Abstract
Objective
To perform a meta-analysis examining the efficacy of phytoestrogens for the relief of menopausal symptoms.
Methods
Medline, Cochrane, EMBASE, and Google Scholar databases were searched until September 30, 2013 using the following key words: vasomotor symptoms, menopausal symptoms, phytoestrogens, isoflavones, coumestrol, soy, red clover. Inclusion criteria were (1) randomized controlled trial (RCT), (2) perimenopausal or postmenopausal women experiencing menopausal symptoms, (3) intervention with an oral phytoestrogen. Outcome measures included Kupperman index (KI) changes, daily hot flush frequency, and the likelihood of side-effects.
Results
Of 543 potentially relevant studies identified, 15 RCTs meeting the inclusion criteria were included. The mean age of the subjects ranged from 49 to 58.3 and 48 to 60.1 years, respectively, in the placebo and phytoestrogen groups. The number of participants ranged from 30 to 252, and the intervention periods ranged from 3 to 12 months. Meta-analysis of the seven studies that reported KI data indicated no significant treatment effect of phytoestrogen as compared to placebo (pooled mean difference = 6.44, p = 0.110). Meta-analysis of the ten studies that reported hot flush data indicated that phytoestrogens result in a significantly greater reduction in hot flush frequency compared to placebo (pooled mean difference = 0.89, p < 0.005). Meta-analysis of the five studies that reported side-effect data showed no significant difference between the two groups (p = 0.175).
Conclusion
Phytoestrogens appear to reduce the frequency of hot flushes in menopausal women, without serious side-effects.
Keywords: CLIMACTERIC, ESTROGEN, HOT FLUSH, ISOFLAVONE, LIGNAN, MENOPAUSE, META-ANALYSIS
INTRODUCTION
Menopause is characterized by a decrease in estrogen, which triggers the uncomfortable symptoms of hot flushes, night sweats, sleep disturbances, and vaginal dryness. Among these menopausal symptoms, hot flushes are reported by many women to be the most bothersome1. The symptoms of menopause as a result of decreasing estrogen levels can significantly affect quality of life2. While hormone replacement therapy (HRT) effectively reduces vasomotor symptoms associated with the decrease of estrogen levels during menopause, results of the Women's Health Initiative (WHI) trial indicated that the benefits of HRT did not outweigh the risks3, as estrogen alone would increase risks of stroke and venous thromboembolism (VTE), and together with progestin could incur additional risks of causing breast cancer and heart attack. As a result, the role of HRT has been limited to treat postmenopausal symptoms at minimal dose and duration, and more efficacious and better tolerated alternatives to decrease menopausal symptoms are still being sought4–6.
Phytoestrogens are plant compounds with estrogen-like properties7. The two major classes of phytoestrogens are isoflavones and lignans; soybeans are rich in isoflavones, and lignans are found in flaxseed, whole grains, legumes, fruits, and vegetables8. The chemical structures of isoflavones and lignans are similar to that of estradiol, and these compounds appear to exert an estrogenic or antiestrogenic effect depending on the circulating estrogen level (i.e. they exert an antiestrogenic effect when the circulating estrogen level is high, but when the estrogen level is low, their effect becomes more estrogenic)7. There is much interest in the use of phytoestrogens to treat menopausal symptoms, in part because vasomotor symptoms are much less frequently experienced by Asian women than by women in America or Europe9, and because the Asian diet being rich in phytoestrogens may be a contributing factor10. Though there has been a large amount of research devoted to determine whether phytoestrogens are well tolerated and effective for the treatment of menopausal symptoms, study results have been inconclusive and no consensus on their utility has been reached7,11,12. Conflicting data may be due to multiple factors including variations in studies' inclusion criteria, types and dosages of consumed phytoestrogens, the lack of appropriate study controls, control for the consumption of phytoestrogens from other sources, and differences in the outcome measures used7,11,12. There is also a need for more well-funded studies on potential long-term adverse effects of phytoestrogens such as heart diseases, breast cancer, VTE and stroke.
The purpose of this study was to perform a meta-analysis of high-quality, randomized, controlled trials (RCTs), to evaluate the effectiveness and short-term side-effects of phytoestrogens in alleviating menopausal symptoms and improving quality of life.
METHODS
Literature search strategy
Medline, Cochrane, EMBASE and Google Scholar databases were searched until September 30, 2013 using combinations of the following key words: vasomotor symptoms, menopausal symptoms, phytoestrogens, isoflavones, coumestrol, soy, red clover. Reference lists of relevant studies were hand-searched.
Inclusion criteria for this meta-analysis were (1) RCT, (2) subjects were perimenopausal or postmenopausal women experiencing menopausal symptoms, (3) the intervention was a phytoestrogen (e.g. isoflavone, genistein, soy extract), and (4) the intervention was in oral form. Studies were excluded from the analysis if (1) there was no placebo control group, (2) there was only an active control group (e.g. hormone replacement therapy), and (3) numerical outcome data were not provided.
Study selection, data extraction and quality assessment
Studies were identified via the search strategy by two independent reviewers. When there was uncertainty regarding eligibility, a third reviewer would be consulted. The following information/data were extracted from studies that met the inclusion criteria: the name of the first author, year of publication, study design, participants' age and gender, diagnosis, number of participants in each treatment group, treatment, length of follow-up, function and quality-of-life outcomes, and side-effects. The Delphi list was used to assess the quality of the included studies13.
