Abstract
Recombination between moderately divergent DNA sequences is impaired compared with identical sequences. In yeast, an HO endonuclease-induced double-strand break can be repaired by single-strand annealing (SSA) between flanking homologous sequences. A 3% sequence divergence between 205-bp sequences flanking the double-strand break caused a 6-fold reduction in repair compared with identical sequences. This reduction in heteroduplex rejection was suppressed in a mismatch repair-defective msh6Δ strain and partially suppressed in an msh2 separation-of-function mutant. In mlh1Δ strains, heteroduplex rejection was greater than in msh6Δ strains but less than in wild type. Deleting PMS1, MLH2,or MLH3 had no effect on heteroduplex rejection, but a pms1Δ mlh2Δ mlh3Δ triple mutant resembled mlh1Δ. However, correction of the mismatches within heteroduplex SSA intermediates required PMS1 and MLH1 to the same extent as MSH2 and MSH6. An SSA competition assay in which either diverged or identical repeats can be used for repair showed that heteroduplex DNA is likely to be unwound rather than degraded. This conclusion is supported by the finding that deleting the SGS1 helicase also suppressed heteroduplex rejection.
Genetic recombination depends on the efficient and accurate search for homology between recipient and donor DNA substrates. Studies in both prokaryotes and eukaryotes have shown that mismatch repair proteins play a critical role in regulating this homology search during strand invasion (1, 2). A role for mismatch repair proteins in regulating recombination was first obtained in transformation studies performed in Pneumococcus. A small number of base–base differences between donor and recipient molecules significantly decreased the formation of stable transformants. This decrease, known as heteroduplex rejection, was suppressed by mutations in hexA and hexB, homologs of the Escherichia coli mismatch repair proteins MutS and MutL, respectively (3, 4). The MutS and MutL proteins play key roles in the repair of base pair mismatches; MutS binds to mispairs and MutL appears to interact with MutS-mispair complexes to initiate downstream mismatch repair steps (5–8). Subsequent studies in bacteria, yeast, and humans showed that mismatch repair plays a critical role in repressing recombination between moderately divergent (homeologous) sequences (9–12).
In Saccharomyces cerevisiae repair of mismatches arising during DNA replication or through heteroduplex DNA formation during recombination depends on the activity of several MutS and MutL homologs. Msh2p, Msh3p, Msh6p, and two MutL homologs, Mlh1p and Pms1p, have been shown to play major roles in mismatch repair, whereas two other MutL homologs, Mlh2p and Mlh3p, play specialized roles (13–19). These proteins appear to function as heterodimers in mismatch repair, because Msh2p–Msh3p, Msh2p–Msh6p, Mlh1p–Pms1p, Mlh1p–Mlh3p, and Mlh1p–Mlh2p complexes have been identified (20). Furthermore, the Msh2p–Msh6p complex shows a strong selectivity for base pair substitutions, whereas Msh2p–Msh3p preferentially recognizes loop insertion/deletion heterologies up to 12 nucleotides in size (14–16, 20, 21). msh2Δ, pms1Δ, and mlh1Δ mutants are equally defective in mismatch correction during DNA replication and in repairing heteroduplex DNA in meiosis (22, 23). Double mutants, such as pms1Δ mlh1Δ or mlh1Δ msh2Δ, exhibit epistasis, implying that these genes work in the same repair pathway (24).
In both vegetative and meiotic yeast cells, the presence of even a few mismatches can markedly reduce recombination (12, 25–31). These studies have also shown that mismatch repair mutations including msh2, msh6, pms1, and mlh1 can elevate the level of recombination events involving homeologous sequences (32, 33).
In addition to their role in mismatch correction and reduction in homeologous recombination, Msh2p and Msh3p play a third role in recombination, independent of Mlh1p, Pms1p, and Msh6p. During both gene conversion and single-strand annealing (SSA), nonhomologous 3′-ended single-strand DNA ends must be clipped off before a 3′ end is exposed that can be used to prime new DNA synthesis (34–36). Removal of nonhomologous tails at the junction between base-paired and unpaired DNA requires the Rad1p–Rad10p endonuclease and the Msh2p–Msh3p complex.
For this study we used S. cerevisiae to examine the effect of mismatch repair on the formation and repair of heteroduplex DNA that results by means of the SSA pathway. This process involves the repair of a single double-strand break (DSB) induced in vivo that occurs between repeated sequences. DNA resection occurs at the 5′ ends resulting in single-stranded tails that can anneal and ultimately create a deletion (Fig. 1A). A decrease in SSA is observed when a 3% sequence divergence is introduced within 205-bp repeats flanking the DSB (34). We show that the decrease in SSA because of sequence divergence depends much more on Msh6p than on the Mlh proteins, of which only Mlh1p has a discernable effect. In contrast, mismatches formed during SSA are still subject to mismatch correction by both the Msh2p–Msh6p and Mlh1p–Pms1p complexes. Evidence suggesting that heteroduplexed DNA strands are unwound rather than degraded is supported by the finding that heteroduplex rejection also requires the Sgs1 helicase. We propose that the decrease in SSA as the result of sequence divergence results from a heteroduplex DNA rejection mechanism that is distinct from the repair of mismatches in heteroduplex DNA.
