Abstract
Purpose of review
Endoscopic eradication therapy is considered a safe and effective alternative to esophagectomy for a select patient population with high-grade Barrett’s esophagus and intramucosal adenocarcinoma. This review highlights available eradication techniques (resection and ablation) with emphasis on factors that influence choice of therapy.
Recent findings
Long-term follow-up of patients treated with endoscopic eradication therapies demonstrate high rates of complete remission of dysplasia and intestinal metaplasia with overall survival comparable to subjects treated surgically. Cohort studies also report that recurrence following successful ablation occurs in a significant proportion of subjects, making careful surveillance an indispensable component following successful endoscopic therapy. Endoscopic eradication therapy is also effective for treatment of recurrent dysplasia and intestinal metaplasia. Ablative therapies may lead to buried metaplasia in a small proportion of subjects. The long-term clinical implications of buried metaplasia are unclear.
Summary
Patients undergoing endoscopic eradication therapy should be enrolled in a comprehensive surveillance and staging program that offers both resection and ablative techniques. Complete remission of dysplasia and intestinal metaplasia can be achieved in the vast majority of patients undergoing endoscopic therapy. Surveillance should continue after treatment with close monitoring for recurrent dysplasia.
Keywords: Barrett’s esophagus, Endoscopic Eradication Therapy
INTRODUCTION
Barrett’s esophagus (BE) progresses to esophageal adenocarcinoma (EA) through stepwise transformation of intestinal metaplasia to dysplasia to cancer.[1*] The absolute risk of EA increases in proportion to the grade of dysplasia with high-grade dysplasia (HGD) carrying a 30% five year risk of EA and a 12% risk of associated invasive EA.[2, 3] Esophagectomy, which was the conventional treatment of choice for patients with HGD and intramucosal carcinoma (IMC), was the only modality to both reduce cancer risk (in subjects with HGD), induce remission (in subjects with IMC) and improve overall as well as cancer free survival. This approach, however, is associated with substantial mortality (2–5%) and morbidity (30–40%) as well as decreased quality of life even in high volume centers (>10/year).[4, 5, 6] Intramucosal carcinoma (T1a EA) is associated with low rates of metastatic lymphadenopathy (<5%) as shown in surgical series, providing a rationale for non surgical therapy. [7, 8]Endoscopic therapy was developed in an attempt to provide a therapeutic modality with minimal mortality and morbidity risks, but with equivalent survival benefits, in a cost effective manner.[6]
ENDOSCOPIC THERAPEUTIC MODALITIES
Endoscopic eradication therapy can be broadly divided into tissue acquiring (resection) and ablative techniques. Resection of Barrett’s mucosa can be achieved through endoscopic mucosal resection (EMR) (focal or circumferential) and more recently described, through endoscopic submucosal dissection (ESD). Both techniques provide tissue for histopathologic analysis. Ablative techniques, on the other hand, achieve eradication of Barrett’s mucosa using thermal, photochemical or cryo energy without the acquisition of tissue for histopathologic analysis.
TISSUE ACQUIRING/RESECTION TECHNIQUES
Resection techniques play both a diagnostic and a therapeutic role in the management of dysplastic BE. These techniques allow for greater diagnostic accuracy (via assessment of the degree of dysplasia and the presence of carcinoma) by providing better interobserver agreement amongst gastrointestinal pathologists when compared to routine biopsies.[9] Histopathological evaluation following EMR leads to upstaging of the degree of dysplasia (compared to biopsies) in 30% of patients with HGD.[10] Presence of the lamina propria, muscularis mucosa (which can be duplicated in patients with BE) and the submucosa in EMR specimens allows for precise determination of the depth of invasion when carcinoma is present. (figure 1) This has important therapeutic implications given that the rate of metastatic lymphadenopathy is substantially higher in subjects with submucosal invasion (20–30%).[7] Staging with EMR has been shown to correlate well with pathology at esophagectomy.[11] EMR specimens also help in determining the completeness of resection by assessment of lateral and deep margins. In specimens with negative margins the procedure may be considered curative.
FIGURE 1. Endoscopic Mucosal Resection Histopathology.
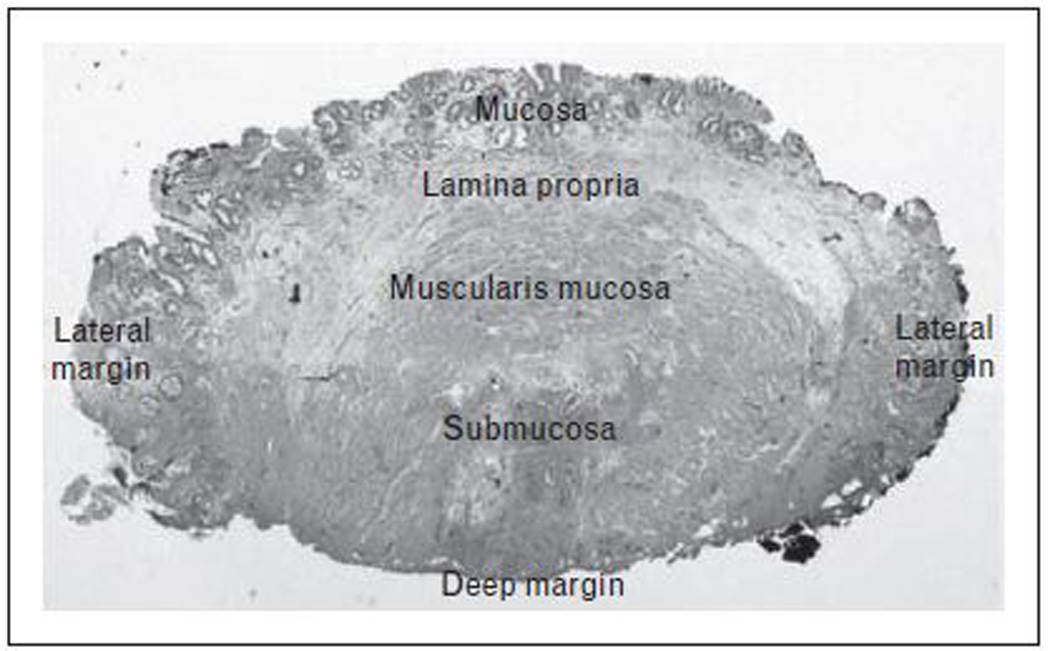
Histopathology of cross-section of endoscopic mucosal resection specimen showing mucosa, lamina propria, muscularis mucosa and submucosa. Focal high-grade dysplasia within the mucosal layer with clear deep and lateral margins.