Outcome measures
Outcome measures included changes in Kupperman index (KI)14, changes in daily hot flush frequency, and the likelihood of side-effects. Briefly, the Kupperman index covers 11 menopausal symptoms including hot flushes (vasomotor), paresthesia, insomnia, nervousness, melancholia, vertigo, weakness, arthralgia or myalgia, headache, palpitations, and formication. Each symptom is rated on a scale from 0 to 3 for having no, slight, moderate or severe complaints, respectively, with the highest possible total score being 51.
Statistical analysis
The mean differences between treatment groups in changes in KI and daily hot flush frequency were calculated to evaluate the efficacy of phytoestrogens, and the odds ratios (ORs) of side-effects occurring were calculated to evaluate the safety of phytoestrogens. A χ2-based test of homogeneity was performed using Cochran's Q statistic and I2. The percentage of the total variability in effect estimates among trials that are due to heterogeneity rather than chance is expressed by I2. Random-effects models of analysis were used if heterogeneity was detected (Q statistic with p < 0.1 or I2 > 50%). The pooled estimates of mean differences in KI change, mean differences in change of daily hot flush frequency, and the pooled estimates of ORs of side-effects were calculated. Sensitivity analysis was performed based on the leave-one-out approach. The one-sided Egger's test and Funnel plots were performed to evaluate publication bias. The homogeneity test, pooled estimates, and sensitivity analysis were performed by using Comprehensive Meta-Analysis, version 2.0 (Biostat, Englewood, NJ, USA). A value of p < 0.05 indicates statistical significance.
RESULTS
Literature search and study characteristics
A flow diagram of study selection is shown in Figure 1. A total of 543 potentially relevant articles were initially identified and screened, and 488 of them were subsequently excluded. Of the 55 full-text articles reviewed, 40 were excluded and ultimately 15 studies11,15–28 were included in the meta-analysis.
Figure 1.
Flow diagram of study selection
The characteristics and outcomes of the 15 studies are summarized in Tables 1 and 2.
Table 1.
General characteristics of the 15 studies included in the meta-analysis. Age is presented as mean (standard deviation)
| 1st author, year of publication | Number of women | Inclusion criteria | Intervention (compound, daily dose) | Intervention period (months) |
Age (years) |
|
|---|---|---|---|---|---|---|
| Placebo | Phytoestrogen | |||||
| Aso, 201215 | 160 | Postmenopausal | S-(−) equol, 5 mg | 3 | 53.9 (3.4) | 53.2 (3.6) |
| Atkinson, 200416 | 205 | Wolfe P2 or DY mammographic breast patterns | Isoflavones | 12 | 55.2 (4.9) | 55.1 (4.7) |
| Cancellieri, 200717 | 142 | Postmenopausal | Isoflavones, 72 mg | 6 | 54.4 (4.2) | 54.1 (5.2) |
| Del Giorno, 201018 | 120 | Postmenopausal | Trifolium, 40 mg | 12 | 55.14 (4.97) | 55.78 (4.93) |
| Ferrari, 200919 | 180 | Minimum of five moderate-to-severe hot flushes in last 7 days at baseline | Isoflavones, 80 mg | 3 | 54.9 (4.6) | 53.2 (4.3) |
| Han, 200220 | 80 | Menopause of at least 12 months | Isoflavones, 100 mg | 4 | 49 (1.3) | 48 (1.1) |
| Lewis, 200611 | 99 | Natural menopause with last menses 1–8 years before recruitment | Isoflavones, 42 mg | 4 | 52.9 (3.6) | 53.3 (3.1) |
| Nahas, 200721 | 80 | Postmenopausal | Isoflavones, 100 mg | 10 | 56.2 (7.7) | 55.1 (6.0) |
| Penotti, 200322 | 62 | Postmenopausal | Isoflavones, 36 mg | 6 | 52.5 (2.3) | 52.5 (2.5) |
| Petri Nahas, 200423 | 50 | Menopausal | Isoflavones, 60 mg | 6 | 52.9 (5.11) | 53.7 (5.45) |
| Riesco, 201124 | 56 | Postmenopausal | Isoflavones, 25 mg | 6 | 58.3 (5.84) | 60.1 (3.67) |
| Sammartino, 200625 | 80 | Postmenopausal | Isoflavones 60 mg; lignans 100 mg | 3 | 50.6 (1.75) | 50.9 (1.85) |
| Tice, 200326 | 252 | Menopausal; 45–60 years of age; experiencing at least 35 hot flushes per week | Isoflavones, 82 mg (Promensil); Isoflavones, 57 mg (Rimostil) | 3 | 52.3 (3.4) | 52.3 (2.9) |
| Van de Weijer, 200227 | 30 | Postmenopausal | Isoflavones, 82 mg | 3 | 52.5 (5.2) | 54.2 (7.4) |
| Van Patten, 200228 | 157 | Postmenopausal with breast cancer | Isoflavones, 90 mg | 3 | 54.9 (6.5) | 55.5 (6.3) |
Table 2.
