Abstract
Allogeneic hematopoietic cell transplantation (allo HCT) is the only curative therapy for the myelodysplastic syndromes (MDS) and myeloproliferative neoplasms (MPN), but treatment toxicity has been a barrier to its more widespread use. The nonmyeloablative regimen of total lymphoid irradiation (TLI) and antithymocyte globulin (ATG) permits the establishment of donor hematopoiesis necessary for the graft-versus-malignancy effect and is protective against acute graft-versus-host disease (aGVHD), but it has minimal direct cytotoxicity against myeloid diseases. We explored the use of TLI-ATG conditioning to treat 61 patients with allo HCT for MDS (n = 32), therapy-related myeloid neoplasms (n = 15), MPN (n = 9), and chronic myelomonocytic leukemia (n = 5). The median age of all patients was 63 years (range, 50 to 73). The cumulative incidence of aGVHD grades II to IV was 14% (95% confidence interval [CI], 4% to 23%) and for grades III to IV, 4% (95% CI, 0 to 9%), and it did not differ between patients who received allografts from related or unrelated donors. The cumulative incidence of nonrelapse mortality (NRM) at 100 days, 12 months, and 36 months was 0%, 7%, and 11%. Overall survival and progression-free survival were 41% (95% CI, 29% to 53%) and 35% (95% CI, 23% to 48%), respectively. The safety and tolerability of TLI-ATG, as exemplified by its low NRM, provides a foundation for further risk-adapted or prophylactic interventions to prevent disease progression.
Keywords: Myelodysplastic syndrome, Myeloproliferative neoplasm, Nonmyeloablative conditioning
Introduction
Allogeneic hematopoietic cell transplantation (allo HCT) is the only curative therapy for the myelodysplastic syndromes (MDS) and the myeloproliferative neoplasms (MPN). MDS and, to a lesser extent MPN, affects older patients who are often deemed ineligible for myeloablative preparative regimens because of the regimen-related toxicity. Increasingly, MDS and MPN patients are offered reduced-intensity conditioning (RIC) with the goal of shifting the burden of disease control from the preparative regimen to the donor immune system. Nonetheless, allo HCT carries intrinsic risk of therapy-related mortality that limits its more widespread use.
The optimal timing of allo HCT in MDS and MPN is difficult to discern, as the risk of disease progression is highly variable. Recently, therapies for MDS, such as DNA methyltransferase inhibitors (DNMTi), have been shown to alter the natural course of the disease in 40% to 50% of high-risk patients, yet responses are often incomplete and rarely durable [1,2]. Prognostic scoring systems have been valuable for the determination of early versus deferred allo HCT. In the absence of randomized controlled trials, decision analytic tools have suggested that for patients with an HLA-matched sibling donor, early myeloablative allo HCT prolongs life expectancy for eligible patients with International Prognostic Scoring System (IPSS) intermediate 2– (Int-2) and high-risk disease, but it shortens life expectancy for patients with lowor intermediate 1– (Int-1) risk disease [3]. Similar statistical techniques were applied to analyze patients older than 60 years [4]. Again, early transplantation maximized life expectancy only for patients with IPSS Int-2 and high-risk disease. However, the generalizability of these conclusions is limited by the variety of conditioning regimens available to older patients. Treatment-related mortality among MDS patients receiving RIC-based transplants has historically ranged from 25% to 40% [5-7].
We and, more recently, others have reported the outcomes of patients treated with a nonmyeloablative regimen of total lymphoid irradiation (TLI) and antithymocyte globulin (ATG) for the treatment of myeloid and lymphoid malignancies [8-11]. This regimen has minimal, if any, direct cytotoxic effects towards myeloid neoplasms. Rather, it is primarily immune modulatory, resulting in the enrichment of regulatory lymphocytes in the recipient [8,12]. As a consequence, allo HCT after TLI-ATG depends on an effective graft-versus-malignancy (GVM) response for control of disease. The objectives of the current study were to determine the tolerability, nonrelapse mortality (NRM), and relapse-free survival (RFS) for patients with MDS and MPN receiving allogeneic HCT after TLI-ATG conditioning.
Materials and Methods
Patients and Donors
The study group included 61 consecutive patients with MDS/MPN treated between August 1, 2004 and December 31, 2011 on a trial of nonmyeloablative conditioning with allogeneic transplantation. All patients provided written informed consent and were treated at Stanford University Medical Center. The protocol and informed consent documents were approved by the institutional review board of Stanford University in accordance with the Declaration of Helsinki (http://clinicaltrials.gov NCT00185796). The censoring date was the last clinic visit before July 1, 2012, allowing for a minimal follow-up of 18 months.