-ENDOSCOPIC MUCOSAL RESECTION
EMR targets macroscopically visible mucosal irregularities. This treatment modality is best suited for discrete lesions that are less than 1.5–2 cm in greatest diameter. The identified visible lesion is first injected with saline or diluted epinephrine at the level of the submucosa. This allows the mucosa to lift away from the muscularis propria, creating a fluid cushion protecting from deeper injury and perforation during the application of electrocautery. A ‘lift sign’ also signals that the lesion is superficial to the muscularis propria. Resection can be performed using two methods: (figure 2)
EMR-Cap: The endoscope is fitted with a plastic cap (Olympus, Tokyo, Japan) that allows a snare to be positioned along an internal circumferential groove. The mucosa is retracted into the cap by suction and the snare is closed around the target site at which point electrocautery is applied for resection.
EMR-Ligation: Involves the use of a band ligation device (Wilson Cook, Wilson-Cook Medical, Winston-Salem, North Carolina, USA) to create a pseudopolyp while aspirating the mucosa. This technique can also be performed without prior submucosal injection but this alternative may be associated with a higher risk of perforation.[12] Once the mucosa has been ligated, a hexagonal snare is used to resect the lesion with electrocautery. This technique has the advantage of allowing multiple resections (up to 6) with a single intubation.
FIGURE 2. Endoscopic Mucosal Resection Techniques.
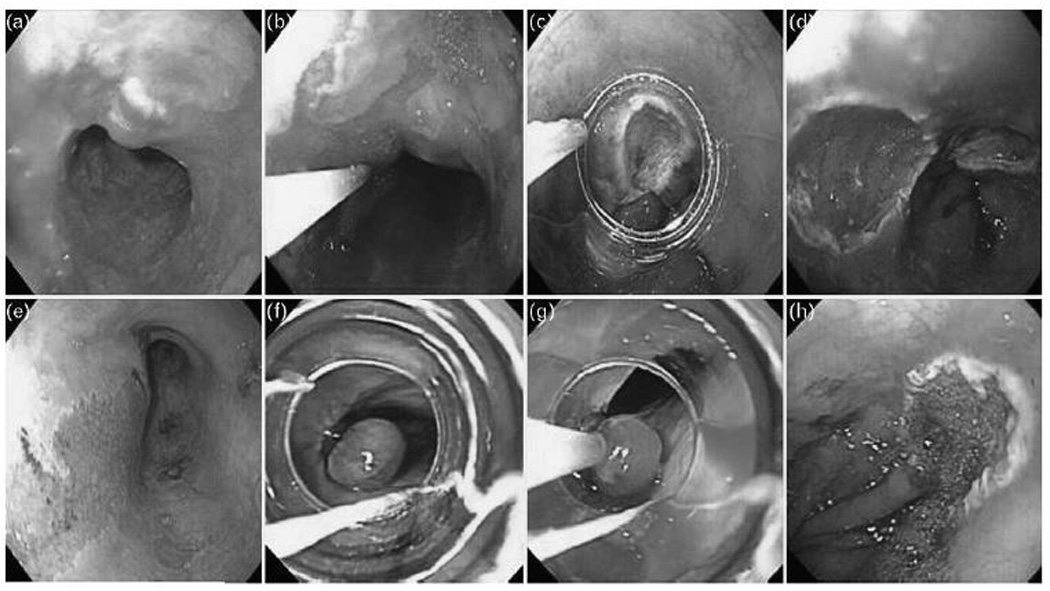
EMR-Cap (A) A high-resolution white light endoscopy photograph shows a nodular lesion within a section of Barrett’s mucosa in the distal esophagus. (B) Saline epinephrine solution is injected submucosally under the lesion (C) A cap with a snare positioned on its rim is placed over the lesion. The lesion is suctioned into the cap and resected with the use of electrocautery leaving a (D) clean base ulcer. EMR-Ligation (E) A high-resolution white light endoscopy photograph shows a nodular lesion within Barrett’s mucosa. The lesion is suctioned into a band ligation device and a band is deployed to create a (F) pseudopolyp. (G) A hexagonal snare is positioned over the pseudopolyp and electrocautery is applied for resection leaving a (H) clean base ulcer.