Summary of Kupperman index and hot flush frequency reported in the studies included in the meta-analysis. Data are given as mean (standard deviation), except for side-effects which are reported as a number
|
Number of women |
Baseline KI |
KI after intervention |
Baseline hot flush frequency (/day) |
Hot flush frequency after intervention (/day) |
Side-effect number |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Placebo | Phyto | Placebo | Phyto | Placebo | Phyto | Placebo | Phyto | Placebo | Phyto | Placebo | Phyto |
| Aso15 | 60 | 66 | 2.9 (2.1) | 3.2 (2.4) | 1.9 (NA) change from baseline: −1.0 (2.0) |
1.3 (NA) change from baseline: −1.9 (1.8) |
||||||
| Atkinson16 | 103 | 102 | 2.5 (3.0) | 2.1 (2.7) | 1.5 (2.0) change from baseline: −1.0 (1.8) |
1.2 (2.3) change from baseline: −0.8 (2.1) |
||||||
| Cancellieri17 | 65 | 60 | 19.6 (8.5) | 20.8 (9.1) | 12.2 (7.2) | 9.6 (5.7) | ||||||
| Del Giorno18 | 50 | 50 | 25.12 (9.02) | 25.34 (10.17) | 12.01 (9.01) | 11.12 (8.68) | ||||||
| Ferrari19 | 94 | 82 | 23.5 (7.1) | 23.4 (8.3) | 16.1 (7.6) | 17.5 (10.0) | 7.5 (2.8) | 8.0 (3.3) | 5.3 (3.8) | 4.7 (4.2) | 8 | 6 |
| Han20 | 40 | 40 | 40.3 (7.6) | 44.6 (6.3) | 41.6 (7.0) | 24.9 (10.8) | ||||||
| Lewis11 | 33 | 33 | 4.72 (3.04) | 4.08 (2.38) | 3.79 (3.00) | 3.37 (2.55) | ||||||
| Nahas21 | 38 | 38 | 10.1 (4.9) | 9.6 (3.9) | 5.9 (4.3) | 3.1 (2.3) | 4 | 7 | ||||
| Penotti22 | 34 | 28 | 8.6 (2.9) | 9.9 (4.5) | 4.0 (3.9) | 4.6 (3.8) | ||||||
| Petri Nahas23 | 25 | 26 | 20.05 (6.14) | 21.10 (6.11) | 13.5 (5.0) | 12 (6.0) | 6.4 (2.4) | 7 (1.8) | 5.2 (2.8) | 3.0 (2.1) | 2 | 7 |
| Riesco24 | 21 | 19 | 17.0 (9.35) | 13.3 (9.9) | 15.7 (9.0) | 12.3 (9.56) | ||||||
| Sammartino25 | 36 | 39 | 31 (2.5)∗ | 31 (3)∗ | 26 (1.5)∗ | 8 (1)∗ | ||||||
| Tice26 | 85 | 167 | 7.8 (2.35) | 8.3 (3.83) | 5.0 (3.76) | 5.25 (4.17) | 33 | 59 | ||||
| van de Weijer27 | 14 | 16 | 5.75 (5) | 5.43 (2.6) | 6.04 (5.5) | 3.35 (3) | ||||||
| Van Patten28 | 64 | 59 | 7.4 (6.4) | 7.1 (4.3) | 4.9 (3.9) | 5.3 (4.1) | 14 | 28 | ||||
KI, Kupperman index; Phyto, phytoestrogen; NA, not available
∗, Mean and standard deviation were estimated from median and range29
The mean age of the subjects ranged from 49 to 58.3 and 48 to 60.1 years, in the placebo and phytoestrogen groups, respectively. The number of participants in the studies ranged from 30 to 252, and the intervention periods ranged from 3 to 12 months. Seven studies reported the KI at baseline and after intervention17–20,23–25, while ten studies reported hot flush frequency at baseline and after intervention, or change from baseline11,15,16,19,21–23,26–28. As expected, there was no significant difference in baseline KI and hot flush frequency between treatment and placebo groups, since the participants were randomly allocated. Only five studies reported the number of side-effects19,21,23,26,28.
Change in Kupperman index
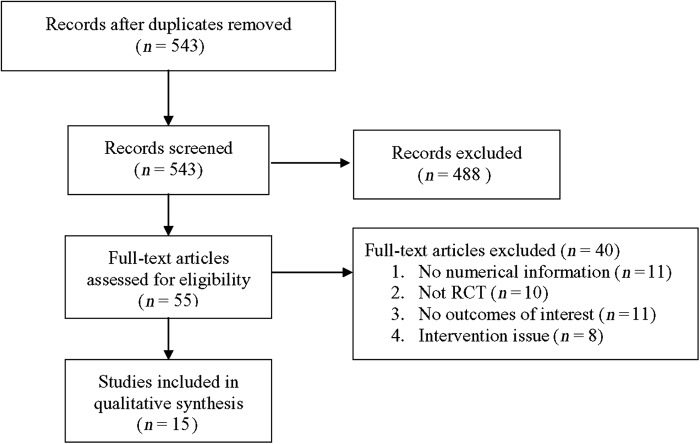
Of the seven studies that reported KI data17–20,23–25, three reported a significant reduction of KI in the phytoestrogen group when compared with the placebo group17,20,25, while the other four18,19,23,24 reported no difference between the groups. Meta-analysis of the seven studies indicated no significant treatment effect of phytoestrogen when compared to placebo (pooled mean difference = 6.4, p = 0.110, Figure 2a). The pooled estimate remained positive and non-significant after sensitivity analysis based on the leave-one-out approach was made, indicating there was no influence of individual studies on the pooled estimate (Figure 2b).
Figure 2.
