Abstract
Objective
To identify changes in incidence of cutaneous melanoma over time in the fastest-growing segment of the US population, middle-aged adults.
Patients and Methods
Using the Rochester Epidemiology Project resource, we identified patients aged 40 to 60 years who had a first lifetime diagnosis of melanoma between January 1, 1970, and December 31, 2009, in Olmsted County, Minnesota. Incidence of melanoma and overall and disease-specific survival rates were compared by age, sex, year of diagnosis, and stage of disease.
Results
From 1970 through 2009, age- and sex-adjusted incidence increased significantly over time (P<.001) from 7.9 to 60.0 per 100,000 person-years, with a 24-fold increase in women and a 4.5-fold increase in men. Although not significant (P=.06), incidence of melanoma increased with age. Overall and disease-specific survival improved over time, with hazard ratios of 0.94 (P<.001) and 0.93 (P<.001) for each 1-year increase in year of diagnosis, respectively. Each 1-year increase in age at diagnosis was associated with increased risk of death from any cause (hazard ratio, 1.07; P=.01) but was not significantly associated with disease-specific death. Sex was not significantly associated with death from any cause or death from disease. No patient with malignant melanoma in situ died from disease. Patients with stage II, III, and IV disease were over 14 times more likely to die from disease compared with patients with stage 0 or I disease (P<.001).
Conclusion
The incidence of cutaneous melanoma among middle-aged adults increased over the past 4 decades, especially in middle-aged women, while mortality decreased.
Keywords: cutaneous melanoma, epidemiology, incidence, mortality
Introduction
Skin cancer affects more people in the United States than any other malignancy, with cutaneous melanoma (hereafter called melanoma) being particularly deadly. Melanoma is diagnosed in more than 75,000 Americans each year, and more than 9,000 die from it annually.1 Melanoma is the sixth most frequently diagnosed cancer in developed countries,2 with the burden carried mainly by fair-skinned populations. It is within this demographic that melanoma incidence is the highest and is well documented across Australia and New Zealand,3,4 Europe,5-9 and North America.10,11 In the United States, the incidence for many common cancers, including prostate, colon, and breast cancer, either remains steady or has declined over time.12 Melanoma, however, steadily increases in incidence from year to year in non-Hispanic white men and women of all age groups and tumor thickness categories.12,13 In addition, melanoma has previously been shown to have a higher incidence rate among women younger than 45 years and in men older than 45 years.14,15
The US Census Bureau in 2010 estimated that the group of residents between 45 and 64 years of age has grown more rapidly during the preceding decade than any other age group. Interestingly, this group of individuals have more than 50% of the invasive melanomas diagnosed each year in the United States.15 To our knowledge, there have been few true population-based epidemiology studies looking at the incidence of malignant melanoma in middle-aged men and women. The studies used to estimate the natural history, distribution of subtypes, incidence, and disease-related mortality of melanoma among the middle-aged population have been so far limited to those performed in populations that were not very well defined16 or were biased by the underreporting and delayed reporting that characterize registry-based epidemiology studies.17-19 Jemal et al13 recently reported incidence rates of melanoma between 2002 and 2006 in the United States using data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. They estimated incidence rates at 43.5 and 34.0 per 100,000 persons for men and women between 40 and 64 years old, respectively. The calculated annual percent change in incidence rates from 1992 and 2006 was 3.0%.13 In 2012, Reed et al14 estimated the true age- and sex-specific incidence of melanoma in Olmsted County, Minnesota, in patients aged 18 to 39 years from 1970 through 2009. They found that melanoma among young adults is increasing rapidly, especially among women. The current study aims to estimate the true age- and sex-specific incidence of melanoma in adults aged 40 to 60 years in Olmsted County over the same period. Univariable and multivariable associations of select features with death from melanoma were assessed in this same population.