A recent randomized control trial looking at EMR-Cap versus EMR-Ligation for piecemeal resection of HGD BE and IMC showed EMR-Ligation to be faster and more economical compared to EMR-Cap. [13*] EMR-Ligation led to smaller resection specimens but with comparable depth of resection. No significant higher risk of perforations was associated with EMR-Ligation in this trial.
EMR may be performed to remove focal nodular lesions with the remaining BE mucosa being targeted by ablative techniques. An alternative approach is to perform EMR to remove focal visible lesions as well as the remaining BE segment; this approach is termed wide (WEMR) or circumferential EMR (CEMR). This technique, though effective in achieving complete remission of dysplasia (CRD) and intestinal metaplasia (CRIM), is associated with a higher rate of complications including stricture formation (37%).[14] A recent randomized study compared stepwise WEMR with focal EMR followed by radiofrequency ablation (RFA) in subjects with 5cm or less of BE, and concluded that the latter approach is associated with lower complication rates with equivalent results in terms of achieving remission from neoplasia/dysplasia and intestinal metaplasia.[15**] The focal EMR followed by RFA arm also needed fewer procedures to achieve CRIM. Recurrence rates at 2 years were low and comparable in both approaches.
CRD rates are reported to be high (>90%) in patients undergoing EMR with or without subsequent ablation.[16, 17, 18] EMR is associated with an overall low rate of complications (13–17%), including minor bleeding.[19] Serious complications such as clinically significant bleeding (3.8%) and esophageal strictures (6%) are rare when this technique is performed by an experienced endoscopist. If recognized during EMR, perforations and bleeding may be treated endoscopically with hemoclips or stents.[20, 21]
-ENDOSCOPIC SUBMUCOSAL DISSECTION
Endoscopic submucosal dissection (ESD) is a newer excisional technique, initially developed in Japan, which allows for resection of large specimens (up to 10 cm in diameter). Similar to EMR, the submucosa is injected with fluid to elevate the lesion of interest. A variety of endoscopic instruments (knives) are then used to carefully dissect in the submucosal plane using electrocautery. ESD allows for complete en-bloc resection which can lead to more accurate pathological assessment of deep and lateral margins, compared to piecemeal resection.[22] This technique, however, is technically demanding, time consuming and is associated with a higher rate of perforations and strictures, even in the hands of highly trained endoscopists.[23] ESD appears to be associated with high curative resection rates and low recurrence rates.[22] Treatment efficacy data, however, is limited in patients with HGD BE and IMC, with most case series describing the results of ESD in subjects with squamous cell carcinoma or dysplasia of the esophagus.[24, 25, 26]
ABLATIVE TECHNIQUES
Ablation refers to the destruction of neoplastic esophageal mucosa followed by regrowth of normal squamous mucosa in an esophageal environment in which acid reflux is reduced by treatment with proton pump inhibitors (PPIs) or antireflux surgery. This is thought to lead to the lowering of the future risk of dysplasia and EA.[27, 28] Careful endoscopic examination and accurate staging is crucial prior to ablative therapy, given that ablation can only treat superficial disease. Staging should begin with careful inspection of the esophageal mucosa under high-definition white light endoscopy (WLE). Enhanced endoscopic imaging techniques such as narrow-band imaging (NBI) and confocal endomicroscopy can also be used to highlight irregularities in the mucosa, but should not replace careful inspection under WLE. EMR should be targeted to discrete areas of abnormal mucosa to exclude invasive malignancy. Metastatic disease should be excluded with a combination of computed tomography imaging as well as endoscopic ultrasound.
The principle behind all ablative techniques is the superficial induction of tissue necrosis. Cellular damage can be produced through thermal injury (radiofrequency ablation), photochemical injury (photodynamic therapy) or exposure to cold temperatures (cryotherapy). Optimal dosimetry (number of applications, time of exposure) aims at limiting tissue damage to the mucosal layer in order to avoid complications such as stricture formation and perforation.
-RADIOFREQUENCY ABLATION (RFA)
RFA is an emerging therapeutic modality that uses a high frequency alternating electrical current to generate thermal energy. Commercially available RFA devices include the HALO90 and HALO360 (BARRX Medical Inc, Sunnyvale, CA), designed for focal and circumferential ablation respectively. HALO systems contain bipolar electrode arrays that produce radiofrequency energy. In the HALO90, the ablation array (20 by 13 mm) is fitted on the tip of an endoscope. In the HALO360, the array (3cm) encircles a 4 cm (axial length) balloon. Direct contact between the array and the esophageal mucosa is required for effective ablation. The device delivers a preset amount of radiofrequency energy (typically 12 joules/cm2).
When performing focal ablation using the HALO90, a total of four applications are recommended. In order to ensure adequate contact, cellular debris generated from the first two applications is cleaned prior to performing the remaining two treatments. When performing circumferential ablation using the HALO360, a sizing balloon must be used to determine the approximate inner diameter of the esophagus. The smallest esophageal diameter measured by the balloon is used to select the treatment balloon. Two applications at each level/segment are recommended with cleaning of coagulation debris between applications.
Several prospective trials have shown RFA to be an effective treatment modality for the eradication of BE with HGD. A recent randomized, multicenter, sham-controlled trial demonstrated a rate of CRD in 81% and CRIM in 75% of participants with HGD receiving RFA compared to 19% and 2% in the sham arm. [28] (figure 3). The risk of progression to EA was also reduced in the treatment arm (4%) compared to the sham arm (16%). In this study, RFA was associated with an approximately 6% rate of stricture formation. However, stricture rates may be somewhat higher with RFA administered following EMR (13%).[29*]
FIGURE 3. Radiofrequency Ablation in Barrett’s Esophagus with High-grade Dysplasia.
