Abstract
Objective
To assess the efficacy and safety of abatacept for secondary Sjögren's syndrome (SS) associated with rheumatoid arthritis (RA).
Methods
The primary endpoint of this 1-year, open-labeled, prospective, observational multicenter study of RA-associated secondary SS was the rate of SDAI remission at 52 weeks after initiation of abatacept therapy. The secondary endpoints included that of Saxson's test and Schirmer's test. Adverse events during the study period were also analyzed.
Results
Thirty-two patients (all females) were enrolled in this study. Interim analysis at 24 weeks included assessment of efficacy (n = 31) and safety (n = 32). The mean SDAI decreased from 19.8 ± 11.0 (± SD) at baseline to 9.9 ± 9.9 at 24 weeks (P < 0.05). Patients with clinical remission, as assessed by SDAI, increased from 0 patient (0 week) to 8 patients (25.8%) at 24 weeks. Saliva volume (assessed by Saxson's test) increased slightly from 2232 ± 1908 (0 week) to 2424 ± 2004 (24 weeks) mg/2 min (n = 29). In 11 patients with Greenspan grading 1/2 of labial salivary glands biopsy, saliva volume increased from 2945 ± 2090 (0 week) to 3419 ± 2121 (24 weeks) mg/2 min (P < 0.05). Schirmer's test for tear volume showed increase from 3.6 ± 4.6 (0 week) to 5.5 ± 7.1 (24 weeks) mm/5 min (n = 25; P < 0.05). Five adverse events occurred in five of 32 patients (15.6%), and three of these events were infections.
Conclusion
Abatacept seems to be effective for both RA and RA-related secondary SS.
Keywords: Sjögren's syndrome, Rheumatoid arthritis, Abatacept
Introduction
Sjögren's syndrome (SS) is an autoimmune disease that affects exocrine glands including salivary and lacrimal glands. It is characterized pathologically by lymphocytic infiltration into the exocrine glands, and clinically by dry mouth and dry eyes. SS is subcategorized into primary SS, which is not associated with any other well-defined connective tissue disease (CTD), and secondary SS, which is associated with other well-defined CTD [1]. Primary SS is further subdivided into the glandular form, with involvement of the exocrine glands only, and the extra-glandular form, with involvement of organs other than exocrine glands. With regard to secondary SS, it was reported that rheumatoid arthritis (RA) was diagnosed in 38.7%, and systemic lupus erythematosus in 22.2% of secondary SS patients by all Japan survey on the epidemiology of SS [2].
In the selection of treatment for SS, it is important to distinguish the type of SS (i.e., primary or secondary) as well as the subtype (i.e., primary SS glandular or extra-glandular form). For secondary SS, the associated CTDs or organ involvements should be the main target of treatment. For primary SS, moderate to high doses of corticosteroids or immunosuppressant are used mainly against severe organ involvement, such as active interstitial lung disease, interstitial nephritis, and neuropathy. For primary SS without severe organ involvement, palliative therapies are used for dry mouth, dry eye, and arthralgia [3]. Although TNF blockers (infliximab and etanercept) have been used for dryness, which is main symptom of SS, they do not result in sufficient improvement [3]. Currently, rituximab, a chimeric anti-CD20 monoclonal antibody, seems to be the most effective for dryness of SS among various biologics [3–5]. However, a recent retrospective report from Autoimmune and Rituximab registry showed that rituximab was used mainly for systemic organ involvement in primary SS, but rarely for glandular involvements in primary SS patients [6]. Collectively, the use of biologics for SS is limited at the present time; the efficacy of TNF blockers remains questionable, and rituximab is used mainly for systemic organ involvement and rarely for glandular involvement.
Abatacept (CTLA4-Ig) is a unique biologic agent that binds to CD80 and CD86 on antigen-presenting cells (APCs), blocking the binding of these molecules with CD28 on T cells. Consequently, abatacept inhibits co-stimulation required for complete T cell activation. Inhibition of co-stimulatory molecules by abatacept is a new therapeutic strategy in autoimmune diseases, such as RA [7]. Previous clinical studies have confirmed the effectiveness of abatacept on undifferentiated arthritis, very early RA [8], methotrexate (MTX)-naïve early RA [9], MTX inadequate response established RA [10], and TNF blocker inadequate response established RA [11].
Because CD4+T cells play crucial roles in the pathogenesis of SS [12,13] as well as in RA, abatacept could be useful for SS. However, only few studies have tested the usefulness of abatacept in primary SS [14,15] and SS mouse model [16]. Although these studies established the value of abatacept in sialadenitis of SS [14] and SS mouse model [16], the results need to be confirmed in a larger number of patients, for longer period, and in various SS models. Moreover, no study has reported the efficacy of abatacept on secondary SS associated with other CTDs.
We designed an open-labeled, one-year, prospective, observational, and multicenter study (Rheumatoid Arthritis with Orencia Trial toward Sjögren's syndrome Endocrinopathy [ROSE] trial) to establish the efficacy of abatacept in RA and RA-related secondary SS and its safety in patients with RA-associated secondary SS.
Patients and methods
Patients
Patients aged more than 20 years diagnosed with RA according to the American College of Rheumatology (ACR) 1987 [17] or ACR/European League Against Rheumatism (EULAR) 2010 criteria [18], and SS according to the Japanese Ministry of Health criteria for the diagnosis of SS 1999 [19], who presented with dryness were eligible for this study. The Japanese Ministry of Health criteria for the diagnosis of SS 1999 include four clinicopathological findings: lymphocytic infiltration of the salivary or lacrimal glands, dysfunction of salivary secretion, keratoconjunctivitis sicca, and presence of anti-SS-A or SS-B antibodies. The diagnosis of SS was based on the presence of two or more of the above four items [19]. The patients were followed up at three hospitals in Japan (University of Tsukuba hospital, University of Occupational and Environmental Health hospital, and Nagasaki University hospital). The target number of enrolled patients was 30. Approval for this study was obtained from the local ethics committees of each study site and a signed informed consent was obtained from each subject (approval number of University of Tsukuba: H23–29, certification date of approval: 28th July, 2011). This study was registered in University hospital Medical Information Network (UMIN)–Clinical Trials Registry (CTR; UMIN-ID: UMIN000005724).
