,48,50,61,68,69].
Histological analysis was mostly carried out at 2–500 × magnification, using a light or fluorescence microscope. Polarized light, electron or transmission electron microscopy was used to identify various sizes of wear particles and the intracellular pathology induced by prosthetic by-products [70]. Polyethylene wear debris is strongly birefringent in polarized light, unlike ceramic or metal particles.
3.3. Distinction between aseptic and septic failure
This analysis is based on the detection of the number of polymorphonuclear neutrophil leukocytes (PMNs) in the examined tissues. The higher the number of PMNs observed, the higher the probability that the examined tissue is affected by infection. Generally, the minimum criterion for infection is more than five PMNs in five separate high-power fields, using 400 or 500 × magnification [71]. Others have proposed a criterion of 23 PMNs in 10 high-power fields [72]. In fact, the key prerequisite for accurate interpretation is a very experienced pathologist, ideally blinded.
To identify PMNs, routine hematoxylin-eosin staining (of polymorphic nuclei) and immunostains using CD15 are performed on FFPE or frozen sections. Using the classification system of Morawietz et al. (Table 3) [56], analysis of frozen sections has a high correlation to results from FFPE specimens [73]. Hence, the intraoperative histological analysis of frozen sections is recommended as a valid diagnostic tool for the prompt confirmation of infection [74–76]. Although sensitivity of frozen sections can vary, the specificity of intraoperative examination is excellent [73,75,76]. In addition, a number of studies examined the contribution of Gram staining to the diagnosis of prosthetic joint infection [77,78]. However, the evidence for the routine use of this method is limited [79,80].
Table 3.
Histopathological classification of synovial-like interface membrane.
| Moravietz et al. [56] | Krenn et al. [108]: the revised classification with the additional pathologies | ||
|---|---|---|---|
| Type I Wear particle induced |
- abundance of macrophages and multinuclear giant cells |
Allergic alteration | - lymphocytic infiltration |
| - positive allergy tests | |||
| - rarely lymphocytes | Type I | - abundance of macrophages and multinuclear giant cells |
|
| Wear particle induced | |||
| - rarely lymphocytes | |||
| +Necrosis | - wear debris, metal on metal implant | ||
| Type II | - activated fibroblasts, proliferation of small blood ves- sels, oedema |
Type II | - activated fibroblasts, proliferation of small blood ves- sels, oedema |
| Infectious type | Infectious type | ||
| - abundance of neutrophilic granulocytes | - abundance of neutrophilic granulocytes | ||
| - plasma cells | - plasma cells | ||
| Type III | - combination of the histomorphological changes in types I and II |
Type III | - combination of the histomorphological changes in types I and II |
| Combined type | Combined type | ||
| Type IV | - connective tissue rich in collagen fibers | Type IV | - connective tissue rich in collagen fibers |
| Indetermine type | - fibrin or fibroblasts and macrophages sometimes on the membrane surface |
Indetermine type | - fibrin or fibroblasts and macrophages sometimes on the membrane surface |
| Implant-associated arthrofibrosis |
- fibrosis and β-catenin positivity | ||
| Osseous pathologies | - osteomyelitis, osteonecrosis, ossification, osteopenia | ||
Some investigators have shown that “particle disease” is more likely when bacterial molecules are absorbed on the prosthetic particles [81]. Participation of bacterial substances in the processes of aseptic loosening can be demonstrated either by finding these products (e.g. teichoic acids, endotoxins, protein A) or signs of a bacteria-specific host response in periprosthetic tissues. In favor of this hypothesis, several studies have shown bacterial by-products, including bacterial DNA, in periprosthetic tissue homogenates [82]. Additionally, septic membranes have a significantly higher number of CD45+ leukocytes, while aseptic membranes show a preponderance of CD68+ macrophages and histiocytes [57,69]. However, tissues with low-grade infection can appear like tissues retrieved from patients with aseptic loosening.
Bacteria and bacterial products have been found to cause up-regulation of interleukin-1 receptor-associated kinase (IRAK-M), which regulates toll-like receptor (TLR) signaling [51]. Several studies show that TLRs, sensors for detection of bacteria, are activated in periprosthetic tissues [57,83]. However, microbe-oriented sensors like TLRs, nucleotide-binding oligomerization-domain protein-like receptors, RIG-like receptors and AIM-2-like receptors are stimulated not only by exogenous pathogens, but also by intrinsic signals of tissue/cell damage serving generally as inducers of inflammation [84]. The detection of specific antibacterial proteins secreted by innate immunity cells after contact with bacteria seems to be a promising approach [85,86].
However, to date, no one study has analyzed the microbe-specific responses in aseptic periprosthetic tissues in detail. As a result, the histological evidence of the participation of bacteria in aseptic loosening and periprosthetic osteolysis is still considered insufficient.
3.4. Evidence for aseptic inflammatory response
Local tissue resident cells orchestrate the tissue response to prosthetic by-products via regulation of macrophage polarization into an M1 or M2 phenotype, and balance between fibrocytes and fibroblasts [16,87]. The foreign body response to wear debris and the abundance of macrophages and giant cells show a direct relationship to the degree of bone resorption [37,42,88]. Polyethylene particles smaller than 5–7 µm can be observed within macrophages [42,47], while larger particles induce a foreign body giant cell reaction – with the amount of particles correlating with the number of foreign body giant cells [19,32]. Generally, tissues with increased numbers of wear particles have the appearance of foreign body granuloma [19,66,69,88,89].
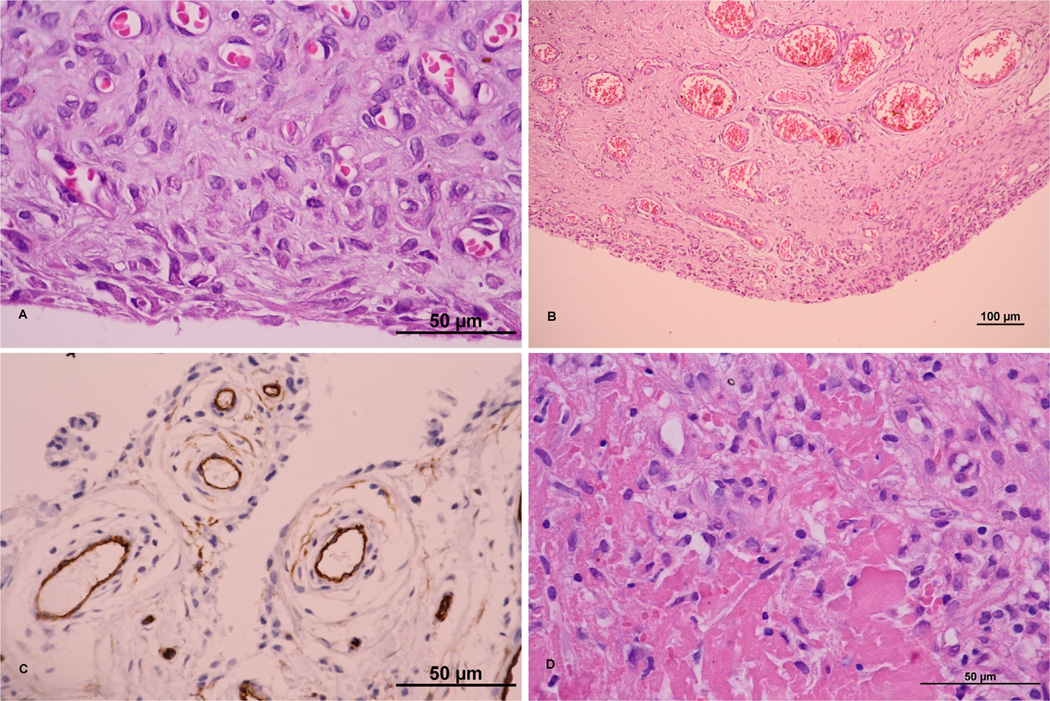
Samples from osteolytic tissue show higher proliferative cellular responses, including predominantly macrophages, fibroblasts, highly vascularized fibrous tissue with rare occurrence of PMNs (Fig. 4) and high expression of inflammatory cytokines [37,43,88,90,91]. Histological findings show various differences in the profiles of cell populations and biological mediators dependent on the type of fixation and articulation bearings (Table 4), which may reflect different mechanisms of aseptic loosening and osteolysis [92–95]. The amount and distribution of T cells, and their specific role in aseptic loosening, were explained by the cytokines which they produce, the most important ones being interferon (IFN)-γ and interleukin (IL)-17. However, the former can also be produced by other cells, such as natural killer T cells [39], and IL-17A and IL-17F are also produced, for instance, by mast cells [96]. Much less is known about the other important cell types, such as fibroblasts [97], mast cells [44,90], eosinophils, platelets, adipocytes, vascular endothelial cells [98], lymphatics [60] and neurons [99]. Several histological studies show that fibroblasts release similar factors as macrophages [32,41,42,48,50,63,100,101].
Fig. 4.