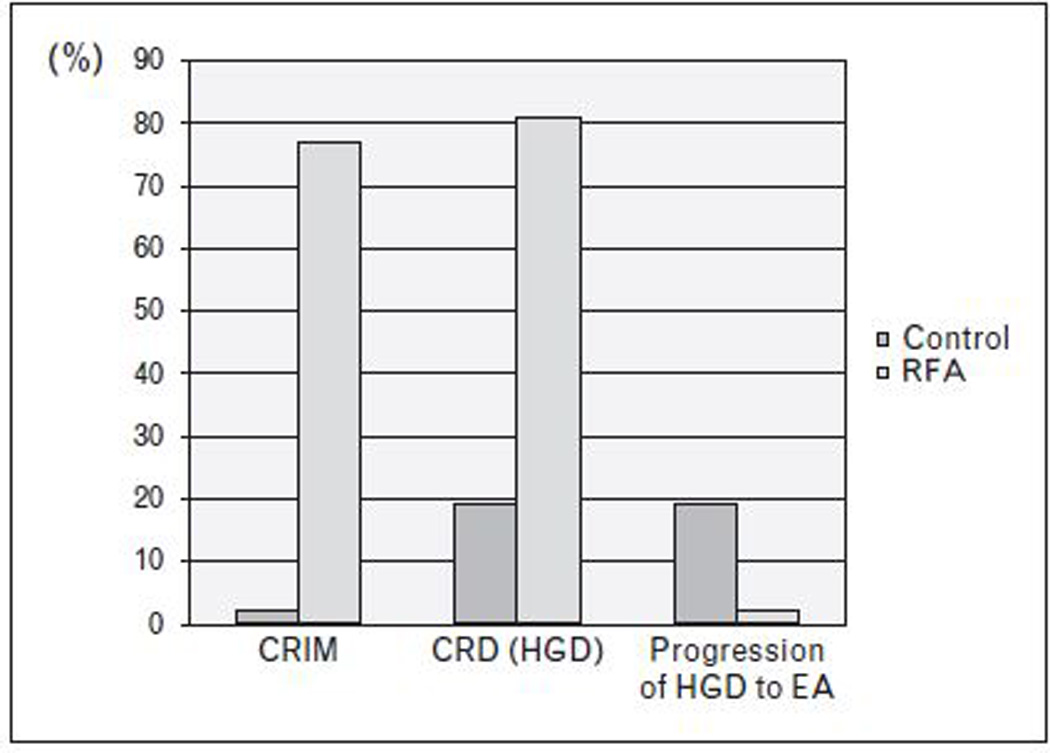
Study by Shaheen et al (28) demonstrating significant increase in complete eradication of high-grade dysplasia (CRD) (p<0.001) and intestinal metaplasia (CRIM) (p<0.001) with radiofrequency ablation (RFA) compared to a sham control group. Decrease in progression of high-grade dysplasia (HGD) to esophageal adenocarcinoma (EA) was also observed as a secondary outcome (p=0.04).
-CRYOTHERAPY
Endoscopic cyrotherapy exposes the esophageal mucosa to freezing temperatures through the application of liquid nitrogen or carbon dioxide. This technique is applied in repeated cycles of rapid freezing and slow thawing in order to maximize tissue damage. Two endoscopic cryotherapy systems are commercially available. The CryoSpray Ablation System (CSA Medical, Inc, Baltimore) delivers liquid nitrogen at low pressure (2–4 PSI) through a catheter that is passed through the working channel of the endoscope. A decompression tube placed alongside the endoscope with the distal end in the gastric antrum prevents overdistention. The Polar Wand (GI Supply, Camp Hill, Pennsylvania) delivers carbon dioxide (6–8 L/min) that rapidly expands and produces cooling. In both systems the endoscopist controls the time and number of exposures. Cryotherapy is delivered by spraying, thus circumventing the need for mucosal contact and allowing application of cryogen to irregular surfaces.
Cryotherapy for the treatment of BE with HGD and IMC has shown promising results in preliminary studies. A recent multicenter cohort study demonstrated CRD in 88% of patients with HGD and 53% of patients with IMC treated with liquid nitrogen.[30] The most common reported complication was chest pain and dysphagia in 17% and 13% of participants, respectively. Minor strictures requiring dilatation were seen in 3 patients. Gastric perforation secondary to overdistention occurred in a single patient who had Marfan syndrome. Carbon dioxide cyrotherapy appears to share similar efficacy rates to liquid nitrogen although, at this time, data are available only in abstract form.[31]
-PHOTODYNAMIC THERAPY
Photodynamic therapy uses photochemical energy to induce tissue damage. A photosensitizer administered systemically is activated locally by exposure to light of a particular wavelength within the esophageal lumen. Activation of the photosensitizer leads to a cytotoxic cascade mediated by the generation of oxygen radicals. The photosensitizers used in PDT include porfimer sodium and 5-aminolevulinic acid. A large multicenter, partially blinded, randomized controlled trial that enrolled 208 patients with HGD shows a CRD of 77% in PDT treated patients compared to 39% in the control group receiving omeprazole alone.[32] The PDT group also experienced a statistically significant decrease in the rate of progression to EA (13%) compared to the control group receiving only surveillance endoscopy and omeprazole (27%). Remission remained higher in the treatment group at 5 year follow-up (77% in PDT group, 39% in control group).[33] Progression to cancer was also significantly lower in the PDT group (15%) compared to the control group (29%). The most common adverse events included photosensitivity reactions (69%), esophageal strictures (36%), chest pain (20%) and dysphagia (19%). Photosensitivity can last for 30–90 days, along with significant chest pain, nausea and vomiting.