Patients with contraindication for abatacept, aged more than 75 or less than 20 years, leukopenia (leukocyte count, ≤ 3000/mm2), severe liver or kidney diseases, severe hematological disorders, negative for both anti-SS-A and SS-B antibody and positive for anti-centromere antibody were excluded from this study. Patients who were pregnant, nursing, wanted to get pregnant, and were treated with palliative therapies for dryness including cevimeline, anetholtritione, and pilocarpine within the last 4 weeks were also excluded. We also excluded patients who were considered not suitable for this study by the attending physician.
Medications
The dosing regimen approved for the treatment of RA was used in this study. The weight-adapted dose of abatacept (500 mg for patients weighing less than 60 kg, and 750 mg for those weighing ≥ 60 kg) was administrated intravenously at weeks 0, 2, 4, and every 4 weeks, for 1 year. Other disease-modifying anti-rheumatic drugs (DMARDs), corticosteroids, and non-steroidal anti-inflammatory drugs were also used during the 1-year observational period based on the clinical judgment of the attending physician.
Analysis of effects of abatacept
The study design was an open-labeled, 1-year, prospective, and observational study. For RA, the number of tender and swollen joints, physicians' global visual analog scale (VAS), patients' global VAS, Simplified Disease Activity Index (SDAI), disease activity score (DAS) 28-ESR and CRP, serum CRP, ESR, and rheumatoid factor (RF) were assessed at weeks 0 (baseline), 4, 12, 24, and 52. For SS, patients' VAS (dry mouth, dry eye, and parotid pain), physicians' VAS (dry mouth, keratoconjunctivitis sicca, and general condition), saliva volume by Saxon's test, tear volume by Schirmer's test, anti-SS-A/SS-B antibody, and serum IgG level were examined at weeks 0, 12, and 24. The primary endpoint was the percentage of patients who achieved clinical remission by SDAI at 52 weeks. Secondary endpoints included CRP, ESR, RF, Saxon's test, Schirmer's test, anti-SS-A/SS-B antibody, IgG, patients' VAS, and physicians' VAS.
Analysis for safety of abatacept
Adverse events (AEs) during observational periods were analyzed at each visit. The type of AE, onset, concomitant drugs used, treatment for AEs, cessation of use of abatacept, and outcome were recorded.
Statistical analysis
Data are expressed as mean ± SD. Differences between baseline and after treatment with abatacept were examined for statistical significance using the Wilcoxon signed-rank test. Differences between groups were examined using Mann–Whitney U test. Correlation between improvement of secretory function and SDAI was assessed by Spearman's rank correlation. A P value less than 0.05 denoted the presence of a statistically significant difference. The deficit of data was compensated by the last observation carried forward (LOCF) method.
Results
Clinicopathological features of enrolled patients at baseline
Thirty-two patients (all females) were enrolled in this study. This interim analysis for 24 weeks included assessment of the effect of abatacept in 31 patients and safety in 32 patients. One patient was excluded from such assessment due to missing data at baseline. The baseline clinicopathological features of the 32 patients are summarized in Table 1. The mean age was 55.3 ± 14.4 years, and RA disease duration was 129.7 ± 140.5 months. More than half of the patients were assessed as Stage I/Stage II, and Class 1 and Class 2. The RA disease activity assessed by DAS28 and SDAI was moderate. Saxon's test was 2232 ± 1908 mg/2 min, Schirmer's test 3.6 ± 4.6 mm/5 min, Greenspan grading of labial salivary gland (LSG) biopsy was 1/2 in 12 patients and 3/4 in 17 patients. Corticosteroids were used in 15 of 32 (46.9%) patients, and the mean dose of prednisolone was 5.2 ± 2.7 mg/day. MTX was administered in 75.0% of patients (24/32 patients), at a mean dose of 9.4 ± 4.0 mg/week. Furthermore, 21.9% of patients (7/32 patients) were treated previously with biologics other than abatacept, while 25 patients were biologics-naïve. Collectively, the enrolled patients had secondary SS with moderate dryness, in addition to moderately active RA.
Table 1.
Clinicopathological features of enrolled patients at baseline (N = 32).