Meta-analysis (a) with sensitivity evaluation (b) for change in Kupperman index between placebo and phytoestrogen groups (seven studies included). The random-effects approach was used due to significant heterogeneity (Q = 370.03, I2 = 98.38, p < 0.001). CI, confidence interval
Daily hot flush frequency
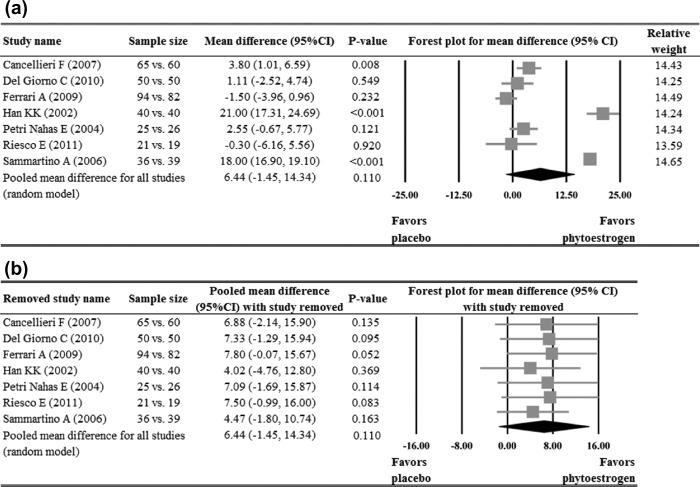
Of the ten studies that reported hot flush frequency data11,15,16,19,21–23,26–28, four reported a significant reduction of hot flush frequency in the phytoestrogen group when compared to the placebo group15,19,21,23, while the other six11,16,22,26–28 reported no significant difference between the groups. Meta-analysis of the ten studies indicated that the phytoestrogen group had a significant reduction in hot flush frequency when compared with the placebo (pooled mean difference = 0.89, p < 0.005, Figure 3a). The pooled estimate remained positive and significant after sensitivity analysis based on the leave-one-out approach was made, indicating there was no influence of individual studies on the pooled estimate (Figure 3b).
Figure 3.
Meta-analysis (a) with sensitivity evaluation (b) for change of hot flush frequency between placebo and phytoestrogen groups (10 studies included). The random-effects approach was used due to significant heterogeneity (Q = 22.75, I2 = 60.43, p = 0.007). CI, confidence interval
Likelihood of side-effects
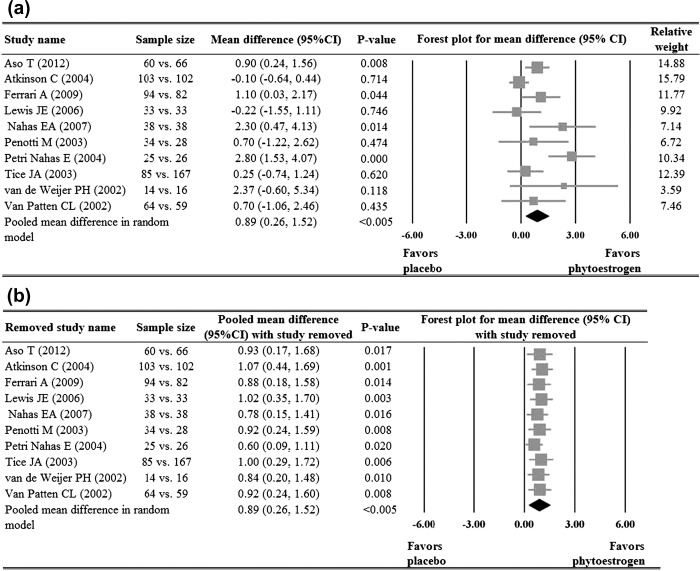
Four19,21,23,26 of the five studies that reported the number of side-effects showed no significant difference in the numbers of side-effects between groups. The study by Van Patten and colleagues28 reported that the subjects in phytoestrogen group were less likely to experience side-effects than those in the placebo group (OR = 0.31, p = 0.003). Meta-analysis of the five studies showed no significant difference in side-effects between the two groups (p = 0.175, Figure 4a). With the exception of the study by Tice and colleagues26, when each of the other studies was removed in turn, the pooled ORs remained < 1 and non-significant. When the study by Tice and colleagues26 was removed, the pooled OR reached statistical significance (p = 0.034) (Figure 4b).
Figure 4.