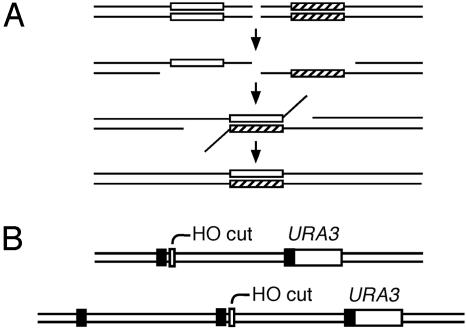
Fig. 1.
SSA by using a homeologous substrate. (A) An in vivo DSB created between two repeated sequences initiates the 5′ to 3′ resection of one DNA strand, creating 3′ single-strand DNA tails. Annealing of single-strand DNA at complementary sequences creates an intermediate with mismatched base pairs. Nonhomologous tails are removed by Rad1p–Rad10p endonuclease with the assistance of Msh2p–Msh3p, and the gaps are filled in and ligated. If mismatches are not corrected by mismatch repair, progeny containing both genotypes (sectored colony) will result. (B) SSA substrates contain an HO cut site flanked by two or three 205-bp sequences derived from URA3. Black boxes indicate the repeated sequences.
Materials and Methods
Strains. Strain tNS1379 carries a duplication of 205-bp URA3 sequences (designated A-A) separated by 178 bp of pUC9 DNA, the HO cut site (117 bp) derived from MATa, and 2.3-kb λ DNA (Fig. 1B; described in refs. 34 and 37). In strain tNS1357, the leftward URA3 repeat was replaced by sequences derived from strain FL100 and differs by seven single-site mutations (Fig. 4, which is published as supporting information on the PNAS web site); this arrangement is designated F-A (34). The A-A-A (tNS2038) and A-F-A (tNS2041) strains (Fig. 1B) were made by respectively transforming tNS1366 and tNS1116 with pNSU255 cut with PvuI, so that there are two repeats, separated by 2.9 kb, to the left of the HO cleavage site. These strains contained pJH727 (LEU2 GAL::HO ARS CEN) or GAL10::HO integrated into the ADE3 locus (38). Additional details are provided in Supporting Materials, which is published as supporting information on the PNAS web site.
Analysis of SSA. HO was induced by addition of galactose (2% wt/vol final concentration) to cultures grown in yeast extract/peptone/lactate medium (37). DNA was extracted at intervals after induction, digested with BglII, and analyzed by Southern hybridization (37). The efficiency of SSA was also determined by comparing the viability of cells after GAL::HO induction to those of the preinduced culture, corrected for cells that had lost the GAL::HO TRP1 plasmid pFH800, as described in ref. 34. Cell viabilities in the competition assay were determined by plating equal numbers of cells on selective medium lacking leucine (to maintain the GAL::HO plasmid pJH727) and containing either galactose or glucose. The PCR assay of SSA product was carried out by adjusting the genomic DNA so that equal amounts of final SSA product were formed. Primers (5′ to 3′) were TGAGTAGCAGCACGTTCC and GCACCATATGCGGTGTG (to assay SSA) and AGAAAGGGGGTATTATCAATGGCTC and AGGAAAATCACGGCGCAAAA (arg5,6).
Mismatch repair was assayed by inducing cells for 1 hr with galactose (2% wt/vol) and extracting DNA from cultures derived from unbudded cells that had been isolated by micromanipulation within a 30-min period. DNA was analyzed by Southern analysis for the presence of the MspI site in ura3-FL100 and absence in ura3-A (Fig. 4) (39).
Results
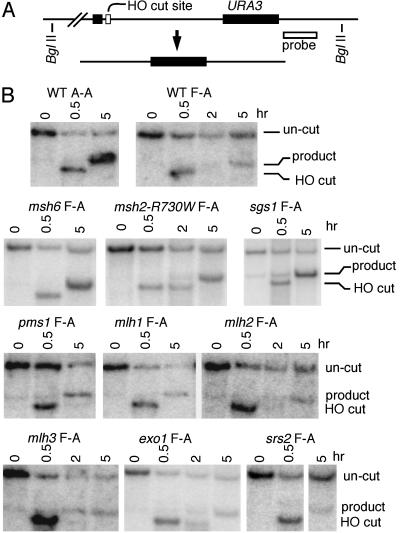
The Effect of Mismatch Repair-Null Mutations on SSA Between Homeologous DNA Sequences. We developed an assay to monitor SSA between homologous and homeologous repeats (Fig. 1 A). In this system, HO endonuclease was induced to create a single DSB between 205-bp repeated segments near the URA3 gene. Induction of a GAL::HO gene carried on a centromeric plasmid efficiently induced SSA and created deletion products that could be monitored by Southern hybridization (Fig. 2). When the repeat regions were identical (A-A substrate), SSA occurred efficiently, as shown by densitometric analysis of deletion products (Fig. 2B and Table 1). In contrast, when the flanking regions differed by seven single base pair heterologies (F-A), consisting of six single base pair substitutions and one 1-bp insertion/deletion, SSA was reduced about 6-fold. As shown in Fig. 2 and Table 1, the reduction in SSA observed with homeologous substrates (F-A) was largely suppressed in msh6Δ strains (P < 0.001). Similar results were obtained by assaying cell viability, which measures how often SSA repairs the DSB. Whereas the ratio of cell survival for F-A vs. A-A strains was 0.20 for wild type, it was 0.77 for msh6Δ. We were unable to determine the effect of msh2Δ or msh3Δ mutations because Msh2p–Msh3p is required for the excision of nonhomologous single-strand tails during SSA (34).
