Abstract
Purpose
The objectives of this study were (i) to characterize the hemodynamic responses caused by controlled hemorrhage (HEM) in pentobarbital-anesthetized rats, and (ii) to determine the responses elicited by systemic bolus injections of isotonic saline (0.15 M) or hypertonic saline (3 M) given 5 min after completion of HEM.
Results
Controlled HEM (4.3 ± 0.2 ml/rat at 1.5 ml/ min) resulted in a pronounced and sustained fall in mean arterial blood pressure (MAP) to about 40 mmHg. The fall in MAP was associated with a reduction in hindquarter vascular resistance (HQR) but no changes in renal (RR) or mesenteric (MR) vascular resistances. Systemic injections of isotonic saline (96–212 µmol/kg iv, in 250–550 µl) did not produce immediate responses but promoted the recovery of MAP to levels below pre-HEM values. Systemic injections of hypertonic saline (750–3000 µmol/kg, i.v., in 250–550 µl) produced immediate and pronounced falls in MAP, RR, MR and especially HQR of 30–120 sec in duration. However, hypertonic saline prompted a full recovery of MAP, HQR and RR to pre-HEM levels and an increase in MR to levels above pre-HEM values.
Conclusions
This study demonstrates that (i) HEM induced a pronounced fall in MAP which likely involved a fall in cardiac output and HQR, (ii) isotonic saline did not fully normalize MAP, and (iii) hypertonic saline produced dramatic initial responses, and promoted normalization of MAP probably by restoring blood volume and cardiac output through sequestration of fluid from intracellular compartments.
Index terms: hemorrhage, hemodynamics, arterial blood pressure, vascular resistances, renal, mesenteric, hindquarter, isotonic saline, hypertonic saline, rats
INTRODUCTION
Moderate to severe hemorrhage (HEM) in humans and animals results in a fall in cardiac output (CO) and mean arterial blood pressure (MAP) but an increase in total peripheral resistance (TPR), which limits the fall in MAP (Brooks, 1935; Baue et al., 1967, 1991; Boyd and Mansberger, 1968; Jarhult, 1973; Mittman et al., 1976; Pirkle and Gann, 1976; Drucker et al., 1981; Traveso et al., 1987; Wade et al., 1997). The falls in CO and MAP during HEM are partially reversed by the movement of fluid and protein from the interstitium into capillaries (Starling, 1896; Guyton, 1965; Boyd and Mansberger, 1968; Pirkle and Gann, 1976; Drucker et al., 1981). The movement of fluid and proteins is initiated by a fall in capillary hydrostatic pressure, which promotes a rapid shift of interstitial fluid into the capillaries and a less rapid shift of interstitial albumin into the plasma, which helps to support plasma oncotic pressure (Drucker et al., 1981). Intracellular fluid drawn down an osmotic gradient facilitates restitution of plasma volume after HEM by replenishing interstitial fluid volume. The increase in interstitial volume and pressure provides the driving force for transcapillary movement of fluid and albumin into plasma (Drucker et al., 1981). The driving force responsible for movement of fluid into the interstitial space and transcapillary movement of albumin, is regulated by circulating factors and especially glucose derived from the splanchnic circulation. The release of these factors is triggered by HEM-induced increases in circulating hormones levels (Stone et al., 1977; Drucker et al., 1981; Friedman et al., 1982).
The major objective of providing fluid therapy to subjects with hemorrhagic shock is to restore circulating volume and thereby support tissue perfusion (Drucker et al., 1981; Falk et al., 1983; Dubick et al., 2013; Thongrong et al., 2013). Fluids such as physiological salt solutions, Ringer’s lactate, hydroxyethyl starch, albumin and dextrans have been employed in clinical and experimental settings (Falk et al., 1983; Moss and Gould, 1988; Shires et al., 1995; Dubic et al., 2013). Administration of small volumes of hypertonic saline (H-saline) to subjects in hemorrhagic shock restores MAP by increasing circulating volume (De Felippe and Timoner, 1980; Nakayama et al., 1984; Traveso et al., 1987; Baue et al., 1991; Dontigny, 1992; Coimbra et al., 1997; Shackford et al., 1998), most likely due to the movement of intracellular fluid into the vascular space (Drucker et al., 1981). The use of reduced volume H-saline therapy has the advantage of reducing the potential development of third space fluid sequestration (such as cerebral edema in patients with head injury), which may occur with large volume therapy (Drucker et al., 1981). In addition, there are several reports that H-saline directly increased cardiac contractility in patients with moderate to marked HEM (Ogino et al., 1998). Several studies have also examined the effects of mild to severe HEM on MAP, CO and TPR in experimental subjects (Drucker et al., 1981). It should be noted however that in recent trials, low volume H-saline resuscitation did not improve outcomes in either hypovolemic shock or traumatic head injury patients (Dubick et al., 2013), thus calling into question the benefit of such therapy for these broadly defined patient populations. A more detailed understanding of the underlying pharmacology could aid in defining a patient population that might be better served by such therapy.