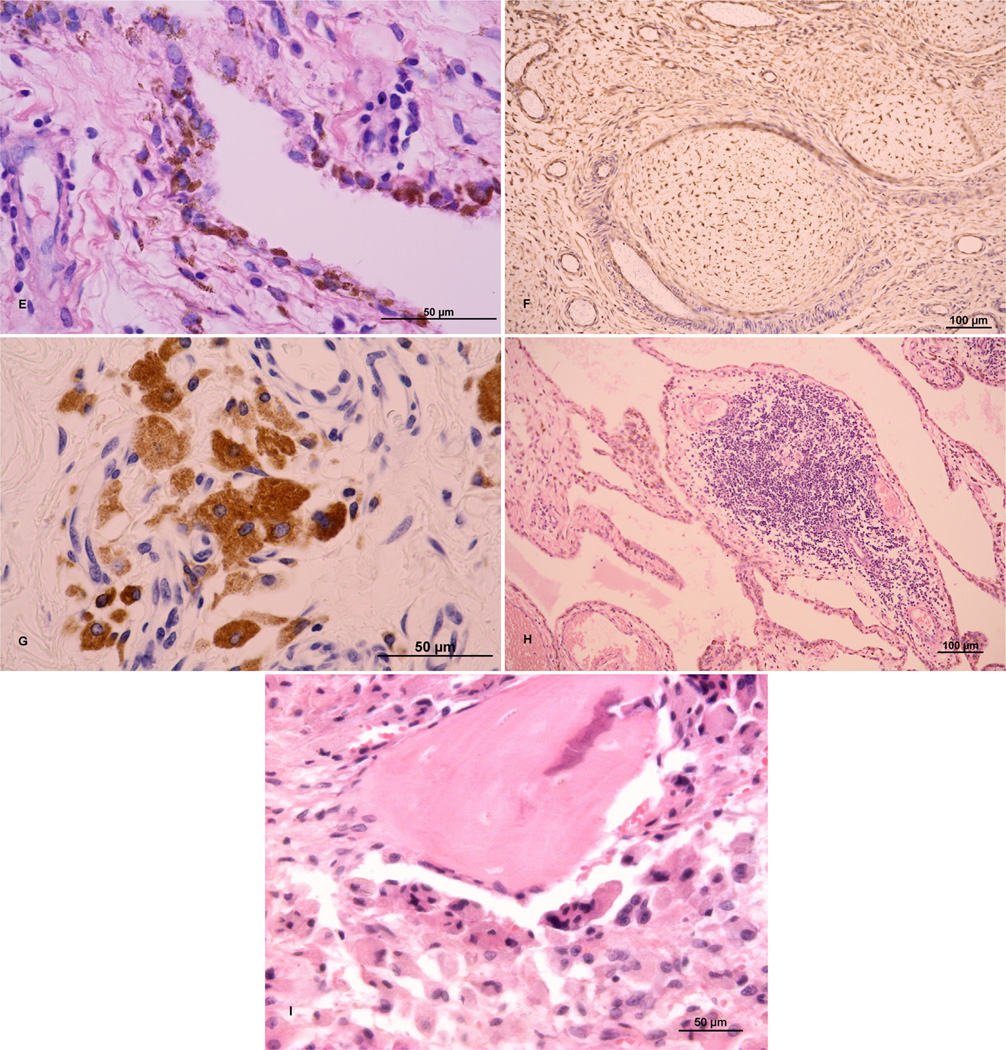
(A) Hyperplasia of synovial membrane, fibroproliferative changes in subsynovial stroma, reactive neovascularization; hematoxylin and eosin (H&E); ×1000. (B) “Neovascularization” in the synovial membrane; H&E; ×200. (C) Highly vascularized synovial membrane; CD34-positive vessels; ×1000. (D) Fibroproliferative changes with fibrinoid degeneration of connective tissue; H&E; ×1000. (E) Synovial-like cells containing metallic debris; H&E; ×1000. (F) Nodular hyperplasia with reactive changes; vimentin-positive mesenchymal cells; v200. (G) Reactive changes with macrophages containing granules of exogenous material; CD68; ×600. (H) Lymphocytic infiltration typical for chronic inflammatory changes around an implant; H&E; ×1000. (I) Bone covered by osteoblasts and osteoclasts with multiple nuclei; HE; ×200. A scale bar of 50 or 100 µm is included in each particular figure. Sources of figures: (A–H) Department of Pathology, University Hospital Ostrava, Czech Republic; (I) Department of Pathology, Faculty of Medicine and Dentistry, University Hospital, Palacky University Olomouc, Czech Republic.
Table 4.
Histopathological differences associated with type of prosthesis.
| Type of fixation | cemented [18,24,35,41,42,48,50,52,62,74,77,82,90,91,100,106,120] |
- abundance of macrophages, greater histiocytic reaction |
| - well-developed synovial-like interface membrane | ||
| - wear debris | ||
| cementless [28,40–42,47,52,61,62,66,74,82,88] |
- organized fibroblastic tissue | |
| - fibroblasts forming bundles or sheets associated with collagen fibrils | ||
| Articulation surface |
metal-on-metal [29,30,47,70,92,93,89,104,111,113,122,127,128,130– 132,134] |
- more ulcerated, granulomatous pseudotumors and connective tissue necrosis |
| - perivascular and diffuse lymphocytic infiltration | ||
| - predominantly T lymphocytes | ||
| - macrophages phagocytosing metallic wear-debris particles | ||
| metal-on-polyethylene [38,63,93,94,133,135] |
- predominantly histiocytic inflammation, loose connective tissue, fibrous and syno- vial cells |
|
| ceramic-on-ceramic [58,94,112] |
- necrosis, abundance of macrophages, neutrophils and lymphocytes | |
| - particles in agglomerates | ||
| ceramic-on-polyethylene [92,132] |
- lower degree of diffuse perivascular lymphocytic infiltration than in metal on metal bearing |
|
| � extensive necrosis |
Periprosthetic tissue was found to express increased levels of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-1α, IL-1β, IL-6, IL-8 and macrophage colony-stimulating factor (M-CSF) [18,42,88,102]. The most commonly observed cytokines expressed in periprosthetic tissue are IL-1β, IL-6 and TNF-α. Some studies showed high levels of prostaglandin E2 (PGE2), inducible nitric oxide synthase (iNOS) and other mediators of inflammation in periprosthetic tissues [40,66]. Also investigated were regulators of tissue homeostasis participating potentially in development of osteolysis and aseptic loosening, such as vascular endothelial factors, IL-10 or transforming growth factor (TGF)-β [95,103–106]. Importantly, it was shown how wear debris up-regulates IRAK-M, which plays a critical role in innate immunity and the down-regulation of foreign body reactions [51].
In aseptic loosening, a periprosthetic membrane develops at the interface between the implant and the bone bed. A wide variation was observed in the macroscopic appearance of this membrane. Many authors use the term “synovial-like interface membrane” (SLIM), while others prefer the term “granulomatous tissue” [18,107,108]. The term “synovial-like” simply refers to the presence of synovial lining on the interface membrane as a result of the expansion of the “effective joint space” [26]. Hyaluronan-containing synovial fluid induces a connective tissue synovial lining composed of macrophage-like type A cells and fibroblast-like type B cells [25]. The synovial-like membrane has a villous architecture and contains three distinct zones: first, a layer adjacent to the implant consisting of synovial-like cells supported by fibrovascular tissue; second, a vascular layer composed of dense fibrous tissue with sheets of activated macrophages, foreign body giant cells, polymorphonuclear leukocytes and a large accumulation of wear debris; and third, the poorly vascularized fibrous layer adjacent to the bone, in part covered by osteoclasts and osteoblasts, and showing signs of accelerated bone remodeling [42,90,109,110].
Together, these observations provide strong evidence that macrophages and fibroblasts orchestrate inflammatory, immunomodulatory and osteolytic pathways, leading to the formation of chronic fibrosis and periprosthetic osteolysis in aseptically loosened THA. Histopathological studies have demonstrated a number of factors typical of chronic inflammation and fibrosis, including chemokines, cytokines, nitric oxide metabolites and metalloproteinases.
3.5. Influence of biomaterial combinations on histology of periprosthetic tissues
A number of studies investigated the specific biomaterial-dependent variability in the periprosthetic tissues trying to clarify how the type of prosthetic particles or ions (in the case of metal components) influences the structure of periprosthetic membranes [18,24,93,94,109,111–114]. However, the answer to this question is not straightforward because the status of periprosthetic tissues at the time of revision is not simply a function of the prosthetic particles [16]. There is some evidence for the difference between polyethylene-on-zirconia-, CoCr-on-CoCr- and ceramic-on-ceramic-bearing materials not being substantial 1 year after surgery [67]. However, with the amount of particles and tissue damage growing with time after surgery, a slightly wider spectrum of histological findings has been observed even with identical implants and in different areas of individual patients [100,115]. Undoubtedly, the “non-material” characteristics of the particles (their size, number, shape, charge, etc.) play a key role in the development of histological findings, together with other factors not related to biomaterial particles, like the stability of an implant, parameters of the effective joint space, the volume of joint fluid and its pressure patterns [116,117]. In relation to the latter, the synovial lining layer develops at the sites in contact with fluid and/or relative motion. Taken together, tissues retrieved during revision arthroplasty exhibit a relatively limited macroscopic variability, at least in terms of tissue color (basically metallic vs. non-metallic), tissue consistency (ranging from rigid firm fibrotic to soft fragile tissue) and volume (atrophy vs. abundant hypertrophic periprosthetic tissues). In this line, histopathological studies have revealed a trend suggesting that there could be differences associated with particular biomaterial combinations in terms of inflammatory response (in relation to the presence of specific cell populations, mediators, etc.), type of fibrosis, extent of tissue necrosis or presence of apoptotic events (Table 4). For example peri-implant tissues from cemented THA (a polyethylene cup and a stainless steel or CoCr alloy stem) often consist of a dense fibrotic tissue with variable areas of necrosis. With regard to the cellular profile, macrophages predominate, together with fibroblasts and myofibroblasts, followed by the scattered to frequent presence of foreign body multinuclear cells and perivascular infiltrates of lymphocytes [5,110]. Membranes retrieved from uncemented CoCr or titanium alloy femoral components are very similar. Both types of membrane were composed of a dense fibrous tissue stroma with a moderate to large number of macrophages and fibroblasts [90,109]. This is due at least in part to very similar bearing materials (here metal on polyethylene). Ceramic-on-ceramic THAs exhibit very low wear rates, and ceramic wear particles also have much lower specific and functional biological activity than polyethylene particles [118]. Therefore, under normal conditions (i.e. without gross surface damage or fracture of ceramic components associated with metallosis), membranes taken from loosened ceramic-on-ceramic THA are generally hypotrophic in comparison to the former ones and contain decreased numbers of macrophages and lymphoplasmacytic cells, with the scattered presence of foreign body multinuclear cells [94,112]. Adverse reaction to metal particles/ions is described elsewhere in this review.