COMBINATION ENDOTHERAPY
Non-randomized, retrospective cohort studies have compared the outcomes of subjects who had HGD or IMC treated by EMR and ablation (PDT, APC) with those treated by esophagectomy, and have reported comparable outcomes in terms of overall survival, but higher rates of cancer-free survival in the esophagectomy groups.[6, 19, 34] Recurrences in the endotherapy groups were endoscopically treatable, highlighting the importance of continued endoscopic surveillance. Data on non-invasive squamous cell esophageal carcinoma suggest that RFA with our without EMR is effective in the treatment of recurrent disease (100% CRD in 13 patients with median follow-up of 17 months). [35]
FACTORS INFLUENCING CHOICE OF ENDOSCOPIC THERAPY
A decision on endoscopic therapy versus surgery should be made following thorough discussion of the advantages and disadvantages of each approach. The need for continued surveillance following endoscopic therapy should be emphasized given that this is vital in order to detect recurrent neoplasia. Institutional endoscopic and surgical expertise as well as patient preferences and risk tolerance will likely influence choice of therapy.
The choice of endoscopic eradication therapy starts with careful examination of the BE segment. (figure 4) Discrete nodular disease can be targeted with EMR. Assessment of margins (lateral to determine remission and deep margins to assess appropriateness of endoscopic therapy) is important in determining the next step. The remaining BE mucosa is usually targeted using ablative techniques especially with longer residual segments.[15*]
FIGURE 4. Algorithm of approach to Endoscopic Eradication Therapy of HGD/IMC in BE.
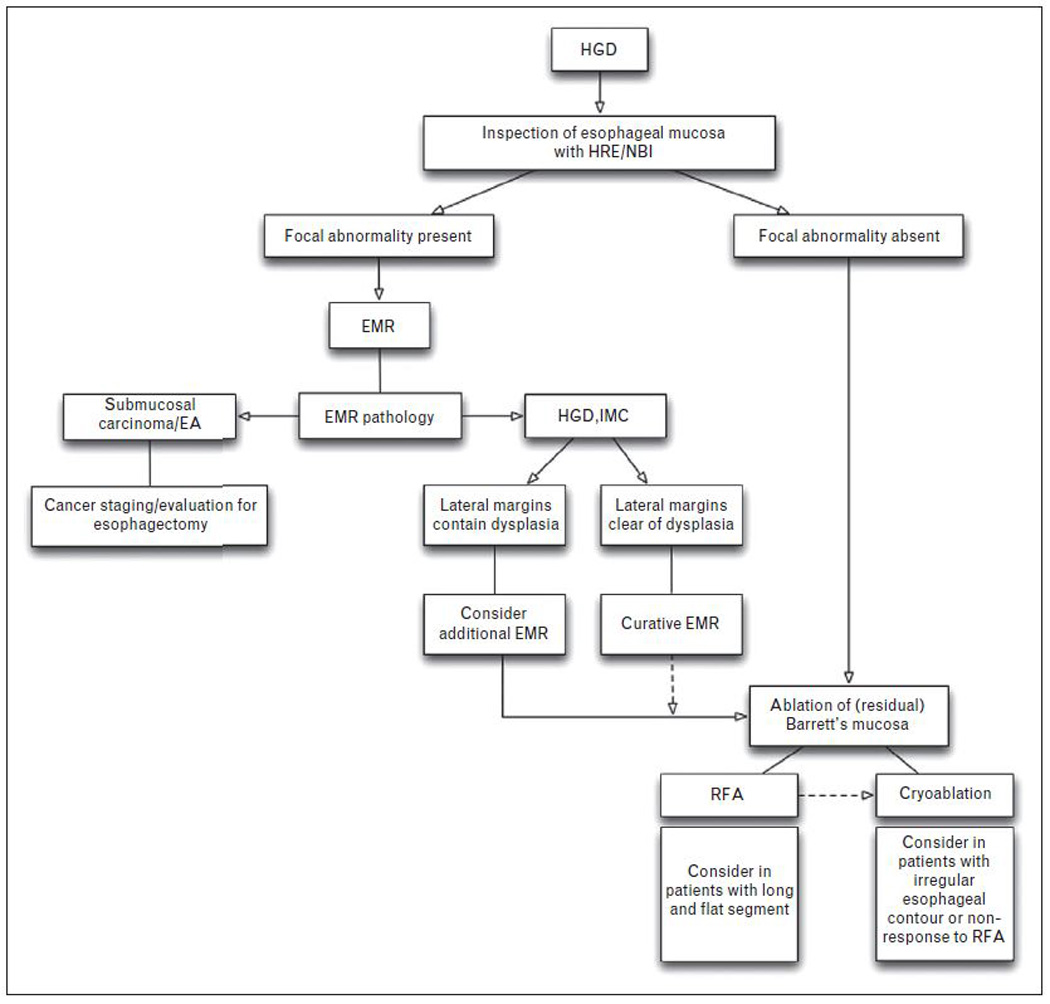
Approach to endoscopic eradication therapy in patients with Barrett’s esophagus (BE) with high-grade dysplasia (HGD) and intramucosal carcinoma (IMC). HRE: high resolution endoscopy; NBI: narrow band imaging; EMR: endoscopic mucosal resection; RFA: radiofrequency ablation
In general, ablative therapies are safe following EMR and do not necessarily increase the risk of strictures.[29*] The choice of ablative therapy is somewhat empiric given the lack of comparative studies looking at long-term outcomes with different techniques. RFA may be best suited for flat mucosa in esophagi with preserved contours where the ablation catheter can establish direct contact with the entire mucosa. Treatment in subjects with scarring and distorted anatomy which makes contact challenging, may be approached with cryoablation where the cryogen is sprayed over the mucosal area. In general, PDT use has declined due to prolonged photosensitivity, moderate to severe discomfort following the procedure and a high rate of stricture formation. Further studies are needed to help determine which technique is associated with the lowest rate of recurrence when targeting specific esophageal findings.