Fig. 2.
Southern hybridization analysis of SSA. (A) HO endonuclease cleaves at its recognition site between 205-bp repeats of ura3 sequence (solid boxes) leading to a deletion. The ura3 sequence on the left consists of either the ura3-A or ura3-FL100 allele, whereas the right sequence contains ura3-A. The probe used for Southern blotting is a HindIII–BamHI fragment downstream of URA3. (B) DNA was extracted and digested with BglII from wild-type strains, tNS1379 and tNS1357 (A-A and F-A respectively), and the following derivatives of tNS1357: msh6Δ (tNS1600), msh2-R730W (tNS1826), sgs1Δ (EAY994), pms1Δ (tNS1394), mlh1Δ (tNS1396), mlh2Δ (tNS1916), mlh3Δ (tNS1909), exo1Δ (tNS1678), and srs2Δ (tNS1631). Southern blots show the uncut band before induction, the HO-cleaved band (4.8 kb) at 0.5 h, and the product band (5.5 kb) after 5 h of induction.
Table 1. Effect of mismatch repair and sgs1Δ mutations on the efficiency of SSA.
| Product formation
|
||
|---|---|---|
| Genotype | A-A substrate | F-A substrate |
| Wild type | 0.92 (0.23) | 0.15 (0.05) |
| msh6Δ | 1.11 (0.27) | 0.71 (0.20) |
| msh2-R730W (pEAA83)* | 0.92 (0.18) | 0.40 (0.09) |
| MSH2 (pEAA54)* | 1.03 (0.13) | 0.23 (0.03) |
| mlh1::LEU2 | 0.86 (0.06) | 0.26 (0.10) |
| mlh1Δ::KANMX | 0.79 (0.21) | 0.38 (0.09) |
| pms1Δ | 0.87 (0.10) | 0.13 (0.02) |
| mlh2Δ | 1.25 (0.39) | 0.13 (0.02) |
| mlh3Δ | 1.07 (0.18) | 0.12 (0.04) |
| mlh1::LEU2 pms1Δ | n.d. | 0.17 (0.01) |
| mlh1::LEU2 mlh2Δ | n.d. | 0.23 (0.04) |
| mlh1::LEU2 mlh3Δ | n.d. | 0.37 (0.10) |
| mlh2Δmlh3Δ | n.d. | 0.15 (0.03) |
| pms1Δmlh3Δ | 0.82 (0.01) | 0.22 (0.03) |
| pms1Δmlh2Δ | n.d. | 0.08 (0.02) |
| pms1Δmlh2Δmlh3Δ | n.d. | 0.39 (0.04) |
| pms1Δmlh1Δ::KANMX | 0.79 (0.19) | n.d. |
| pms1Δmlh1Δ::KANMX mlh3Δ | 0.69 (0.10) | n.d. |
| sgs1Δ | 0.72 (0.13) | 0.75 (0.06) |
| srs2Δ | 0.38 (0.18) | 0.06 (0.03) |
| exo1Δ | 0.73 (0.20) | 0.11 (0.02) |
The intensity of the product band 5 hr after HO induction was divided by the intensity of the 0-h uncut band. This ratio was normalized to the same ratio derived from an uncut control sequence and corrected for the fraction of colonies containing pFH800 (GAL10::HO TRP1) prior to induction. One SD of the mean is shown in parentheses. n.d., not determined.
Centromere plasmids pEAA83 and pEAA54 bearing msh2-R730W or MSH2, respectively, were transformed into a msh2Δ strain
Unlike msh6Δ, single deletions of MutL-homolog genes had little or no effect on SSA of either the A-A or F-A constructs. Surprisingly, pms1Δ was not different from wild type in the level of SSA product formed (Fig. 2 and Table 1). The increase in SSA product formation for the F-A substrate in mlh1 strains is statistically significantly different from wild-type strains based on a t test (P < 0.01) but also statistically different from msh6Δ (Table 1). We analyzed mlh1Δ::KANMX, a complete deletion, and mlh1::LEU2, a deletion of the MLH1 promoter and the first 100 aa (17), where the remaining segment might still be fortuitously transcribed and translated. The mlh1::LEU2 allele appears to retain some activity in suppressing spontaneous homeologous recombination (S. Jinks-Robertson, personal communication). Consistent with this activity, the mlh1::LEU2 allele was similar to wild type when measured by cell survival, yielding a (F-A)/(A-A) ratio of 0.21 (wild type = 0.20).
A previous study indicated that Mlh3p preferentially acts in the correction of insertions/deletions in runs of mononucleotides, particularly in runs of Ts (21). One of the seven heterologies in the 205-bp substrates is an insertion/deletion in a run of Ts (10 vs. 11 bp) (Fig. 4). However, neither the mlh3Δ nor mlh2Δ mutations affected SSA in the A-A or F-A strains (Table 1). None of the double-mutant combinations of pms1Δ, mlh2Δ, or mlh3Δ exhibited any suppression of heteroduplex rejection (Table 1); however, the triple mutant pms1Δ mlh2Δ mlh3Δ was similar to mlh1Δ::KANMX. This result suggests that Mlh1p might act with any of three heterodimeric partners to carry out heteroduplex rejection; alternatively, the absence of Mlh1p or all of its Mlh/Pms partners might have an indirect effect on the abundance or activity of Msh2p–Msh6p.
