Abstract
Many orally administered, small-molecule, targeted anticancer drugs, such as dasatinib, exhibit pH-dependent solubility and reduced drug exposure when given with acid-reducing agents. We previously demonstrated that betaine hydrochloride (BHCl) can transiently re-acidify gastric pH in healthy volunteers with drug-induced hypochlorhydria. In this randomized, single-dose, three-way crossover study, healthy volunteers received dasatinib (100 mg) alone, after pretreatment with rabeprazole, and with 1500 mg BHCl after rabeprazole pretreatment, to determine if BHCl can enhance dasatinib absorption in hypochlorhydric conditions. Rabeprazole (20 mg b.i.d.) significantly reduced dasatinib Cmax and AUC0-∞ by 92 and 78%, respectively. However, coadministration of BHCl significantly increased dasatinib Cmax and AUC0-∞ by 15- and 6.7-fold, restoring them to 105 and 121%, respectively, of the control (dasatinib alone). Therefore, BHCl reversed the impact of hypochlorhydria on dasatinib drug exposure and may be an effective strategy to mitigate potential drug-drug interactions for drugs that exhibit pH-dependent solubility and are administered orally under hypochlorhydric conditions.
KEY WORDS: betaine hydrochloride, dasatinib, drug-drug interactions, pH-dependent solubility, proton pump inhibitors
INTRODUCTION
Drug-drug interactions due to changes in gastric pH typically result in alterations of drug solubility, which can subsequently impact drug absorption and exposure. These interactions can have clinically significant consequences, as increased or reduced systemic concentrations can result in drug toxicity or decreased therapeutic efficacy. For orally administered drugs that exhibit a pH-dependent solubility over the range of pH 1–4, the likelihood for an interaction of this nature to occur is higher, as normal fasting gastric pH ranges between pH 1 and 3 (1) while gastric pH becomes elevated to ≥4 during the administration of acid-reducing agents (ARAs) such as proton-pump inhibitors (PPIs) or H2-receptor antagonists (H2-RAs). ARAs are commonly used to manage the symptoms of gastroesophageal reflux disease (GERD) or peptic ulcer disease. In the USA, it was estimated that PPIs were prescribed for greater than 50% of digestive diseases, which resulted in an estimated health-care cost of more than $11 billion (2), and does not include ARAs purchased over-the-counter (OTC). Therefore, the wide usage of ARAs together with the increasing frequency of polypharmacy (3) increases the likelihood that drug-drug interactions associated with a drug-induced increase in gastric pH may occur.
For many orally administered, weakly basic compounds with pH-dependent solubility, coadministration with ARAs has been shown to lower drug solubility at the time of their administration, thereby reducing systemic concentrations and drug exposure (4–6). Although the severity of this interaction can depend on the therapeutic window of the drug and on the degree to which drug solubility is pH-dependent, small molecule anticancer agents are at risk for having much lower oral bioavailability and subtherapeutic concentrations when coadministered with ARAs (7). The significance of the interaction between these anticancer agents and gastric pH is further emphasized by the finding that approximately 33% of patients receiving an anticancer agent are also prescribed an ARA (8). As such, for a number of orally administered anticancer agents, it is advised to avoid concomitant administration with ARAs due to potentially clinically significant reductions in drug exposure and therapeutic effect (9–13).
One such compound affected by this gastric pH interaction is dasatinib (Sprycel®), which is a tyrosine kinase inhibitor used in the treatment of chronic phase, Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) and imatinib-resistant/intolerant CML (9). Dasatinib is weakly basic in nature and exhibits a pH-dependent solubility, becoming practically insoluble in water above pH 6 (solubility <0.008 mg/mL) (9). In a study published in 2009, dasatinib area under the concentration versus time curve (AUC) and maximum plasma concentration (Cmax) were reduced by approximately 60% when given following a single oral dose of famotidine (40 mg), an H2-RA, administered 10 h prior (5). Although it has been proposed to further separate the time the two drugs are administered as a way to possibly prevent this interaction, a dose timing strategy might not always be clinically feasible. In addition for more potent ARAs such as PPIs, where gastric acid inhibition is more potent and prolonged, a dose timing strategy would not work. Therefore, a transient gastric reacidification at the time of drug dosing may aid in mitigating this gastric pH-drug-drug interaction.
Previously, oral acidic solutions (i.e., cola beverages) have been used in an attempt to raise the extent of drug absorption following the administration of a weakly basic drug with an ARA (4, 14, 15). However, data from these studies suggest that this strategy may be inconsistent for various weakly basic drugs, as cola was unable to consistently reverse the reduced absorption caused by the ARA. Furthermore, the use of colas to improve drug absorption can be hampered by the harm that may result from a large volume of acidic fluid that may come into contact with the esophagus, as well as an increased risk of dental damage due to the formation of caries. For this purpose, gastric reacidification in the form of a solid oral dosage form may prove to have a higher utility.