Meta-analysis (a) with sensitivity evaluation (b) for the likelihood of side-effects between the placebo and phytoestrogen groups (five studies included). The random-effects approach was used due to significant heterogeneity (Q = 10.15, I2 = 60.60, p = 0.038). OR, odds ratio; CI, confidence interval
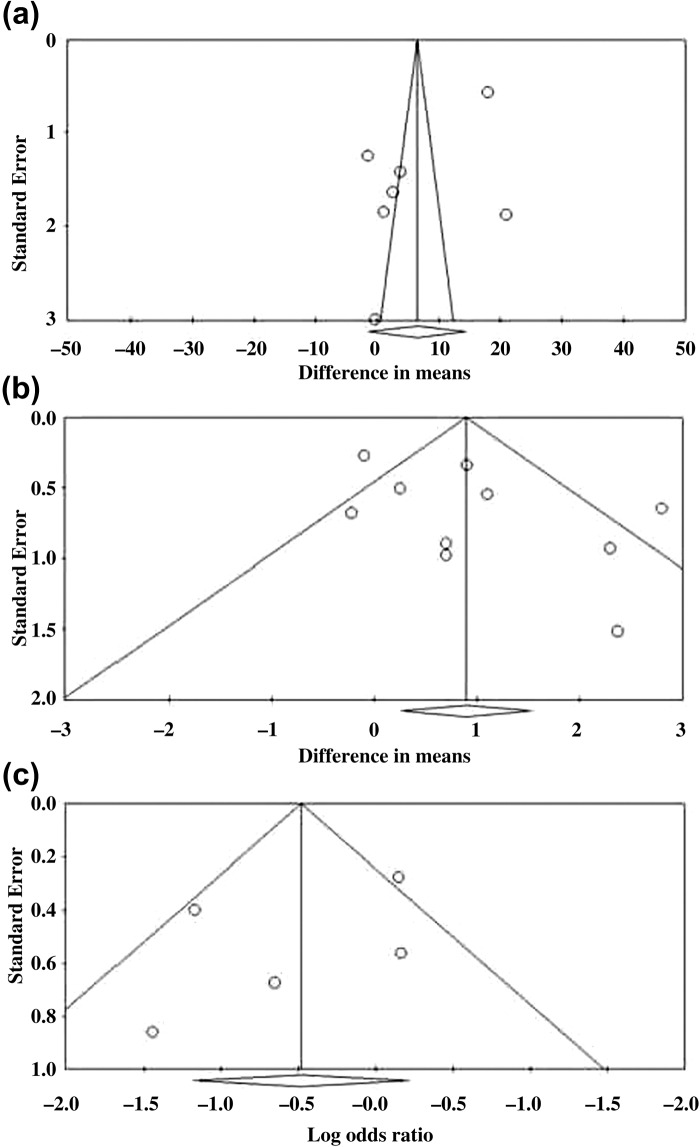
Evaluation of publication bias
Egger's test for KI (seven studies)17–20,23–25 showed the estimated intercept to be −10.01, with a one-tailed p value = 0.043, which indicated a significant asymmetry in the funnel plot (Figure 5a). Egger's test for hot flush frequency (ten studies)11,15,16,19,21–23,26–28 showed the estimated intercept to be 2.99, with a one-tailed p value = 0.036, which indicated a significant asymmetry in the funnel plot (Figure 5b). Egger's test for side-effects (five studies)19,21,23,26,28 showed the estimated intercept to be −1.92 with one-tailed p value of 0.185 (non-significant) which indicated no significant asymmetry in the funnel plot (Figure 5c).
Figure 5.
Evaluation of publication bias for (a) Kupperman index, (b) hot flush frequency, and (c) side-effects
Quality assessment
Results of the analysis of quality assessment are shown in Figure 6.
Figure 6.
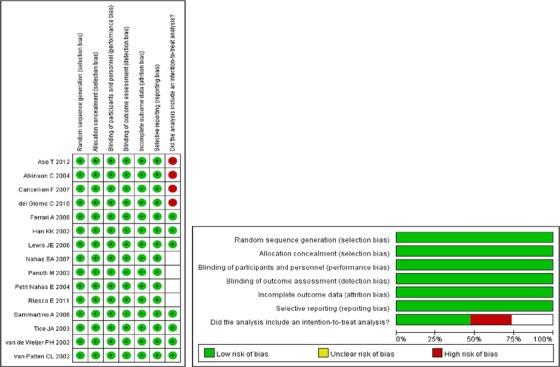
Quality assessment
DISCUSSION
Phytoestrogens are commonly used for the relief of menopausal symptoms, but studies have provided conflicting results of their efficacy. The results of this meta-analysis including 15 high-quality RCTs showed that, although the use of phytoestrogens did not result in a change of KI when compared to the use of placebo, they did significantly reduce the frequency of hot flushes as compared with the placebo. In addition, there was no difference in the occurrence of side-effects between patients who received phytoestrogens and those who received placebo.
There is a large body of research devoted to determine the efficacy of phytoestrogens in treating menopausal symptoms, and a search of PubMed using the terms “phytoestrogen, menopause” produces well over 800 results. However, results of many studies are conflicting and there is still a lack of consensus on their efficacy7,12,30. Some of the reasons for these conflicting data include variations in the study design, variable types and dosage of consumed phytoestrogen, lack of appropriate study controls, a lack of controls for the consumption of phytoestrogens from other sources, and differences in the outcome measures used7,11,12. Although many studies have shown phytoestrogens can reduce the vasomotor symptoms of menopause15,17,19–23,25,27, many others have not11,16,18,22,24,26,28. Two recent reviews and meta-analyses have found little conclusive evidence on the effectiveness of phytoestrogens for treating vasomotor symptoms associated with menopause12,30. In 2009, Jacobs and colleagues30 performed a systematic review of the literature including 17 randomized and placebo-controlled trials that investigated soy isoflavones and reported that the interventions and outcome measures in those studies were so highly heterogeneous that a meta-analysis could not be made. Their systematic review decided that there were qualitative deficiencies in the studies and a consistent reduction in hot flushes was not present. A more recent (2013) Cochrane review of the literature of phytoestrogens for menopausal vasomotor symptoms had included 43 RCTs with a total of 4364 participants, but found that very few studies actually provided data suitable for meta-analysis, so they too were unable to reach a conclusion on the efficacy of phytoestrogens12.