Separation of Function Mutations in MSH2 Partially Suppress Heteroduplex Rejection. We isolated the msh2-R730W mutant that is defective in mismatch repair, as judged by an elevated spontaneous mutation frequency, but is capable of removing nonhomologous ends of DNA in an SSA assay (40). The defect conferred by this mutation was independent of whether it was on a plasmid or integrated into its endogenous chromosomal location (data not shown). Thus, SSA in msh2-R730W strains with the A-A substrate occurred at ≈90% of the wild-type level (Table 1). With the F-A repeats, msh2-R730W strains displayed a 2-fold increase in SSA compared with wild type (Table 1), indicating that the msh2-R730W mutation can partially suppress heteroduplex rejection.
MLH1, PMS1, MSH2, and MSH6 Are Necessary for Mismatch Repair of F-A Heteroduplex DNA. When SSA occurs between the F and A repeats, heteroduplex DNA will contain several mispairs. If these mismatches are corrected, then descendants of the original cell will have the same genotype, but if not, each strand will be used as a template for DNA replication and both alleles will be found among the progeny. One of the seven heterologies contains an MspI restriction site in the F sequence. Hence, the progeny from each SSA event from an F-A strain can be scored to determine whether they are all MspI+ (F), all MspI- (A), or a mixture (F and A) by analyzing MspI -digested DNA extracted from a culture derived from a G1 cell. Cells were grown in liquid medium, induced for HO expression for 1 hr, and then streaked on agar plates so that unbudded (G1) cells could be isolated by micromanipulation and grown into colonies.
In a wild-type strain, 81% of SSA colonies were homozygous for MspI+ (F) or MspI- (A), whereas 13% were mixed (F-A) (Table 2). Similar ratios of mixed and unmixed colonies were also found for mlh2Δ, mlh3Δ, and sgs1Δ strains, indicating that mismatch repair is proficient in these strains. In contrast, ≈70% of the colonies were mixed for the F and A alleles in msh6Δ, pms1Δ, mlh1::LEU2, and msh2-R730W strains, which signifies a defect in mismatch repair. We conclude that Pms1p is required for mismatch repair, although it is not used in heteroduplex rejection.
Table 2. Mismatch correction of heteroduplexes arising during SSA between F and A flanking sequences.
| No. of colonies
|
||||
|---|---|---|---|---|
| Mutant | MspI+ (F) | MspI- (A) | Mixed (F-A) | Percent mixed |
| Wild type | 22 | 4 | 4 | 13 |
| msh6Δ | 3 | 3 | 14 | 70 |
| msh2-R730W | 4 | 4 | 17 | 68 |
| pms1Δ | 2 | 2 | 9 | 69 |
| mlh1::LEU2 | 4 | 3 | 16 | 70 |
| mlh2Δ | 16 | 3 | 1 | 5 |
| mlh3Δ | 20 | 6 | 4 | 13 |
| mlh1Δmsh6Δ | 5 | 3 | 21 | 72 |
| pms1Δmsh6Δ | 0 | 2 | 8 | 80 |
| sgs1Δ | 12 | 4 | 1 | 6 |
| exo1Δ | 21 | 5 | 4 | 13 |
Colonies were derived from unbudded cells after 1 h of galactose induction and analyzed for the presence of the MspI site (see Materials and Methods) present in the ura3-FL100 (F) allele. Mixed colonies contain cells with and without the MspI site.
Among the colonies that accomplished mismatch repair, there was a 6:1 bias in favor of the F allele. This ratio may reflect a repair bias intrinsic to the C/A mismatch in this context, or it could reflect the influence of nearby heterologies. Alternatively, it could be caused by a directed bias in mismatch correction in much the same way as mismatch repair is biased during strand invasion during mitotic DSB-induced gene conversion (41, 42). No apparent preference for the A or F allele was observed in strains defective in mismatch repair, suggesting that a residual repair pathway is unlikely to possess such a bias.
A Competition Assay Suggests Heteroduplex Rejection Occurs by DNA Unwinding. Heteroduplex rejection could occur in several different ways. One process would be similar to mismatch repair itself, in that excision of DNA on one strand of the heteroduplex could destroy the SSA intermediate if the excision of the mismatch extends to the end of the annealed sequence. Similarly, two independent resections beginning on different strands could destroy the intermediate entirely. Alternatively the two annealed strands could simply be unwound.
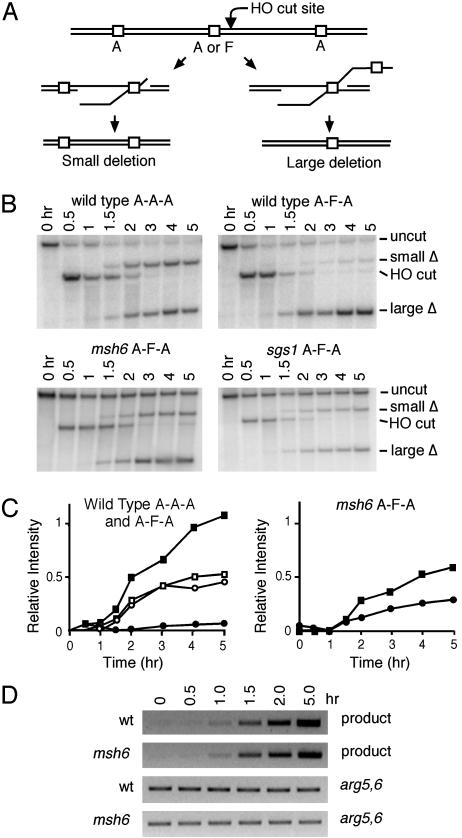
To distinguish among these mechanisms, we constructed two strains that have three regions of 205-bp homology in the vicinity of the DSB (Fig. 3A). The first strain carries three identical A repeats (A-A-A). When a DSB is created, SSA can occur with either of the two A sequences on the left side of the DSB, creating two different-sized deletions that can be distinguished on a Southern blot (Fig. 3B). Densitometric analysis showed that ≈50% of the deletions use the closer homology (Table 3). In the second strain, the middle sequence was changed to F (designated A-F-A). In this instance, nearly all of the deletions were formed between the identical A sequences, (93%, Table 3 and Fig. 3B). Significantly, cell viability was statistically indistinguishable (74%) from the case in which all three sequences were identical (79%; P > 0.05).