There is considerable information as to effects of small volumes of isotonic saline (I-saline) and H-saline on MAP, CO and TPR in low CO-induced hypotension during HEM (Brooks, 1935; Nakayama et al., 1984; Smith et al., 1985; Maningas et al., 1986; Traveso et al., 1987; Hannon et al., 1989; Barbosa et al., 1990, 1992). However, although the changes in systemic vascular resistances during HEM in animals have received attention (see Liu et al., 2003; Whalen et al., 2007), nothing is known about the changes in systemic resistances elicited by administration of I-saline or H-saline in these rats. Such vital data would help us to understand how vascular beds subserving different physiological roles respond to HEM and to H-saline. Moreover, the rat is an ideal species to perform pharmacological studies designed to develop therapeutic strategies to treat hemorrhagic shock and other conditions associated with severe hypotension.
The first aim of this study was to determine the changes in MAP and systemic vascular resistances resulting from mild HEM in pentobarbital-anesthetized rats, in which the falls in MAP during HEM are comparable to those of conscious rats (Soucy et al., 1995a,b). The second aim was to determine the changes in MAP and vascular resistances elicited by bolus injections of I- and H-saline in these HEM rats. The changes in hindquarter (HQR), renal (RR) and mesenteric (MR) vascular resistances elicited by mild HEM were examined because of the key roles these vascular beds play in the circulatory adjustments to hemodynamic challenges, and because endocrine cells in the kidneys and mesentery release factors known to directly affect fluid movement across capillary walls and overall body fluid homeostasis (see Drucker et al., 1981).
Methods and materials
Rats and surgical procedures
All studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 1996. The protocols were approved by the University of Iowa Institutional Animal Care and Use Committee. Male Sprague Dawley rats (250–300g) from Harlan (Madison, WI) were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). A catheter was placed into a femoral vein to administer drugs. Supplemental doses of pentobarbital (5 mg/kg, i.v.) were given as necessary to maintain anesthesia. A catheter was inserted into a femoral artery to measure pulsatile arterial blood pressure (PP) and MAP, and to determine heart rate (HR). A midline laparotomy was then performed and miniature pulsed Doppler flow probes were placed on (i) the superior mesenteric artery to measure mesenteric blood flow velocities (MF) and to determine MR, (ii) a renal artery to measure renal blood flow velocities (RF) and RR, and (iii) on the descending aorta (below the level of the kidneys) to measure hindquarter blood flow velocities (HQF) and to determine HQR (Whalen et al., 1999, 2000). Vascular resistances were determined by dividing MAP by blood flow velocity. The body temperature of each rat was maintained at 37°C via a thermostatically-controlled heating pad.
Experimental protocols
The arterial catheter was connected to a Beckman Dynograph-coupled pressure transducer to measure PP and MAP. HR was determined from PP by a cardiotachometer. The wire leads from the flow probes were connected to a Doppler flowmeter (Department of Bioengineering, University of Iowa) to continuously record blood flow velocities. The rats were allowed 15–20 min to stabilize before commencement of the HEM. Protocol 1 - In each group, blood was withdrawn to obtain a MAP value of about 40 mmHg. In the first group of rats (n=5), blood was withdrawn (4.3 ± 0.2 ml/rat at 1.5 ml/min) and parameters were monitored for 20 min after completion of HEM. Protocol 2 - In the second group (n=5), blood was withdrawn (5.9 ± 0.5 ml/rat at 1.5 ml/min) and after 5 min, 100, 200 and 400 µl injections of H-saline (17.5% NaCl, 3 M) were given 3–5 min apart (at which time the responses had subsided or reached plateau values). The doses of NaCl (including the extra 150 µl volumes of isotonic NaCl used to flush the H-saline into the rats) were 750, 1500 and 3000 µmol/kg, i.v. Resting parameters were monitored for 20 min after completion of HEM. Protocol 3 - In the third group (n=5), blood was withdrawn (6.2 ± 0.5 ml/rat at 1.5 ml/min) and after 5 min, i.v. injections (250, 350 and 550 µl) of I-saline (0.9% NaCl, 154 mM) were given 3–5 min apart. The doses of NaCl were 96, 135 and 212 µmol/kg, i.v., respectively. Resting parameters were monitored for 20 min after completion of HEM.