Betaine hydrochloride (BHCl) is an OTC natural supplement that can be commonly found in digestive aids. As a solid oral dosage form, BHCl acidifies gastric fluid by dissociating into free betaine and hydrochloric acid, thus lowering gastric pH. We recently characterized the ability of BHCl to reacidify gastric pH in healthy volunteers pretreated with rabeprazole, a PPI, reporting that BHCl was able to safely, significantly, and transiently lower gastric pH under rabeprazole-induced hypochlorhydria. A 1500 mg dose of BHCl resulted in a decrease in gastric pH on average from pH 5 to 1, with a gastric pH <3 achieved on average for 73 min (16). Therefore, BHCl coadministration may be a potential strategy to mitigate drug-drug interactions with gastric pH. The goal of this randomized, single-dose, three-treatment cross-over study reported here was to investigate whether BHCl can mitigate the reduced dasatinib absorption observed in healthy volunteers with rabeprazole-induced hypochlorhydria.
MATERIALS AND METHODS
Subjects
Ten self-declared nonsmoking subjects (nine male, one female) between 23 and 59 years of age, with body mass indexes ranging from 21.6 to 29.1, and taking no concomitant medications were enrolled in this three-treatment crossover study. After providing written informed consent, subjects underwent a screening visit, which occurred at least 1 week prior to the start of the study, consisting of a medical history review, physical examination, electrocardiogram, and standard clinical blood and urine laboratory tests, to determine eligibility to participate in the study. Screening visits also included baseline gastric pH measurements through the use of the Heidelberg pH Diagnostic System (Heidelberg Medical Inc., Mineral Bluff, GA) to confirm normochlorhydria (fasting gastric pH <4), as previously described (16). All subjects enrolled in this study did not have hypo- or achlorhydria, nor did they have any prior reported history of gastrointestinal diseases. A summary of patient-enrolled demographics can be found in Table I.
Table I.
Summary of Enrolled Subject Demographics
| Total N | 10 |
|---|---|
| Male | 9 |
| Female | 1 |
| Race/ethnicity | |
| Caucasian | 6 |
| Asian | 3 |
| African-American | 1 |
| Age (years) | |
| Mean ± SD | 38 ± 11 |
| Range | 23–59 |
| Baseline gastric pH | |
| Median | 0.6 |
| Range | 0.5–1.8 |
Subjects had no prior history of gastrointestinal diseases as determined by a medical history review. Baseline gastric pH measurements were taken during the initial screening visit, and represents the median gastric pH observed in a 15-min interval after placement of the Heidelberg capsule
This study was approved by the Committee on Human Research of the University of California, San Francisco and registered on the US National Institutes of Health Clinical Trials Database (NCT01398046; http://clinicaltrials.gov/ct2/show/NCT01398046).
Study Design
This open-label, three-treatment, randomized clinical pharmacokinetic crossover study was conducted at the Clinical & Translational Science Institute’s Clinical Research Center (CCRC) located at the University of California, San Francisco. All enrolled subjects received the first treatment (treatment A), which was a single 100 mg dose of dasatinib (Bristol-Myers Squibb, New York City, NY) given orally with 250 mL of water. Subjects were then block-randomized using a random number generator (N = 6 subjects per block) for the order in which they would receive treatments B and C. Both treatments began with a pretreatment phase during which subjects self-administered 20 mg of rabeprazole (Eisai Co., Ltd., Woodcliff Lake, NJ) with food, twice daily for 3 days. An additional 20 mg dose was given on the morning of the fourth study day, and once gastric pH remained above pH ≥4 for at least 15 min, subjects then received either a single 100 mg dose of dasatinib with 250 mL of water (treatment B), or 1500 mg of betaine HCl, followed by 100 mg dose of dasatinib 5 min later with a total of 250 mL of water (treatment C). Heidelberg pH capsules were administered to subjects on the morning of each study day, and gastric pH measurements were taken until 3 h post dasatinib dosing. All subjects fasted from midnight the night prior and remained fasted until gastric pH measurements were completed. The subjects were also required to record the dates and times of all self-administered rabeprazole doses in addition to any adverse events experienced, in a verified medication diary. All dasatinib doses were separated by at least a 7-day washout period, and subjects completed all three treatments by the end of study.
Venous blood samples were drawn at the following times with respect to dasatinib dosing: pre-AM rabeprazole dose (treatments B and C only), 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 22 h. Blood samples were centrifuged within 30 min at 4°C. Plasma was then separated and stored in aliquots at −80°C until bioanalysis. Plasma concentrations of dasatinib and rabeprazole were measured using a validated LC-MS/MS method (Covance, Inc., Madison, WI), as described below.
Outcome Measures
The primary outcome measures for this study included plasma dasatinib Cmax and AUCs (AUC0–22, AUC0−∞) for each treatment period. Dasatinib Tmax, t1/2, and oral clearance (CL/F) for all treatment arms were included as secondary outcomes for this study.
Pharmacokinetic Analyses
Dasatinib and rabeprazole pharmacokinetic parameters for each treatment period were estimated from measured plasma concentrations using noncompartmental analyses in Phoenix WinNonlin 5 (Pharsight®, Sunnyvale, CA). Cmax and Tmax were estimated from the observed data. The terminal rate constant (λz) was calculated using a linear regression of the terminal phase of the log-linear concentration curve, and the terminal half-life was calculated using ln 2/λz. Dasatinib and rabeprazole AUCs were calculated using the logarithmic-linear trapezoidal method, and extrapolated to infinity by dividing the last measured concentration by λz. Oral dasatinib and rabeprazole clearances (CL/F) were obtained by dividing the dose by AUC0−∞.