Fig. 3.
A competition assay to examine the mechanism of heteroduplex rejection. (A) A DSB was created so that the URA3 sequence to the right of the break can anneal with one of two 205-bp segments to the left to form a small or a large deletion. The three repeats are either all identical A sequences (A-A-A) or the middle repeat contains the 3% mismatched F sequence (A-F-A). (B) Southern blots (as described in Fig. 2) show that nearly all of the deletions in the A-F-A substrate (EAY1139) are to the distal, perfectly matched partner, in contrast to the A-A-A substrate in the wild-type (EAY1137), msh6Δ A-F-A, or sgs1Δ A-F-A strains. (C) Densitometric analysis of the kinetics of forming large (▪) and small (•) deletions in wild-type and msh6Δ A-F-A strains and large (□) and small (○) deletions in a wild-type A-A-A strain. (D) Wild-type (tNS1357) and msh6Δ (tNS1600) F-A strains were induced and DNA was extracted at the time points shown. SSA product was formed with the same kinetics when assayed by PCR with primers flanking the repeats. Reverse images are shown. Quantities of genomic DNA were adjusted so that equal amounts of final SSA product were formed by using DNA from wild-type and msh6Δ strains.
Table 3. Effect of mismatch repair and sgs1Δ mutations on the distribution of A-A and F-A SSA events.
| A-A-A substrate
|
A-F-A substrate
|
|||
|---|---|---|---|---|
| Genotype | Small Δ | Large Δ | Small Δ | Large Δ |
| Wild type | 47.4 (1.7) | 52.6 (1.7) | 7.1 (2.6) | 92.9 (2.6) |
| msh6Δ | 49.1 (1.2) | 50.9 (1.2) | 37.8 (2.1) | 62.2 (2.1) |
| sgs1Δ | 49.9 (0.3) | 50.1 (0.3) | 32.7 (2.3) | 67.2 (2.3) |
| mlh1Δ::KANMX | 44.9 (3.0) | 55.1 (3.0) | 11.8 (1.2) | 88.2 (1.2) |
| msh2-R730W | 45.1 (1.5) | 54.9 (1.5) | 16.3 (0.6) | 83.7 (0.6) |
The relative intensities (±SD) of the small and large deletion products are shown 5 h after inducing HO expression. In all experiments, the overall product formation was similar to the intensity of the starting substrate prior to HO induction. The (total product):(initial substrate) ratio for A-A-A strains was 1.0 ± 0.07 for wild type, 0.92 ± 0.21 for msh6Δ, and 0.90 ± 0.21 for sgs1Δ. For the A-F-A strains, the ratio was 1.1 ± 0.03 for wild type, 0.65 ± 0.16 for msh6Δ, and 0.74 ± 0.06 for sgs1Δ.
These data support models in which the annealed intermediate is unwound rather than largely destroyed by nucleases. We assume that 50% of the initial encounters between single strands were between the closer pair, as seen when all sequences are identical. If this scenario also occurred in the case in which the middle sequence is divergent, then unwinding the sequence would allow the centromere proximal A segment to continue its search for a partner until the more distal A region was found. If the heteroduplex formed between F and A led to its complete destruction by nucleases, then there would no longer be a chance for SSA to occur, and viability should have dropped by 50% relative to the A-A-A strain. If only one of the two strands were degraded at random, then there should have been a 25% decrease, where the inviability results from removal of the A repeat. This decrease was not observed.
By using 30-min time points, we were unable to see a difference in the time of appearance of the large and small deletion products in A-A-A or A-F-A strains (Fig. 3B) in wild-type, msh6Δ, or sgs1Δ backgrounds. The effect of msh6Δ is shown quantitatively in Fig. 3C. We also observed similar kinetics when comparing F-A wild-type and msh6Δ strains in a PCR assay for which the amounts of genomic DNA were adjusted so that the amount of final product formation was the same in the wild-type and msh6 strains (Fig. 3D). This result suggests that the mechanisms of heteroduplex rejection and mismatch repair do not alter the initial kinetics of product formation; instead, they limit the amount of product formed.