| Clinicopathological features | |
|---|---|
| Age (years) | 55.3 ± 14.4 |
| Gender | Male 0/Female 32 cases |
| Disease duration of RA (months) | 129.7 ± 140.5 |
| Stage of RA | Stage I: 6/Stage II: 16/Stage III: 4/Stage IV: 6 cases |
| Functional class of RA | Class1: 10/Class 2: 19/Class 3: 3/Class 4: 0 cases |
| DAS28-ESR/DAS28-CRP | 4.6 ± 1.2/3.8 ± 1.1 (31 cases) |
| SDAI/CDAI | 19.8 ± 11.0/18.7 ± 11.0 (31 cases) |
| IgG (mg/dl) | 1782 ± 551 (31 cases) |
| Autoantibodies (positivity) | |
| RF/Anti-CCP antibodies | 90.6% (29/32 cases)/78.6% (22/28 cases) |
| Anti-SS-A/SS-B antibodies | 80.0% (24/30 cases)/10.7% (3/28 cases) |
| Organ involvements (prevalence) | |
| Interstitial lung disease (ILD) | 12.5% (4/32 cases) |
| Others | 12.5% (4/32 cases, Kidney 2, AR 1, PBC 1 case) |
| Saxon's test (mg/2 min) | 2232 ± 1908 (29 cases) |
| Schirmer's test (mm/5 min) | 3.6 ± 4.6 (25 cases) |
| Greenspan grading in LSG | 1: 8/2: 4/3: 9/4: 8 (29 cases) |
| Corticosteroid (prevalence) | 46.9% (15/32 cases) |
| Mean dose (15 cases; mg/day) | PSL 5.2 ± 2.7 |
| MTX (prevalence) | 75.0% (24/32 cases) |
| Mean dose (24 cases) (mg/week) | 9.4 ± 4.0 |
| DMARDs other than MTX (prevalence) | 34.4% (11/32 cases) |
| Previous Biologics (prevalence) | 21.9% (Bio-switch, 7; Bio-naïve, 25 cases) |
| IFX | 5 cases |
| ETN | 3 cases |
| ADA | 2 cases |
| TCZ | 1 case |
| GLM | 0 case |
| CER | 0 case (including overlap) |
RA rheumatoid arthritis, DAS28 disease activity score, SDAI Simplified Disease Activity Index, CDAI Clinical Disease Activity Index, AR aortic regurgitation, PBC primary biliary cirrhosis, LSG labial salivary gland, MTX, methotrexate, DMARDs disease-modifying anti-rheumatic drugs, IFX infliximab, ETN etanercept, ADA adalimumab, TCZ tocilizumab, GLM golimumab, CER certolizumab
Effectiveness of abatacept on RA
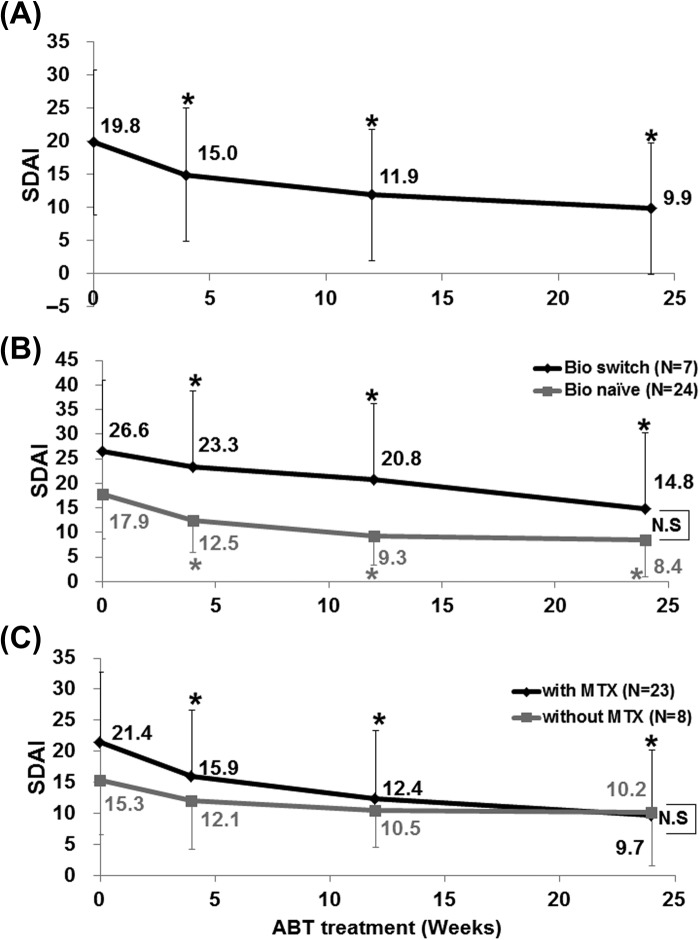
Analysis of data of 31 patients showed that SDAI decreased significantly after treatment with abatacept from 19.8 ± 11.0 (0 week, baseline) to 9.9 ± 9.9 (24 weeks) (P < 0.05). Significant reduction of SDAI relative to baseline (P < 0.05) was noted at 4 weeks and maintained to 24 weeks (Figure 1A). Comparison of bio-switch patients with bio-naïve patients showed significant falls in SDAI after initiation of abatacept in both groups (P < 0.05; Figure 1B). Although the disease activity by SDAI was higher in bio-switch patients than in bio-naïve patients, there was no significant difference between two groups at each time point (Figure 1B).
Figure 1.
Effect of abatacept on RA. (A) Effects of abatacept treatment on Simplified Disease Activity Index (SDAI) in 31 patients. Data deficit was compensated by the last observation carried forward (LOCF) method (∗P < 0.05 vs. 0 week [baseline]), Wilcoxon signed-rank test. ABT, abatacept. (B) Effects of abatacept treatment on SDAI in 7 bio-switch patients and 24 bio-naïve patients. Data deficit compensated by the LOCF method (∗P < 0.05 vs. 0 week [baseline]), Wilcoxon signed-rank test. The difference between two groups was examined using Mann–Whitney U-test. ABT, abatacept; N.S, not significant. (C) Effects of abatacept and methotrexate combination treatment on SDAI in 23 patients and of abatacept alone in 8 patients. Data deficit was compensated by the LOCF method (∗P < 0.05 vs 0 week [baseline]), Wilcoxon signed-rank test. Difference between two groups was examined using Mann–Whitney U test. ABT, abatacept; N.S, not significant.