Patients from 50 to 75 years old were eligible. Exclusion criteria were creatinine clearance of <60 mL/min or serum creatinine > 2 mg/dL, left ventricular ejection fraction < 30% or uncontrolled congestive heart failure, diffusing capacity of the lungs for carbon monoxide < 40% predicted, liver dysfunction as indicated by total bilirubin > 3 mg/dL, or aspartate aminotransferase or alanine aminotransferase > 4 times the upper limits of normal, Karnofsky performance status < 60%, active central nervous system involvement with disease, and treatment-refractory fungal or bacterial infections.
Eligible diagnoses included MDS (including the FAB subtypes refractory anemia, refractory anemia with excess blasts, refractory anemia with ring sideroblasts, or chronic myelomonocytic leukemia [CMML]), therapy-related myeloid neoplasms (t-MN), or advanced MPN excluding Philadelphia chromosome–positive chronic myelogenous leukemia. Patients with polycythemia vera or essential thrombocythemia were eligible if they had progressed to a fibrotic stage or experienced persistent complications (eg, hemorrhage or thrombosis) despite conventional therapy. Patients with marrow blast percentage of greater than or equal to 10% required cytoreduction before the preparative regimen to achieve a marrow blast percentage of less than 10%. Patients with prior acute myelogenous leukemia (AML) required cytoreduction to achieve a marrow blast percentage of less than 5%. Cytoreductive regimens were chosen and administered by referring physicians.
Patients with de novo MDS were assigned to a revised IPSS (IPSS-R) risk group from laboratory features at diagnosis and within 30 days before allo HCT [13]. Patients with t-MN, CMML, and MPN were not assigned IPSS-R scores. Status at transplantation was assessed by bone marrow evaluation with conventional metaphase cytogenetics. The hematopoietic cell transplantation comorbidity index (HCT-CI) score was assigned according to clinical features and history at the time of allo HCT [14]. All donor-recipient pairs were HLA matched or single antigen/allele mismatched after high resolution typing for HLA-A, B, C, DRB1, and DQB1.
Treatment and Evaluations
The preparative regimen consisted of TLI with a cumulative dose of 12 Gy in 10 fractions as previously described [10]. Rabbit ATG (Thymoglobulin, Genzyme or Sanofi) was administered day -11 to day -7 in doses of 1.5 mg/kg for a total dose of 7.5 mg/kg. Unmanipulated granulocyte-colony stimulating factor–mobilized peripheral blood mononuclear cells were infused on day 0. GVHD prophylaxis consisted of cyclosporine A (CsA) and mycophenolate mofetil (MMF). CsA began day -3 at a dose of 5 mg/kg orally twice daily with a target trough level of 350 to 450 ng/mL. Oral MMF administration began on day 0 after HCT at a dose of 15 mg/kg. Patients who received related donor grafts received MMF twice daily and those who received unrelated donor grafts received MMF 3 times daily.
Immune-suppressant medication was tapered in the absence of GVHD as follows: MMF was discontinued for patients with matched related donors on day 28. For patients with unrelated donors, MMF was tapered by approximately 10% weekly beginning on day 40 with the taper completing by day 96. The CsA taper commenced for patients with matched related donors on day 56 and after completion of the MMF taper for patients with unrelated donors.
Anti-infective prophylaxis and treatment were administered per institutional standards. Patients at risk for cytomegalovirus (CMV) reactivation were screened with quantitative PCR of peripheral blood weekly beginning on day -11 until day 100. Pre-emptive therapy for CMV viremia was instituted per institutional guidelines. All patients were screened for Epstein-Barr virus viremia by quantitative PCR every other week.
Chimerism, Lymphocyte Subset Analysis, and Response Assessments
Donor cell chimerism analysis was determined by quantitative PCR of polymorphic short tandem repeats from unfractionated peripheral blood leukocytes and immunomagnetically purified CD3+, CD15+, CD19+, and CD56+ cells [15]. These tests were performed monthly for the first 3 months after allo HCT and then annually or more frequently at the discretion of the treating physician. Engraftment was characterized per standard criteria [16]. Specifically, primary graft failure was defined as failure to achieve greater than 5% donor CD3+ cells at any time point. Secondary graft failure was defined as donor CD3+ cells less than 5% after prior chimerism of greater than 5%. Mixed chimerism was defined as between 5% and 95% peripheral blood donor CD3+ cells. Full chimerism was defined as greater than 95% donor CD3+. Flow cytometry of peripheral blood mononuclear cells was performed monthly for the first 3 months after allo HCT, as previously described [10].
Bone marrow aspirates and biopsies were obtained at 3, 6, and 12 months and yearly until year 5 after transplantation. Disease relapse was defined as recurrence or progression of morphologic abnormalities and/or by recurrence or persistence of a previously identified cytogenetic abnormality. All occurrences of acute or chronic GVHD (cGVHD) were graded according to established criteria [17,18]. Corticosteroids were added for patients with grade II to IV aGVHD at the discretion of the treating physician. Treatment of relapse or progression was administered by the referring hematologist with or without donor lymphocyte infusions (DLI). Relapse, but not isolated low chimerism, was the only indication for DLI.