3.6. Significance of necrosis/apoptosis ratio around aseptically failed THA
Apoptosis can contribute to the resolution of inflammation, the normalization of tissue turnover and the processes of cell renewal accompanying tissue proliferation [69,84]. In contrast, insufficient apoptosis could contribute to the growth of granulomatous tissue and prolonged production of proteolytic enzymes. In fact, a number of studies have shown apoptotic activity of various cells in peri-implant tissues around loosened THAs [59,62,63,66,69]. The low immunopositivity of the apoptotic markers in fact correlated with the degree of osteolysis, and with the proliferation of macro-phages and fibroblasts [63]. Importantly, the type of implant, and the gender and age of patients did not influence the level of apoptosis or the number of T lymphocytes [61].
Apoptosis is characterized in hematoxylin–eosin staining by dark eosinophilic and dense cytoplasm, dense purple condensation of nuclear chromatin, DNA fragmentation and the formation of apoptotic bodies, which are quickly phagocytosed by macrophages or adjacent cells. Current studies were designed to identify the intrinsic and extrinsic pathways of apoptosis by immunostaining (Table 5). The strong expression of iNOS, cyclooxygenase-2, nitrotyrosine, caspase-4, GRP78 and GADD153 was demonstrated in macrophages [66]. Macrophages, giant cells and fibroblasts also expressed important apoptotic regulators, including p53 and BAK [62].
Table 5.
Immunohistochemical analysis of apoptosis.
Immunostaining of tissues from aseptic loosened THAs showed DNA-cleaving active caspase-3 and pro-apoptotic BAK positive macrophages, fibroblasts, giant cells and T lymphocytes [62,63]. The immunopositivity of Fas ligand (FasL), which is able to bind to its death receptor FasR, was found in macrophages, fibroblasts and giant cells [62]. The precursor and active forms of caspase-1 (an interleukin-converting enzyme) were found in the macrophage-like, fibroblast-like cells and some vascular endothelial cells in the synovial-like membrane [101]. Fibroblasts showed caspase-8 positivity located downstream from FasL and FasR, and no reaction with caspase-6 [69,119].
TUNEL assays (TdT-mediated dUTP–biotin nick-end labeling) can be used to detect cells undergoing apoptosis. This method in fact also detects necrotic cells in some cases because it extends and labels the free terminal ends of the cleaved deoxyribonucleic acid. The results of TUNEL analysis suggest high numbers of apoptotic macrophages in the interface membrane with metal and polyethylene debris [66], but only a few TUNEL-positive fibroblasts in aseptic loosening of cementless implants [69]. On the other hand, the anti-apoptotic marker Bcl-2 was not found in macrophages, giant cells or fibroblasts [62,69].
Taken the above findings together, it appears that apoptosis may be a key element in understanding the mechanism of aseptic loosening and periprosthetic osteolysis.
3.7. Evidence for osteoclast-driven periprosthetic bone resorption
To determine whether osteoclasts play a key role in periprosthetic osteolysis requires their identification in areas of bone resorption, together with a demonstration of increased concentrations of the chemokines that can attract osteoclast precursors and promote their differentiation in the periprosthetic tissues. M-CSF has been detected in macrophages, fibroblasts and vascular endothelial cells in periprosthetic tissues [50]. High levels of receptor activator of nuclear factor kappa-B ligand (RANKL) were detected in macrophages, multinucleated giant cells adjacent to wear particles and endothelial cells [38,39]. RANKL was also found in fibroblast-like stromal cells in periprosthetic tissues and soluble RANKL was found to bind RANK+ cells [34]. In another study, histiocytes, multinucleated giant cells and fibroblast-like cells in the membranous tissue expressed RANKL and TNF-α converting enzyme [32]. Fibroblasts and macrophages also expressed IL-11, which participated in osteoclast formation [48].
An opposite role is played by osteoprotegerin (OPG), which can down-regulate osteoclastogenesis by binding to and neutralizing RANKL. OPG was reduced in the tissues located near failed implants [38]. As a result, the RANKL/OPG ratio was increased in periprosthetic tissues from sites with osteolysis and with a high number of wear particles [34,53,65].
An important role in the destructive bone process is attributed to collagenases and other members of the matrix metalloproteinase (MMP) family [46,120]. The expression of collagenase-3 (MMP-13) was found in endothelial cells, macrophages and fibroblasts of the synovial-like membrane [41]. Local mRNA expression profile showed that MMP-1, −9, −10, −12 and −13 were strongly elevated in aseptic loosening compared to controls; MMP-2, −7, −8, −11, −14, −15, −16, −17 and −19 were moderately expressed, whereas MMP-3 expression was lower and MMP-20 very low [121]. Furthermore, collagen degradation in periprosthetic tissue correlated significantly with the number of local MMP-1, MMP-13 and cathepsin K-positive cells [54].
Most studies report data on the cellular responses in soft periprosthetic tissue, rather than in bone samples. A key histomorphometric work in this field showed a high turnover in periprosthetic bone remodeling and immature bone formation around loosened cemented THA [27]. The total eroded surface was increased at the implant–bone interface in loose compared to well-fixed implants (14.7 ± 5.1 vs. 9.8 ± 2.4%, p = 0.027). However, the osteoid surface was increased in cases with loosening (25.8 ± 8.7 vs. 16.8 ± 3.4%, p = 0.017). In addition, in loosened implants, the volume value of the mature mineral was low (54.6 ± 12.9 vs. 80.6 ± 10.0%, p < 0.001), whereas the volume of low-mineralized bone was increased (39.3 ± 12.2 vs. 17.6 ± 9.1%, p < 0.001), which might be related to the low pH and high cathepsin K values in the interface membrane [33]. Shen et al. [45] examined specimens of soft tissue and demineralized bone to analyze the phenotype of cells associated with polyethylene particles or the bone surface. Expression of β3-integrin and calcitonin receptors was predominantly found in cells associated with the bone surface, and their activity rose after induction of cathepsin K and tartrate-resistant acid phosphatase (TRAP). Although TRAP and cathepsin K are used as markers of osteoclast lineage, several studies detected high activity of these enzymes in macrophages and giant cells located at the bone-oriented surface of the interface tissue [32,33,45,52].
Taken together, there is growing evidence for the role of osteoclasts in periprosthetic osteolysis and aseptic loosening in THA. On the other hand, the contribution of other cells of the periprosthetic bone multicellular unit to bone resorption still remains to be elucidated.
3.8. Evidence for localized delayed-type hypersensitivity reaction
Some authors believe that painful THA and aseptic loosening could be explained, at least partially, by hypersensitivity to chemicals or metals used in the implant [122–124]. Prosthetic particles and metallic ions can indeed induce type IV hypersensitivity reactions, as exemplified by allergic contact dermatitis overlying the implant. However, the putative participation in tissue damage and eventually in implant loosening is currently unclear. Metal ions, or haptens such as acrylates, primarily form covalent bonds between the electrophilic components of the hapten and the amino acid nucleophilic side chains of the target proteins. This complex then binds to the surface of dendritic cells, which migrate to the draining local lymph nodes via the lymphatic vessels and present the antigen to T cells, leading to sensitization [125]. Memory T cells then migrate back to the site of the implant and cause an immune reaction, in particular with (antigen-responsive) lymphocyte infiltrates in the deep fibrous tissue and high endothelial venules for lymphocyte recruitment from the circulation [39,111,122,126– 128]. Pertinently, direct non-peptide-dependent activation of TLRs can also occur with some metals [129]. If this lymphocyte-mediated hypersensitivity response occurs in peri-implant tissues, which are already affected by a chronic foreign body reaction, multinuclear foreign body giant cells or granulomas can also be seen [123]. The typical histopathological picture in metal-on-metal arthroplasties demonstrates a layer of fibrin on the synovial lining, granulomatous inflammation and connective tissue necrosis [111,130,131]. The widespread infiltration in areas of tissue necrosis has been described as adverse reactions to metal debris – metallosis participating at least partially in the development of pseudotumors [30,132].
Key cell populations associated with hypersensitivity include lymphocytes and dendritic cells. The presence of large numbers of lymphocytes forming perivascular cuffs and synovial ulceration is called lymphocytic vasculitis-associated lesion (ALVAL), and demonstrates a lymphocyte-dominant delayed-hypersensitivity-type immunopathological reaction. The highest ALVAL score is found in association with suspected metal hypersensitivity [29,111,132]. The diffuse perivascular lymphocytic infiltration is associated with the presence of visible metal particles and aseptic loosening [89,133]. Importantly, cell-mediated immune responses have not been confirmed to be involved in aseptic loosening in metal-on-polyethylene THAs [59,91]. Other cell groups participating in tissue hypersensitivity are mast cells and eosinophils. The former were detected in periprosthetic tissues less frequently compared to in control knee synovium, despite their being more often degranulated in aseptic loosening [44]. However, mast cells play a role in immediate type I hypersensitivity reactions, which are characterized by Th2 skewing of the immune system, as seen in the atopic disorders.