Statistical analyses
The data are expressed as the mean ± SEM. The data were tested and found to be normally distributed (BMDP Statistical Package, Statistical Solutions, Boston, MA). The data were then analyzed by one-way or repeated-measures analysis of variance (ANOVA) using the above statistical package, followed by Student's modified t test with Bonferroni corrections for multiple comparisons between means using the modified error mean square term from the ANOVA (Whalen et al., 1999, 2000). A value of P < 0.05 was taken to denote statistical difference.
RESULTS
Hemodynamic responses produced by HEM
Resting hemodynamic parameters recorded prior to beginning the HEM protocol in the three groups are summarized in Table 1. As can be seen, there were no between-group differences in these parameters. A typical example of the responses during HEM (4.2 ml) in a rat, which did not receive subsequent injections of I- or H-saline, is shown in Fig. 1. HR, MAP and blood flow velocities began to fall about half way through HEM. At the completion of HEM (0 min post-HEM), there was a reduction in HR (−22%) and a pronounced decrease in MAP (−61%). Blood flow velocities were also reduced at the end of the HEM. The reduction in HQF (−7%) was smaller than the fall in MAP so that there was a substantial reduction in HQR (−36%), that is, a pronounced vasodilation. The decrease in RF (−44%) was somewhat less than the reduction in MAP such that there was minor increase in RR (+5%), that is, a minor vasoconstriction. The reduction in MF (−51%) was somewhat less than the reduction in MAP and so there was a minor increase in MR (+18%). These resting hemodynamic parameters remained at these levels over the following 20 min.
Table 1.
Resting hemodynamic parameters prior to hemorrhage
| Treatment group | |||
|---|---|---|---|
| Parameter | Control | I-Saline | H-saline |
| HR, beats/min | 332 ± 16 | 292 ± 17 | 306 ± 18 |
| MAP, mmHg | 123 ± 4 | 115 ± 4 | 116 ± 4 |
| HQR, mmHg/kHz | 59 ± 12 | 76 ± 15 | 72 ± 18 |
| RR, mmHg/kHz | 17 ± 2 | 16 ± 2 | 15 ± 3 |
| MR, mmHg/kHz | 18 ± 2 | 17 ± 2 | 15 ± 2 |
The data are presented as mean ± SEM. The group designated “Control” did not receive injections of either hypertonic or isotonic saline after hemorrhage. The group designated I-saline received injections of isotonic saline after hemorrhage. The group designated H-saline received injections of hypertonic saline after hemorrhage.
HR = heart rate. MAP = mean arterial blood pressure. HQR = hindquarter vascular resistance. RR = renal vascular resistance. MR = mesenteric vascular resistance.
Note that there were no between-group differences for any parameter (P > 0.05, for all comparisons).
Fig. 1.