Statistical Analysis
Overall survival (OS) was defined as time from transplantation to death from any cause. RFS was defined as time from transplantation to relapse, progression, or death from nonrelapse causes. Living patients without progression/relapse were censored at the date of last contact. Univariate survival probabilities were generated by the product-limit method [19]. The cumulative incidence estimates for aGVHD, cGVHD, and NRM were calculated with relapse and graft failure as competing risks [20]. The log-rank test was used to compare univariate survival probabilities. Univariate analysis of risk factors for all outcomes was performed using Cox regression. Approximate 95% confidence intervals (CIs) are provided for point estimates of OS, RFS, relapse incidence, GVHD incidence, and NRM. The relationship between donor-host chimerism and relapse probability was analyzed by generation of receiver operating characteristic (ROC) curves.
Results
Patient Characteristics
Pretransplantation characteristics for all patients are indicated in Table 1. To summarize, 61 patients underwent allo HCT from August 2004 through December 2011, inclusive. The median age was 63 years and 26% had an HCT-CI score of 3 or greater. De novo MDS was the most frequent diagnosis (52%), followed by therapy-related MDS (24%), MPN (15%), and CMML (8%). Thirty-five percent of patients had been diagnosed with AML at some point in their disease course before allo HCT. All but 9% of patients received chemotherapy before allo HCT, including AML-type induction chemotherapy, DNMTi such as 5-azacytidine or decitabine, or immunomodulatory agents, such as lenalidomide or thalidomide. IPSS-R scores at diagnosis were assessed for the 32 patients with de novo MDS. Fourteen had high or very high risk, 7 had intermediate, and 11 were good or very good risk. Of this last group of good- and very good–risk patients, before transplantation, 3 had progressed to AML, 1 to refractory anemia with excess blasts-2, and 5 were transfusion dependent. At the time of transplantation, 14% of patients had 5% or more bone marrow blasts, and 38% had persistent cytogenetic abnormalities. According to the IPSS-R criteria, 4 patients had features consistent with high- and very high–risk disease, 10 met criteria for intermediate-risk disease, and the remaining 18 were characterized as having good- or very good–risk status. Donor types were matched related (41%), matched unrelated (44%), and mismatched unrelated (15%). All patients received granulocyte-colony stimulating factor–mobilized peripheral blood grafts. The median CD34+ cell dose was 6.1 ×106/kg (range,.6 to 16.5) and the median CD3+ cell dose was 3.2 × 108/kg (range, 1.4 to 6.3).
Table 1. Patient Characteristics.
| Characteristic | Value (range) |
|---|---|
| Male | 39 (64) |
| Female | 22 (36) |
| Age at transplantation, median, yr | 63 (50-73) |
| Time from diagnosis to HCT, median, mo | 11 (3-160) |
| HCT-comorbidity index | |
| 0 | 26 (43) |
| 1-2 | 19 (31) |
| ≥3 | 16 (26) |
| Diagnosis | |
| De novo MDS | 32 (52) |
| t-MN | 15 (24) |
| MPN | 9 (15) |
| CMML | 5 (8) |
| Ever AML | |
| Yes | 18 (35) |
| Prior therapy, n (%) | |
| Cytotoxic only | 16 (26) |
| DNMTi and/or IMID | 28 (46) |
| Cytotoxic +DNMTI/IMID | 8 (13) |
| None/supportive care | 9 (15) |
| Blast percentage at transplantation | |
| ≤5 | 54 (86) |
| >5 | 7 (14) |
| Abnormal BM cytogenetics at transplantation | |
| Yes | 23 (38) |
| No | 32 (52) |
| Unknown/indeterminate | 6 (10) |
| Donor | |
| Matched related | 25 (41) |
| Matched unrelated | 27 (44) |
| Mismatched unrelated | 9 (15) |
MDS indicates myelodysplasia; t-MN, therapy-related myeloid neoplasm; MPN, myeloproliferative neoplasm; CMML, chronic myelomonocytic leukemia; DNMTI, DNA methyltransferase inhibitor; IMID, immunomodulatory drug; BM, bone marrow; AML, acute myelogenous leukemia; HCT, hematopoietic cell transplantation.
Data presented are n (%) unless otherwise indicated.