In summary, the concept of local hypersensitivity remains to be proved in prospective studies with greater numbers of patients with aseptic loosening of different implants. In addition, rigorous standardized rules for tissue processing and interpretation of immunostainings have to be established [108]. Notably, metal hypersensitivity can be a secondary phenomenon due to inflammation-based corrosion of the metal alloys.
3.9. Limitations of histopathological studies of aseptic loosening and periprosthetic osteolysis
The obvious limitations include the origin and location of tissue samples, the size of the observed groups, their homogeneity, at least in terms of age, gender, primary diagnosis, type of implant and type of samples (pseudosynovial, interface membrane, osteolytic tissue, etc.), and the lack of appropriate control groups. The control samples involved in almost all studies are synovial tissues from primary osteoarthritis taken before THA/total knee arthroplasty surgery, rarely synovial tissue from around a well-fixed THA, tissues from mandibular or maxilla fractures or biological ingrowth membranes [27,31,38,40,41,48,50,52,53,66,68,88,90– 92,101,106,134]. The periprosthetic tissue from deceased patients with THA was also used [5,23,24,61–63,130].
One important factor potentially influencing the results from tissue analysis is the location of the harvested specimen [115]. Some studies report an increased cellular response in synovial-like membrane of periprosthetic tissue and suggest that SLIM is the best material available for histopathological analysis [50,73]. However, some studies show that there is a large variability within the sampled tissues even when the same kind of retrieved tissue is sampled [42,115,135]. Discrepancies may be even in the single sample [42]. This points to the vast diversity in the process of aseptic loosening even in a single patient, at least in terms of the cell types and numbers present and the different levels of activation among cells, which may represent different stages of loosening in different topological areas.
The process of harvesting tissue and the type of fixation play crucial roles in the results of the diagnostic methods involved [136–138]. The samples used for immunostaining may be frozen and paraffin-embedded sections harvested right at the time of surgery. Although FFPE sections are suitable for immunohistochemical analysis of most antigens, many authors prefer snap-frozen cryostat sections [25,33,35,37–44,48,50,54,64,88,91,98,100,101,106,115, 126,139]. The preparation of fresh-frozen sections takes less time than FFPE sections; moreover, the technique of processing is simpler and the activity and epitope of antigens is better preserved. However, the use of frozen sections also has disadvantages: (i) the tissue morphology is not as clear as it is in the FFPE section; (ii) the fresh tissue for other diagnostic purposes must be stored, preferably at −70/80 °C; and (iii) freezing must be done very rapidly to minimize the formation of ice crystals. Immunostaining of FFPE samples after defrosting may show incorrect results and is of low histomorphological value. The tissue to be processed as an FFPE sample must be fixed in buffered formalin immediately after harvesting. The advantages of FFPE sections are: (i) applicability to routinely processed material, even if it is stored for long period; (ii) possibility of correlation with the morphologic parameters; and (iii) workability even in decalcified material (bone).
The critical factor for the interpretation of the results of immunostaining is their validity and verification of antibody specificity and sensitivity [136,137,140]. Despite the high popularity of immunostaining, there are also potential limitations with respect to limited availability of some sensitive or specific antibodies to the required antigens [136,137]. These may be demonstrable by PCR or ISH. Therefore, molecular techniques can provide superior diagnostic information and should be used together with immunostaining.
The results of immunohistochemical staining are mostly reported qualitatively, though some specific antibodies and numerous regulatory molecules require quantitative analysis [141,142]. Even though well-established criteria often exist for characterization of individual cells under light microscopy, inter-individual variation is also of concern. Computer-assisted analysis of digital images might prove to be more reliable [141–143].
3.10. Directions of future research
On the basis of the above limitations, one may speculate that further models of the bone-implant interface and periprosthetic tissues may be more descriptive of the mechanical and biological processes responsible for integration and loosening of THA in humans.
The clinical criteria for inclusion and exclusion of surgical cases and specimens need to be further defined. Other factors contributing to aseptic loosening, such as mechanical factors and/or implant deficiencies, must be elucidated. The key problem lies in the complexity of THA because a number of patient-, surgery- and implant-related variables could potentially influence the stability of the THA, making the interpretation of the cause of failure difficult in an individual case.
Further progress in developing valid control groups is needed. Samples from all patients with functional and stable THA could be collected and offered for research, for instance, via international collaboration (analogous to a tissue bank). If possible, samples should be collected from both capsular and interface membranes. Perhaps the optimal method for archiving of periprosthetic tissues is deep-freezing, for future distribution to specialized centers of excellence.
Research in fields related to material science, biochemistry, physiology, and cellular and molecular immunology could stimulate further investigation of periprosthetic tissues in terms of target cells, pathways and proteins. For instance, there is a lack of information on the role of fibroblasts, mast cells, dendritic cells and osteocytes, as well as lining cells of pseudosynovial membrane in periprosthetic tissue homeostasis and aseptic loosening.
The role of hypersensitivity and adverse tissue reactions to metal debris in aseptic loosening needs further investigation. First, it is necessary to identify the mechanisms of tolerance to metallic implants induced in most patients with THA. Secondly, the evaluation of hypersensitivity reactions should be based on more specific morphologic criteria, using immunostaining and the analysis of lymphocyte clonality and specificity and their cytokines. There is a need to clarify the role and presence of lymphocytes, their subsets and products. The relationship between delayed lymphocyte-mediated hypersensitivity reactions and in vivo and in vitro allergy tests, such as cutaneous skin patch tests (eliciting skin induration/lymphocyte infiltrates and erythema) or the ELISPOT test of peripheral blood lymphocytes (production of IFN-γ or other relevant cytokines), upon exposure to specific metals is extremely controversial. More definitive proof would require the demonstration of an oligoclonal (antigen- or allergen-driven) lymphocyte activation pattern locally in peri-implant tissues without any in vitro cellular expansion. This could be used to demonstrate discriminative antigen-driven local expansion of antigen-specific T cells in situ.
4. Conclusion
Histology and immunostaining of periprosthetic tissue have contributed significantly to the knowledge of the mechanisms of aseptic loosening and periprosthetic osteolysis. The key role of innate immunity sentinel cells and their receptors has emphasized the host response to prosthetic by-products and subsequent tissue damage. Histology and immunostaining also contribute to the understanding of the mechanisms of prosthetic integration vs. the adverse outcomes associated with chronic inflammation, fibrosis and allergy in the processes of periprosthetic tissue maladaptation.
Limitations of histological analysis of retrieved periprosthetic tissues include: (i) the heterogeneity in patient, surgeon and implant characteristics; (ii) the inability to harvest tissues at different stages of tissue maladaptation before aseptic loosening; (iii) the lack of standardized sampling and processing methodologies; and (iv) the absence of true control tissues (tissues loaded by implant-related signals but without even early signs of tissue maladaptation). Further research in multiple specialized collaborative centers could address these issues more effectively.
Acknowledgements
We apologize to many authors whose important works could not be cited owing to space limitations. Funding was obtained from the Czech Ministry of Health (IGA MZ CR NT/11049).
Footnotes
Conflict of interest statement
The work has not been supported by commercial sources and we are not aware of any potential conflict of interests.