BURIED METAPLASIA
Endoscopic ablation aims at the eradication of metaplastic mucosa to allow for squamous re-epithialization. When ablation does not destroy the entire mucosal layer, overgrowth by squamous epithelium may bury the remaining metaplastic glands. Buried metaplasia is not visible endoscopically and may carry malignant potential. A recent systematic review shows the overall prevalence of buried metaplasia to be from 0 to 28%.[36*] PDT is associated with a higher rate of buried metaplasia compared to RFA (14.2% compared to 0.9% respectively). EA arising from buried metaplasia has been described in case reports following PDT but not RFA. The author’s emphasize that this may be secondary to a shorter time of follow-up with RFA (RFA being a newer technique, compared to PDT). This study also identified that not all reports on this subject provide information on adequate biopsy specimen size. A recent study raises concerns on the inadequate depth of routinely acquired post ablation biopsies; with most not containing lamina propria consistently to reliably exclude subsquamous BE [37**] though this has been contested by other studies.[38**] Only a few studies have evaluated the biological properties of buried metaplasia. Buried metaplasia following PDT appears to have a lower crypt proliferation rate and neoplastic potential compared to pre-treatment BE. [39] Further investigation in this area, with appropriate biopsy specimens and long-term follow-up, is warranted in order to understand the clinical implications of buried metaplasia.
RECURRENCE OF DYSPLASIA AND TREATMENT STRATEGIES
Endoscopic eradication therapy should always include plans for a comprehensive surveillance program. Following CRIM, surveillance is currently performed every 6 months for a year, followed by annually thereafter. Risk factors for recurrence include long-segment BE, piecemeal EMR and mutifocal disease.[40] Recurrence rates for intestinal metaplasia and dysplasia range from 17% to 22 % over 1–3 years of follow up following PDT and RFA.[41*, 42**] The therapeutic approach to recurrent dysplasia is similar to that of primary dysplasia. Discrete nodular disease should continue to be targeted with EMR unless these are multiple or extensive. With regard to ablative therapy, if a patient tolerated mucosal ablation with no complications, it may not be unreasonable to reattempt the same technique if indicated. Whether switching to a different mucosal ablative technique is beneficial in the eradication of recurrent dysplasia is unclear. Patients who have not responded to RFA may benefit from cryoablation (Figure 4) If ablative therapy is performed following EMR it should be done 4–6 weeks following resection in order to allow mucosal healing.[29*] The decision to pursue esophagectomy as a curative outcome if endoscopic eradication therapy fails should be considered and discussed with the patient.
CONCLUSION
Endoscopic eradication therapy is considered a safe and effective alternative to esophagectomy for a select patient population who have BE with HGD or IMC. Patients undergoing endoscopic eradication therapy should be enrolled in a comprehensive surveillance and staging program. The endoscopist should be familiar with both resection and ablative therapies, given their complimentary roles in treatment. Surveillance should continue after endoscopic eradication therapy, with close monitoring for recurrent dysplasia. The clinical implications of buried metaplasia will become more apparent once long-term follow-up data are available on patients treated with ablative therapy.
KEY POINTS.
Complete remission of dysplasia and intestinal metaplasia can be achieved in the vast majority of patients undergoing endoscopic eradication therapy for HGD or IMC in BE.
Surveillance endoscopy should continue after eradication therapy, with close monitoring for recurrent dysplasia, which can usually be targeted endoscopically.
The clinical implications of buried metaplasia will become more apparent once long-term follow-up data are available on patients treated with ablative therapy.
Acknowledgments
FINANCIAL SUPPORT: Supported by a Junior Faculty Development Award from the American College of Gastroenterology, the NIDDK (RC4DK090413) and the Mayo Foundation.
Footnotes
CONFLICT OF INTEREST : None
References
- 1. Spechler SJ, Fitzgerald RC, Prasad GA, et al. History, molecular mechanisms, and endoscopic treatment of Barrett's esophagus. Gastroenterology. 2010;138:854–869. doi: 10.1053/j.gastro.2010.01.002. This comprehensive review discusses the history, implicated molecular mechanisms of BE genesis (transdifferentiation and proximal migration from cardia), influence of obesity on BE genesis and methods and outcomes of endoscopic therapy of BE related neoplasia.