Statistical Analyses
Based on a 60% reduction in dasatinib AUC when administered in healthy volunteers pretreated with famotidine (5), it was determined that 18 subjects would be necessary to show a 50% increase in dasatinib AUC when coadministered with betaine HCl in healthy volunteers pretreated with rabeprazole, with greater than 80% power and a two-sided α of 0.05, but subject number was decreased based on an interim analysis described below.
All statistical analyses were performed using GraphPad Prism 5.02 (La Jolla, CA). A repeated measures analysis of variance (ANOVA) with the Tukey’s test for multiple comparisons was used to determine statistical significance across all treatments for all dasatinib pharmacokinetic parameters except Tmax (Cmax, AUC0–22, AUC0−∞, CL/F, and t1/2). Differences in dasatinib Tmax values were tested for statistical significance using the Friedman test for multiple comparisons with α = 0.05, and a Dunn’s posttest for all-by-all comparisons. With the exception of Tmax, results of all pharmacokinetic parameters are reported as means ± SD. Results for Tmax are reported as medians, with ranges in parentheses. A two-tailed, paired t test with α = 0.05 was used to test for statistically significant differences in rabeprazole pharmacokinetic parameters. Logarithmic transformation of all pharmacokinetic parameters (except Tmax) was performed prior to statistical analyses. Additionally, the geometric mean ratios and 90% confidence intervals of dasatinib Cmax, AUC0–22, AUC0−inf, CL/F, and t1/2 were calculated for all treatment comparisons (B to A, B to C, and C to A), while the ratios and confidence intervals of rabeprazole Cmax, AUC0-22, AUC0-inf, CL/F, and t1/2 were calculated only for rabeprazole pretreatment periods (C to B).
Interim Analysis
An interim analysis was performed after the first block of subjects completed all treatments of the study to assess if the study should be terminated due to a lack of effect and further risk of administering an anticancer agent to healthy volunteers. Surprisingly, results of this interim analysis showed statistically significant differences in all primary outcome measures between treatments B and C, with P < 0.05. As such, it was determined that a modified sample size of ten subjects would be sufficient to complete the study, without unnecessary exposure to dasatinib in other healthy volunteers. Despite the initial block-randomization, an equal number of subjects received treatment B→C and C→B (N = 5) at the conclusion of the study.
Quantitative Determination of Dasatinib and Rabeprazole
Qualified high-performance liquid chromatography tandem mass spectrometry (LC-MS/MS) methods were used for the analysis of dasatinib and rabeprazole in human plasma. Dasatinib plasma samples were cleaned using supported liquid extraction before analysis. Fifty microliters of dasatinib plasma samples, calibration standards, or quality control (QC) samples were added to a 96-well plate followed by the addition of 50 μL of internal standard (d8-dasatinib, 200 ng/mL) and 100 μL of water. Following vortexing and centrifugation at 1640×g for a minute, 200 μL of each sample mixture was loaded onto an Isolute SLE + plate (Biotage, Charlotte, NC). After equilibrating for 5 min, the samples were eluted with 800 μL of tert-butyl methyl ether, dried under nitrogen and reconstituted in a methanol/water mixture (1:2 v/v) with 0.1% formic acid. For rabeprazole, 25 μL of plasma samples, calibration standards, or quality control (QC) samples were added to a 96-well plate followed by the addition of 25 μL of internal standard (d3-rabeprazole, 200 ng/mL). Proteins were precipitated with the addition of 200 μL of methanol. Following vortexing and centrifugation at 1640×g for 5 min, 150 μL of each supernatant was mixed with 150 μL of water. Processed samples were then injected into the LC-MS/MS for analysis.
The LC-MS/MS system consisted of an LC-20AD and SIL-20AC Prominence HPLC system (Shimadzu, Columbia, MD) and an AB Sciex API 4000 mass spectrometer (AB Sciex, Foster City, CA) equipped with a turbo-ionspray source operating in the positive ionization mode. Separation was achieved using a reverse-phase liquid chromatography with a 3 μm 50 × 2.1 mm Pursuit PFP column (Varian, Palo Alto, CA) for dasatinib and 3 μm 50 × 2.1 mm BetaBasic C18 column (Thermo, Waltham, MA) for rabeprazole. Gradient elution was used at a flow rate of 0.7 mL/min for dasatinib and 0.6 mL/min for rabeprazole, with a retention time of 1.3 min for both dasatinib and rabeprazole. Mobile phases consisted of (A) water and (B) methanol, each containing 0.1% formic acid. Quantitation was performed using multiple reaction monitoring (MRM) for the following mass transitions: m/z 488 to 401 for dasatinib, m/z 496 to 406 for d8-dasatinib, m/z 360 to 242 for rabeprazole, and m/z 363 to 245 for d3-rabeprazole. Accuracy and precision (relative standard deviation) were within 75 to 125% (70 to 130% for LLOQ).