Appendix A. Figures with essential colour discrimination
Certain figures in this article, particularly Figs. 2–4, are difficult to interpret in black and white. The full color images can be found in the on-line version, at http://10.1016/j.actbio.2014.02.003
References
- 1.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am Vol. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 2.Vanhegan IS, Malik AK, Jayakumar P, Ul Islam S, Haddad FS. A financial analysis of revision hip arthroplasty: the economic burden in relation to the national tariff. J Bone Joint Surg Am Br Vol. 2012;94:619–623. doi: 10.1302/0301-620X.94B5.27073. [DOI] [PubMed] [Google Scholar]
- 3.Patil S, Garbuz DS, Greidanus NV, Masri BA, Duncan CP. Quality of life outcomes in revision vs primary total hip arthroplasty: a prospective cohort study. J Arthroplasty. 2008;23:550–553. doi: 10.1016/j.arth.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 4.Inacio MC, Ake CF, Paxton EW, Khatod M, Wang C, Gross TP, et al. Sex and risk of hip implant failure: assessing total hip arthroplasty outcomes in the United States. JAMA Intern Med. 2013;173:435–441. doi: 10.1001/jamainternmed.2013.3271. [DOI] [PubMed] [Google Scholar]
- 5.Willert HG, Ludwig J, Semlitsch M. Reaction of bone to methacrylate after hip arthroplasty: a long-term gross, light microscopic, and scanning electron microscopic study. J Bone Joint Surg Am Vol. 1974;56:1368–1382. [PubMed] [Google Scholar]
- 6.Willert HG, Semlitsch M. Reactions of the articular capsule to wear products of artificial joint prostheses. J Biomed Mater Res. 1977;11:157–164. doi: 10.1002/jbm.820110202. [DOI] [PubMed] [Google Scholar]
- 7.Gallo J, Raska M, Mrazek F, Petrek M. Bone remodeling, particle disease and individual susceptibility to periprosthetic osteolysis. Physiol Res. 2008;57:339–349. doi: 10.33549/physiolres.931140. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Bai C, Xie L, Zhang Y, Wang K. Inflammatory response to orthopedic biomaterials after total hip replacement. J Orthop Sci. 2012;17:407–412. doi: 10.1007/s00776-012-0234-8. [DOI] [PubMed] [Google Scholar]
- 9.Yang SY, Yu H, Gong W, Wu B, Mayton L, Costello R, et al. Murine model of prosthesis failure for the long-term study of aseptic loosening. J Orthop Sci. 2007;25:603–611. doi: 10.1002/jor.20342. [DOI] [PubMed] [Google Scholar]
- 10.Green TR, Fisher J, Matthews JB, Stone MH, Ingham E. Effect of size and dose on bone resorption activity of macrophages by in vitro clinically relevant ultra high molecular weight polyethylene particles. J Biomed Mater Res. 2000;53:490–497. doi: 10.1002/1097-4636(200009)53:5<490::aid-jbm7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Huang Z, Ma T, Ren PG, Smith RL, Goodman SB. Effects of orthopedic polymer particles on chemotaxis of macrophages and mesenchymal stem cells. J Biomed Mater Res, Part A. 2010;94:1264–1269. doi: 10.1002/jbm.a.32803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingham E, Green TR, Stone MH, Kowalski R, Watkins N, Fisher J. Production of TNF-alpha and bone resorbing activity by macrophages in response to different types of bone cement particles. Biomaterials. 2000;21:1005–1013. doi: 10.1016/s0142-9612(99)00261-6. [DOI] [PubMed] [Google Scholar]
- 13.Ma GK, Chiu R, Huang Z, Pearl J, Ma T, Smith RL, et al. Polymethylmethacrylate particle exposure causes changes in p38 MAPK and TGF-beta signaling in differentiating MC3T3-E1 cells. J Biomed Mater Res, Part A. 2010;94:234–240. doi: 10.1002/jbm.a.32686. [DOI] [PubMed] [Google Scholar]
- 14.Rao AJ, Nich C, Dhulipala LS, Gibon E, Valladares R, Zwingenberger S, et al. Local effect of IL-4 delivery on polyethylene particle induced osteolysis in the murine calvarium. J Biomed Mater Res, Part A. 2013;101:1926–1934. doi: 10.1002/jbm.a.34486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y, Jia T, Gong W, Wooley PH, Yang SY. Titanium particle-challenged osteoblasts promote osteoclastogenesis and osteolysis in a murine model of periprosthestic osteolysis. Acta Biomater. 2013;9:7564–7572. doi: 10.1016/j.actbio.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo J, Goodman SB, Konttinen YT, Raska M. Particle disease: biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013;19:213–224. doi: 10.1177/1753425912451779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nich C, Takakubo Y, Pajarinen J, Ainola M, Salem A, Sillat T, et al. Macrophages-Key cells in the response to wear debris from joint replacements. J Biomed Mater Res, Part A. 2013;101:3033–3045. doi: 10.1002/jbm.a.34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldring SR, Schiller AL, Roelke M, Rourke CM, O’Neil DA, Harris WH. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg Am Vol. 1983;65:575–584. [PubMed] [Google Scholar]
- 19.Ito S, Matsumoto T, Enomoto H, Shindo H. Histological analysis and biological effects of granulation tissue around loosened hip prostheses in the development of osteolysis. J Orthop Sci. 2004;9:478–487. doi: 10.1007/s00776-004-0808-1. [DOI] [PubMed] [Google Scholar]
- 20.Meinecke I, Pap G, Mendoza H, Drange S, Ender S, Strietholt S, et al. Small ubiquitin-like modifier 1 [corrected] mediates the resistance of prosthesis-loosening fibroblast-like synoviocytes against Fas-induced apoptosis. Arthritis Rheum. 2009;60:2065–2070. doi: 10.1002/art.24633. [DOI] [PubMed] [Google Scholar]
- 21.Koreny T, Tunyogi-Csapo M, Gal I, Vermes C, Jacobs JJ, Glant TT. The role of fibroblasts and fibroblast-derived factors in periprosthetic osteolysis. Arthritis Rheum. 2006;54:3221–3232. doi: 10.1002/art.22134. [DOI] [PubMed] [Google Scholar]
- 22.Shi Q, Lajeunesse D, Reboul P, Martel-Pelletier J, Pelletier JP, Dehnade F, et al. Metabolic activity of osteoblasts from periprosthetic trabecular bone in failed total hip arthroplasties and osteoarthritis as markers of osteolysis and loosening. J Rheumatol. 2002;29:1437–1445. [PubMed] [Google Scholar]
- 23.Engh CA, Hooten JP, Jr, Zettl-Schaffer KF, Ghaffarpour M, McGovern TF, Bobyn JD. Evaluation of bone ingrowth in proximally and extensively porous-coated anatomic medullary locking prostheses retrieved at autopsy. J Bone Joint Surg Am Vol. 1995;77:903–910. doi: 10.2106/00004623-199506000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Jasty M, Maloney WJ, Bragdon CR, Haire T, Harris WH. Histomorphological studies of the long-term skeletal responses to well fixed cemented femoral components. J Bone Joint Surg Am Vol. 1990;72:1220–1229. [PubMed] [Google Scholar]
- 25.Konttinen YT, Li TF, Xu JW, Tagaki M, Pirila L, Silvennoinen T, et al. Expression of laminins and their integrin receptors in different conditions of synovial membrane and synovial membrane-like interface tissue. Ann Rheum Dis. 1999;58:683–690. doi: 10.1136/ard.58.11.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmalzried TP, Kwong LM, Jasty M, Sedlacek RC, Haire TC, O’Connor DO, et al. The mechanism of loosening of cemented acetabular components in total hip arthroplasty. Analysis of specimens retrieved at autopsy. Clin Orthop Relat Res. 1992:60–78. [PubMed] [Google Scholar]
- 27.Takagi M, Santavirta S, Ida H, Ishii M, Takei I, Niissalo S, et al. High-turnover periprosthetic bone remodeling and immature bone formation around loose cemented total hip joints. J Bone Miner Res. 2001;16:79–88. doi: 10.1359/jbmr.2001.16.1.79. [DOI] [PubMed] [Google Scholar]
- 28.Boehler M, Knahr K, Plenk H, Jr, Walter A, Salzer M, Schreiber V. Long-term results of uncemented alumina acetabular implants. J Bone Joint Surg Br. 1994;76:53–59. [PubMed] [Google Scholar]
- 29.Browne JA, Bechtold CD, Berry DJ, Hanssen AD, Lewallen DG. Failed metal-on-metal hip arthroplasties: a spectrum of clinical presentations and operative findings. Clin Orthop Relat Res. 2010;468:2313–2320. doi: 10.1007/s11999-010-1419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh C, Kaplan A, Pambuccian SE. Necrotic granulomatous pseudotumor following metal-on-metal hip arthroplasty: a potential mimic of sarcoma on fine needle aspiration cytology. Diagn Cytopathol. 2012;40(Suppl 2):E104–E108. doi: 10.1002/dc.21605. [DOI] [PubMed] [Google Scholar]
- 31.Clarke MT, Roberts CP, Lee PT, Gray J, Keene GS, Rushton N. Polymerase chain reaction can detect bacterial DNA in aseptically loose total hip arthroplasties. Clin Orthop Relat Res. 2004:132–137. doi: 10.1097/01.blo.0000136839.90734.b7. [DOI] [PubMed] [Google Scholar]
- 32.Horiki M, Nakase T, Myoui A, Sugano N, Nishii T, Tomita T, et al. Localization of RANKL in osteolytic tissue around a loosened joint prosthesis. J Bone Miner Metab. 2004;22:346–351. doi: 10.1007/s00774-003-0493-8. [DOI] [PubMed] [Google Scholar]
- 33.Konttinen YT, Takagi M, Mandelin J, Lassus J, Salo J, Ainola M, et al. Acid attack and cathepsin K in bone resorption around total hip replacement prosthesis. J Bone Miner Res. 2001;16:1780–1786. doi: 10.1359/jbmr.2001.16.10.1780. [DOI] [PubMed] [Google Scholar]