Patients and Methods
Patient Selection
After approval by the institutional review boards of Olmsted Medical Center and Mayo Clinic, 383 adults aged 40 to 60 years who were residents of Olmsted County, Minnesota, at their first lifetime diagnosis of cutaneous melanoma between January 1, 1970, through December 31, 2009, were identified using the resources of the Rochester Epidemiology Project (REP).20 The REP was created in 1966, when indexes of diagnoses were created for use by the medical professionals in Olmsted County, Minnesota. The result is linkage of medical data from almost all sources of medical care available to the local population of the county. Olmsted County had a population of 144,000 people in 2010, according to the US Census. The majority are non-Hispanic white persons who are socioeconomically similar to the general white population, despite a higher percentage of college graduates in Olmsted County.21 Approximately 95% of the residents of Olmsted County have given permission to use their medical records for research purposes. The REP allows researchers to estimate true incidence in the population for almost any disease.22
Statistical Methods
Incidence rates per 100,000 person-years were calculated overall and by decade using incident cases of melanoma as the numerator and age- and sex-specific estimates of the population of Olmsted County, Minnesota, as the denominator. The population at risk was estimated using US Census data from 1970, 1980, 1990, and 2000, with linear interpolation for intercensal years. Incidence rates were age- and sex-adjusted to the structure of the 2000 US white population.
The relationships between the incidence of melanoma and age at diagnosis, sex, and calendar year of diagnosis were assessed by fitting generalized linear models using the SAS procedure GENMOD (SAS Institute Inc, Cary, North Carolina). Incident cases were grouped into 4 age intervals (40-44, 45-49, 50-54, and 55-60 years) and 4 calendar year intervals (1970-1979, 1980-1989, 1990-1999, and 2000-2009). The models fit the natural logarithm of crude incidence rates as a linear function of age at diagnosis, sex, and calendar year of diagnosis, with a Poisson distribution used to model the error structure. The significance of the linear trends for the features of interest and interaction terms among these features was assessed using likelihood ratio statistics.
Changes in features by decade of diagnosis were evaluated using Kruskal-Wallis and trend tests. Overall survival and disease-specific survival were estimated using the Kaplan-Meier method. The duration of follow-up was calculated from the date of diagnosis to the date of death or last follow-up. Univariable and multivariable associations with death from any cause and death from disease were evaluated using Cox proportional hazards regression models and summarized with hazard ratios and 95% confidence intervals (CIs). The covariates used in the multivariable models included year of diagnosis, age at diagnosis, sex, and pathologic stage.
All analyses were performed using the SAS software package (version 9.2) and P<.05 was considered statistically significant.
Results