RESULTS
Gastric Reacidification Using Betaine HCl
The time course and potency of BHCl on gastric pH in rabeprazole pretreated subjects are summarized in Table II. The onset of the effect of BHCl, measured as the time necessary to achieve gastric pH <3, was quick, averaging 12 min. Gastric pH was significantly lower after BHCl (ΔpH −3.4 ± 0.9; P < 0.001). In addition, a return to a gastric pH >3 required an average of 69 min after BHCl administration, indicating that the gastric reacidification period was temporary. All subjects achieved a gastric pH <3 for at least 26 min.
Table II.
Time Course and Potency of Gastric Reacidification Using BHCl in Healthy Subjects with Rabeprazole-Induced Hypochlorhydria
| Time course of BHCl | Mean ± SD (min) | ||
| Onset (time to pH <3) | 12 ± 21 | ||
| Rebound time to pH >3 | 69 ± 77 | ||
| Potency of BHCl | |||
| pH before BHCl | pH after BHCl | ΔpH | |
| Mean ± SD | 4.1 ± 0.9 | 0.74 ± 0.54 | 3.4 ± 0.9 |
| Range | 2.8–5.2 | 0.50–3.6 | 0.61–4.7 |
Gastric pH data at 1-min intervals from treatment C were used to calculate the time course and potency of BHCl. The time course is described by the onset of effect (time to pH <3) and rebound time to pH >3, relative to BHCl administration, while the potency is described by the ΔpH calculated before and after BHCl administration
Dasatinib Pharmacokinetics
The mean plasma concentration-time curves of dasatinib for ten subjects following a 100 mg oral dose of dasatinib on each of the three treatment days are shown in Fig. 1. Dasatinib pharmacokinetics in treatment A were comparable to those previously reported in healthy volunteers receiving the same dose (9). When subjects were pretreated with rabeprazole prior to dasatinib dosing (treatment B), significant reductions (P < 0.001) in dasatinib Cmax, AUC0–22, and AUC0−∞ of 92, 84, and 78% were observed relative to dasatinib alone (treatment A; Table III). However, with the addition of BHCl in rabeprazole pretreated subjects (treatment C), dasatinib Cmax, AUC0–22, and AUC0−∞ were significantly increased relative to rabeprazole pretreatment alone by 15-, 6.5-, and 6.7-fold, respectively (P < 0.001 for all comparisons). Coadministration of BHCl with dasatinib under hypochlorhydric conditions was able to restore dasatinib Cmax, AUC0–22, and AUC0−∞ to 105, 110, and 121% of their respective values on dasatinib alone “control” treatment days (treatment A).
Fig. 1.
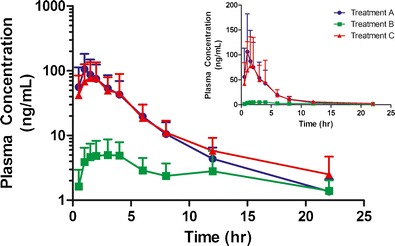
Effects of gastric pH modulators on plasma concentrations of dasatinib in healthy volunteers. A single-oral dose of 100 mg dasatinib was given on each study day either alone (treatment A), after pretreatment with rabeprazole (treatment B), or after pretreatment with rabeprazole followed by betaine HCL given 5 min prior to dasatinib (treatment B). Points represent the mean of ten subjects (error bars indicating the SD) plotted on a logarithmic scale, with the inset graphs plotted on a linear scale
Table III.
Dasatinib Pharmacokinetics in Ten Healthy Volunteers Following a 100 mg Oral Dose Given with and Without Gastric pH Modulators
| Parameter | DAS alone (treatment A) | DAS + RAB (treatment B) | DAS + RAB + BHCl (treatment C) | Geometric mean ratios (90% CI) | ||
|---|---|---|---|---|---|---|
| B to A | C to B | C to A | ||||
| Cmax (ng/mL) | 115 ± 68 | 6.87 ± 3.57***;††† | 103 ± 58 | 0.0757 (0.0342–0.168) | 14.0 (7.7–25.4) | 1.05 (0.48–2.30) |
| AUC0-22 (ng/mL*h) | 395 ± 196 | 57.9 ± 28.7***;††† | 379 ± 161 | 0.165 (0.090–0.303) | 6.66 (4.23–10.5) | 1.10 (0.623–1.92) |
| AUC0−∞ (ng/mL*h)a | 405 ± 200 | 70.5 ± 24.8***;††† | 469 ± 233 | 0.217 (0.113–0.417) | 5.57 (3.81–8.14) | 1.21 (0.671–2.17) |
| Tmax (h) | 1.0 (1.0–4.0) | 2.0 (1.5–12) | 2.0 (0.50–4.0) | N/A | N/A | N/A |
| CL/F (mL/min/kg) | 107 ± 114 | 376 ± 127***;††† | 67.8 ± 42.0 | 4.62 (2.41–8.85) | 0.180 (0.123–0.264) | 0.835 (0.470–1.48) |
| t1/2 (h) | 4.43 ± 0.64 | 11.2 ± 7.2***;†† | 5.29 ± 1.04 | 2.10 (1.42–3.11) | 0.553 (0.391–0.781) | 1.16 (1.03–1.31) |
DAS dasatinib, RAB rabeprazole, BHCl betaine HCl, C max maximum plasma concentration, T max time to maximum plasma concentration, t 1/2 terminal half-life, AUC area under the plasma concentration-time curve, CL/F oral clearance, N/A not available
Statistical results for treatment B versus A comparisons and Treatment C versus B comparisons are reported in each row of the Treatment B column. No significant differences were observed for all pharmacokinetic parameters in treatment C versus A comparisons, and in Tmax across all treatments
**P < 0.01; ***P < 0.001; ††P < 0.01; †††P < 0.001
aData from one subject was excluded from analysis as the terminal phase of dasatinib pharmacokinetics could not be estimated
In addition to the changes observed in dasatinib Cmax, AUC0–22, and AUC0−∞, oral clearance (CL/F) and half-life (t1/2) of dasatinib were also sensitive to changes in gastric pH. In subjects with rabeprazole-induced hypochlorhydria, CL/F and t1/2 were increased by over 3.5- and 2.5-fold, respectively (P < 0.001), when compared to dasatinib given alone. However, the coadministration of BHCl lowered dasatinib CL/F and t1/2 by 82 and 45%, respectively, thereby reversing the effects of the elevated gastric pH caused by rabeprazole. Furthermore, no significant differences in dasatinib CL/F and t1/2 were observed between subjects in treatment C versus treatment A.