Sgs1 Helicase Is Required for Heteroduplex Rejection During SSA. To test whether heteroduplex rejection occurs through an unwinding mechanism, we examined SSA in sgs1Δ, srs2Δ, and exo1Δ strains. The Sgs1 helicase was previously implicated in suppressing homeologous recombination between inverted repeated sequences occurring by spontaneous gene conversion (43). The Srs2 helicase facilitates the removal of nonhomologous DNA ends during DSB-induced gene conversion (35) and appears to have overlapping functions with Sgs1p (44, 45). Exo1p has been implicated in mismatch repair through interactions with Msh2p. In addition, exo1Δ strains are moderately defective in preventing homeologous recombination between inverted repeat sequences (32, 46–48). As shown in Table 1 and Fig. 2, the sgs1Δ mutation suppressed heteroduplex rejection at a level similar to that seen in msh6Δ. The srs2Δ and exo1Δ mutations showed no effect in our assay.
We also examined msh6Δ and sgs1Δ for their effect on SSA in A-F-A and A-A-A strains (Table 3 and Fig. 3). Both msh6Δ and sgs1Δ restored the fraction of F-A annealings to nearly the level observed with the wild-type A-A-A strain. In contrast, the mlh1Δ and msh2-R730W mutants behaved similarly to wild type in preventing homeologous recombination (Table 3). These data provide additional evidence that MSH6 and SGS1 play a primary role in preventing homeologous recombination, whereas MLH1 plays a much less significant role. One explanation for why the mlh1Δ and msh2-R730W mutants display nearly wild type phenotypes in A-F-A strains is that, in contrast to strains with only F-A, the A-F-A strains have a homologous sequence available that can be used for repair after an initial rejection/unwinding event. In F-A strains, only a homeologous substrate is available, but it might be used in repeated rounds of annealing/rejection before the cells die.
Discussion
Efficient recombination begins with a search for a homologous partner. Important steps in this process are the assessment of sequence identity and then the rejection or correction of mismatched heteroduplex intermediates. In the SSA process described here, Sgs1p and Msh6p (and presumably Msh2p) play important roles in heteroduplex rejection. In contrast, Pms1p and Mlh1p are very important for mismatch repair of SSA intermediates but play a much less prominent role in heteroduplex rejection. Surprisingly pms1Δ had no effect on heteroduplex rejection. A partial deficiency in heteroduplex rejection was only observed when PMS1, MLH2, and MLH3 were all deleted. Mlh1p has been previously shown to form heterodimers with Pms1p, Mlh2p, and Mlh3p (20). Either Mlh1p functions indiscriminately with any heterodimeric partner in this process, or the absence of all three partner proteins reduces the abundance of Mlh1p. We conclude that mismatch repair and heteroduplex rejection are distinctly different processes and that heteroduplex rejection occurs by unwinding a heteroduplex intermediate rather than by destroying the intermediate by excision repair.
SSA is a nonconservative process that appears to play a role in repairing DSBs arising within repeated tandem arrays, such as in the rRNA-encoding DNA locus of S. cerevisiae (49). Such arrays maintain a very high degree of sequence homogeneity. Here we investigated how the processes of heteroduplex rejection and mismatch repair act to conserve homogeneity when repairing a DSB by SSA. SSA is a unique process for investigating mismatch repair and heteroduplex rejection because it provides a straightforward way of generating heteroduplex DNA in vivo. In contrast, other studies of the role of mismatch repair proteins in homeologous recombination in mitotic cells have analyzed spontaneous gene conversion and crossing-over that presumably involve the encounter of a single strand with an intact double-stranded DNA template. In these studies, msh2Δ exhibits a 3-fold stronger suppression of homeologous recombination than mlh1Δ (S. Jinks-Robertson, personal communication); similarly, when sequence divergence was 6–9%, msh2Δ increased homeologous recombination more than pms1Δ (12). In our study, PMS1 appears to have no antirecombination role in SSA.
The clear distinction between msh6Δ and pms1Δ in our study suggests that there could be additional steps in the assessment of heterology in the more complicated case where a single strand invades duplex DNA and where Pms1p clearly plays a role (12, 30, 31). One difference between our assays and those used in previous studies is that SSA occurs without the assistance of the strand exchange protein Rad51 (50, 51), whereas HO-induced gene conversions primarily proceed by strand exchanges mediated by Rad51p (52–54). There may be additional complexities when mismatches are encountered in the context of a Rad51 protein filament involving both the single-stranded invading strand and the template duplex DNA. The idea that mismatch repair proteins may operate in the context of such a filament was raised by the studies of E. coli MutS protein associated with the Rad51p homolog, RecA (55). We note also that the 6-fold inhibition of SSA caused by 3% heterology in our study is less than the 14- to 50-fold inhibition caused by 1% heterology in spontaneous recombination assays of gene conversion and crossing-over (12, 30, 31).
How heteroduplex rejection is accomplished is not fully understood. Our data suggest strongly that it does not occur simply by the normal mismatch repair process in which a mismatched base and a substantial amount of surrounding DNA are removed nucleolytically. Given that SSA must occur within a 205-bp region, extensive excision of DNA around mismatched bases during an attempt at homeologous SSA would preclude subsequent annealing at a more distant homologous locus. Rather, our data suggest that the mismatched DNA strands are unwound so that they can continue to search for homology. We also considered the possibility that the F sequence in the A-F-A strain was preferentially degraded, allowing only the A sequences to anneal. Our results from the F-A strains, however, showed that there was a strong bias to repair in favor of the F sequence rather than the A sequence, which would not occur if the F sequence were preferentially degraded. In support of the unwinding model, we found that the Sgs1p helicase was essential for heteroduplex rejection but not mismatch repair and that the Msh2-associated exonuclease Exo1p does not play an important role in heteroduplex rejection. In addition, sgs1-hd, which disrupts the helicase activity of SGS1 (56), confers a phenotype in the SSA F-A assay that is indistinguishable from the sgs1Δ mutation (T.G. and E.A., unpublished data). It is possible that there could be redundant exonucleases, as there are in mismatch repair in bacteria (57), but studies of Amin et al. (48), which looked for additional genes involved in mismatch repair, failed to support this possibility.