Tolerability of TLI-ATG
The preparative regimen of TLI-ATG included a planned 5-day hospitalization for administration of ATG but was otherwise designed for the outpatient setting. Fifty-nine of 61 patients (97%) received donor cell infusions as outpatients. Twenty-nine (48%) patients were admitted to the hospital before day 100 after transplantation and the median duration of hospitalization was 5 days (range,1 to 114.) The most frequent indication for admission was fever (n = 12), including 7 patients with febrile neutropenia. Four patients were readmitted for management of nausea or abdominal pain. Fifty patients (82%) were at risk for CMV and 20 patients reactivated CMV within the first 100 days, with a median time to reactivation of 45 days (range, 17 to 88). Two patients developed CMV disease (enteritis and pneumonitis) and both cases were treated to resolution with antiviral medications. Seven patients demonstrated asymptomatic Epstein-Barr virus reactivation and none required therapy.
Engraftment
All patients underwent evaluation of donor chimerism. Three patients (5%) had primary graft failure, as indicated by failure to achieve donor CD3 chimerism of greater than 5%. Each of these patients had marrow hypercellularity at the initiation of the conditioning regimen, and 1 had 8% myeloid blasts. Thirty patients achieved full CD3 chimerism with a median time of 90 days (range, 28 to 730). Thirty-seven patients achieved full CD15 chimerism with a median time of 56 days (range, 28 to 730). Five patients, 2 with MDS, and 3 with MPN, experienced secondary graft failure and 4 have been diagnosed with overt relapse. One patient with secondary graft rejection remains alive without evidence of disease. Secondary graft rejection was observed as early as 2 months and as late as 1 year after transplantation. T cell reconstitution was evaluable at day 28 in 49 of 61 patients. The median CD3 count was 199/mm3 (range,12 to 1268). The median donor CD3 count was 141/mm3 (range, 2 to 986) and recipient, 43/mm3 (range, 1 to 560).
Survival
Thirty-six month OS and RFS were 41% (95% CI, 29% to 53%) and 35% (95% CI, 23% to 48%), respectively (Figure 1A). Donor source did not appear to significantly impact overall outcomes (Figure 1B). NRM at 100 days, 12 months, and 36 months was 0, 7, and 11%, respectively (Figure 1C). Causes of NRM were cGVHD (n = 3), infections (n = 2), and liver failure not attributable to GVHD (n = 1). The 36-month cumulative incidence of relapse was 61% (95% CI, 47% to 74%). The median time to relapse was 5.6 months (range,.4 to 36.5). Of the 38 patients who relapsed after allo HCT, 25 received subsequent therapy, including 2 patients who received unfractionated DLI and 1 who received allogeneic cytokine-induced killer cells [21]. Five patients with relapse remain alive, with a median time from relapse to censoring date of 26 months (range, 16 to 31).
Figure 1.
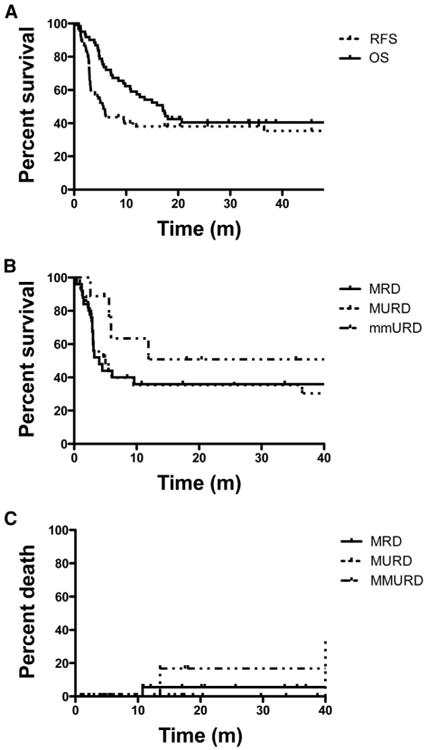
Kaplan Meier estimates of (A) overall survival (OS) and relapse-free survival (RFS) for all patients, (B) RFS and (C) nonrelapse mortality (NRM) stratified according to donor.
GVHD
The cumulative incidence of aGVHD (grade II to IV) was 14% (95% CI, 4% to 23%) and grade III and IV was 4% (95% CI, 0 to 9%). The median time to the onset of aGVHD was 51 days. There was no difference in aGVHD according to donor type (P =.25). No patients died from aGVHD. The cumulative incidence of cGVHD was 33% (95% CI, 20% to 47%) with median time to onset of 199 days (range, 112 to 475). There was no significant difference in the incidence of cGVHD according to donor status (P =.52). Of the patients with cGVHD, 71% had no prior history of aGVHD.
Univariate Analysis of Factors for Association with Outcome
The 36-month RFS was not significantly different for patients with MDS, MPN, t-MN, or CMML (data not shown). Univariate analysis of pretransplantation characteristics failed to reveal a significant association between relapse and blast percentage in the bone marrow >5% at transplantation (P =.67) or the presence of abnormal cytogenetics at transplantation (P =.54). There was no difference in OS, RFS, relapse, or treatment-related mortality for patients with related versus unrelated donors. IPSS-R was calculated for features present at diagnosis and at transplantation among the patients with de novo MDS. Patients with good and very good IPSS-R scores at diagnosis had a 36-month cumulative incidence of relapse of 43%, which was not significantly different from that of patients with intermediate-risk disease (Figure 2A). Those patients with high and very high–risk disease at diagnosis had a relapse risk of 78% (P =.04).