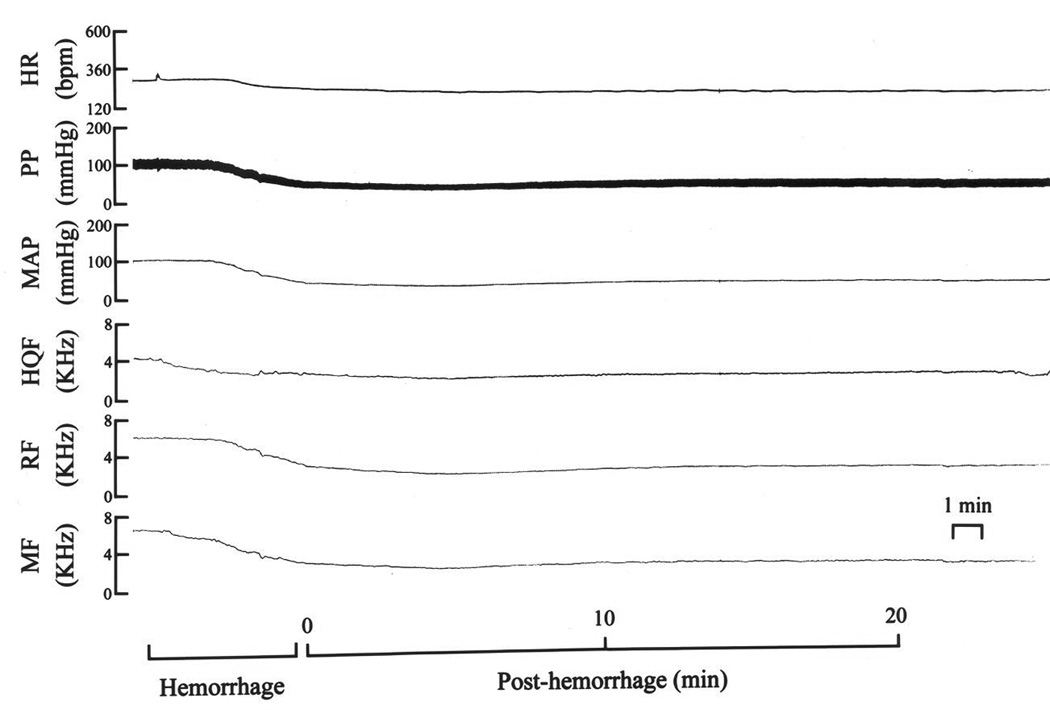
A typical example of the changes in heart rate (HR), pulsatile (PP) and mean (MAP) arterial blood pressures, and hindquarter (HQF), renal (RF) and mesenteric (MF) blood flow velocities produced by hemorrhage (5 ml at 1.5 ml/min) in a pentobarbital-anesthetized rat.
The responses produced by HEM (withdrawal of 4.3 ± 0.2 ml of blood) in rats that did not receive subsequent injections of I- or H-saline are summarized in Table 2. HEM elicited reductions in HR, MAP and HQR, but no changes in RR or MR (0 min post-HEM, i.e., immediately following withdrawal of blood). These parameters remained constant over the subsequent 20 min. The responses elicited by HEM (withdrawal of 5.9 ± 0.2 ml of blood) in the group of rats that subsequently received injections of H-saline are summarized in Table 3. This HEM also produced falls in HR, MAP and HQR, but no changes in RR or MR. The responses elicited by HEM (withdrawal of 6.2 ± 0.5 ml of blood) in the group of rats, which subsequently received injections of I-saline are summarized in Table 4. This HEM also produced falls in HR and MAP whereas there were increases in HQR, RR and MR. The falls in MAP and HR in all three groups of rats were similar to one another (P > 0.05, for all comparisons).
Table 2.
Changes in hemodynamic parameters following hemorrhage
| Post-hemorrhage (min) | |||||
|---|---|---|---|---|---|
| Parameter | 0 | 5 | 10 | 15 | 20 |
| ΔHR (%) | −16 ± 3* | −20 ± 4* | −21 ± 2* | −20 ± 2* | −20 ± 3* |
| ΔMAP (%) | −69 ± 2* | −63 ± 4* | −56 ± 3* | −60 ± 4* | −60 ± 3* |
| ΔHQR (%) | −38 ± 5* | −30 ± 9* | −23 ± 10* | −32 ± 5* | −32 ± 6* |
| ΔRR (%) | −24 ± 10 | −5 ± 12 | −7 ± 8 | −21 ± 10 | −17 ± 10 |
| ΔMR (%) | −16 ± 9 | −10 ± 7 | +2 ± 12 | +4 ± 10 | +17 ± 20 |
The data are presented as mean ± SEM.
HR = heart rate. MAP = mean arterial blood pressure. HQR = hindquarter vascular resistance. RR = renal vascular resistance. MR = mesenteric vascular resistance.
P < 0.05, significant change form pre-values. Note that the values recorded between 15-45 min were similar to those recorded at 5 min (P > 0.05, for all comparisons).
Table 3.