Using REP resources, we identified 383 middle-aged adults aged 40 to 60 years at their first lifetime diagnosis of melanoma between 1970 and 2009. Characteristics of the 383 adults under study are summarized in Table 1. Demographics include body distribution of melanoma, with the trunk (back) being the most commonly affected region of the body in men and the lower limbs (leg) in women. For this study, histologic slides were not examined to confirm diagnosis, as histologic examination of melanomas from prior REP incidence studies of young adults showed no discrepancies.14
Table 1. Summary of Features (N=383)a.
| Feature | Value |
|---|---|
| Age at diagnosis, y | 49.6 (49; 40-60) |
| Exact Breslow thickness (n=288), mm | 0.95 (0.54; 0.10-14.00) |
| Sex and site | |
| Women | 181 (47) |
| Head/neck | 19 (11) |
| Trunk | 51 (28) |
| Upper limb | 54 (30) |
| Lower limb | 57 (31) |
| Men | 202 (53) |
| Head/neck | 46 (23) |
| Trunk | 84 (42) |
| Upper limb | 49 (24) |
| Lower limb | 20 (10) |
| Unknown | 3 (1) |
| Decade of diagnosis | |
| 1970-1979 | 13 (3) |
| 1980-1989 | 41 (11) |
| 1990-1999 | 102 (27) |
| 2000-2009 | 227 (59) |
| Location (n=378) | |
| Left | 173 (46) |
| Right | 170 (45) |
| Central | 34 (9) |
| Bilateral | 1 (<1) |
| Breslow thickness (n=362) mm | |
| ≤1.00 | 304 (84) |
| 1.01-2.00 | 35 (10) |
| 2.01-4.00 | 15 (4) |
| >4.00 | 8 (2) |
| Base transected | 13 (3) |
| Clark level (n=368) | |
| I | 76 (21) |
| II | 177 (48) |
| III | 66 (18) |
| IV | 44 (12) |
| V | 5 (1) |
| Margins at initial incision (n=325) | |
| Negative | 221 (68) |
| Positive | 104 (32) |
| Histogenic type (n=318) | |
| SS | 209 (66) |
| SS in situ | 3 (1) |
| Nodular | 21 (7) |
| LM | 18 (6) |
| LM in situ | 12 (4) |
| MMIS | 52 (16) |
| Acral lentiginous | 3 (1) |
| Preexisting nevus (n=311) | |
| Absent | 209 (67) |
| Nevus NOS | 62 (20) |
| CN | 22 (7) |
| DN | 18 (6) |
| Pathologic stage (n=373) | |
| 0 | 69 (19) |
| IA | 239 (64) |
| IB | 33 (9) |
| IIA | 7 (2) |
| IIB | 8 (2) |
| IIC | 1 (<1) |
| III | 1 (<1) |
| IIIA | 5 (1) |
| IIIB | 3 (1) |
| IIIC | 1 (<1) |
| IV | 6 (2) |
Abbreviations: CN, compound nevus; DN, dermal nevus; LM, lentigo maligna; MMIS, malignant melanoma in situ; NOS, not otherwise specified; SS, superficial spreading.
Values are mean (median; range) or number (percentage).
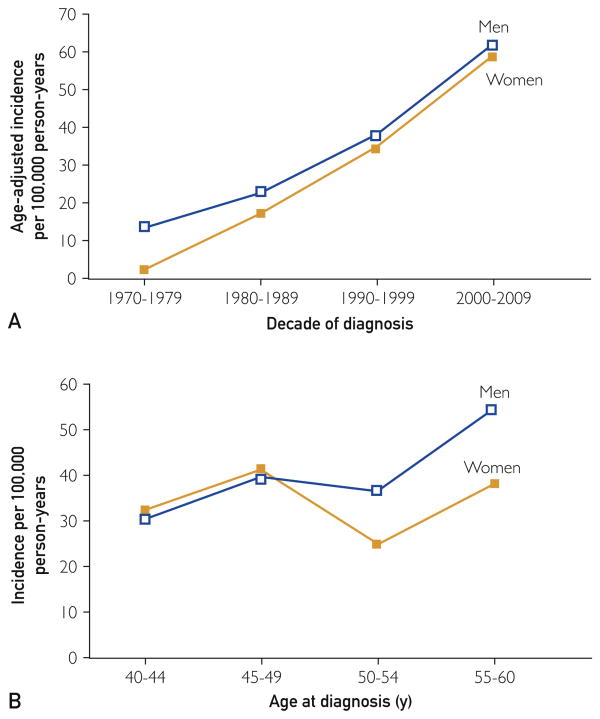
The overall age- and sex-adjusted melanoma incidence for the adults studied was 37.1 per 100,000 person-years. Incidence rates by age and decade at diagnosis are illustrated in Figure 1. Age-adjusted incidence was similar for women and men (34.5 and 39.8 per 100,000 person-years, respectively; P=.16). The incidence appeared to increase with age but was not statistically significant (P=.06). Interestingly, however, the incidence increased significantly by decade of diagnosis (P<.001) for both women and men. Age- and sex-adjusted incidence increased from 7.9 per 100,000 person-years in 1970-1979 to 60.0 per 100,000 person-years in 2000-2009, a 7.6-fold increase, with a 24-fold increase in women and a 4.5-fold increase in men. There were no statistically significant interactions among age at diagnosis, sex, and decade of diagnosis.
Figure 1.
Incidence per 100,000 person-years age-adjusted to 2000 US white population rates. A, By decade of diagnosis; B, by age.
Among the 373 patients with pathologic stage available, 69 (19%) were classified as stage 0 at diagnosis, 272 (73%) as stage I, and 32 (9%) as stage II, III, or IV disease. Incidence rates by stage are summarized and illustrated in Figure 2 and Supplemental Table 1.
Figure 2.
Age-adjusted incidence per 100,000 person-years by pathologic stage.