Figure 2.
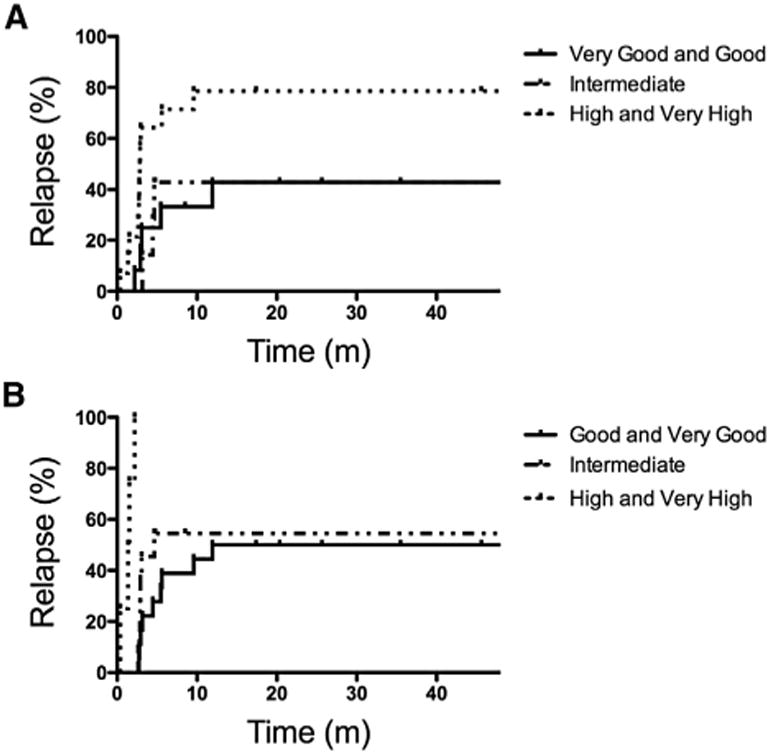
Relapse proportion according to IPSS-R (A) at diagnosis or (B) at the time of allo HCT.
Reassessment at the time of allogeneic transplantation was also performed and showed that patients with high and very high–risk disease all succumbed to early relapse (Figure 2B). Outcomes among patients with HCT-CI scores of ≥3 tended to have worse outcomes, although this did not reach statistical significance (data not shown).
Chimerism and Relapse Risk
To analyze early determinants of relapse, we performed ROC analysis of donor chimerism at days 28 and 56 after transplantation. CD15+ cell chimerism (a continuous random variable) at day 28 provided the best discrimination of no relapse (true positives) versus relapse (false positive). The median day 28 CD15+ cell chimerism was 72% (range, 0 to 99%) versus 90% (range, 29% to 100%) among patients who did and did not relapse, respectively (Figure 3A). Establishing a cutoff of 90% provided 77% sensitivity and 61% specificity for relapse. Patients who achieved donor CD15+ of ≥90% at day 28 had a 2-fold relative risk (95% CI, 1.05 to 3.94) of achieving full CD3+ cell chimerism. Patients with day 28 CD15+cell chimerism of ≥90% had significantly lower risk of relapse (hazard ratio,.38, 95% CI,.20 to.72) (Figure 3B). By contrast, the median day 28 CD3+ cell chimerism was 70% (range, 0 to 98%) versus 80% (range, 25% to 98%) among patients who did and did not relapse (Figure 3C). Patients with day 28 CD3+ chimerism of ≥90% also had reduced risk of relapse (hazard ratio,.49, 95% CI,.25 to.98) (Figure 3D).
Figure 3.
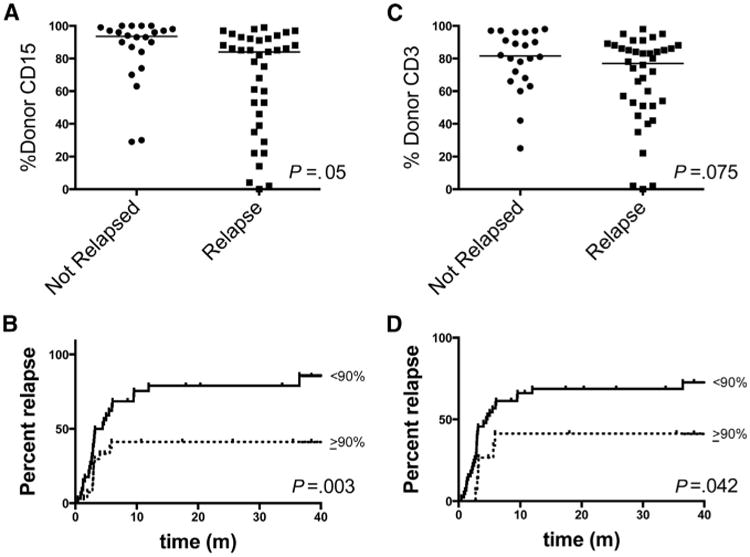
(A,C) Day 28 CD15+ or CD3+ cell donor chimerism among patients according to relapse. (B,D) Cumulative incidence of relapse among patients stratified according to day 28 CD15+ or CD3+ cell chimerism.