No significant differences were observed in dasatinib Tmax across all treatments.
Correlation of Dasatinib Cmax and AUC Ratios with Gastric Acid Exposure
Gastric acid exposure (AUCpH<3) was calculated as previously described (16) for treatment C, as a secondary measure of BHCl efficacy. As shown in Fig. 2, significant positive correlations (P < 0.05; Spearman r > 0.70) were observed between AUCpH<3 and the increases in dasatinib Cmax and AUC when given with BHCl under rabeprazole-induced hypochlorhydria (treatment C).
Fig. 2.
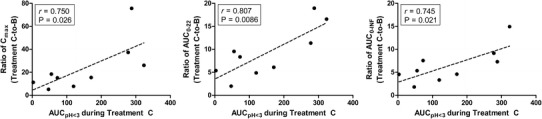
Relationship between gastric acid exposure (AUCpH<3) during betaine HCl treatment and change in dasatinib Cmax and AUC during rabeprazole-induced hypochlorhydria. The changes in dasatinib Cmax, AUC0–22, and AUC0−∞were calculated by taking the ratio of each parameter during treatment C (dasatinib + rabeprazole + betaine HCl) relative to treatment B (dasatinib + rabeprazole). AUCpH<3, a measure of overall gastric acid exposure, was calculated as previously described (16). Data from one subject was excluded from these comparisons as the terminal phase of dasatinib pharmacokinetics could not be estimated during treatment B
Rabeprazole Pharmacokinetics
Noncompartmental analyses of rabeprazole plasma concentrations for treatments B and C demonstrated that no difference in rabeprazole pharmacokinetics was observed with the addition of BHCl (Table IV).
Table IV.
Rabeprazole Pharmacokinetics in Ten Healthy Volunteers During Rabeprazole Pretreatment Study Days
| Parameter | Treatment B | Treatment C | Geometric mean ratio (90% CI) |
|---|---|---|---|
| Cmax (ng/mL) | 538 ± 210 | 413 ± 150 | 0.755 (0.507–1.12) |
| AUC0–22 (ng/mL*h) | 968 ± 319 | 825 ± 312 | 0.843 (0.694–1.03) |
| AUC0−∞ (ng/mL*h) | 970 ± 320 | 830 ± 311 | 0.847 (0.699–1.03) |
| Tmax (h) | N/A | N/A | N/A |
| CL/F (mL/min/kg) | 5.33 ± 1.88 | 6.34 ± 2.28 | 1.19 (0.978–1.44) |
| t1/2 (h) | 2.57 ± 1.63 | 2.39 ± 1.75 | 0.863 (0.646–1.15) |
No significant differences were observed in rabeprazole pharmacokinetics with the addition of BHCl. Tmax values were unable to be estimated as plasma concentrations were measured at time points relative to dasatinib administration
C max maximum plasma concentration, T max, time to maximum plasma concentration, t 1/2 terminal half-life, AUC area under the plasma concentration-time curve, CL/F oral clearance, N/A not available
Safety
All ten subjects enrolled received and completed all treatments. No adverse effects were reported due to dasatinib, rabeprazole, or BHCl dosing. Additionally, no gastrointestinal side effects were reported due to rabeprazole or BHCl administration. Furthermore, subjects experienced no adverse events using the Heidelberg pH capsule during all phases of the study.
DISCUSSION
In this three-treatment, randomized, pharmacokinetic, crossover study, we demonstrated that a 1500 mg oral dose of BHCl was able to safely and significantly reverse the effect of rabeprazole-induced hypochlorhydria on the absorption of dasatinib in healthy volunteers. Dasatinib, a BCR-ABL tyrosine kinase inhibitor, is a weak base with pH-dependent solubility, and therefore is a prime substrate for drug-drug interactions due to changes in gastric pH. As many small-molecule, molecular-targeted anticancer agents share similar physicochemical properties with dasatinib and display marked reductions in drug exposure when coadministered with ARAs (7), the addition of BHCl may prove to be a clinically valuable strategy to mitigate this significant drug-drug interaction by transiently reacidifying gastric pH at the time of drug dosing.