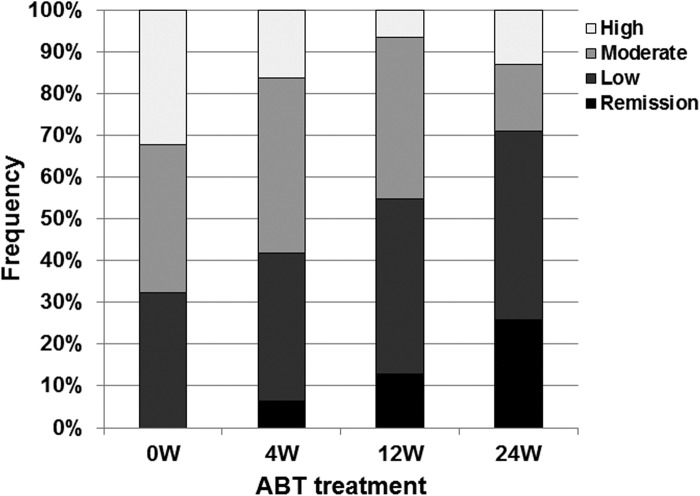
We also examined the effect of concomitant use of MTX. SDAI decreased significantly in patients treated with the combination of abatacept and MTX (P < 0.05; Figure 1C). In comparison, only a moderate decrease was noted in SDAI of patients who were not treated with MTX (Figure 1C). Patients with clinical remission, as assessed by SDAI, increased from 0 patient at 0 week to 8 patients (25.8%) at 24 weeks (Figure 2). In contrast, the number of patients with moderate or high disease activity, as assessed by SDAI, decreased from 21 patients (67.7%) at 0 week to 9 patients (29.0%) at 24 weeks (Figure 2). These findings confirmed the effectiveness of abatacept against RA and RA-related SS.
Figure 2.
Effect of abatacept treatment on RA disease activity. Effects of abatacept treatment on disease activity as assessed by SADI in 31 patients. Data deficit was compensated by the LOCF method. ABT, abatacept.
Effectiveness of abatacept for SS
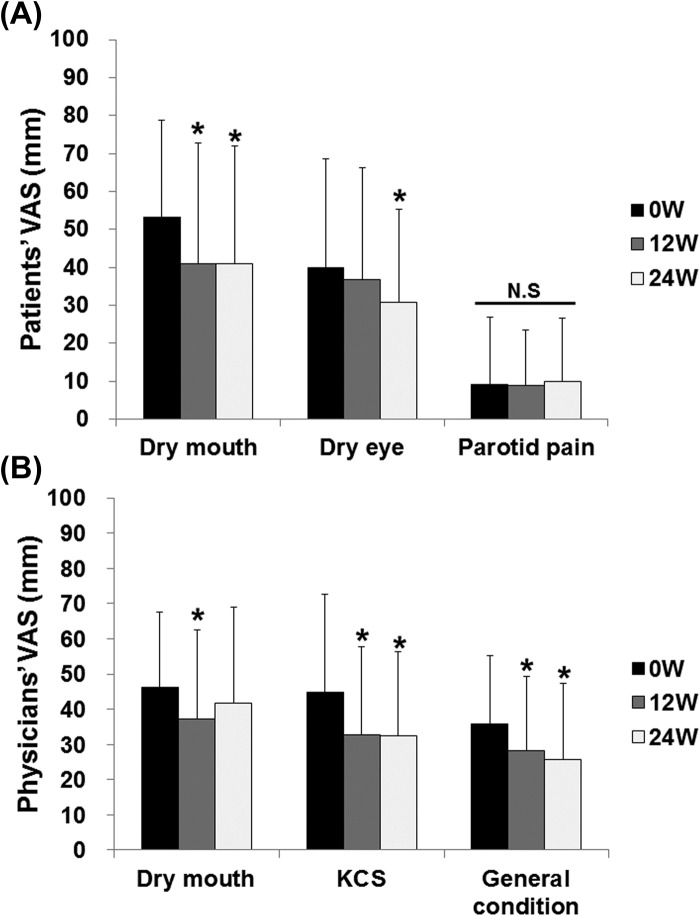
Abatacept significantly decreased patients' VAS for dry mouth and dry eye from 53.3 ± 25.4 and 39.9 ± 28.6 mm at 0 week to 41.0 ± 30.8 and 30.8 ± 24.4 mm at 24 weeks (P < 0.05), respectively (Figure 3A). However, abatacept did not change patients' VAS for parotid pain (Figure 3A). Abatacept significantly decreased physicians' VAS for keratoconjunctivitis sicca (KCS) and general condition from 44.7 ± 28.0 and 35.9 ± 19.3 mm at 0 week to 32.5 ± 23.8 and 25.8 ± 21.6 mm at 24 weeks (P < 0.05), respectively (Figure 3B).
Figure 3.
Effect of abatacept on VAS in SS. (A) Effects of abatacept treatment on patients' VAS for dry mouth, dry eye, and parotid pain in 29 patients. Data deficit was compensated by the LOCF method (∗P < 0.05 vs 0 week [baseline]), Wilcoxon signed-rank test. VAS, visual analog scale; N.S, not significant. (B) Effects of abatacept treatment on physicians' VAS for dry mouth, keratoconjunctivitis sicca, and general condition in 28 patients. Data deficit was compensated by the LOCF method (∗P < 0.05 vs 0 week [baseline]), Wilcoxon signed-rank test. VAS, visual analog scale; KCS, keratoconjunctivitis sicca.