A comparison of select features by decade is shown in Supplemental Table 2. The first 2 decades were combined because only 13 patients received a diagnosis of melanoma in 1970-1979 (and fewer than 13 had complete data). There was evidence that Breslow thickness and pathologic stage decreased significantly over time.
At last follow-up, 52 patients had died at a mean of 7.1 years after diagnosis (median, 3.2 years; range, 0.1-30.6 years). Among the 331 patients still alive at last follow-up, the mean duration of follow-up was 9.9 years (median, 7.6 years; range, 0.1-37.1 years). Estimated overall survival rates (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis were 92% (89%-95%; 239), 87% (83%-91%; 140), 82% (76%-88%; 75), and 77% (70%-85%; 40), respectively. Univariable and multivariable associations of select features with death from any cause are summarized in Table 2. In a multivariable setting, each 1-year increase in calendar year of diagnosis was associated with a statistically significantly decreased risk of death from any cause (hazard ratio, 0.94; P<.001), while each 1-year increase in age at diagnosis was associated with a 7% increased risk of death from any cause (hazard ratio, 1.07; P=.01). There was no significant difference in death from any cause between women and men (P=.81).
Table 2. Associations With Death From Disease (n=382) and Death From Any Cause (N=383).
| Univariable | Multivariable | |||
|---|---|---|---|---|
|
|
|
|||
| Feature | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value |
| Associations with death from disease | ||||
| Year of diagnosis | 0.92 (0.89-0.95)a | <.001 | 0.93 (0.90-0.96)a | <.001 |
| Age at diagnosis | 1.02 (0.96-1.08)a | .53 | 1.00 (0.94-1.06)a | .98 |
| Sex | ||||
| Female | 1.0 (reference) | 1.0 (reference) | ||
| Male | 2.16 (1.03-4.52) | .04 | 1.58 (0.75-3.35) | .23 |
| Pathologic stage (n=372) | ||||
| 0, I | 1.0 (reference) | 1.0 (reference) | ||
| II, III, IV | 17.76 (8.92-35.35) | <.001 | 14.40 (7.10-29.23) | <.001 |
|
| ||||
| Associations with death from any cause | ||||
| Year of diagnosis | 0.93 (0.91-0.96)a | <.001 | 0.94 (0.91-0.97)a | <.001 |
| Age at diagnosis | 1.07 (1.02-1.12)a | .004 | 1.07 (1.02-1.12)a | .01 |
| Sex | ||||
| Female | 1.0 (reference) | 1.0 (reference) | ||
| Male | 1.49 (0.85-2.63) | .16 | 1.07 (0.60-1.91) | .81 |
| Pathologic stage (n=373) | ||||
| 0 | 1.0 (reference) | 1.0 (reference) | ||
| I | 6.53 (0.89-48.10) | .07 | 5.38 (0.73-39.82) | .10 |
| II, III, IV | 63.78 (8.54-476.45) | <.001 | 43.74 (5.80-330.14) | <.001 |
Abbreviation: CI, confidence interval.
Hazard ratio represents a 1-year increase.