A recent review of five meta-analyses and one review investigating the impact of phytoestrogens on menopausal symptoms concluded that isoflavones did not relieve menopausal vasomotor symptoms31. Our meta-analysis indicated that phytoestrogens did not result in a significant decrease of the KI, but did reduce the frequency of hot flushes compared with placebo. Hot flushes occur in up to 74% of postmenopausal women and can have a negative impact on quality of life; they can still be experienced by some women in their seventies32. The Kupperman index represents more comprehensive grouping of 11 menopausal symptoms which include not only hot flushes (vasomotor), but also paresthesia, insomnia, nervousness, melancholia, vertigo, weakness, arthralgia/myalgia, headache, palpitations, and formication. The lack of significant reduction in KI found in our analysis does cast doubt on the utility of phytoestrogens in alleviating menopausal symptoms other than hot flushes. It is also important to note the high heterogeneity among the included studies in this meta-analysis. There were differences in participant numbers, ages, doses used and outcomes which were recorded. Only seven studies reported KI data, while five studies recorded side-effects and ten studies reported frequency of hot flushes. This wide heterogeneity makes interpretation of data challenging.
A review by Bolanos and colleagues33 in 2010 specifically examined soy isoflavones versus placebo in the treatment of menopausal vasomotor symptoms and found that, although there was a tendency to favor the effectiveness of soy isoflavones, the heterogeneity of studies was high. In the aforementioned Cochrane review12, three placebo-controlled studies reported a significant reduction in the frequency of hot flushes from the use of soy extracts as compared with placebo (21%, 43% and 38% reduction, respectively). Few studies have compared the effect of phytoestrogens or soy products with HRT. In a double-blind, RCT of low-dose hormone therapy, Carmignani and colleagues34 treated symptomatic postmenopausal women with either a regimen of 1 mg estradiol and 0.5 mg norethisterone acetate, daily dietary soy supplementation containing 90 mg of isoflavone, or placebo for 16 weeks and reported that patients who received soy supplementation and hormone therapy had a 49.8% and 45.6% reduction in hot flushes, respectively. Similarly, Crisafulli and colleagues35 evaluated the effects of the phytoestrogen genistein (an isoflavone), estrogen–progesterone HRT, and placebo on hot flushes in postmenopausal women and reported a 22% daily reduction in hot flushes in the genistein group after 12 weeks of treatment when compared with placebo, and a 53% reduction after 12 weeks when compared with placebo in the HRT group. Interestingly, decreased estrogen levels at menopause were accompanied by a significant decrease in BRCA1 and BRCA2 mRNA levels in placebo-treated women, but this was reversed in genistein-treated women36. Bone mineral density for femoral neck and lumbar spine was higher in genistein-treated women compared to placebo36, and genistein did not significantly increase the risk of hypothyroidism compared to placebo37. Furthermore, a randomized trial of 224 postmenopausal women showed no significant difference in endometrial thickness or rates of endometrial hyperplasia or cancer in women receiving an isoflavone soy protein supplement compared to placebo-treated women38.
Lignans have not been as extensively studied as isoflavones, but the currently available data suggest that their effectiveness for reducing the vasomotor symptoms of menopause was no better than placebo. A double-blind, placebo-controlled RCT performed in 2010 by Simbalista and colleagues39 studied two groups of postmenopausal women with one group consuming two slices of bread containing 25 g of flaxseed (46 mg lignans) and the other group consuming wheat bran (< 1 mg lignans; control group) daily for 12 weeks, and reported similar reduction in hot flush frequency and KI in both groups. Likewise, Pruthi and colleagues40 randomized 188 postmenopausal women to consume a flaxseed bar (410 mg of lignan) or a placebo bar daily for 6 weeks, and found that in both groups there were approximately one-third of women reported a 50% reduction in hot flushes.
The majority of studies examining the roles of phytoestrogens in alleviating menopausal symptoms do not investigate their potential side-effects; of the 15 RCTs included in this meta-analysis, only five reported side-effect data. However, these studies were consistent with the results of this analysis, because they also found side-effects of phytoestrogen consumption to be no different from that with placebo. Most studies indicate that phytoestrogens are well tolerated without serious side-effects12,31,41. Our side-effect data were consistent with a recent meta-analysis which reviewed 174 RCTs and reported that phytoestrogens had a safe side-effect profile42. The rates of hormonal side-effects such as endometrial hyperplasia, endometrial cancer and breast cancer were no higher among phytoestrogen-treated women than placebo-treated women42. Though data are not conclusive, consumption of isoflavones by menopausal women7 may have a positive impact on vaginal atrophy, sleep disturbances, bone mineral density, and cognition. In addition, phytoestrogens do not seem to result in breast cancer or endometrial hyperplasia7, and may actually exert a positive effect on lipid profile23.
There are limitations of this study that should be considered. As with other similar meta-analyses, this meta-analysis is limited by the heterogeneity of the included studies. There was a lack of standardization in dosage used, and the compliance data were not available. In addition, isoflavones were the phytoestrogens used in the majority of the studies, and few studies with other phytoestrogens were included; outcomes with other phytoestrogens were therefore not examined. Furthermore, publication biases detected in the Egger's tests for KI as well as frequency of hot flushes might be due to the small number of studies included in this analysis. Despite these shortcomings, the similarities in inclusion criteria and outcome measures among the included studies aided the comparison and analysis.
In conclusion, results of this meta-analysis indicate that, while phytoestrogens did not bring a decrease in KI compared to placebo, their use was associated with a reduction in the hot flush frequency and their side-effects were no more common than those with placebo. While the available data do not support the recommendation of phytoestrogens for relief of all menopausal symptoms, some patients may benefit from their use in reducing hot flushes as these compounds also seem to be well tolerated. As the current data are inconclusive, further study of phytoestrogens for the relief of menopausal symptoms and their potential long-term adverse effects is warranted.