Drug-drug interactions due to ARAs, particularly the PPIs, can involve significant reductions in drug absorption due to elevations in pH in the stomach and upper gastrointestinal regions (17), or increased drug exposure due to inhibition of the cytochrome P450 (CYP) metabolizing enzymes (18). Most PPIs are primarily metabolized in the liver by the CYP2C19 and CYP3A4 isoforms (19), and have the potential to cause drug-drug interactions with other drugs metabolized by the same CYPs. However, rabeprazole is unique as a PPI in that it is primarily metabolized by non-P450-mediated mechanisms (20). Therefore, in the current study, metabolic interactions with dasatinib, which is primarily metabolized by CYP3A4 (21), were minimized and our ability to focus on the effects of gastric pH was enhanced. Although dasatinib CL/F was shown in our study to be sensitive to changes in gastric pH, these differences can be attributed to dasatinib bioavailability (F), which decreases as gastric pH increases due to the reduced dasatinib solubility at elevated gastric pH (pH >4). In a similar fashion, dasatinib t1/2 was also sensitive to changes in gastric pH, as dasatinib elimination appears to become rate-limited by drug absorption during rabeprazole-pretreatment days.
The effects of elevated gastric pH on drug absorption of weakly basic drugs due to concomitant administration of ARAs have been widely studied, and it is well known that significant reductions in systemic concentrations and drug exposures are observed as a result (4–6). Interestingly, in the current study when subjects were administered with dasatinib after rabeprazole pretreatment (treatment B), the reductions in dasatinib Cmax and AUC were far greater than previously reported in studies using famotidine (5) or omeprazole (22), in which reductions of approximately 60% were seen. The more pronounced impact on PK seen in our study might be partially attributed to the use of twice daily dosing of rabeprazole as opposed to the once daily dosing of famotidine or omeprazole used in the prior studies. In addition, rabeprazole may provide a higher degree of acid secretion inhibition compared to other ARAs (23), resulting in greater alterations in gastric pH and a larger impact on dasatinib solubility and absorption. Importantly, our study design was optimized based on a previous methodology study (16) to achieve timely and maximum gastric pH alterations and included the utilization of continuous gastric pH measurements (i.e., Heidelberg capsules) on the study days. Therefore, we were able to confirm an elevated gastric pH in all patients at the time of dasatinib dosing.
To mitigate gastric pH interactions for weakly basic compounds such as dasatinib, strategies might include separating the times at which the ARAs are administered, increasing the dose of a potential victim drug, or transient gastric reacidification. The separation of dosing times of a victim drug and weak ARAs, such as antacids, has been shown to mitigate the reduction in drug exposure caused by the elevated gastric pH (5). However, for H2-RAs and PPIs, this may not be feasible as both drugs have the potential to inhibit acid secretion for a much longer time in a 24-h period. Alternatively, increasing the dose of a victim drug is not likely to overcome this interaction, particularly if drug absorption is limited by its solubility. In this case, the maximum solubility of the drug would already have been achieved and further dose escalations would have no benefit. Lastly, transient gastric reacidification is a strategy that artificially lowers gastric pH through the use of oral acidifying agents, such as acidic beverages or hydrochloric acid salts. However, as described above, the use of acidic beverages does not completely reverse the effects of hypochlorhydria and is limited by the pH of the solutions used, the large volumes necessary to sufficiently acidify gastric pH, and the potential dental and esophageal damage that may result from chronic administration. As a solid, oral dosage form, BHCl can improve gastric reacidification strategies due to the ease of administration and minimal direct exposure of acid to the mouth and esophagus.
The use of BHCl in this study proved to be a safe and successful way to mitigate the reduction in dasatinib absorption during rabeprazole-induced hypochlorhydria, nearly raising Cmax and AUC to levels observed during treatment with dasatinib alone (i.e., under normochlorhydric conditions; treatment A). This effect can be attributed to the large change in gastric pH (mean ΔpH −3.4) that was of rapid onset (mean time to pH <3 was 12 min) after a 1500 mg dose of BHCl. In addition, the time period of reacidification was also favorable with a mean time of pH <3 of 69 min, indicating that dasatinib exposure to highly acidic gastric pH was of sufficient duration to allow drug dissolution yet still temporary. The time course and magnitude of gastric pH change was similar to what was found in our previous methodology study (16). These characteristics with which BHCl lowers gastric pH make it a promising strategy for patients to coadminister BHCl with dasatinib, as greater than 90% of a 150 mg Sprycel tablet was reported to be dissolved within 10 min at pH 3 in a previous dissolution study (24).
The increases in dasatinib Cmax and AUC within an individual subject during BHCl treatment correlated well with the subject’s AUCpH<3 during BHCl treatment (Fig. 2). Higher values of AUCpH<3 indicate a lower gastric pH over a longer period of time and represent a greater efficacy of BHCl in rabeprazole-induced hypochlorhydria (16). Therefore, confirming sufficient gastric acid exposure in subjects may be useful in predicting improvement in dasatinib absorption during rabeprazole-induced hypochlorhydria. However, it is worth noting that large interindividual variability (coefficient of variation >40%) was observed in the pharmacokinetics of dasatinib across all treatments. The variability in dasatinib pharmacokinetics was also observed in the geometric mean ratios, reported in Table III. Although the ratios for the treatment comparisons were expected due to the significant differences in gastric pH at the time of dasatinib dosing, the 90% confidence intervals for dasatinib Cmax and AUCs in the C to A comparison fall well outside of the FDA recommended 80–125% confidence interval of no effect. Previous reports have described that the large interindividual variability may be mostly attributed to drug absorption, more specifically oral bioavailability, that can range from 32 to 118% (25).