- 2.Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 2008;67:394–398. doi: 10.1016/j.gie.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Konda VJ, Ross AS, Ferguson MK, et al. Is the risk of concomitant invasive esophageal cancer in high-grade dysplasia in Barrett's esophagus overestimated? Clin Gastroenterol Hepatol. 2008;6:159–164. doi: 10.1016/j.cgh.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Edwards MJ, Gable DR, Lentsch AB, et al. The rationale for esophagectomy as the optimal therapy for Barrett's esophagus with high-grade dysplasia. Ann Surg. 1996;223:585–589. doi: 10.1097/00000658-199605000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng EE, Wu TT, Yeo CJ, et al. Barrett's esophagus with high grade dysplasia: surgical results and long-term outcome--an update. J Gastrointest Surg. 2003;7:164–170. doi: 10.1016/s1091-255x(02)00153-1. [DOI] [PubMed] [Google Scholar]
- 6.Prasad GA, Wang KK, Buttar NS, et al. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett's esophagus. Gastroenterology. 2007;132:1226–1233. doi: 10.1053/j.gastro.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566–573. doi: 10.1097/01.sla.0000184211.75970.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pennathur A, Farkas A, Krasinskas AM, et al. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg. 2009;87:1048–1054. doi: 10.1016/j.athoracsur.2008.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wani S, Puli SR, Shaheen NJ, et al. Esophageal adenocarcinoma in Barrett's esophagus after endoscopic ablative therapy: a meta-analysis and systematic review. Am J Gastroenterol. 2009;104:502–513. doi: 10.1038/ajg.2008.31. [DOI] [PubMed] [Google Scholar]
- 10.Mino-Kenudson M, Hull MJ, Brown I, et al. EMR for Barrett's esophagus-related superficial neoplasms offers better diagnostic reproducibility than mucosal biopsy. Gastrointest Endosc. 2007;66:660–666. doi: 10.1016/j.gie.2007.02.063. [DOI] [PubMed] [Google Scholar]
- 11.Prasad GA, Buttar NS, Wongkeesong LM, et al. Significance of neoplastic involvement of margins obtained by endoscopic mucosal resection in Barrett's esophagus. Am J Gastroenterol. 2007;102:2380–2386. doi: 10.1111/j.1572-0241.2007.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerke H, Siddiqui J, Parekh KR, et al. Esophageal perforation complicating band ligator-assisted mucosal resection. Gastrointest Endosc. 2009;69:153–154. doi: 10.1016/j.gie.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 13. Pouw RE, van Vilsteren FG, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett's neoplasia. Gastrointest Endosc. 2011;74:35–43. doi: 10.1016/j.gie.2011.03.1243. In this prospective randomized study, EMR-L was found to be faster and less expensive than EMR-L. EMR-L produced smaller pieces than EMR-C, but with comparable depth of resection. Complication rates with both groups were comparable.
- 14.Chennat J, Konda VJ, Ross AS, et al. Complete Barrett's eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol. 2009;104:2684–2692. doi: 10.1038/ajg.2009.465. [DOI] [PubMed] [Google Scholar]
- 15. van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765–773. doi: 10.1136/gut.2010.229310. In this multicenter prospective study, complications following SRER versus RFA for the treatment of residual dysplastic BE were compared in subjects with a BE segment less than 5 cm. SRER lead to a high stricture rate with more endoscopic sessions than RFA. Recurrence rates were comparable in both arms after short term follow up.
- 16.Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer) Gastrointest Endosc. 2007;65:3–10. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 17.May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett's oesophagus: acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol. 2002;14:1085–1091. doi: 10.1097/00042737-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett's esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65:185–195. doi: 10.1016/j.gie.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 19.Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology. 2009;137:815–823. doi: 10.1053/j.gastro.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu Y, Kato M, Yamamoto J, et al. Endoscopic clip application for closure of esophageal perforations caused by EMR. Gastrointest Endosc. 2004;60:636–639. doi: 10.1016/s0016-5107(04)01960-1. [DOI] [PubMed] [Google Scholar]
- 21.Leers JM, Vivaldi C, Schafer H, et al. Endoscopic therapy for esophageal perforation or anastomotic leak with a self-expandable metallic stent. Surg Endosc. 2009;23:2258–2262. doi: 10.1007/s00464-008-0302-5. [DOI] [PubMed] [Google Scholar]
- 22.Yoshinaga S, Gotoda T, Kusano C, et al. Clinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junction. Gastrointest Endosc. 2008;67:202–209. doi: 10.1016/j.gie.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 23.Ogura K, Okamoto M, Sugimoto T, et al. Efficacy and safety of endoscopic submucosal dissection for gastric cancer in patients with liver cirrhosis. Endoscopy. 2008;40:443–445. doi: 10.1055/s-2007-995650. [DOI] [PubMed] [Google Scholar]
- 24.Teoh AY, Chiu PW, Yu Ngo DK, et al. Outcomes of endoscopic submucosal dissection versus endoscopic mucosal resection in management of superficial squamous esophageal neoplasms outside Japan. Journal of clinical gastroenterology. 2010;44:e190–e194. doi: 10.1097/MCG.0b013e3181ce52fb. [DOI] [PubMed] [Google Scholar]
- 25.Repici A, Hassan C, Carlino A, et al. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: results from a prospective Western series. Gastrointestinal endoscopy. 2010;71:715–721. doi: 10.1016/j.gie.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointestinal endoscopy. 2010;72:960–966. doi: 10.1016/j.gie.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 27.Overholt BF, Panjehpour M, Halberg DL. Photodynamic therapy for Barrett's esophagus with dysplasia and/or early stage carcinoma: long-term results. Gastrointest Endosc. 2003;58:183–188. doi: 10.1067/mge.2003.327. [DOI] [PubMed] [Google Scholar]
- 28.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 29. Okoro NI, Tomizawa Y, Dunagan KT, et al. Safety of Prior Endoscopic Mucosal Resection in Patients Receiving Radiofrequency Ablation of Barrett's Esophagus. Clin Gastroenterol Hepatol. 2012;10:150–154. doi: 10.1016/j.cgh.2011.10.030. In this retrospective cohort study, the rates of complications (primarily stricture) and histologic outcomes were compared in two cohorts of subjects undergoing RFA : those with preceding EMR and those without. Stricture rates were numerically higher in the RFA+EMR group but not statistically different between the two groups. Histologic outcomes were similar between the two groups after adjusting for baseline differences.