Discussion
This study demonstrates that for patients with MDS and MPN, nonmyeloablative conditioning with TLI-ATG is associated with a low incidence of transplantation-related complications and mortality. Our study included only patients 50 years old or older, a cohort for whom, until recently, had few therapeutic options other than supportive care. Thirty-five percent of patients had prior evolution to AML, and 26% had an HCT-CI of 3 or greater. Most patients did not require hospital admission after allo HCT. NRM at 100 days was 0%. The estimated 36-month RFS and OS were 35% and 41%, comparable to that reported by for myeloablative or RIC conditioning using other more aggressive regimens [22].
The low incidence of NRM reported here (8%) is due in part to the infrequent occurrence of aGVHD (II to IV) (14%) after TLI-ATG. In most studies of MDS and MPN, for patients treated on RIC regimens, the risk of aGVHD is comparable to that observed for patients treated with myeloablative regimens. Laport et al. reported the outcome of a multicenter trial with a preparative regimen of low-dose total body irradiation with or without fludarabine [23]. The rate of aGVHD (II to IV) was 38%, 3-year NRM was 32%, and OS was 27%. Nakamura et al. reported that a RIC regimen of fludarabine and melphalan, followed by GVHD prophylaxis with sirolimus and tacrolimus, was associated with a 35% rate of aGVHD (grades II to IV), and approximately 70% risk of cGVHD, although NRM was only 10.5% at 2 years. At 2 years, only 21% of patients experienced relapse, resulting in OS of 75% [24]. Potter et al. described the single-center experience fludarabine, busulfan, and alemtuzumab conditioning for patients with MDS. AGVHD (II to IV) and cGVHD were 20% and 19% with 5-year NRM of 26%, relapse of 51%, and OS of 44% [25].
Registry data provides comparable rates of aGVHD to the Laport and Nakamura studies. In a retrospective analysis of the Center for International Blood and Marrow Transplant Research data of MDS patients receiving various RIC regimens for transplantation, the incidence of aGVHD grades II to IV was 31%, NRM at 1 year was 29% to 35%, and OS was 34% [5]. In a retrospective analysis of the European Society for Blood and Marrow Transplantation registry, NRM was 32% and OS was 32% after RIC conditioning, with 40% of patients experiencing relapse and 32% with NRM. By comparison, patients undergoing myeloablative conditioning had NRM of 44% and OS 30%. Luger et al., in a review of the Center for International Blood and Marrow Transplant Research registry, reported OS of 34% for patients receiving myeloablative conditioning or RIC conditioning, but 26% for those receiving nonmyeloablative conditioning [26-28]. Therefore, the results achieved with TLI-ATG are comparable in overall outcome; however, they are associated with lower NRM and acute GVHD.
ATG has the potential to deplete aGVHD-causing donor T cells, and randomized trial data have demonstrated that ATG decreases aGVHD after myeloablative conditioning [29,30]. No randomized trial of ATG after RIC has been reported. Analysis of registry data has failed to show decreased incidence of aGVHD with pretransplantation ATG when compared with T cell–replete regimens [31]. Because RIC regimens rely on the GVM effect mediated primarily by donor T cells, the potential impact of ATG on relapse remains an area of great interest. We do not believe that ATG, administered on days -11 to -8 in this regimen, has a direct impact on donor T cells, because active ATG, ie, capable of binding CD3+ T cells, is nearly undetectable in serum on the day of transplantation [10].
The low incidence of aGVHD reported here may be attributable to the enrichment of regulatory lymphocytes, as has been shown in preclinical murine models [32]. It is also possible that the low incidence of aGVHD is attributable to delayed achievement of full donor CD3+ chimerism, as it is generally believed that full donor CD3+ chimerism is necessary for the development of GVHD [33]. Interestingly, achievement of full donor chimerism among patients with MDS and MPN receiving TLI-ATG is not as prevalent as those with AML and non-Hodgkin lymphoma [8], yet the incidences of aGVHD among these different populations are comparable. It has been observed previously that achievement of full donor chimerism is less frequent among patients with MDS compared with those with other hematologic malignancies [34]. It is unclear whether this is due to the intensity, or lack thereof, of previous myelosuppressive or immunosuppressive chemotherapy, or whether it reflects a property of the underlying disease and the bone marrow stroma.