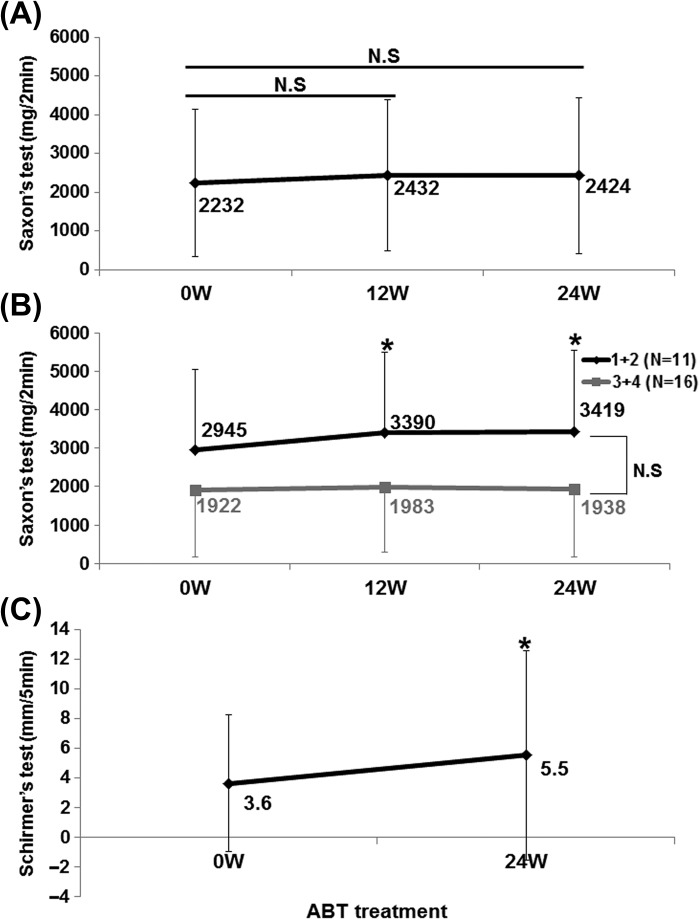
Saliva volume by Saxson's test increased slightly from 2232 ± 1908 mg/2 min at 0 week to 2424 ± 2004 mg/2 min at 24 weeks in 29 patients (Figure 4A). Interestingly, in 11 patients with Greenspan grading 1/2 of LSG biopsy, saliva volume significantly increased from 2945 ± 2090 mg/2 min at 0 week to 3419 ± 2121 mg/2 min at 24 weeks (P < 0.05; Figure 4B). Tear volume by Schirmer's test significantly increased from 3.6 ± 4.6 mm/5 min at 0 week to 5.5 ± 7.1 mm/5 min at 24 weeks in 25 patients (P < 0.05; Figure 4C).
Figure 4.
Effects of abatacept on secretory function in SS. (A) Effects of abatacept treatment on saliva volume assessed by Saxson's test in 29 patients. Data deficit was compensated by the LOCF method. N.S, not significant vs. 0 week (baseline); Wilcoxon signed-rank test; ABT, abatacept. (B) Effect of abatacept treatment on saliva volume assessed by Saxson's test in 11 patients with Greenspan grading 1/2 of labial salivary gland (LSG) biopsy and in 16 patients with grade 3/4. Data deficit was compensated by the LOCF method (∗P < 0.05 vs 0 week [baseline]), Wilcoxon signed-rank test. Difference between two groups was examined using Mann–Whitney U test. ABT, abatacept; N.S, not significant. (C) Effects of abatacept treatment on tear volume assessed by Schirmer's test in 25 patients. Data deficit was compensated by the LOCF method (∗P < 0.05 vs. 0 week [baseline]), Wilcoxon signed-rank test. ABT, abatacept.
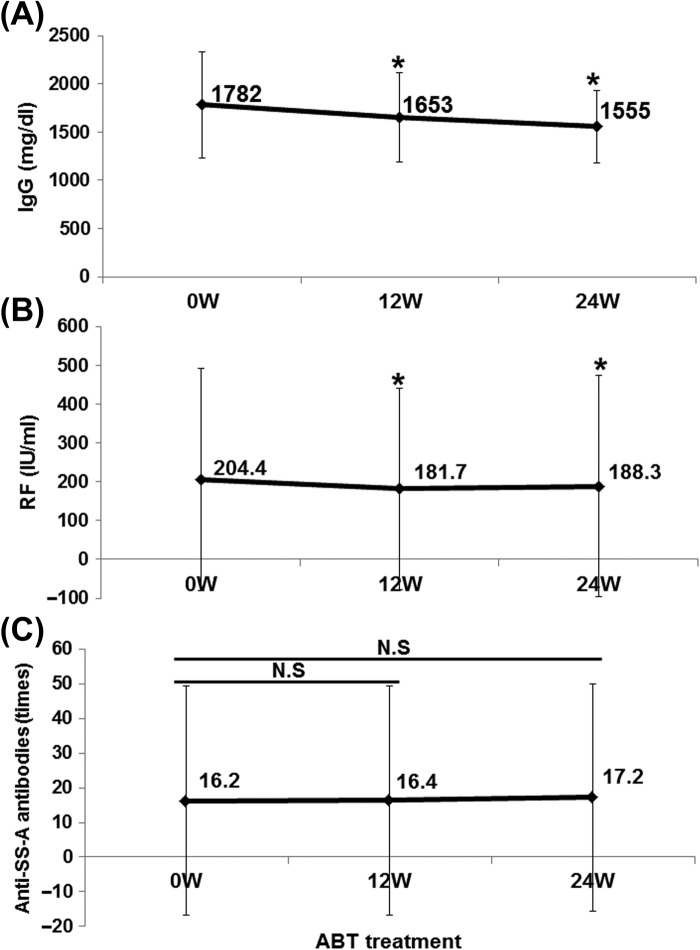
Abatacept significantly decreased serum IgG and RF levels from 1782 ± 551 mg/dl and 204 ± 286 IU/ml at 0 week to 1555 ± 379 mg/dl and 188 ± 285 IU/ml at 24 weeks, respectively (P < 0.05; Figure 5A and B). On the other hand, abatacept had no effect on anti-SS-A antibody titer (Figure 5C). These findings suggest the effectiveness of abatacept for RA-related SS, especially SS-dryness, secretory dysfunction, and production of antibodies.
Figure 5.