- 30.Greenwald BD, Dumot JA, Horwhat JD, et al. Safety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Dis Esophagus. 2010;23:13–19. doi: 10.1111/j.1442-2050.2009.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canto MIGE, Shin EJ. Carbon dioxide cryotherapy is a safe and effective treatment of Barrett's eosphagus (BE) with HGD/intramucosal carcinoma. Gastrointestinal endoscopy. 2009:AB341. [Google Scholar]
- 32.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–498. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 33.Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc. 2007;66:460–468. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 34.Schembre DB, Huang JL, Lin OS, et al. Treatment of Barrett's esophagus with early neoplasia: a comparison of endoscopic therapy and esophagectomy. Gastrointest Endosc. 2008;67:595–601. doi: 10.1016/j.gie.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 35.van Vilsteren FG, Alvarez Herrero L, Pouw RE, et al. Radiofrequency ablation for the endoscopic eradication of esophageal squamous high grade intraepithelial neoplasia and mucosal squamous cell carcinoma. Endoscopy. 2011;43:282–290. doi: 10.1055/s-0030-1256309. [DOI] [PubMed] [Google Scholar]
- 36. Gray NA, Odze RD, Spechler SJ. Buried metaplasia after endoscopic ablation of Barrett's esophagus: a systematic review. Am J Gastroenterol. 2011;106:1899–1908. doi: 10.1038/ajg.2011.255. In this systematic review, the prevalence of subsquamous BE after endoscopic therapy is reviewed. Implications and potential significance of subsquamous BE are discussed.
- 37. Gupta N, Mathur SC, Dumot JA, et al. Adequacy of esophageal squamous mucosa specimens obtained during endoscopy: are standard biopsies sufficient for postablation surveillance in Barrett's esophagus? Gastrointest Endosc. 2012;75:11–18. doi: 10.1016/j.gie.2011.06.040. In this elegant study, the depth of biopsies taken from neo-squamous mucosa after ablation and normal squamous mucosa was carefully evaluated. Primary outome was the proportion of biopsies containing any lamina propria (to detect subsquamous BE). Biopsy specimens of neo-squamous mucosa obtained after endoscopic therapy appeared to be inadequate to exclude subsquamous intestinal metaplasia/dysplasia because lamina propria is not present in more than 60% of specimens.
- 38. Shaheen NJ, Peery AF, Overholt BF, et al. Biopsy depth after radiofrequency ablation of dysplastic Barrett's esophagus. Gastrointestinal endoscopy. 2010;72:490–496. e1. doi: 10.1016/j.gie.2010.04.010. In a secondary analysis of the AIM Dysplasia trial biopsies, the proportion of biopsies with any subepithelial component was assessed. In both squamous (78%) and columnar tissue (99%), endoscopic biopsy samples after RFA were as likely to demonstrate subepithelium as untreated controls. Biopsy samples after RFA appeared to be of adequate depth to assess response to therapy.
- 39.Hornick JL, Mino-Kenudson M, Lauwers GY, et al. Buried Barrett's epithelium following photodynamic therapy shows reduced crypt proliferation and absence of DNA content abnormalities. Am J Gastroenterol. 2008;103:38–47. doi: 10.1111/j.1572-0241.2007.01560.x. [DOI] [PubMed] [Google Scholar]
- 40.Esaki M, Matsumoto T, Hirakawa K, et al. Risk factors for local recurrence of superficial esophageal cancer after treatment by endoscopic mucosal resection. Endoscopy. 2007;39:41–45. doi: 10.1055/s-2006-945143. [DOI] [PubMed] [Google Scholar]
- 41. Badreddine RJ, Prasad GA, Wang KK, et al. Prevalence and predictors of recurrent neoplasia after ablation of Barrett's esophagus. Gastrointestinal endoscopy. 2010;71:697–703. doi: 10.1016/j.gie.2009.08.031. In this study, the incidence of recurrent IM following successful eradication of dysplasia or intestinal metaplasia with PDT was studied. Over three years of follow up, cumulative recurrence rate of IM was 17%, of which 29% had HGD and 4% had intramucosal carcinoma. Predictors of dysplastic recurrence included older age, presence of residual BE and current smoking.
- 42. Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology. 2011;141:460–468. doi: 10.1053/j.gastro.2011.04.061. In this study, the results of the AIM Dysplasia study were extended to 3 years. Kaplan-Meier analysis showed that dysplasia remained eradicated in >85% of patients and intestinal metaplasia in >75%, without maintenance RFA. Additional RFA was hence required in a significant proportion of subjects after CRIM was achieved initially. With additional RFA, CRIM and CRD were achieved in >95% of subjects at 3 years. Stricture rate was 7.6%. The rate of esophageal adenocarcinoma was 1 per 181 patient-years (0.55%/patient-years) after successful ablation.