Despite the low incidence of aGVHD, cGVHD was observed in one third of patients, most of whom had no prior history of aGVHD. Although it is widely accepted that a history of aGVHD is the strongest predictor for the development of cGVHD [35], there are precedents of alternative GVHD prophylaxis resulting in much lower rates of aGVHD than cGVHD [36,37].
For both myeloablative and RIC, decision analysis has suggested that the benefit of frontline allo HCT outweighed the risk of treatment-related toxicity for those with Int-2 or high-risk disease. In contrast, patients in IPSS low or Int-1 risk groups may have shortened survival when treated with up front allo HCT [3]. Among low and Int-1 patients, allo HCT was best deferred until disease progression. Nonetheless, the overall tolerability of the TLI-ATG regimen and the low NRM suggest that allo HCT may afford survival benefit compared with conventional therapies when offered to patients with lower risk disease earlier in their treatment course.
Nonetheless, the majority of patients in the current study either presented with high-risk disease or had evidence of disease progression before transplantation, including 35% who had progressed to AML. Patients with low-risk disease by IPSS or IPSS-R criteria and who had not progressed were mostly red blood cell transfusion dependent, a high-risk feature according to the World Health Organization Prognostic Scoring System [38]. The vast majority had received therapy with DNMTi, immunomodulatory, or cytotoxic agents, with most having been effectively “downstaged” by prior therapy so that few patients had more than 5% marrow blasts at the time of transplantation. Despite favorable responses to prior therapy, it was anticipated that without additional therapy, their disease would soon progress. For instance, of the 40% of patients who may respond to azacytidine, the median duration of response is only 8 to 10 months [39] and the median survival for high- and low-risk patients after azacytidine failure is only 5.6 and 17 months, respectively [40]. Likewise, for patients receiving decitabine, the median duration of response is 9 months. Survival after decitabine treatment failure is approximately 4 months [41]. There is no standard treatment after DNMTi treatment failure. Among patients with azacytidine treatment failure, allo HCT afforded median OS of 19 months, significantly longer than that seen with intensive chemotherapy, low-dose chemotherapy, or best supportive care [42]. Of course, the ability to proceed to allo HCT is limited to patients with an adequate performance status, available donors, and some measure of disease control.
As with other RIC regimens for patients with MDS/MPN, disease relapse remains the most vexing problem after TLI-ATG–based conditioning and suggests that post-transplantation prophylaxis or risk-adapted therapy are warranted. We sought to understand the impact of disease characteristics at diagnosis and disease burden at transplantation on outcome. Univariate analysis of factors, such as cytogenetic abnormalities or blast percentage, failed to reveal a significant association with relapse or survival, but the small number of patients with these abnormalities may not have provided adequate statistical power to detect an association. We found that a composite measure, the IPSS-R, as assessed immediately before allo HCT, was associated with relapse. As with the IPSS, the IPSS-R was derived to assign prognosis from features present at diagnosis and is not a dynamic scoring system. It is not to be inferred that the IPSS-R immediately before transplantation confers equivalent prognostic information as the IPSS-R as assessed at diagnosis, but, rather, it is a marker of disease burden. Of note, among patients with IPSS-R high- or very high–risk scores at the time of diagnosis, approximately 20% experienced long-term disease-free survival. However, all patients with high- or very high–risk scores at the time of allo HCT had early relapse or death. We conclude from this observation that patients with a high IPSS-R at transplantation require either additional therapy before TLI-ATG or more aggressive transplantation conditioning regimens.
To further identify patients with a high risk of relapse, we studied earlier chimerism. Using ROC analysis, we found that day 28 CD15+ cell donor–specific chimerism provided the best discrimination of no relapse versus relapse. In our analysis, failure to achieve 90% donor CD15+ chimerism by day 28 was 77% sensitive and 61% specific for relapse. It is generally held that achievement of full donor CD3+ cell chimerism is associated with GVM effects. It is conceivable that persistent recipient CD15+ cells represent residual malignancy, although this is unclear, as immunophenotype of the malignant clone was not always available.
Most of the patients who relapse after allo HCT retained a performance status that allowed for additional post-transplantation therapy, including DLI. Although most patients who relapse ultimately succumbed to their disease, 5 patients demonstrated achieved durable remissions with post-transplantation therapy. The low incidence of early NRM and the overall tolerability of TLI-ATG support the feasibility of post-transplantation intervention for patients with markers of impending relapse.