Effect of abatacept on IgG, RF, and anti-SS-A antibody in SS. Effects of abatacept treatment on (A) serum IgG level (n = 31), (B) serum RF level (n = 30 patients), (C) titer of anti-SS-A antibody (n = 20 patients). Data deficit was compensated by the LOCF method (∗P < 0.05 vs 0 week [baseline]), N.S; not significant vs baseline, Wilcoxon signed-rank test. ABT; abatacept.
Correlation between improvement of SS and RA
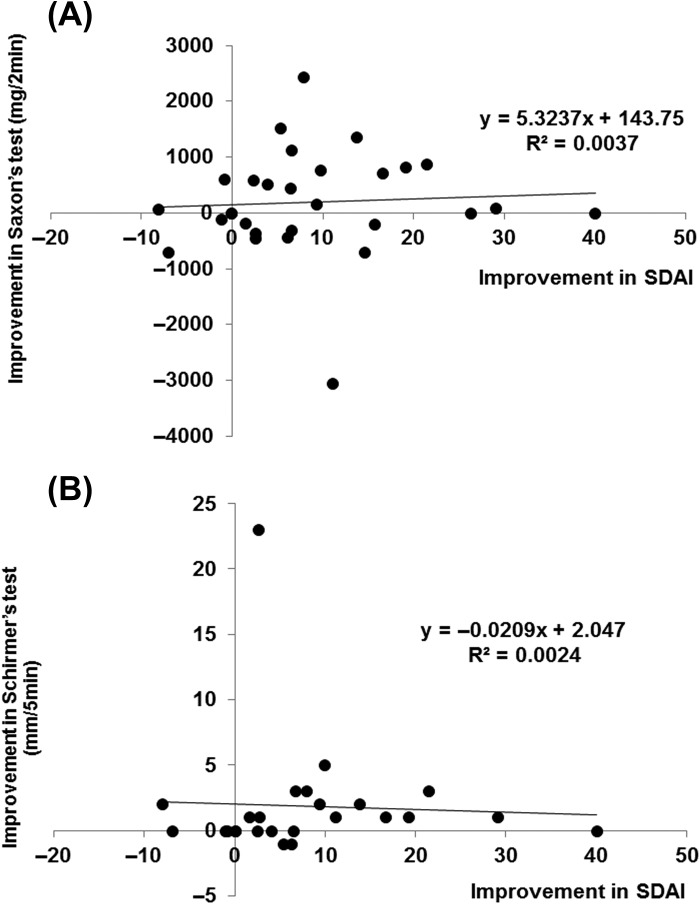
We analyzed the correlation between the increase in saliva volume by Saxon's test and tear volume by Schirmer's test, and the fall in SDAI. There was no significant correlation between the increase in saliva volume and reduction of SDAI (Spearman's rank correlation coefficient = 0.221; Figure 6A), as well as between the increase in tear volume and reduction of SDAI (Spearman's rank correlation coefficient = 0.333; Figure 6B). However, saliva volume and SDAI improved simultaneously in 13 of 29 (44.8%) patients, and tear volume and SDAI in 13 of 25 (52.0%) patients, respectively.
Figure 6.
Correlation between improvement in SS and RA. Correlation between (A) increase in saliva volume (assessed by Saxon's test) and fall in SDAI (n = 29 patients). Spearman's rank correlation coefficient = 0.211, not significant, and (B) between increase of tear volume (assessed by Schirmer's test) and fall in SDAI (n = 25 patients). Data deficit was compensated by the LOCF method. Spearman's rank correlation coefficient = 0.333, not significant.
Safety of abatacept
Five AEs occurred in 5 out of 32 patients (15.6%) during the 24-week period, and three of them were infections (urinary tract infection, infected corneal ulceration, and bronchitis). Importantly, all 5 patients were on corticosteroids treatment. Two of the five patients were admitted to the hospital for treatment of the above AEs. Although abatacept was withheld in three patients, it was resumed after recovery of AEs (Table 2).
Table 2.
Adverse events in 32 cases during 24 weeks.
| Adverse event (5 events, 5 cases, 15.6%) |
Onset (Week) | PSL (mg/d) | MTX (mg/w) | Admission | Treatment for AE | Administration of ABT | Outcome |
|---|---|---|---|---|---|---|---|
| Urinary tract infection | 7 | 5 | None | Yes | Antibiotics div | Cessation | Recovery |
| Infectious cornea ulcer | 24 | 2.5 | None | No | Antibiotics eye drop | Continue | Recovery |
| Compression fracture of lumber spine | 12 | 5 | None | Yes | Operation | Cessation | Recovery |
| Bronchitis | 4 | 3.75 | 8 | No | Antibiotics p.o | Continue | Unknown |
| Vomit and diarrhea | 8 | 5 | None | No | Antiemetics and antidiarrheal | Cessation | Recovery |
AE, adverse event, PSL prednisolone, MTX methotrexate, ABT abatacept, div drip infusion into vein, p.o per oral
Discussion
SS is an autoimmune disease and a variety of immunosuppressant therapies are used for sialadenitis and dacryoadenitis, which are the main organ involved in SS. However, clinical evidence suggests failure of treatment with corticosteroids, MTX, cyclosporine A, and azathioprine to improve objective sicca findings [3]. With regard to biologic agents, TNF blockers (infliximab and etanercept) do not produce sufficient alleviation of dryness symptoms [3]. At present, rituximab seems to be the most promising biologics in ameliorating glandular involvements of SS as well as systemic involvement [3–5]. Importantly, a recent retrospective report from Autoimmune and Rituximab registry has shown that rituximab was administered mainly for systemic organ involvement of primary SS and rarely for glandular involvements [6]. Thus, there are currently no effective disease-modifying drugs for glandular involvement of SS, and standard therapeutic approaches for dryness currently include palliative treatment using secretagogues and topical medications [3].