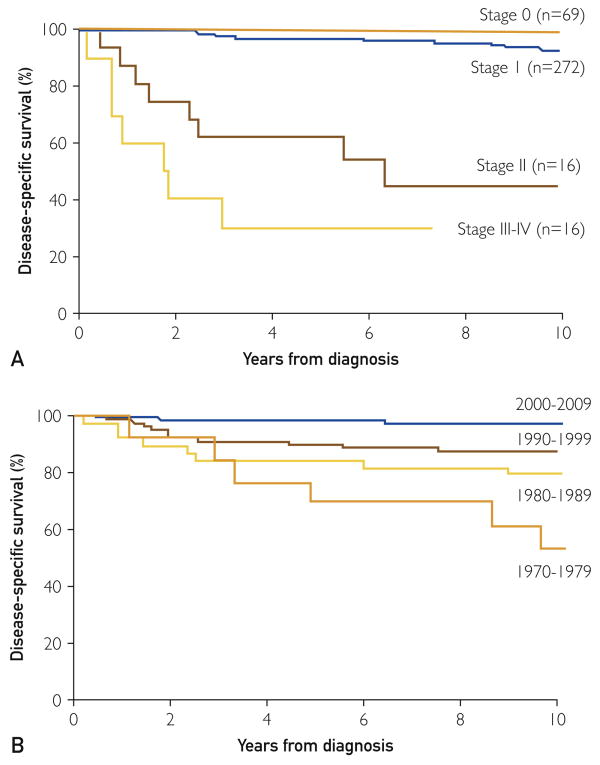
Among the 52 patients who died, 34 died from melanoma, 17 died from other causes, and 1 had an unknown cause of death and was therefore excluded from analyses of disease-specific survival. Estimated disease-specific survival rates (95% CI; number still at risk) at 5, 10, 15, and 20 years after diagnosis were 93% (91%-96%; 239), 90% (86%-94%; 140), 88% (84%-92%; 75), and 86% (81%-92%; 40), respectively. Univariable and multivariable associations of select features with death from disease are summarized in Table 2. In a multivariable setting, each 1-year increase in calendar year of diagnosis was associated with a statistically significantly decreased risk of death from melanoma (hazard ratio, 0.93; P<.001). Age (P=.98) and sex (P=.23) were not significantly associated with death from disease in a multivariable setting. No patients with stage 0 melanoma died from their disease, therefore stage 0 could not be used as reference group in a Cox proportional hazard regression model. As such, patients with stage 0 and stage I disease were combined for analysis. Patients with disease stage II, III, or IV were more than 14 times more likely to die from melanoma than patients with stage 0 or I disease (hazard ratio, 14.40; P<.001). Disease-specific survival by stage and decade of diagnosis is illustrated in Figure 3.
Figure 3.
A, Disease-specific survival by stage (P<.001, log-rank test). B, Disease-specific survival by decade (P<.001, log-rank test).
Discussion
The results of this population-based study confirm that the incidence of melanoma is increasing in the middle-aged population, the fastest growing segment of our society, despite reports to the contrary.18,23 The incidence rose 7.6-fold from the 1970s to the 2000s in Olmsted County, Minnesota, with increasing rates for all tumor thickness categories among our study population. The dramatic rise in incidence of melanoma was seen mainly in stage 0 and I tumors for both men and women during the study period, which is in line with data reported for non-Hispanic white persons across Europe and Australia.7,24 Women had 3-fold higher incidence rates of malignant melanoma in situ in the last decade. Stage I melanomas were equivalent between sexes, and the deeper Breslow depth lesions tended to occur more frequently in men. However, our study did not have the numbers to make statistically meaningful comparisons between men and women for deeper melanomas. As has been published in the young adult population,14 rates rose more dramatically among women, where the incidence increased by 24-fold; for men, the incidence increased by 4.5-fold. Although not statistically significant (P=.06), the incidence of melanoma increased with age in both men and women; rates were higher in women up to 50 years old and much higher in men after this age (Figure 1).
The convergence and parallel nature of incidence rates in the middle-aged population for women and men was not entirely expected (Figure 1), as recently published data suggest a divergence of incidence rates among women and men in the middle-aged population, with men demonstrating age-related increases with time.13,15 The higher rates of melanoma in women tend to parallel the years prior to menopause.14,25 In the current study, women aged 50 years or younger tended to have higher rates of melanoma, suggesting a potential hormonal influence during these years. The reason(s) behind this is not clear. However, these are the years when previous tanning bed use and UV exposure in the preceding 1 or 2 decades may have influenced the development of melanoma. The relationship between UV exposure and melanoma is well documented in the literature. A recent meta-analysis confirmed the association between the use of tanning beds and melanoma.26 Studies are ongoing at our institution to better define and explore potential hormonal influences in the development of melanoma.