Effects of hypertonic saline on hemodynamic parameters in hemorrhaged rats
| Post-saline values | ||||
|---|---|---|---|---|
| Parameter | post-HEM, 0 min pre-sal1 |
post-sal1 pre-sal2 |
post-sal2 pre-sal3 |
post-sal3 |
| ΔHR (%) | −18 ± 4* | −17 ± 2* | −15 ± 3* | −9 ± 6† |
| ΔMAP (%) | −68 ± 3* | −54 ± 3*,† | −31 ± 7*,† | +2 ± 3† |
| ΔHQR (%) | −23 ± 7* | −30 ± 7* | −9 ± 6† | −3 ± 4† |
| ΔRR (%) | 0 ± 10 | −9 ± 6 | −19 ± 7* | +13 ± 5* |
| ΔMR (%) | −11 ± 11 | −3 ± 8 | +29 ± 10*,† | +57 ± 6*,† |
The data are shown as mean ± SEM of the percent changes from pre-hemorrhage (HEM) values. The column designated as post-HEM/pre-sal1 shows the hemodynamic changes in response to HEM. The columns designated post-sal1/pre-sal2, post-sal2/pre-sal3 and post-sal3,summarize the hemodynamic status after the first, second and third injections of hypertonic saline, respectively.
HR = heart rate. MAP = mean arterial blood pressure. HQR = hindquarter vascular resistance. RR = renal vascular resistance. MR = mesenteric vascular resistance.
P < 0.05, significant change from pre-HEM values.
P < 0.05, pre-sal2, pre-sal3,orpost-sal3 versus pre-sal1.
Table 4.
Effects of isotonic saline on hemodynamic parameters in hemorrhaged rats
| Post-saline values | ||||
|---|---|---|---|---|
| Parameter | post-HEM, 0 min pre-sal1 |
post-sal1 pre-sal2 |
post-sal2 pre-sal3 |
post-sal3 |
| ΔHR (%) | −15 ± 4* | −10 ± 3* | −6 ± 4 | −7 ± 4 |
| ΔMAP (%) | −63 ± 4* | −40 ± 6*,† | −32 ± 6*,† | −23 ± 3*,† |
| ΔHQR (%) | +41 ± 11* | +55 ± 11* | +69 ± 13*,† | +86 ± 22*,† |
| ΔRR (%) | +242 ± 32* | +77 ± 15*,† | +68 ± 12*,† | +44 ± 13*,† |
| ΔMR (%) | +46 ± 8* | +60 ± 13* | +67 ± 16* | +49 ± 14* |
The data are mean ± SEM of the percent changes from pre-hemorrhage (HEM) values. The column designated as post-HEM/pre-sal1 shows the hemodynamic changes in response to HEM. The columns designated post-sal1/pre-sal2, post-sal2/pre-sal3 and post-sal3, summarize the hemodynamic status after the first, second and third injections of isotonic saline, respectively.
HR = heart rate. MAP = mean arterial blood pressure. HQR = hindquarter vascular resistance. RR = renal vascular resistance. MR = mesenteric vascular resistance.
P < 0.05, significant change from pre-HEM values.
P < 0.05, pre-sal2, pre-sal3, or post-sal3 versus pre-sal1.
Effects of bolus injections of hypertonic saline in rats subjected to HEM
A typical example of the changes in hemodynamic parameters produced by three successive injections of H-saline (750, 1500 and 3000 µmol/kg, i.v.) is shown in Fig. 2. The injections of H-saline elicited pronounced hemodynamic responses. The 750 µmol/kg dose produced a reduction in MAP that was accompanied by a minor increase in HR. The decrease in MAP was associated with relatively minor reductions in HQF and RF and no change in MF. These hemodynamic changes resulted in reductions in HQR, RR and MR (i.e., vasodilator responses). As MAP returned toward pre-injection levels, an increase in HQF occurred such that there was an even more pronounced reduction in HQR. The 1500 µmol/kg dose of H-saline produced a pronounced fall in MAP that was associated with a minor increase in HR. The reduction in MAP was associated with pronounced reduction in HQF and RF and a minor reduction in MF. These changes resulted in minor reductions in HQR and RR but a relatively pronounced reduction in MR. As MAP returned toward pre-injection levels, an increase in HQF occurred such that there was now a pronounced reduction in HQR. The 3000 µmol/kg dose of H-saline produced a pronounced fall in MAP and a minor increase in HR. The reduction in MAP was associated with minor reductions in HQF and MF but a substantial reduction in RF. These changes resulted in reductions in HQR and MR but no change in RR. As MAP returned toward pre-injection levels, a pronounced increase in HQF occurred such that there was now a substantial reduction in HQR. However, the injections of H-saline promoted a full recovery of MAP to pre-HEM levels (compare the end of the tracings in Fig. 2 to pre-HEM values).
Fig. 2.