Histopathological and immunological studies have demonstrated the important roles of CD4-positive T cells in the pathogenesis and development of SS [12,13]. Recently, various helper T cell subsets, such as Th1, Th2, Th17, and Tfh, and related cytokines have been reported to be involved in the development of SS [12,13]. M3 muscarinic acetylcholine receptor (M3R), which is expressed in exocrine glands (e.g., salivary glands and lacrimal glands) and plays an important role in exocrine secretion, is considered a candidate receptor for auto-antigen recognized by T cells in patients with SS [13]. Previous studies indicated the presence of M3R-reactive T cells that produce IFNγ in peripheral blood of 40% of patients with SS [13]. Moreover, it has been reported that salivary gland epithelial cells could act as APCs through the expression of both MHC class II molecules and co-stimulatory molecules CD80/CD86 [12]. These findings encouraged us to postulate the potential of abatacept in the treatment of glandular involvement of SS. To our knowledge, two open-label pilot studies have so far examined the effectiveness of abatacept against primary SS [14,15]. Adler et al. showed that abatacept significantly reduced salivary gland inflammation and increased saliva production when adjusted for disease duration [14]. On the other hand, Meiners et al. reported that while abatacept improved disease activity, laboratory tests, and fatigue in patients with early and active primary SS, it had a minor beneficial effect on preservation of salivary and lacrimal gland functions [15]. In the present study, we demonstrated that treatment with abatacept resulted in amelioration of both RA and RA-related SS, including dryness, secretory dysfunction, and production of antibodies. The findings are important not only clinically but also pathophysiologically.
With regard to the clinical aspects of treatment, our results have raised five important contentions. First, secondary SS associated with RA is the main treatment target in rheumatology. The reported prevalence of SS in RA patients ranges from 3.5% to 31% [20]. Furthermore, RA was diagnosed in 38.7% of secondary SS patients by all Japan survey on epidemiology of SS [2]. Thus, RA patients with secondary SS seem to be the most common population among RA patients complicated with other CTDs and also among all secondary SS patients. Second, the treatment strategy for RA-related SS remains to be established. Importantly, a recent study showed that patients with RA and secondary SS develop more severe arthritis and higher RA disease activity than those with RA without SS [21]. Moreover, in patients with RA, the presence of anti-SS-A antibody and secondary SS might be related to the lesser clinical response to TNF inhibitors [22]. In this respect, we confirmed in the present study the effectiveness of abatacept against RA-related SS and SS-free RA, suggesting that abatacept is a potentially useful therapeutic option for RA-related secondary SS. Third, we demonstrated that abatacept improved secretory dysfunctions of SS in a larger number of patients (29 patient for saliva volume and 25 patients for tear volume) compared with previous studies [14,15]. Therefore, abatacept can probably be regarded a new disease modifying drug for glandular involvements of secondary and primary SS based on more strict evidence from the present study. Fourth, we showed that volume of saliva significantly increased only in patients with Greenspan grading 1/2 of LSG biopsy. This finding indicates that early intervention against glandular involvements is important for recovery of secretory function in SS patients, adding support to the previous report by Adler et al. [14]. Fifth, no unexpected serious AEs of abatacept were encountered in the present study, and abatacept seems to be tolerable in patients with RA-related secondary SS.
Three important findings related to the pathophysiological features of SS were identified in the present study. First, our study confirmed the crucial roles of T cells in the pathogenesis of SS. This is based on the fact that abatacept, a T-cell-targeting therapy, improved various SS involvements, such as dryness, secretory dysfunction, and production of antibodies. Second, our results provided support for the roles of co-stimulatory molecules in the development of SS. As mentioned above, salivary gland epithelial cells in SS can act as APCs through the expression of MHC class II and co-stimulatory molecules CD80/CD86 [12] as well as macrophages. Importantly, B cells might play an important role by not only producing autoantibodies but also acting as APCs through the expression of MHC class II and various co-stimulatory molecules, such as CD80/86, B7RP1, CD27, CD137L, OX40L, and CD40 in some autoimmune conditions [23,24]. Therefore, abatacept might suppress co-stimulatory molecules CD80/86 on multiple cells including epithelial cells and B cells in addition to macrophages, resulting in T cell inhibition through multiple targets for SS. Third; we clarified the effectiveness of abatacept in both RA and RA-related secondary SS. Although there was no significant correlation between the improvement in secretory function and SDAI, both secretory function and SDAI improved simultaneously in about 50% of patients. These findings suggest that T cells play a common pathogenic role in development of SS and RA.
In conclusion, the present study suggested the potential therapeutic usefulness of abatacept for both RA and RA-related secondary SS. Particularly, we demonstrated that abatacept improved secretory dysfunction of SS in more patients than those in previous studies. Importantly, early intervention against glandular involvement might be necessary for proper recovery of secretory function in SS patients. Abatacept seems to be a promising new therapeutic agent for SS-glandular involvement, as well as for RA complicated with SS. The results need to be confirmed in a large population sample and in randomized controlled trials that include and control and placebo groups.
Acknowledgments
We thank Dr. F. G. Issa for the critical reading of the manuscript. This work was supported by Health and Labour Sciences Research Grants for research on intractable diseases (The Research Team for Autoimmune Diseases) from the Ministry of Health, Labour and Welfare of Japan.
Authors' contributions
All authors contributed to the study design, data collection and participated in the writing of the manuscript and all agree to accept equal responsibility for the accuracy of the contents of this paper.
Conflict of interest
This study was supported in part by Bristol-Myers Squibb (BMS); H.T. received lecture fees and/or honoraria from BMS; Y.T. received consulting fees, speaking fees, and/or honoraria from Abbvie, Chugai, Astellas, Takeda, Santen, Mitsubishi-Tanabe, Pfizer, Janssen, Eisai, Daiichi- Sankyo, UCB, GlaxoSmithKline, BMS, and received research grants from Mitsubishi-Tanabe, Chugai, MSD, Astellas, Novartis.