The severity of a drug-drug interaction due to changes in gastric pH can be highly drug dependent and governed by a drug’s physicochemical properties and the pH range over which its solubility is sensitive. As demonstrated in this study, dasatinib absorption is highly sensitive to gastric pH changes that occur during ARA therapy (pH 4 to 6) since its solubility is dramatically reduced from 18.4 mg/mL at pH 2.6 to 0.008 mg/mL at pH 6.0 (9). Therefore, dasatinib may serve as an example of what could be considered a worst-case scenario, and not all small molecule, targeted anticancer agents may experience this degree of an interaction, if at all. Other small molecule, targeted anticancer agents that exhibit a pH-dependent solubility include erlotinib (Tarceva®), gefitinib (Iressa™), and nilotinib (Tasigna®), all of which have significant reductions in AUC and Cmax during concomitant ARA therapy (7), though not to same extent as dasatinib. Vandetanib (Caprelsa®) is a tyrosine kinase inhibitor used to treat advanced medullary thyroid cancer (26), and although vandetanib also exhibits a pH-dependent solubility (increased solubility at low pH), its highest dose strength of 300 mg is highly soluble in aqueous media until pH 6 at 37°C (27), suggesting that a clinically significant drug-drug interaction between vandetanib and ARAs is not likely. Therefore, we hypothesize that weakly basic drugs with a dynamic pH-dependent solubility between pH 1 and 4 will be most affected by elevations in gastric pH during concomitant ARA therapy.
Though we demonstrate the success of BHCl in enhancing dasatinib absorption under rabeprazole-induced hypochlorhydria, the impact of repeated BHCl coadministration in a patient managing GERD or other gastrointestinal diseases for which ARAs are prescribed still remains to be investigated as there may be a potential risk of having repeated exposure to a high gastric acidity, even for short periods of time. Further studies are also warranted to investigate the impact that repeated BHCl coadministration will have on dasatinib efficacy in patients with CML also taking ARAs to manage GERD symptoms. It was recently reported that dasatinib major cytogenetic response, defined as having ≤35% of cells test positive for the Philadelphia chromosome (28), was significantly associated with dasatinib steady-state concentrations and maintenance of uninterrupted dosing (29). Thus, a clinical study comparing dasatinib major cytogenetic responses in CML patients taking ARAs with and without gastric acid modulation would be needed to describe the clinical significance of BHCl coadministration to improve drug absorption.
CONCLUSION
Drug-drug interactions between gastric pH and weakly basic drugs with pH-dependent solubility can significantly impact systemic concentrations and drug exposures. However, we report for the first time herein that the use of BHCl, a gastric acid supplement in a solid oral dosage form, can vastly improve dasatinib absorption in healthy volunteers with rabeprazole-induced hypochlorhydria. While the magnitude of the effect may be drug-dependent, the results of this study suggest that coadministration of BHCl can be a viable strategy to enhance the absorption of other small molecule, targeted anticancer agents that have pH-dependent solubility in the range of pH 1–4 and are given during ARA therapy.
Acknowledgments
The authors would like to acknowledge the staff and nurses of the UCSF-CCRC for their assistance in this study. The UCSF-CCRC is supported by an NIH/NCRR grant (UL1 RR0224131). This study was supported by a grant from Genentech, Inc., and M. R. Yago was supported in part by NIH Training Grant T32 GM007175.
Conflict of Interest
L. Z. Benet is a consultant for Genentech, Inc., and X. Ding, B. Dean, L. Salphati, N. Budha, J. Y. Jin, M. J. Dresser, and J. A. Ware are employees of Genentech, Inc.
References
- 1.Dressman JB, Vertzoni M, Goumas K, Reppas C. Estimating drug solubility in the gastrointestinal tract. Adv Drug Deliv Rev. 2007;59:591–602. doi: 10.1016/j.addr.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL. Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med. 2013;28:930–937. doi: 10.1007/s11606-013-2345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics. Health, United States, 2012: with special feature on emergency care. 2013. [PubMed]
- 4.Chin TW, Loeb M, Fong IW. Effects of an acidic beverage (Coca-Cola) on absorption of ketoconazole. Antimicrob Agents Chemother. 1995;39:1671–1675. doi: 10.1128/AAC.39.8.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eley T, Luo FR, Agrawal S, Sanil A, Manning J, Li T, et al. Phase I study of the effect of gastric acid pH modulators on the bioavailability of oral dasatinib in healthy subjects. J Clin Pharmacol. 2009;49:700–709. doi: 10.1177/0091270009333854. [DOI] [PubMed] [Google Scholar]
- 6.Zhu L, Persson A, Mahnke L, Eley T, Li T, Xu X, et al. Effect of low-dose omeprazole (20 mg daily) on the pharmacokinetics of multiple-dose atazanavir with ritonavir in healthy subjects. J Clin Pharmacol. 2011;51:368–377. doi: 10.1177/0091270010367651. [DOI] [PubMed] [Google Scholar]
- 7.Budha NR, Frymoyer A, Smelick GS, Jin JY, Yago MR, Dresser MJ, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther. 2012;92:203–213. doi: 10.1038/clpt.2012.73. [DOI] [PubMed] [Google Scholar]
- 8.Smelick GS, Heffron TP, Chu L, Dean B, West DA, Duvall SL, et al. Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. Mol Pharm. 2013;10:4055–4062. doi: 10.1021/mp400403s. [DOI] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration. Dasatinib (Sprycel) summary basis of approval. 2006.