The rates of cutaneous melanoma are clearly increasing over time, yet the disease-specific mortality has remained relatively stable and is decreasing in our study population.13 Our results are similar to those reported in central Europe and Australia, namely thinner, less-invasive melanomas, especially since the 1980s.24 Some argue that thin melanomas are the result of more liberal diagnostic criteria and are biologically insignificant, concluding that the melanoma epidemic is simply due to overdiagnosis.27,28 Ackerman et al29 were the first to attribute the increase in melanoma incidence to better diagnostic criteria. That group published the first study to focus on histopathologic criteria for diagnosis of melanoma “formulated on the basis of proven metastatic lesions” in the mid 1970s,30 the same decade in which melanoma rates began to increase substantially. The refinement of these criteria has led to more uniform teaching and application across the world. Moreover, earlier detection through educational programs, more skin cancer screenings, and increased public awareness, together with longer human lifespan and more dermatologists using improved clinical diagnostic algorithms for biopsy (which now includes dermoscopy), provide reasonable explanations for the divergence of incidence and mortality rates.14,31
Identifying melanoma before it has had a chance to invade or early in the invasion process undoubtedly leads to increased survival. Some would highlight this as a reason for overdiagnosis of melanoma, concluding that thin melanomas are morphologically malignant but biologically benign.32 In 1975, Clark et al33 argued that there was no biologic evidence to suggest that malignant melanoma in situ is a malignant disease. Our multivariable models, including year of diagnosis and stage, indicate that even after adjusting for stage, survival improved over time (Table 2). Likewise, after adjusting for improvement in survival over time, patients with high-stage disease were significantly more likely to die. In the middle-aged population, increasing rates are largely confined to melanomas thinner than 1 mm, yet recent data suggest that thin melanomas still represent 24% to 30% of disease-specific deaths.13,34 Our results also showed that one-fourth of melanoma-related deaths occurred in those with thin melanomas. Furthermore, we report an increasing incidence of not only thin melanomas but also all tumor thickness categories and subtypes, which confirms recent SEER program data showing similar increases in the 70,000 new cases of malignant melanoma from 1992 to 2004.11 We believe that all this evidence, together with the data reported here, is sufficient to suggest a true increase in the incidence of melanoma and not a phenomenon of overdiagnosis.
In our study population, men tended to have thicker melanomas and experienced poorer outcomes compared with women. Studies suggest that men are more likely to experience sunburns and have outdoor occupations and are less likely to use sunscreen,35 conduct skin self-examinations,36,37 or use melanoma screening programs.38,39 Invasive tumors are more prevalent in lower socioeconomic populations, in whom access to health care is poor and the availability of skin cancer screening is limited.34 The residents of Olmsted County, Minnesota, have relatively effortless access to health care. This variable remained constant throughout the duration of the study and may help explain why most patients had early-stage disease at the time of presentation. Mortality, as expected, was strongly associated with increased Breslow depth and advanced-stage disease. In our study, the incidence of advanced-stage melanoma remained constant over 4 decades (Figure 2); however, disease-specific mortality decreased significantly with each decade studied, most notably the last 10 years of the study (Figure 3).
A limitation of this study is the incomplete and accurate reporting of melanoma within medical records. However, it is unlikely that incident cases were missed, as final diagnoses from pathologic specimens are input into Olmsted County medical records. The population of Olmsted County may be another potential limitation as the majority of the population studied was white and well educated, with ready access to health care. Thus, care should be taken when extrapolating the results of this study to other subpopulations in the United States.
Conclusion
The incidence of cutaneous melanoma increased significantly over the past 4 decades in Olmsted County, Minnesota, with women experiencing higher rates of increase than men. Although the incidence of melanoma is increasing, death from disease seems to be decreasing with time. Close monitoring of middle-aged patients with regular skin cancer screening examinations is strongly recommended.
Supplementary Material
Acknowledgments
This study was made possible by the Rochester Epidemiology Project (grant number R01-AG034676; Principal Investigators: Walter A. Rocca, MD, and Barbara P. Yawn, MD, MSc).
Abbreviations
- CI
confidence interval
- REP
Rochester Epidemiology Project
Footnotes
Conflict of interest: None.