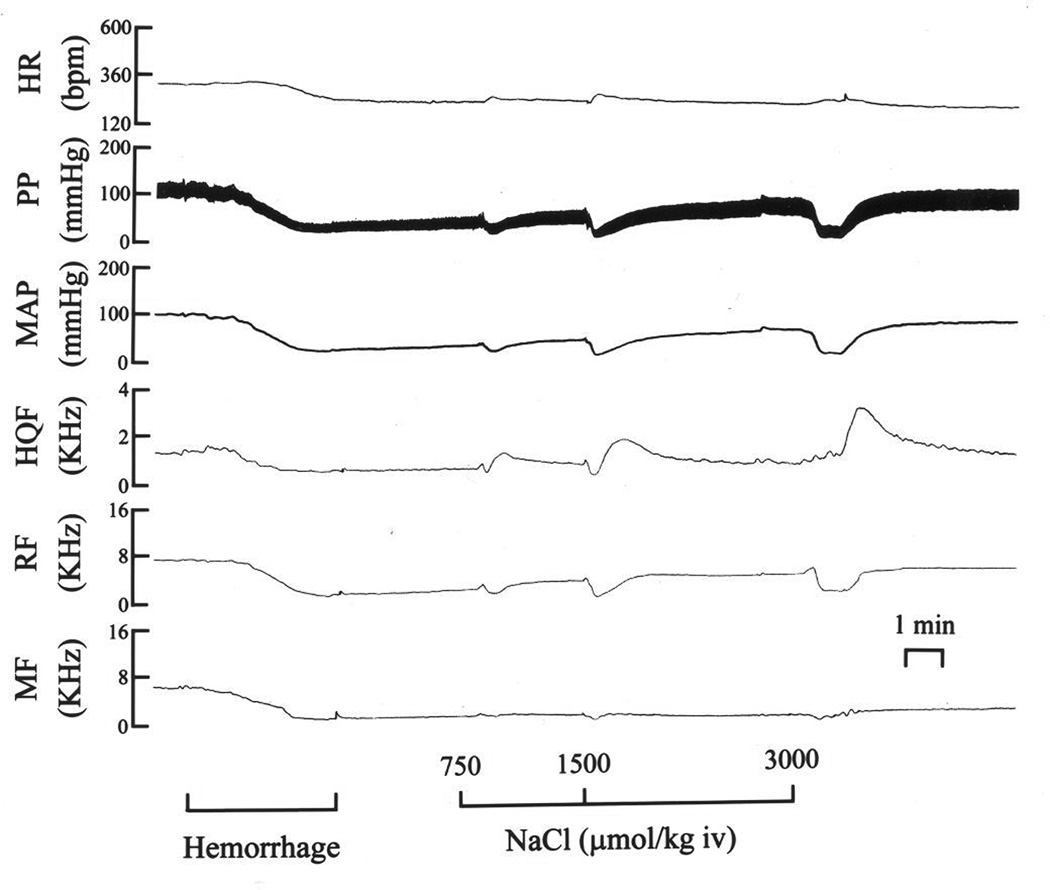
A typical example of the changes in (HR), pulsatile (PP) and mean (MAP) arterial blood pressures, and hindquarter (HQF), renal (RF) and mesenteric (MF) blood flow velocities produced by three bolus injections of isotonic saline (96–212 µmol/kg, i.v.) in a pentobarbital-anesthetized rat subjected to hemorrhage (5 ml at 1.5 ml/min).
The maximal responses (percent change from pre-injection values) elicited by injections of H-saline (750–3000 µmol/kg, i.v.) are summarized in Fig. 3. The Phase I responses are those at the point of maximal reduction in MAP. The Phase II responses occurred at the time of the maximal hindquarter vasodilation, which occurred as MAP began to recover. The next section deals with the Phase I responses. The injections of H-saline elicited dose-dependent reductions in MAP. Each reduction in MAP was associated with a similar increase in HR. The reductions in MAP were associated with dose-dependent reductions in MR. The lowest and highest dose of H-saline produced substantial reductions in HQR. The lowest dose of H-saline produced a relatively minor reduction in RR whereas the higher doses did not affect RR. The next section deals with the Phase II responses. As can be seen, there were pronounced dose-dependent reductions in HQR and MR The reductions in MAP were smaller than during Phase I. Again, there were minimal reductions in RR with the response produced by the second injection reaching statistical significance. The time-dependent responses after each injection of H-saline in HEM rats are summarized in Table 3. The immediate post-HEM changes (see Column designated Pre-sal1) have been described above. The reductions in HR, MAP and HQR subsided toward pre-HEM values after each injection of H-saline. Resting HR, MAP and HQR were equal to pre-HEM values after the third dose of H-saline (~20 min post-HEM, see Table 3). There were minor changes in RR that consisted of a small decrease after the second injection of H-saline and a small increase after the last injection. MR gradually increased after each injection of H-saline. These responses reached significance after the second and third doses of H-saline.