- 34.Mandelin J, Li TF, Liljestrom M, Kroon ME, Hanemaaijer R, Santavirta S, et al. Imbalance of RANKL/RANK/OPG system in interface tissue in loosening of total hip replacement. J Bone Joint Surg Am Br. 2003;85:1196–1201. doi: 10.1302/0301-620x.85b8.13311. [DOI] [PubMed] [Google Scholar]
- 35.Al Saffar N, Revell PA. Interleukin-1 production by activated macrophages surrounding loosened orthopaedic implants: a potential role in osteolysis. Br J Rheumatol. 1994;33:309–316. doi: 10.1093/rheumatology/33.4.309. [DOI] [PubMed] [Google Scholar]
- 36.Baxter RM, Ianuzzi A, Freeman TA, Kurtz SM, Steinbeck MJ. Distinct immunohistomorphologic changes in periprosthetic hip tissues from historical and highly crosslinked UHMWPE implant retrievals. J Biomed Mater Res, Part A. 2010;95:68–78. doi: 10.1002/jbm.a.32813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiba J, Rubash HE, Kim KJ, Iwaki Y. The characterization of cytokines in the interface tissue obtained from failed cementless total hip arthroplasty with and without femoral osteolysis. Clin Orthop Relat Res. 1994:304–312. [PubMed] [Google Scholar]
- 38.Crotti TN, Smith MD, Findlay DM, Zreiqat H, Ahern MJ, Weedon H, et al. Factors regulating osteoclast formation in human tissues adjacent to peri-implant bone loss: expression of receptor activator NFkappaB, RANK ligand and osteoprotegerin. Biomaterials. 2004;25:565–573. doi: 10.1016/s0142-9612(03)00556-8. [DOI] [PubMed] [Google Scholar]
- 39.Hercus B, Revell PA. Phenotypic characteristics of T lymphocytes in the interfacial tissue of aseptically loosened prosthetic joints. J Mater Sci Mater Med. 2001;12:1063–1067. doi: 10.1023/a:1012806409544. [DOI] [PubMed] [Google Scholar]
- 40.Hukkanen M, Corbett SA, Batten J, Konttinen YT, McCarthy ID, Maclouf J, et al. Aseptic loosening of total hip replacement. Macrophage expression of inducible nitric oxide synthase and cyclo-oxygenase-2, together with peroxynitrite formation, as a possible mechanism for early prosthesis failure. J Bone Joint Surg Am Br. 1997;79:467–474. doi: 10.1302/0301-620x.79b3.7469. [DOI] [PubMed] [Google Scholar]
- 41.Imai S, Konttinen YT, Jumppanen M, Lindy O, Ceponis A, Kemppinen P, et al. High levels of expression of collagenase-3 (MMP-13) in pathological conditions associated with a foreign-body reaction. J Bone Joint Surg Am Br. 1998;80:701–710. doi: 10.1302/0301-620x.80b4.7952. [DOI] [PubMed] [Google Scholar]
- 42.Jones LC, Frondoza C, Hungerford DS. Immunohistochemical evaluation of interface membranes from failed cemented and uncemented acetabular components. J Biomed Mater Res. 1999;48:889–898. doi: 10.1002/(sici)1097-4636(1999)48:6<889::aid-jbm19>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 43.Santavirta S, Hoikka V, Eskola A, Konttinen YT, Paavilainen T, Tallroth K. Aggressive granulomatous lesions in cementless total hip arthroplasty. J Bone Joint Surg Am Br. 1990;72:980–984. doi: 10.1302/0301-620X.72B6.2246301. [DOI] [PubMed] [Google Scholar]
- 44.Solovieva SA, Ceponis A, Konttinen YT, Takagi M, Suda A, Eklund KK, et al. Mast cells in loosening of totally replaced hips. Clin Orthop Relat Res. 1996:158–165. [PubMed] [Google Scholar]
- 45.Shen Z, Crotti TN, McHugh KP, Matsuzaki K, Gravallese EM, Bierbaum BE, et al. The role played by cell-substrate interactions in the pathogenesis of osteoclast-mediated peri-implant osteolysis. Arthritis Res Ther. 2006;8:R70. doi: 10.1186/ar1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takagi M, Konttinen YT, Santavirta S, Sorsa T, Eisen AZ, Nordsletten L, et al. Extracellular matrix metalloproteinases around loose total hip prostheses. Acta Orthop Scand. 1994;65:281–286. doi: 10.3109/17453679408995454. [DOI] [PubMed] [Google Scholar]
- 47.Witzleb WC, Hanisch U, Kolar N, Krummenauer F, Guenther KP. Neo-capsule tissue reactions in metal-on-metal hip arthroplasty. Acta Orthop. 2007;78:211–220. doi: 10.1080/17453670710013708. [DOI] [PubMed] [Google Scholar]
- 48.Xu JW, Li TF, Partsch G, Ceponis A, Santavirta S, Konttinen YT. Interleukin-11 (IL-11) in aseptic loosening of total hip replacement (THR) Scand J Rheumatol. 1998;27:363–367. doi: 10.1080/03009749850154393. [DOI] [PubMed] [Google Scholar]
- 49.Xu JW, Konttinen YT, Li TF, Waris V, Lassus J, Matucci-Cerinic M, et al. Production of platelet-derived growth factor in aseptic loosening of total hip replacement. Rheumatol Int. 1998;17:215–221. doi: 10.1007/s002960050037. [DOI] [PubMed] [Google Scholar]
- 50.Xu JW, Konttinen YT, Waris V, Patiala H, Sorsa T, Santavirta S. Macrophage-colony stimulating factor (M-CSF) is increased in the synovial-like membrane of the periprosthetic tissues in the aseptic loosening of total hip replacement (THR) Clin Rheumatol. 1997;16:243–248. doi: 10.1007/BF02238958. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Hou C, Yu S, Xiao J, Zhang Z, Zhai Q, et al. IRAK-M in macrophages around septically and aseptically loosened hip implants. J Biomed Mater Res, Part A. 2012;100:261–268. doi: 10.1002/jbm.a.33258. [DOI] [PubMed] [Google Scholar]
- 52.Gehrke T, Sers C, Morawietz L, Fernahl G, Neidel J, Frommelt L, et al. Receptor activator of nuclear factor kappaB ligand is expressed in resident and inflammatory cells in aseptic and septic prosthesis loosening. Scand J Rheumatol. 2003;32:287–294. doi: 10.1080/03009740310003929. [DOI] [PubMed] [Google Scholar]
- 53.Grimaud E, Soubigou L, Couillaud S, Coipeau P, Moreau A, Passuti N, et al. Receptor activator of nuclear factor kappaB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am J Pathol. 2003;163:2021–2031. doi: 10.1016/s0002-9440(10)63560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma GF, Ali A, Verzijl N, Hanemaaijer R, TeKoppele J, Konttinen YT, et al. Increased collagen degradation around loosened total hip replacement implants. Arthritis Rheum. 2006;54:2928–2933. doi: 10.1002/art.22064. [DOI] [PubMed] [Google Scholar]
- 55.Mandelin J, Li TF, Hukkanen MV, Liljestrom M, Chen ZK, Santavirta S, et al. Increased expression of a novel osteoclast-stimulating factor, ADAM8, in interface tissue around loosened hip prostheses. J Rheumatol. 2003;30:2033–2038. [PubMed] [Google Scholar]
- 56.Morawietz L, Classen RA, Schroder JH, Dynybil C, Perka C, Skwara A, et al. Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J Clin Pathol. 2006;59:591–597. doi: 10.1136/jcp.2005.027458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pajarinen J, Cenni E, Savarino L, Gomez-Barrena E, Tamaki Y, Takagi M, et al. Profile of toll-like receptor-positive cells in septic and aseptic loosening of total hip arthroplasty implants. J Biomed Mater Res, Part A. 2010;94:84–92. doi: 10.1002/jbm.a.32674. [DOI] [PubMed] [Google Scholar]
- 58.Park YS, Moon YW, Lim SJ, Yang JM, Ahn G, Choi YL. Early osteolysis following second-generation metal-on-metal hip replacement. J Bone Joint Surg Am Vol. 2005;87:1515–1521. doi: 10.2106/JBJS.D.02641. [DOI] [PubMed] [Google Scholar]
- 59.Revell PA, Jellie SE. Interleukin 15 production by macrophages in the implant interface membrane of aseptically loosened joint replacements. J Mater Sci Mater Med. 1998;9:727–730. doi: 10.1023/a:1008903018885. [DOI] [PubMed] [Google Scholar]
- 60.Edwards J, Schulze E, Sabokbar A, Gordon-Andrews H, Jackson D, Athanasou NA. Absence of lymphatics at the bone-implant interface: implications for periprosthetic osteolysis. Acta Orthop. 2008;79:289–294. doi: 10.1080/17453670710015175. [DOI] [PubMed] [Google Scholar]
- 61.Landgraeber S, von Knoch M, Loer F, Brankamp J, Tsokos M, Grabellus F, et al. Association between apoptotis and CD4(+)/CD8(+) T-lymphocyte ratio in aseptic loosening after total hip replacement. Int J Biol Sci. 2009;5:182–191. doi: 10.7150/ijbs.5.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Landgraeber S, Toetsch M, Wedemeyer C, Saxler G, Tsokos M, von Knoch F, et al. Over-expression of p53/BAK in aseptic loosening after total hip replacement. Biomaterials. 2006;27:3010–3020. doi: 10.1016/j.biomaterials.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 63.Landgraeber S, von Knoch M, Loer F, Wegner A, Tsokos M, Hussmann B, et al. Extrinsic and intrinsic pathways of apoptosis in aseptic loosening after total hip replacement. Biomaterials. 2008;29:3444–3450. doi: 10.1016/j.biomaterials.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 64.Nakashima Y, Sun DH, Trindade MC, Chun LE, Song Y, Goodman SB, et al. Induction of macrophage C-C chemokine expression by titanium alloy and bone cement particles. J Bone Joint Surg Am Br. 1999;81:155–162. doi: 10.1302/0301-620x.81b1.8884. [DOI] [PubMed] [Google Scholar]
- 65.Veigl D, Niederlova J, Krystufkova O. Periprosthetic osteolysis and its association with RANKL expression. Physiol Res. 2007;56:455–462. doi: 10.33549/physiolres.930997. [DOI] [PubMed] [Google Scholar]
- 66.Yang F, Wu W, Cao L, Huang Y, Zhu Z, Tang T, et al. Pathways of macrophage apoptosis within the interface membrane in aseptic loosening of prostheses. Biomaterials. 2011;32:9159–9167. doi: 10.1016/j.biomaterials.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 67.Nygaard M, Elling F, Bastholm L, Soballe K, Borgwardt A. No difference in early cellular response of the pseudo-synovial membrane after total hip arthroplasty: comparison of 3 combinations of bearing materials. Acta Orthop. 2006;77:402–412. doi: 10.1080/17453670610046325. [DOI] [PubMed] [Google Scholar]