- 10.US Food and Drug Administration. Erlotinib (Tarceva) prescribing information. 2010.
- 11.US Food and Drug Administration. Gefitinib (Iressa) prescribing information. 2004.
- 12.US Food and Drug Administration. Nilotinib (Tasigna) prescribing information. 2013.
- 13.US Food and Drug Administration. Crizotinib (Xalkori) prescribing information. 2013.
- 14.Jaruratanasirikul S, Kleepkaew A. Influence of an acidic beverage (Coca-Cola) on the absorption of itraconazole. Eur J Clin Pharmacol. 1997;52:235–237. doi: 10.1007/s002280050280. [DOI] [PubMed] [Google Scholar]
- 15.Ray JE, Marriott D, Bloch MT, McLachlan AJ. Therapeutic drug monitoring of atazanavir: surveillance of pharmacotherapy in the clinic. Br J Clin Pharmacol. 2005;60:291–299. doi: 10.1111/j.1365-2125.2005.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yago MR, Frymoyer AR, Smelick GS, Frassetto LA, Budha NR, Dresser MJ, et al. Gastric reacidification with betaine HCl in healthy volunteers with rabeprazole-induced hypochlorhydria. Mol Pharm. 2013;10:4032–4037. doi: 10.1021/mp4003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michalek W, Semler JR, Kuo B. Impact of acid suppression on upper gastrointestinal pH and motility. Dig Dis Sci. 2011;56:1735–1742. doi: 10.1007/s10620-010-1479-8. [DOI] [PubMed] [Google Scholar]
- 18.Wedemeyer RS, Blume H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update. Drug Saf. 2014;37:201–211. doi: 10.1007/s40264-014-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer UA. Metabolic interactions of the proton-pump inhibitors lansoprazole, omeprazole and pantoprazole with other drugs. Eur J Gastroenterol Hepatol. 1996;8(Suppl 1):S21–S25. doi: 10.1097/00042737-199610001-00005. [DOI] [PubMed] [Google Scholar]
- 20.Miura M, Satoh S, Tada H, Habuchi T, Suzuki T. Stereoselective metabolism of rabeprazole-thioether to rabeprazole by human liver microsomes. Eur J Clin Pharmacol. 2006;62:113–117. doi: 10.1007/s00228-005-0077-8. [DOI] [PubMed] [Google Scholar]
- 21.Brave M, Goodman V, Kaminskas E, Farrell A, Timmer W, Pope S, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin Cancer Res. 2008;14:352–359. doi: 10.1158/1078-0432.CCR-07-4175. [DOI] [PubMed] [Google Scholar]
- 22.Bristol-Myers Squibb. The effect of omeprazole on the pharmacokinetics of dasatinib (BMS-354825) in healthy subjects. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000–2014. Available from: http://clinicaltrials.gov/show/NCT00655746:NCT00655746. 2009.
- 23.Stedman CA, Barclay ML. Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:963–978. doi: 10.1046/j.1365-2036.2000.00788.x. [DOI] [PubMed] [Google Scholar]
- 24.Fish MP, Young J, Shah P, Gao Z. The use of experimental design principles in dissolution method development: development of a discriminating dissolution method for Sprycel film-coated tablets. J Pharm Innov. 2009;4:165–173. doi: 10.1007/s12247-009-9071-5. [DOI] [Google Scholar]
- 25.van Erp NP, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev. 2009;35:692–706. doi: 10.1016/j.ctrv.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Grande E, Kreissl MC, Filetti S, Newbold K, Reinisch W, Robert C, et al. Vandetanib in advanced medullary thyroid cancer: review of adverse event management strategies. Adv Ther. 2013;30:945–966. doi: 10.1007/s12325-013-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration. Vandetanib (Calpresa) summary basis of approval. 2011.
- 28.Kantarjian HM, Smith TL, O’Brien S, Beran M, Pierce S, Talpaz M. Prolonged survival in chronic myelogenous leukemia after cytogenetic response to interferon-alpha therapy. The Leukemia Service. Ann Intern Med. 1995;122:254–261. doi: 10.7326/0003-4819-122-4-199502150-00003. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Roy A, Hochhaus A, Kantarjian HM, Chen TT, Shah NP. Differential effects of dosing regimen on the safety and efficacy of dasatinib: retrospective exposure-response analysis of a Phase III study. Clin Pharmacol. 2013;5:85–97. doi: 10.2147/CPAA.S42796. [DOI] [PMC free article] [PubMed] [Google Scholar]