Conflict of interest The authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper.
Source of funding Nil.
References
- 1.Avis NE, Stellato R, Crawford S, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc Sci Med. 2001;52:345–56. doi: 10.1016/s0277-9536(00)00147-7. [DOI] [PubMed] [Google Scholar]
- 2.Oldenhave A, Jaszmann LJ, Haspels AA, Everaerd WT. Impact of climacteric on well-being. A survey based on 5213 women 39 to 60 years old. Am J Obstet Gynecol. 1993;168:772–80. doi: 10.1016/s0002-9378(12)90817-0. [DOI] [PubMed] [Google Scholar]
- 3.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Depypere HT, Comhaire FH. Herbal preparations for the menopause: beyond isoflavones and black cohosh. Maturitas. 2014;77:191–4. doi: 10.1016/j.maturitas.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Shou C, Li J, Liu Z. Complementary and alternative medicine in the treatment of menopausal symptoms. Chin J Integr Med. 2011;17:883–8. doi: 10.1007/s11655-011-0932-7. [DOI] [PubMed] [Google Scholar]
- 6.Ismail R, Taylor-Swanson L, Thomas A, et al. Effects of herbal preparations on symptoms clusters during the menopausal transition. Climacteric. 2014. Mar 8. Epub ahead of print. [DOI] [PubMed]
- 7.Bedell S, Nachtigal M, Naftolin F. The pros and cons of plant estrogens for menopause. J Steroid Biochem Mol Biol. 2014;139:225–36. doi: 10.1016/j.jsbmb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Thompson LU, Robb P, Serraino M, Cheung F. Mammalian lignan production from various foods. Nutr Cancer. 1991;16:43–52. doi: 10.1080/01635589109514139. [DOI] [PubMed] [Google Scholar]
- 9.Freeman EW, Sherif K. Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric. 2007;10:197–214. doi: 10.1080/13697130601181486. [DOI] [PubMed] [Google Scholar]
- 10.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JE, Nickell LA, Thompson LU, Szalai JP, Kiss A, Hilditch JR. A randomized controlled trial of the effect of dietary soy and flaxseed muffins on quality of life and hot flashes during menopause. Menopause. 2006;13:631–42. doi: 10.1097/01.gme.0000191882.59799.67. [DOI] [PubMed] [Google Scholar]
- 12.Lethaby A, Marjoribanks J, Kronenberg F, Roberts H, Eden J, Brown J. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;12:CD001395. doi: 10.1002/14651858.CD001395.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235–41. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 14.Kupperman HS, Wetchler BB, Blatt MH. Contemporary therapy of the menopausal syndrome. JAMA. 1959;171:1627–37. doi: 10.1001/jama.1959.03010300001001. [DOI] [PubMed] [Google Scholar]
- 15.Aso T, Uchiyama S, Matsumura Y, et al. A natural S-equol supplement alleviates hot flushes and other menopausal symptoms in equol nonproducing postmenopausal Japanese women. J Womens Health (Larchmt) 2012;21:92–100. doi: 10.1089/jwh.2011.2753. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson C, Warren RM, Sala E, et al. Red-clover-derived isoflavones and mammographic breast density: a double-blind, randomized, placebo-controlled trial [ISRCTN42940165] Breast Cancer Res. 2004;6:R170–9. doi: 10.1186/bcr773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancellieri F, De Leo V, Genazzani AD, et al. Efficacy on menopausal neurovegetative symptoms and some plasma lipids blood levels of an herbal product containing isoflavones and other plant extracts. Maturitas. 2007;56:249–56. doi: 10.1016/j.maturitas.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.del Giorno C, Fonseca AM, Bagnoli VR, Assis JS, Soares JM, Jr, Baracat EC. Effects of Trifolium pratense on the climacteric and sexual symptoms in postmenopause women. Rev Assoc Med Bras. 2010;56:558–62. doi: 10.1590/s0104-42302010000500017. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari A. Soy extract phytoestrogens with high dose of isoflavones for menopausal symptoms. J Obstet Gynaecol Res. 2009;35:1083–90. doi: 10.1111/j.1447-0756.2009.01058.x. [DOI] [PubMed] [Google Scholar]
- 20.Han KK, Soares JM, Jr, Haidar MA, de Lima GR, Baracat EC. Benefits of soy isoflavone therapeutic regimen on menopausal symptoms. Obstet Gynecol. 2002;99:389–94. doi: 10.1016/s0029-7844(01)01744-6. [DOI] [PubMed] [Google Scholar]
- 21.Nahas EA, Nahas-Neto J, Orsatti FL, Carvalho EP, Oliveira ML, Dias R. Efficacy and safety of a soy isoflavone extract in postmenopausal women: a randomized, double-blind, and placebo-controlled study. Maturitas. 2007;58:249–58. doi: 10.1016/j.maturitas.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Penotti M, Fabio E, Modena AB, Rinaldi M, Omodei U, Viganó P. Effect of soy-derived isoflavones on hot flushes, endometrial thickness, and the pulsatility index of the uterine and cerebral arteries. Fertil Steril. 2003;79:1112–17. doi: 10.1016/s0015-0282(03)00158-4. [DOI] [PubMed] [Google Scholar]