- 68.Santavirta S, Xu JW, Hietanen J, Ceponis A, Sorsa T, Kontio R, et al. Activation of periprosthetic connective tissue in aseptic loosening of total hip replacements. Clin Orthop Relat Res. 1998:16–24. [PubMed] [Google Scholar]
- 69.Sabbatini M, Piffanelli V, Boccafoschi F, Gatti S, Reno F, Bosetti M, et al. Different apoptosis modalities in periprosthetic membranes. J Biomed Mater Res, Part A. 2010;92:175–184. doi: 10.1002/jbm.a.32349. [DOI] [PubMed] [Google Scholar]
- 70.Kwon YM, Ostlere SJ, McLardy-Smith P, Athanasou NA, Gill HS, Murray DW. “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty. 2011;26:511–518. doi: 10.1016/j.arth.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 71.Mirra JM, Amstutz HC, Matos M, Gold R. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clin Orthop Relat Res. 1976:221–240. [PubMed] [Google Scholar]
- 72.Morawietz L, Tiddens O, Mueller M, Tohtz S, Gansukh T, Schroeder JH, et al. Twenty-three neutrophil granulocytes in 10 high-power fields is the best histopathological threshold to differentiate between aseptic and septic endoprosthesis loosening. Histopathology. 2009;54:847–853. doi: 10.1111/j.1365-2559.2009.03313.x. [DOI] [PubMed] [Google Scholar]
- 73.Bori G, Munoz-Mahamud E, Garcia S, Mallofre C, Gallart X, Bosch J, et al. Interface membrane is the best sample for histological study to diagnose prosthetic joint infection. Modern Pathol. 2011;24:579–584. doi: 10.1038/modpathol.2010.219. [DOI] [PubMed] [Google Scholar]
- 74.Athanasou NA, Pandey R, de Steiger R, Crook D, Smith PM. Diagnosis of infection by frozen section during revision arthroplasty. J Bone Joint Surg Am Br. 1995;77:28–33. [PubMed] [Google Scholar]
- 75.Tohtz SW, Muller M, Morawietz L, Winkler T, Perka C. Validity of frozen sections for analysis of periprosthetic loosening membranes. Clin Orthop Relat Res. 2010;468:762–768. doi: 10.1007/s11999-009-1102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Musso AD, Mohanty K, Spencer-Jones R. Role of frozen section histology in diagnosis of infection during revision arthroplasty. Postgrad Med J. 2003;79:590–593. doi: 10.1136/pmj.79.936.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Della Valle CJ, Scher DM, Kim YH, Oxley CM, Desai P, Zuckerman JD, et al. The role of intraoperative Gram stain in revision total joint arthroplasty. J Arthroplasty. 1999;14:500–504. doi: 10.1016/s0883-5403(99)90108-0. [DOI] [PubMed] [Google Scholar]
- 78.Spangehl MJ, Masterson E, Masri BA, O’Connell JX, Duncan CP. The role of intraoperative gram stain in the diagnosis of infection during revision total hip arthroplasty. J Arthroplasty. 1999;14:952–956. doi: 10.1016/s0883-5403(99)90009-8. [DOI] [PubMed] [Google Scholar]
- 79.Ghanem E, Ketonis C, Restrepo C, Joshi A, Barrack R, Parvizi J. Periprosthetic infection: where do we stand with regard to Gram stain? Acta Orthop. 2009;80:37–40. doi: 10.1080/17453670902804943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson AJ, Zywiel MG, Stroh DA, Marker DR, Mont MA. Should gram stains have a role in diagnosing hip arthroplasty infections? Clin Orthop Relat Res. 2010;468:2387–2391. doi: 10.1007/s11999-009-1216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bi Y, Collier TO, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin mediates biological responses of titanium particles without stimulating their phagocytosis. J Orthop Sci. 2002;20:696–703. doi: 10.1016/S0736-0266(01)00176-0. [DOI] [PubMed] [Google Scholar]
- 82.Moojen DJ, van Hellemondt G, Vogely HC, Burger BJ, Walenkamp GH, Tulp NJ, et al. Incidence of low-grade infection in aseptic loosening of total hip arthroplasty. Acta Orthop. 2010;81:667–673. doi: 10.3109/17453674.2010.525201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lahdeoja T, Pajarinen J, Kouri VP, Sillat T, Salo J, Konttinen YT. Toll-like receptors and aseptic loosening of hip endoprosthesis-a potential to respond against danger signals? J Orthop Sci. 2010;28:184–190. doi: 10.1002/jor.20979. [DOI] [PubMed] [Google Scholar]
- 84.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 85.Yount NY, Yeaman MR. Peptide antimicrobials: cell wall as a bacterial target. Ann NY Acad Sci. 2013;1277:127–138. doi: 10.1111/nyas.12005. [DOI] [PubMed] [Google Scholar]
- 86.Gollwitzer H, Dombrowski Y, Prodinger PM, Peric M, Summer B, Hapfelmeier A, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am Vol. 2013;95:644–651. doi: 10.2106/JBJS.L.00205. [DOI] [PubMed] [Google Scholar]
- 87.Pajarinen J, Kouri VP, Jamsen E, Li TF, Mandelin J, Konttinen YT. The response of macrophages to titanium particles is determined by macrophage polarization. Acta Biomater. 2013;9:9229–9240. doi: 10.1016/j.actbio.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 88.Goodman SB, Huie P, Song Y, Schurman D, Maloney W, Woolson S, et al. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg Am Br. 1998;80:531–539. doi: 10.1302/0301-620x.80b3.8158. [DOI] [PubMed] [Google Scholar]
- 89.Fujishiro T, Moojen DJ, Kobayashi N, Dhert WJ, Bauer TW. Perivascular and diffuse lymphocytic inflammation are not specific for failed metal-on-metal hip implants. Clin Orthop Relat Res. 2011;469:1127–1133. doi: 10.1007/s11999-010-1649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lennox DW, Schofield BH, McDonald DF, Riley LH., Jr A histologic comparison of aseptic loosening of cemented, press-fit, and biologic ingrowth prostheses. Clin Orthop Relat Res. 1987:171–191. [PubMed] [Google Scholar]
- 91.Li TF, Santavirta S, Waris V, Lassus J, Lindroos L, Xu JW, et al. No lymphokines in T cells around loosened hip prostheses. Acta Orthop Scand. 2001;72:241–247. doi: 10.1080/00016470152846556. [DOI] [PubMed] [Google Scholar]
- 92.Aroukatos P, Repanti M, Repantis T, Bravou V, Korovessis P. Immunologic adverse reaction associated with low-carbide metal-on-metal bearings in total hip arthroplasty. Clin Orthop Relat Res. 2010;468:2135–2142. doi: 10.1007/s11999-009-1187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am Vol. 2005;87:18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 94.Hatton A, Nevelos JE, Nevelos AA, Banks RE, Fisher J, Ingham E. Alumina-alumina artificial hip joints. Part I. A histological analysis and characterisation of wear debris by laser capture microdissection of tissues retrieved at revision. Biomaterials. 2002;23:3429–3440. doi: 10.1016/s0142-9612(02)00047-9. [DOI] [PubMed] [Google Scholar]
- 95.Miyanishi K, Trindade MC, Ma T, Goodman SB, Schurman DJ, Smith RL. Periprosthetic osteolysis: induction of vascular endothelial growth factor from human monocyte/macrophages by orthopaedic biomaterial particles. J Bone Miner Res. 2003;18:1573–1583. doi: 10.1359/jbmr.2003.18.9.1573. [DOI] [PubMed] [Google Scholar]
- 96.Kenna TJ, Brown MA. The role of IL-17-secreting mast cells in inflammatory joint disease. Nat Rev Rheumatol. 2013;9:375–379. doi: 10.1038/nrrheum.2012.205. [DOI] [PubMed] [Google Scholar]
- 97.Santavirta S, Pajamaki J, Eskola A, Konttinen YT, Lindholm T. Proliferative cell response to loosening of total hip replacements: a cytofluorographic cell cycle analysis. Arch Orthop Trauma Surg. 1991;111:43–46. doi: 10.1007/BF00390193. [DOI] [PubMed] [Google Scholar]
- 98.Santavirta S, Ceponis A, Solovieva SA, Hurri H, Jin J, Takagi M, et al. Periprosthetic microvasculature in loosening of total hip replacement. Arch Orthop Trauma Surg. 1996;115:286–289. doi: 10.1007/BF00439055. [DOI] [PubMed] [Google Scholar]
- 99.Niissalo S, Li TF, Santavirta S, Takagi M, Hietanen J, Konttinen YT. Dense innervation in pseudocapsular tissue compared to aneural interface tissue in loose totally replaced hips. J Rheumatol. 2002;29:796–803. [PubMed] [Google Scholar]
- 100.Goodman SB, Huie P, Song Y, Lee K, Doshi A, Rushdieh B, et al. Loosening and osteolysis of cemented joint arthroplasties. A biologic spectrum. Clin Orthop Relat Res. 1997:149–163. doi: 10.1097/00003086-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 101.Li TF, Mandelin J, Hukkanen MV, Liljestrom M, Santavirta S, Westerlund J, et al. Expression of caspase-1 in synovial membrane-like interface tissue around loosened hip prostheses. Rheumatol Int. 2002;22:97–102. doi: 10.1007/s00296-002-0197-8. [DOI] [PubMed] [Google Scholar]
- 102.Neale SD, Athanasou NA. Cytokine receptor profile of arthroplasty macrophages, foreign body giant cells and mature osteoclasts. Acta Orthop Scand. 1999;70:452–458. doi: 10.3109/17453679909000980. [DOI] [PubMed] [Google Scholar]
- 103.Konttinen YT, Waris V, Xu JW, Jiranek WA, Sorsa T, Virtanen I, et al. Transforming growth factor-beta 1 and 2 in the synovial-like interface membrane between implant and bone in loosening of total hip arthroplasty. J Rheumatol. 1997;24:694–701. [PubMed] [Google Scholar]