We imagine heteroduplex rejection requires the Sgs1 helicase that takes its cue from Msh2p–Msh6p. In support of this idea, a physical interaction between Sgs1 and Msh6 proteins was reported using tandem affinity purification analysis (58), and BLM, a RecQ/Sgs1P homolog, was shown to interact with human Msh6p both in vivo and in vitro (59). Alternatively, the Msh2p–Msh6p complex could bind to mismatches as they form in heteroduplex DNA and block further annealing. Evidence for such a mechanism was obtained by Worth and Modrich (55), who showed that mutS prevented RecA-mediated strand transfer between divergent sequences. Although SSA does not require Rad51p, the yeast recA homolog, it is conceivable that Msh2p complexes interfere with the annealing of strands mediated by other gene products. Whether this type of interference would lead to the results seen with the A-F-A competition strain depends on whether the trapped heteroduplex could be reversed.
Supplementary Material
Acknowledgments
We thank members of the Alani and Haber laboratories for their suggestions. This work was supported by a Natural Sciences and Engineering Research Council of Canada Post Graduate Scholarship B Award (to T.G.), National Institutes of Health Grants GM53085 (to E.A.) and GM20056 (to J.E.H.), and a New York State Fellowship and a Cornell University Anonymous Donor Fellowship (to B.S.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SSA, single-strand annealing; DSB, double-strand break.
References
- 1.Evans, E., Sugawara, N., Haber, J. E. & Alani, E. (2000) Mol. Cell 5, 789-799. [DOI] [PubMed] [Google Scholar]
- 2.Pāques, F. & Haber, J. E. (1999) Microbiol. Mol. Biol. Rev. 63, 349-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humbert, O., Prudhomme, M., Hakenbeck, R., Dowson, C. G. & Claverys, J. P. (1995) Proc. Natl. Acad. Sci. USA 92, 9052-9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prudhomme, M., Martin, B., Mejean, V. & Claverys, J. P. (1989) J. Bacteriol. 171, 5332-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grilley, M., Welsh, K. M., Su, S. S. & Modrich, P. (1989) J. Biol. Chem. 264, 1000-1004. [PubMed] [Google Scholar]
- 6.Lahue, R. S., Au, K. G. & Modrich, P. (1989) Science 245, 160-164. [DOI] [PubMed] [Google Scholar]
- 7.Ban, C. & Yang, W. (1998) Cell 95, 541-552. [DOI] [PubMed] [Google Scholar]
- 8.Ban, C., Junop, M. & Yang, W. (1999) Cell 97, 85-97. [DOI] [PubMed] [Google Scholar]
- 9.Matic, I., Taddei, F. & Radman, M. (1996) Trends Microbiol. 4, 69-72. [DOI] [PubMed] [Google Scholar]
- 10.Modrich, P. & Lahue, R. (1996) Annu. Rev. Biochem. 65, 101-133. [DOI] [PubMed] [Google Scholar]
- 11.te Riele, H., Maandag, E. R. & Berns, A. (1992) Proc. Natl. Acad. Sci. USA 89, 5128-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, W. & Jinks-Robertson, S. (1998) Mol. Cell. Biol. 18, 6525-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, R. E., Kovvali, G. K., Prakash, L. & Prakash, S. (1996) J. Biol. Chem. 271, 7285-7288. [DOI] [PubMed] [Google Scholar]
- 14.Habraken, Y., Sung, P., Prakash, L. & Prakash, S. (1997) Curr. Biol. 7, 790-793. [DOI] [PubMed] [Google Scholar]
- 15.Marsischky, G. T., Filosi, N., Kane, M. F. & Kolodner, R. (1996) Genes Dev. 10, 407-420. [DOI] [PubMed] [Google Scholar]
- 16.Prolla, T. A., Christie, D. M. & Liskay, R. M. (1994) Mol. Cell. Biol. 14, 407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prolla, T. A., Pang, Q., Alani, E., Kolodner, R. D. & Liskay, R. M. (1994) Science 265, 1091-1093. [DOI] [PubMed] [Google Scholar]
- 18.Harfe, B. D., Minesinger, B. K. & Jinks-Robertson, S. (2000) Curr. Biol. 10, 145-148. [DOI] [PubMed] [Google Scholar]
- 19.Durant, S. T., Morris, M. M., Illand, M., McKay, H. J., McCormick, C., Hirst, G. L., Borts, R. H. & Brown, R. (1999) Curr. Biol. 9, 51-54. [DOI] [PubMed] [Google Scholar]
- 20.Wang, T.-F., Kleckner, N. & Hunter, N. (1999) Proc. Natl. Acad. Sci. USA 96, 13914-13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flores-Rozas, H. & Kolodner, R. D. (1998) Proc. Natl. Acad. Sci. USA 95, 12404-12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strand, M., Prolla, T. A., Liskay, R. M. & Petes, T. D. (1993) Nature 365, 274-276. [DOI] [PubMed] [Google Scholar]
- 23.Kolodner, R. D. & Marsischky, G. T. (1999) Curr. Opin. Genet. Dev. 9, 89-96. [DOI] [PubMed] [Google Scholar]
- 24.Alani, E., Reenan, R. A. & Kolodner, R. D. (1994) Genetics 137, 19-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers, S. R., Hunter, N., Louis, E. J. & Borts, R. H. (1996) Mol. Cell. Biol. 16, 6110-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter, N., Chambers, S. R., Louis, E. J. & Borts, R. H. (1996) EMBO J. 15, 1726-1733. [PMC free article] [PubMed] [Google Scholar]