- 23.Petri Nahas E, Nahás Neto J, De Luca L, Traiman P, Pontes A, Dalben I. Benefits of soy germ isoflavones in postmenopausal women with contraindication for conventional hormone replacement therapy. Maturitas. 2004;48:372–80. doi: 10.1016/j.maturitas.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 24.Riesco E, Choquette S, Audet M, Tessier D, Dionne IJ. Effect of exercise combined with phytoestrogens on quality of life in postmenopausal women. Climacteric. 2011;14:573–80. doi: 10.3109/13697137.2011.566652. [DOI] [PubMed] [Google Scholar]
- 25.Sammartino A, Tommaselli GA, Gargano V, di Carlo C, Attianese W, Nappi C. Short-term effects of a combination of isoflavones, lignans and Cimicifuga racemosa on climacteric-related symptoms in postmenopausal women: a double-blind, randomized, placebo-controlled trial. Gynecol Endocrinol. 2006;22:646–50. doi: 10.1080/09513590601010722. [DOI] [PubMed] [Google Scholar]
- 26.Tice JA, Ettinger B, Ensrud K, Wallace R, Blackwell T, Cummings SR. Phytoestrogen supplements for the treatment of hot flashes: the Isoflavone Clover Extract (ICE) Study: a randomized controlled trial. JAMA. 2003;290:207–14. doi: 10.1001/jama.290.2.207. [DOI] [PubMed] [Google Scholar]
- 27.van de Weijer PH, Barentsen R. Isoflavones from red clover (Promensil) significantly reduce menopausal hot flush symptoms compared with placebo. Maturitas. 2002;42:187–93. doi: 10.1016/s0378-5122(02)00080-4. [DOI] [PubMed] [Google Scholar]
- 28.Van Patten CL, Olivotto IA, Chambers GK, et al. Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: a randomized, controlled clinical trial. J Clin Oncol. 2002;20:1449–55. doi: 10.1200/JCO.2002.20.6.1449. [DOI] [PubMed] [Google Scholar]
- 29.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;13:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs A, Wegewitz U, Sommerfield C, Grossklaus R, Lampen A. Efficacy of isoflavones in relieving vasomotor menopausal symptoms-A systematic review. Mol Nutr Food Res. 2009;53:1084–97. doi: 10.1002/mnfr.200800552. [DOI] [PubMed] [Google Scholar]
- 31.Eden JA. Phytoestrogens for menopausal symptoms: a review. Maturitas. 2012;72:157–9. doi: 10.1016/j.maturitas.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Villaseca P. Non-estrogen conventional and phytochemical treatments for vasomotor symptoms: what needs to be known for practice. Climacteric. 2012;15:115–24. doi: 10.3109/13697137.2011.624214. [DOI] [PubMed] [Google Scholar]
- 33.Bolanos R, Del Castillo A, Francia J. Soy isoflavones versus placebo in the treatment of climacteric vasomotor symptoms: systematic review and metaanalysis. Menopause. 2010;17:660–6. [PubMed] [Google Scholar]
- 34.Carmignani LO, Pedro AO, Costa-Paiva LH, Pinto-Neto AM. The effect of dietary soy supplementation compared to estrogen and placebo on menopausal symptoms: a randomized controlled trial. Maturitas. 2010;67:262–9. doi: 10.1016/j.maturitas.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Crisafulli A, Marini H, Bitto A, et al. Effects of genistein on hot flushes in early postmenopausal women: a randomized, double-blind EPT- and placebo-controlled study. Menopause. 2004;11:400–4. doi: 10.1097/01.gme.0000109314.11228.e5. [DOI] [PubMed] [Google Scholar]
- 36.Marini H, Bitto A, Altavilla D, et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab. 2008;93:4787–96. doi: 10.1210/jc.2008-1087. [DOI] [PubMed] [Google Scholar]
- 37.Bitto A, Polito F, Atteritano M, et al. Genistein aglycone does not affect thyroid function: results from a three year, randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2010;95:3067–72. doi: 10.1210/jc.2009-2779. [DOI] [PubMed] [Google Scholar]
- 38.Quaas AM, Kono N, Mack WJ, et al. Effect of isoflavone soy protein supplementation on endometrial thickness, hyperplasia, and endometrial cancer risk in postmenopausal women: a randomized, controlled trial. Menopause. 2013;20:840–4. doi: 10.1097/GME.0b013e3182804353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simbalista RL, Sauerbronn AV, Aldrighi JM, Arêas JA. Consumption of a flaxseed-rich food is not more effective than a placebo in alleviating the climacteric symptoms of postmenopausal women. J Nutr. 2010;140:293–7. doi: 10.3945/jn.109.113886. [DOI] [PubMed] [Google Scholar]
- 40.Pruthi S, Qin R, Terstreip SA, et al. A phase III, randomized, placebo-controlled, double-blind trial of flaxseed for the treatment of hot flashes: North Central Cancer Treatment Group N08C7. Menopause. 2012;19:48–53. doi: 10.1097/gme.0b013e318223b021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Adamo CR, Sahin A. Soy foods and supplementation: a review of commonly perceived health benefits and risks. Altern Ther Health Med. 2014;20(Suppl 1):39–51. [PubMed] [Google Scholar]
- 42.Tempfer CB, Froese G, Heinze G, Bentz EK, Hefler LA, Huber JC. Side effects of phytoestrogens: a meta-analysis of randomized trials. Am J Med. 2009;122:939–46. doi: 10.1016/j.amjmed.2009.04.018. [DOI] [PubMed] [Google Scholar]