I.M., Y.H., H.N., A.K., and T.S. received research grants, lecture fees and/or honoraria from BMS.
References
- 1.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuboi H, Asashima H, Takai C, Hagiwara S, Hagiya C, Yokosawa M, et al. Primary and secondary surveys on epidemiology of Sjogren's syndrome in Japan. Mod Rheumatol. 2014;24(3):464–70. doi: 10.3109/14397595.2013.843765. [DOI] [PubMed] [Google Scholar]
- 3.Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, Bosch X, Tzioufas AG. Topical and systemic medications for the treatment of primary Sjögren's syndrome. Nat Rev Rheumatol. 2012;8(7):399–411. doi: 10.1038/nrrheum.2012.53. [DOI] [PubMed] [Google Scholar]
- 4.Meijer JM, Meiners PM, Vissink A, Spijkervet FK, Abdulahad W, Kamminga N, et al. Effectiveness of rituximab treatment in primary Sjögren's syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62(4):960–8. doi: 10.1002/art.27314. [DOI] [PubMed] [Google Scholar]
- 5.Dass S, Bowman SJ, Vital EM, Ikeda K, Pease CT, Hamburger J, et al. Reduction of fatigue in Sjögren syndrome with rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann Rheum Dis. 2008;67(11):1541–4. doi: 10.1136/ard.2007.083865. [DOI] [PubMed] [Google Scholar]
- 6.Gottenberg JE, Cinquetti G, Larroche C, Combe B, Hachulla E, Meyer O, et al. Club Rhumatismes et Inflammations and the French Society of Rheumatology. Efficacy of rituximab in systemic manifestations of primary Sjogren's syndrome: results in 78 atients of the AutoImmune and Rituximab registry. Ann Rheum Dis. 2013;72(6):1026–31. doi: 10.1136/annrheumdis-2012-202293. [DOI] [PubMed] [Google Scholar]
- 7.Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349(20):1907–15. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 8.Emery P, Durez P, Dougados M, Legerton CW, Becker JC, Vratsanos G, et al. Impact of T-cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial) Ann Rheum Dis. 2010;69:510–6. doi: 10.1136/ard.2009.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68(12):1870–7. doi: 10.1136/ard.2008.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144(12):865–76. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 11.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353(11):1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Cohen PL. The T cell in Sjogren's syndrome: force majeure, not spectateur. J Autoimmun. 2012;39(3):229–33. doi: 10.1016/j.jaut.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumida T, Tsuboi H, Iizuka M, Hirota T, Asashima H, Matsumoto I. The role of M3 muscarinic acetylcholine receptor reactive T cells in Sjögren's syndrome: a critical review. J Autoimmun. 2014;51:44–50. doi: 10.1016/j.jaut.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Adler S, Körner M, Förger F, Huscher D, Caversaccio MD, Villiger PM. Evaluation of histological, serological and clinical changes in response to abatacept treatment of primary Sjögren's syndrome: a pilot study. Arthritis Care Res (Hoboken) 2013;65(11):1862–8. doi: 10.1002/acr.22052. [DOI] [PubMed] [Google Scholar]
- 15.Meiners PM, Vissink A, Kroese FG, Spijkervet FK, Smitt-Kamminga NS, Abdulahad WH, et al. Abatacept treatment reduces disease activity in early primary Sjogren's syndrome (open-label proof of concept ASAP study) Ann Rheum Dis. 2014;73(7):1393–6. doi: 10.1136/annrheumdis-2013-204653. [DOI] [PubMed] [Google Scholar]
- 16.Yin H, Nguyen CQ, Samuni Y, Uede T, Peck AB, Chiorini JA. Local delivery of AAV2-CTLA4IgG decreases sialadenitis and improves gland function in the C57BL/6.NOD-Aec1Aec2 mouse model of Sjögren's syndrome. Arthritis Res Ther. 2012;14(1):R40. doi: 10.1186/ar3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, III, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 19.Fujibayashi T, Sugai S, Miyasaka N, Hayashi Y, Tsubota K. Revised Japanese criteria for Sjögren's syndrome (1999): availability and validity. Mod Rheumatol. 2004;14(6):425–34. doi: 10.3109/s10165-004-0338-x. [DOI] [PubMed] [Google Scholar]
- 20.Iaccarino L, Gatto M, Bettio S, Caso F, Rampudda M, Zen M, et al. Overlap connective tissue disease syndromes. Autoimmun Rev. 2013;12(3):363–73. doi: 10.1016/j.autrev.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 21.He J, Ding Y, Feng M, Guo J, Sun X, Zhao J, et al. Characteristics of Sjögren's syndrome in rheumatoid arthritis. Rheumatology (Oxford) 2013;52(6):1084–9. doi: 10.1093/rheumatology/kes374. [DOI] [PubMed] [Google Scholar]
- 22.Matsudaira R, Tamura N, Sekiya F, Ogasawara M, Yamanaka K, Takasaki Y. Anti-Ro/SSA antibodies are an independent factor associated with an insufficient response to tumor necrosis factor inhibitors in patients with rheumatoid arthritis. J Rheumatol. 2011;38(11):2346–54. doi: 10.3899/jrheum.101295. [DOI] [PubMed] [Google Scholar]
- 23.Kow NY, Mak A. Costimulatory pathways: physiology and potential therapeutic manipulation in systemic lupus erythematosus. Clin Dev Immunol. 2013;2013:245928. doi: 10.1155/2013/245928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobón GJ1, Izquierdo JH, Cañas CA. B lymphocytes: development, tolerance, and their role in autoimmunity-focus on systemic lupus erythematosus. Autoimmune Dis. 2013;2013:827254. doi: 10.1155/2013/827254. [DOI] [PMC free article] [PubMed] [Google Scholar]