- 27.Selva, E. M., New, L., Crouse, G. F. & Lahue, R. S. (1995) Genetics 139, 1175-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selva, E. M., Maderazo, A. B. & Lahue, R. S. (1997) Mol. Gen. Genet. 257, 71-82. [DOI] [PubMed] [Google Scholar]
- 29.Chen, W. & Jinks-Robertson, S. (1999) Genetics 151, 1299-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Datta, A., Adjiri, A., New, L., Crouse, G. F. & Jinks, R. S. (1996) Mol. Cell. Biol. 16, 1085-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Datta, A., Hendrix, M., Lipsitch, M. & Jinks, R. S. (1997) Proc. Natl. Acad. Sci. USA 94, 9757-9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholson, A., Hendrix, M., Jinks-Robertson, S. & Crouse, G. F. (2000) Genetics 154, 133-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Welz-Voegele, C., Stone, J. E., Tran, P. T., Kearney, H. M., Liskay, R. M., Petes, T. D. & Jinks-Robertson, S. (2002) Genetics 162, 1131-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugawara, N., Pāques, F., Colaiacovo, M. & Haber, J. E. (1997) Proc. Natl. Acad. Sci. USA 94, 9214-9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pāques, F. & Haber, J. E. (1997) Mol. Cell. Biol. 17, 6765-6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kearney, H. M., Kirkpatrick, D. T., Gerton, J. L. & Petes, T. D. (2001) Genetics 158, 1457-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugawara, N. & Haber, J. E. (1992) Mol. Cell. Biol. 12, 563-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandell, L. L. & Zakian, V. A. (1993) Cell 75, 729-739. [DOI] [PubMed] [Google Scholar]
- 39.Rose, M., Grisafi, P. & Botstein, D. (1984) Gene 29, 113-124. [DOI] [PubMed] [Google Scholar]
- 40.Studamire, B., Price, G., Sugawara, N., Haber, J. E. & Alani, E. (1999) Mol. Cell. Biol. 19, 7558-7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ray, B. L., White, C. I. & Haber, J. E. (1991) Mol. Cell. Biol. 11, 5372-5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haber, J. E., Ray, B. L., Kolb, J. M. & White, C. I. (1993) Proc. Natl. Acad. Sci. USA 90, 3363-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myung, K., Datta, A., Chen, C. & Kolodner, R. D. (2001) Nat. Genet. 27, 113-116. [DOI] [PubMed] [Google Scholar]
- 44.Mankouri, H. W., Craig, T. J. & Morgan, A. (2002) Nucleic Acids Res. 30, 1103-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gangloff, S., Soustelle, C. & Fabre, F. (2000) Nat. Genet. 25, 192-194. [DOI] [PubMed] [Google Scholar]
- 46.Fiorentini, P., Huang, K. N., Tishkoff, D. X., Kolodner, R. D. & Symington, L. S. (1997) Mol. Cell. Biol. 17, 2764-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tishkoff, D. X., Boerger, A. L., Bertrand, P., Filosi, N., Gaida, G. M., Kane, M. F. & Kolodner, R. D. (1997) Proc. Natl. Acad. Sci. USA 94, 7487-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amin, N. S., Nguyen, M. N., Oh, S. & Kolodner, R. D. (2001) Mol. Cell. Biol. 21, 5142-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozenberger, B. A. & Roeder, G. S. (1991) Mol. Cell. Biol. 11, 1222-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ivanov, E. L., Sugawara, N., Fishman, L. J. & Haber, J. E. (1996) Genetics 142, 693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugawara, N. & Haber, J. E. (2000) Mol. Cell. Biol. 20, 5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malkova, A., Ivanov, E. L. & Haber, J. E. (1996) Proc. Natl. Acad. Sci. USA 93, 7131-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Signon, L., Malkova, A., Naylor, M. L., Klein, H. & Haber, J. E. (2001) Mol. Cell. Biol. 21, 2048-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ira, G. & Haber, J. E. (2002) Mol. Cell. Biol. 22, 6384-6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Worth, L., Jr., Clark, S., Radman, M. & Modrich, P. (1994) Proc. Natl. Acad. Sci. USA 91, 3238-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mullen, J. R., Kaliraman, V. & Brill, S. J. (2000) Genetics 154, 1101-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viswanathan, M. & Lovett, S. T. (1998) Genetics 149, 7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gavin, A. C., Bosche, M., Krause, R., Grandi, P., Marzioch, M., Bauer, A., Schultz, J., Rick, J. M., Michon, A. M., Cruciat, C. M., et al. (2002) Nature 415, 141-147. [DOI] [PubMed] [Google Scholar]
- 59.Pedrazzi, G., Bachrati, C. Z., Selak, N., Studer, I., Petkovic, M., Hickson, I. D., Jiricny, J. & Stagljar, I. (2003) Biol. Chem. 384, 1155-1164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.