Publisher: To expedite proof approval, send proof via e-mail to scipubs@mayo.edu.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Erdmann F, Lortet-Tieulent J, Schuz J, et al. International trends in the incidence of malignant melanoma 1953-2008: are recent generations at higher or lower risk? Int J Cancer. 2013;132(2):385–400. doi: 10.1002/ijc.27616. [DOI] [PubMed] [Google Scholar]
- 3.Coory M, Baade P, Aitken J, Smithers M, McLeod GR, Ring I. Trends for in situ and invasive melanoma in Queensland, Australia, 1982-2002. Cancer Causes Control. 2006;17(1):21–27. doi: 10.1007/s10552-005-3637-4. [DOI] [PubMed] [Google Scholar]
- 4.Bulliard JL, Cox B. Cutaneous malignant melanoma in New Zealand: trends by anatomical site, 1969-1993. Int J Epidemiol. 2000;29(3):416–423. [PubMed] [Google Scholar]
- 5.de Vries E, Bray FI, Coebergh JW, Parkin DM. Changing epidemiology of malignant cutaneous melanoma in Europe 1953-1997: rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia. Int J Cancer. 2003;107(1):119–126. doi: 10.1002/ijc.11360. [DOI] [PubMed] [Google Scholar]
- 6.Holterhues C, de Vries E, Louwman MW, Koljenovic S, Nijsten T. Incidence and trends of cutaneous malignancies in the Netherlands, 1989-2005. J Invest Dermatol. 2010;130(7):1807–1812. doi: 10.1038/jid.2010.58. [DOI] [PubMed] [Google Scholar]
- 7.MacKie RM, Bray CA, Hole DJ, et al. Scottish Melanoma Group. Incidence of and survival from malignant melanoma in Scotland: an epidemiological study. Lancet. 2002;360(9333):587–591. doi: 10.1016/S0140-6736(02)09779-9. [DOI] [PubMed] [Google Scholar]
- 8.Downing A, Newton-Bishop JA, Forman D. Recent trends in cutaneous malignant melanoma in the Yorkshire region of England: incidence, mortality and survival in relation to stage of disease, 1993-2003. Br J Cancer. 2006;95(1):91–95. doi: 10.1038/sj.bjc.6603216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansson-Brahme E, Johansson H, Larsson O, Rutqvist LE, Ringborg U. Trends in incidence of cutaneous malignant melanoma in a Swedish population 1976-1994. Acta Oncol. 2002;41(2):138–146. doi: 10.1080/028418602753669508. [DOI] [PubMed] [Google Scholar]
- 10.Bulliard JL, Cox B, Semenciw R. Trends by anatomic site in the incidence of cutaneous malignant melanoma in Canada, 1969-93. Cancer Causes Control. 1999;10(5):407–416. doi: 10.1023/a:1008964621225. [DOI] [PubMed] [Google Scholar]
- 11.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129(7):1666–1674. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62(2):118–128. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S17–S25. doi: 10.1016/j.jaad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 14.Reed KB, Brewer JD, Lohse CM, Bringe KE, Pruitt CN, Gibson LE. Increasing incidence of melanoma among young adults: an epidemiological study in Olmsted County, Minnesota. Mayo Clin Proc. 2012;87(4):328–334. doi: 10.1016/j.mayocp.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson M, Johnson CJ, Chen VW, et al. Melanoma surveillance in the United States: overview of methods. J Am Acad Dermatol. 2011;65(5 suppl 1):S6–S16. doi: 10.1016/j.jaad.2011.04.037. [DOI] [PubMed] [Google Scholar]
- 16.Amerio P, Manzoli L, Auriemma M, et al. Epidemiology and clinical and pathologic characteristics of cutaneous malignant melanoma in Abruzzo (Italy) Int J Dermatol. 2009;48(7):718–722. doi: 10.1111/j.1365-4632.2009.03974.x. [DOI] [PubMed] [Google Scholar]
- 17.Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 18.Hall HI, Jamison P, Fulton JP, Clutter G, Roffers S, Parrish P. Reporting cutaneous melanoma to cancer registries in the United States. J Am Acad Dermatol. 2003;49(4):624–630. doi: 10.1067/s0190-9622(03)00885-5. [DOI] [PubMed] [Google Scholar]