In summary, our results demonstrate that allo HCT after TLI-ATG conditioning can result in long-term RFS with low risk of aGVHD and NRM for elderly patients with MDS and CMML utilizing either matched related or unrelated donors, primarily because of a low incidence of treatment-related complications. Because of the low incidence of treatment-related complications, allogeneic HCT with TLI-ATG conditioning could be utilized with relative safety earlier in the course of disease and may alter treatment algorithms. Relapse is the predominant cause of treatment failure, especially in patients with more advanced disease. After allo HCT, a risk-adapted approach may be based on early chimerism results.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant P01 CA049605-22.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report
References
- 1.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itzykson R, Thepot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–411. doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]
- 3.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–585. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 4.Koreth J, Pidala J, Perez WS, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol. 2013;31:2662–2670. doi: 10.1200/JCO.2012.46.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClune BL, Weisdorf DJ, Pedersen TL, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott BL, Sandmaier BM, Storer B, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20:128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 7.Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12:1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Kohrt HE, Turnbull BB, Heydari K, et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood. 2009;114:1099–1109. doi: 10.1182/blood-2009-03-211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messina G, Giaccone L, Festuccia M, et al. Multicenter experience using total lymphoid irradiation and antithymocyte globulin as conditioning for allografting in hematological malignancies. Biol Blood Marrow Transplant. 2012;18:1600–1607. doi: 10.1016/j.bbmt.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Lowsky R, Takahashi T, Liu YP, et al. Protective conditioning for acute graft-versus-host disease. New Engl J Med. 2005;353:1321–1331. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 11.Arai S, Sahaf B, Narasimhan B, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119:6145–6154. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pillai AB, George TI, Dutt S, Strober S. Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood. 2009;113:4458–4467. doi: 10.1182/blood-2008-06-165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 15.Millan MT, Shizuru JA, Hoffmann P, et al. Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation. 2002;73:1386–1391. doi: 10.1097/00007890-200205150-00005. [DOI] [PubMed] [Google Scholar]
- 16.Baron F, Sandmaier BM. Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia. 2006;20:1690–1700. doi: 10.1038/sj.leu.2404335. [DOI] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 18.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Laport GG, Sheehan K, Baker J, et al. Adoptive immunotherapy with cytokine-induced killer cells for patients with relapsed hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1679–1687. doi: 10.1016/j.bbmt.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott B, Deeg HJ. Hemopoietic cell transplantation as curative therapy of myelodysplastic syndromes and myeloproliferative disorders. Best Pract Res Clin Haematol. 2006;19:519–533. doi: 10.1016/j.beha.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Laport GG, Sandmaier BM, Storer BE, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14:246–255. doi: 10.1016/j.bbmt.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura R, Rodriguez R, Palmer J, et al. Reduced-intensity conditioning for allogeneic hematopoietic stem cell transplantation with fludarabine and melphalan is associated with durable disease control in myelodysplastic syndrome. Bone Marrow Transplant. 2007;40:843–850. doi: 10.1038/sj.bmt.1705801. [DOI] [PubMed] [Google Scholar]
- 25.Potter VT, Krishnamurthy P, Barber LD, et al. Long-term outcomes of alemtuzumab-based reduced-intensity conditioned hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myelogenous leukemia secondary to myelodysplastic syndrome. Biol Blood Marrow Transplant. 2014;20:111–117. doi: 10.1016/j.bbmt.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Martino R, Iacobelli S, Brand R, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108:836–846. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 27.Luger SM, Ringden O, Zhang MJ, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2011 doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim Z, Brand R, Martino R, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28:405–411. doi: 10.1200/JCO.2009.21.8073. [DOI] [PubMed] [Google Scholar]
- 29.Bacigalupo A, Lamparelli T, Bruzzi P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98:2942–2947. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- 30.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 31.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pillai AB, George TI, Dutt S, et al. Host NKT cells can prevent graft- versus-host disease and permit graft antitumor activity after bone marrow transplantation. J Immunol. 2007;178:6242–6251. doi: 10.4049/jimmunol.178.10.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Childs R, Clave E, Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94:3234–3241. [PubMed] [Google Scholar]
- 34.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104:2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 35.Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109:3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ringden O, Remberger M, Dahllof G, et al. Sirolimus and tacrolimus as immune prophylaxis compared to cyclosporine with or without methotrexate in patients undergoing allogeneic haematopoietic stem cell transplantation for non-malignant disorders. Eur J Haematol. 2011;87:503–509. doi: 10.1111/j.1600-0609.2011.01685.x. [DOI] [PubMed] [Google Scholar]
- 38.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–3510. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 39.Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 40.Prebet T, Thepot S, Gore SD, et al. Outcome of patients with low risk myelodysplasia after azacitidine treatment failure. Haematologica. 2013;98:e18–e19. doi: 10.3324/haematol.2012.071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jabbour E, Garcia-Manero G, Batty N, et al. Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer. 2010;116:3830–3834. doi: 10.1002/cncr.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prebet T, Gore SD, Esterni B, et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J Clin Oncol. 2011;29:3322–3327. doi: 10.1200/JCO.2011.35.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