- 104.Waris V, Sillat T, Waris E, Virkki L, Mandelin J, Takagi M, et al. Role and regulation of VEGF and its receptors 1 and 2 in the aseptic loosening of total hip implants. J Orthop Sci. 2012;30:1830–1836. doi: 10.1002/jor.22138. [DOI] [PubMed] [Google Scholar]
- 105.Spanogle JP, Miyanishi K, Ma T, Epstein NJ, Smith RL, Goodman SB. Comparison of VEGF-producing cells in periprosthetic osteolysis. Biomaterials. 2006;27:3882–3887. doi: 10.1016/j.biomaterials.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 106.Arora A, Song Y, Chun L, Huie P, Trindade M, Smith RL, et al. The role of the TH1 and TH2 immune responses in loosening and osteolysis of cemented total hip replacements. J Biomed Mater Res, Part A. 2003;64:693–697. doi: 10.1002/jbm.a.10200. [DOI] [PubMed] [Google Scholar]
- 107.Li TF, Xu JW, Santavirta S, Takagi M, Virtanen I, Pirila L, et al. Expression of vitronectin and its integrin receptors in the synovial membrane-like interface tissue from aseptic loosening of total hip replacement. J Rheumatol. 2000;27:727–734. [PubMed] [Google Scholar]
- 108.Krenn V, Morawietz L, Kienapfel H, Ascherl R, Matziolis G, Hassenpflug J, et al. Revised consensus classification. Histopathological classification of diseases associated with joint endoprostheses. Z. Rheumatol. 2013;72:383–392. doi: 10.1007/s00393-012-1099-0. [DOI] [PubMed] [Google Scholar]
- 109.Kim KJ, Chiba J, Rubash HE. In vivo and in vitro analysis of membranes from hip prostheses inserted without cement. J Bone Joint Surg Am Vol. 1994;76:172–180. doi: 10.2106/00004623-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 110.Goodman SB, Chin RC, Chiou SS, Schurman DJ, Woolson ST, Masada MP. A clinical-pathologic-biochemical study of the membrane surrounding loosened and nonloosened total hip arthroplasties. Clin Orthop Relat Res. 1989:182–187. [PubMed] [Google Scholar]
- 111.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321–2327. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Esposito C, Maclean F, Campbell P, Walter WL, Walter WK, Bonar SF. Periprosthetic tissues from third generation alumina-on-alumina total hip arthroplasties. J Arthroplasty. 2013;28:860–866. doi: 10.1016/j.arth.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 113.von Domarus C, Rosenberg JP, Ruther W, Zustin J. Necrobiosis and T-lymphocyte infiltration in retrieved aseptically loosened metal-on-polyethylene arthroplasties. Acta Orthop. 2011;82:596–601. doi: 10.3109/17453674.2011.625534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carli A, Reuven A, Zukor DJ, Antoniou J. Adverse soft-tissue reactions around non-metal-on-metal total hip arthroplasty – a systematic review of the literature. Bull NYU Hospital Joint Dis. 2011;69(Suppl 1):S47–S51. [PubMed] [Google Scholar]
- 115.Goodman SB, Knoblich G, O’Connor M, Song Y, Huie P, Sibley R. Heterogeneity in cellular and cytokine profiles from multiple samples of tissue surrounding revised hip prostheses. J Biomed Mater Res. 1996;31:421–428. doi: 10.1002/(SICI)1097-4636(199607)31:3<421::AID-JBM17>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 116.Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C. Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop. 2006;77:177–197. doi: 10.1080/17453670610045902. [DOI] [PubMed] [Google Scholar]
- 117.Aspenberg P, Van der Vis H. Migration, particles, and fluid pressure. A discussion of causes of prosthetic loosening. Clin Orthop Relat Res. 1998:75–80. [PubMed] [Google Scholar]
- 118.Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hospital Joint Dis. 2009;67:182–188. [PubMed] [Google Scholar]
- 119.Reno F, Sabbatini M, Masse A, Bosetti M, Cannas M. Fibroblast apoptosis and caspase-8 activation in aseptic loosening. Biomaterials. 2003;24:3941–3946. doi: 10.1016/s0142-9612(03)00276-x. [DOI] [PubMed] [Google Scholar]
- 120.Li TF, Santavirta S, Virtanen I, Kononen M, Takagi M, Konttinen YT. Increased expression of EMMPRIN in the tissue around loosened hip prostheses. Acta Orthop Scand. 1999;70:446–451. doi: 10.3109/17453679909000979. [DOI] [PubMed] [Google Scholar]
- 121.Takei I, Takagi M, Santavirta S, Ida H, Ishii M, Ogino T, et al. Messenger ribonucleic acid expression of 16 matrix metalloproteinases in bone-implant interface tissues of loose artificial hip joints. J Biomed Mater Res. 2000;52:613–620. doi: 10.1002/1097-4636(20001215)52:4<613::aid-jbm5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 122.Thomas P, Braathen LR, Dorig M, Aubock J, Nestle F, Werfel T, et al. Increased metal allergy in patients with failed metal-on-metal hip arthroplasty and peri-implant T-lymphocytic inflammation. Allergy. 2009;64:1157–1165. doi: 10.1111/j.1398-9995.2009.01966.x. [DOI] [PubMed] [Google Scholar]
- 123.Lohmann CH, Meyer H, Nuechtern JV, Singh G, Junk-Jantsch S, Schmotzer H, et al. Periprosthetic tissue metal content but not serum metal content predicts the type of tissue response in failed small-diameter metal-on-metal total hip arthroplasties. J Bone Joint Surg Am Vol. 2013;95:1561–1568. doi: 10.2106/JBJS.L.01273. [DOI] [PubMed] [Google Scholar]
- 124.Thyssen JP, Jakobsen SS, Engkilde K, Johansen JD, Soballe K, Menne T. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009;80:646–652. doi: 10.3109/17453670903487008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martin SF. Contact dermatitis: from pathomechanisms to immunotoxicology. Exp Dermatol. 2012;21:382–389. doi: 10.1111/j.1600-0625.2012.01471.x. [DOI] [PubMed] [Google Scholar]
- 126.Atilla B, Atilla P, Tokgozoglu AM, Cakar AN, Alpaslan AM. Immunohistochemical evaluation of periprosthetic membrane from loose cemented and uncemented total hip arthroplasty. Saudi Med J. 2005;26:429–433. [PubMed] [Google Scholar]
- 127.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, et al. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 128.Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009;80:653–659. doi: 10.3109/17453670903473016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Konttinen YT, Pajarinen J. Adverse reactions to metal-on-metal implants. Nat Rev Rheumatol. 2013;9:5–6. doi: 10.1038/nrrheum.2012.218. [DOI] [PubMed] [Google Scholar]
- 130.Evans EM, Freeman MA, Miller AJ, Vernon-Roberts B. Metal sensitivity as a cause of bone necrosis and loosening of the prosthesis in total joint replacement. J Bone Joint Surg Am Br. 1974;56-B:626–642. doi: 10.1302/0301-620X.56B4.626. [DOI] [PubMed] [Google Scholar]
- 131.Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty. Five to nine-year follow-up. J Bone Joint Surg Am. 2006;88:1183–1191. doi: 10.2106/JBJS.D.02916. [DOI] [PubMed] [Google Scholar]
- 132.Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Am Br. 2010;92:38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- 133.Ng VY, Lombardi AV, Jr, Berend KR, Skeels MD, Adams JB. Perivascular lymphocytic infiltration is not limited to metal-on-metal bearings. Clin Orthop Relat Res. 2011;469:523–529. doi: 10.1007/s11999-010-1570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Milosev I, Trebse R, Kovac S, Cor A, Pisot V. Survivorship and retrieval analysis of Sikomet metal-on-metal total hip replacements at a mean of seven years. J Bone Joint Surg Am Vol. 2006;88:1173–1182. doi: 10.2106/JBJS.E.00604. [DOI] [PubMed] [Google Scholar]
- 135.Baxter RM, Freeman TA, Kurtz SM, Steinbeck MJ. Do tissues from THA revision of highly crosslinked UHMWPE liners contain wear debris and associated inflammation? Clin Orthop Relat Res. 2011;469:2308–2317. doi: 10.1007/s11999-010-1713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dabbs D. Diagnostic immunohistochemistry: theranostic and genomic applications. 3rd ed. Philadelphia, PA: Saunders; 2010. [Google Scholar]
- 137.Burry RW. Immunocytochemistry: a practical guide for biomedical research. New York: Springer; 2010. [Google Scholar]
- 138.Farm AJ, Stead RH. Fixation and processing. In: Kumar GL, Rudbeck L, editors. Education guide: immunohistochemical staining methods. 5th ed. Carpinteria, CA: DAKO North America; 2009. pp. 29–35. [Google Scholar]
- 139.Kim YH, Kim VE. Early migration of uncemented porous coated anatomic femoral component related to aseptic loosening. Clin Orthop Relat Res. 1993:146–155. [PubMed] [Google Scholar]
- 140.Lukas Z, Feit J. Immunohistochemical methods in biology and surgical pathology. Brno: Masaryk University Medical Faculty; 1997. [Google Scholar]
- 141.Cregger M, Berger AJ, Rimm DL. Immunohistochemistry and quantitative analysis of protein expression. Arch Pathol Lab Med. 2006;130:1026–1030. doi: 10.5858/2006-130-1026-IAQAOP. [DOI] [PubMed] [Google Scholar]
- 142.Matos LL, Stabenow E, Tavares MR, Ferraz AR, Capelozzi VL, Pinhal MA. Immunohistochemistry quantification by a digital computer-assisted method compared to semiquantitative analysis. Clinics. 2006;61:417–424. doi: 10.1590/s1807-59322006000500008. [DOI] [PubMed] [Google Scholar]
- 143.Rizzardi AE, Johnson AT, Vogel RI, Pambuccian SE, Henriksen J, Skubitz AP, et al. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn Pathol. 2012;7:42. doi: 10.1186/1746-1596-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]