- 19.Paterson IC, Beer H, Adams Jones D. Under-registration of melanoma in Wales in 1998: use of the capture-recapture method to estimate the ‘true’ incidence. Melanoma Res. 2001;11(2):141–145. doi: 10.1097/00008390-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 23.American Cancer Society. Cancer facts and figures 2008. Vol. 68 Atlanta, GA: American Cancer Society, Inc.; 2008. [Google Scholar]
- 24.Garbe C, McLeod GR, Buettner PG. Time trends of cutaneous melanoma in Queensland, Australia and Central Europe. Cancer. 2000;89(6):1269–1278. doi: 10.1002/1097-0142(20000915)89:6<1269::aid-cncr11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 25.Purdue MP, Freeman LE, Anderson WF, Tucker MA. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J Invest Dermatol. 2008;128(12):2905–2908. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher RP, Spinelli JJ, Lee TK. Tanning beds, sunlamps, and risk of cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev. 2005;14(3):562–566. doi: 10.1158/1055-9965.EPI-04-0564. [DOI] [PubMed] [Google Scholar]
- 27.Swerlick RA, Chen S. The melanoma epidemic: more apparent than real? Mayo Clin Proc. 1997;72(6):559–564. doi: 10.4065/72.6.559. [DOI] [PubMed] [Google Scholar]
- 28.Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331(7515):481. doi: 10.1136/bmj.38516.649537.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ackerman AB, Cavegn BM, Abad-Casintahan F, Robinson MJ. Ackerman's Resolving Quandaries in Dermatology, Pathology, and Dermatopathology. Philadelphia, PA: Promethean Medical Press; 1995. [Google Scholar]
- 30.Price NM, Rywlin AM, Ackerman AB. Histologic criteria for the diagnosis of superficial spreading malignant melanoma: formulated on the basis of proven metastatic lesions. Cancer. 1976;38(6):2434–2441. doi: 10.1002/1097-0142(197612)38:6<2434::aid-cncr2820380631>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Jemal A, Devesa SS, Hartge P, Tucker MA. Recent trends in cutaneous melanoma incidence among whites in the United States. J Natl Cancer Inst. 2001;93(9):678–683. doi: 10.1093/jnci/93.9.678. [DOI] [PubMed] [Google Scholar]
- 32.Glusac EJ. The melanoma ‘epidemic’, a dermatopathologist's perspective. J Cutan Pathol. 2011;38(3):264–267. doi: 10.1111/j.1600-0560.2010.01660.x. [DOI] [PubMed] [Google Scholar]
- 33.Clark WH, Jr, Ainsworth AM, Bernardino EA, Yang CH, Mihm CM, Jr, Reed RJ. The developmental biology of primary human malignant melanomas. Semin Oncol. 1975;2(2):83–103. [PubMed] [Google Scholar]
- 34.Criscione VD, Weinstock MA. Melanoma thickness trends in the United States, 1988-2006. J Invest Dermatol. 2010;130(3):793–797. doi: 10.1038/jid.2009.328. [DOI] [PubMed] [Google Scholar]
- 35.Robinson JK, Rigel DS, Amonette RA. Trends in sun exposure knowledge, attitudes, and behaviors: 1986 to 1996. J Am Acad Dermatol. 1997;37(2 Pt 1):179–186. doi: 10.1016/s0190-9622(97)80122-3. [DOI] [PubMed] [Google Scholar]
- 36.Brady MS, Oliveria SA, Christos PJ, et al. Patterns of detection in patients with cutaneous melanoma. Cancer. 2000;89(2):342–347. doi: 10.1002/1097-0142(20000715)89:2<342::aid-cncr19>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 37.Oliveria SA, Christos PJ, Halpern AC, Fine JA, Barnhill RL, Berwick M. Evaluation of factors associated with skin self-examination. Cancer Epidemiol Biomarkers Prev. 1999;8(11):971–978. [PubMed] [Google Scholar]
- 38.Geller AC, Swetter SM, Brooks K, Demierre MF, Yaroch AL. Screening, early detection, and trends for melanoma: current status (2000-2006) and future directions. J Am Acad Dermatol. 2007;57(4):555–572. doi: 10.1016/j.jaad.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 39.Koh HK, Miller DR, Geller AC, Clapp RW, Mercer MB, Lew RA. Who discovers melanoma? Patterns from a population-based survey. J Am Acad Dermatol. 1992;26(6):914–919. doi: 10.1016/0190-9622(92)70132-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.