Fig. 3.
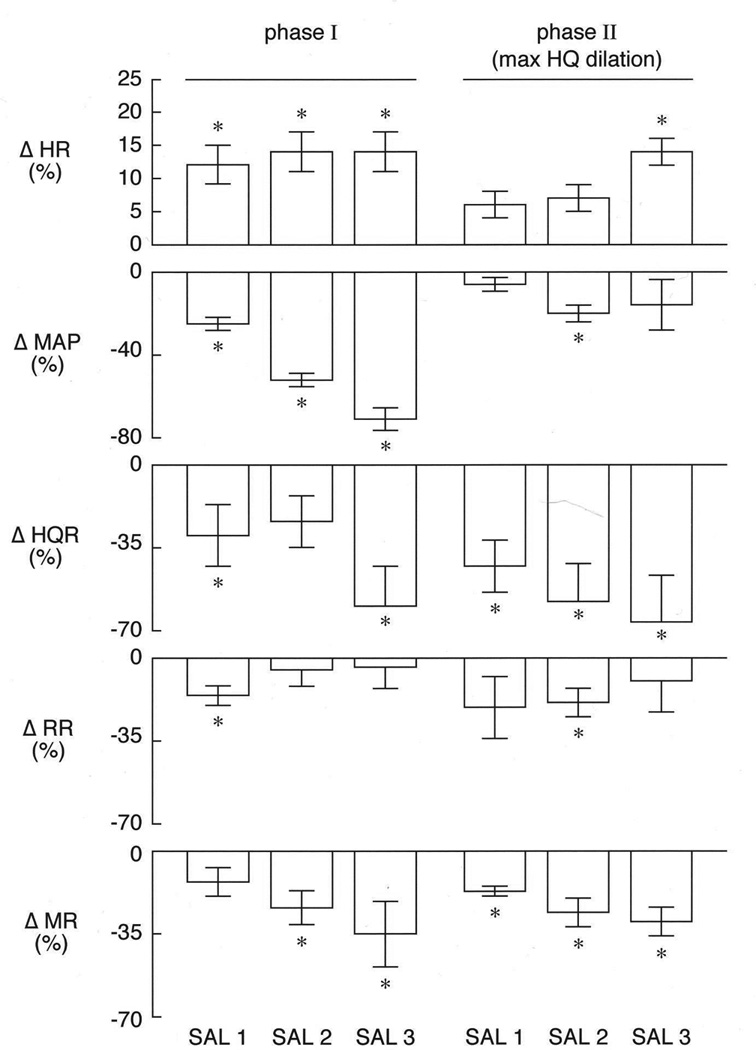
A summary of the maximal initial changes in (HR), mean arterial blood pressure (MAP), and hindquarter (HQR), renal (RR) and mesenteric (MR) vascular resistances produced by three injections of hypertonic saline (750–3000 µmol/kg, i.v.) in pentobarbital-anesthetized rat (n=5) subjected to hemorrhage (5.9 ± 0.5 ml/rat at 1.5 ml/min). The values are expressed as the mean ± SEM of the percent changes form pre-injection baseline values. *P < 0.05, significant response.
Effects of bolus injections of isotonic saline in rats subjected to HEM
A typical example of the changes in hemodynamic parameters produced by three injections of I-saline (96–212 µmol/kg, i.v.) in a HEM rat is shown in Fig. 4. The injections of I-saline did not elicit immediate hemodynamic responses. However, the injections of I-saline promoted the time-dependent recovery of MAP, although not to pre-HEM levels over the 20 min post-HEM observation period. The time-dependent responses following each injection of I-saline (96–212 µmol/kg, i.v.) in rats subjected to HEM are summarized in Table 4. Again, the immediate post-HEM changes (see Column designated Pre-sal1) have been described above. MAP recovered somewhat after each injection of I-saline but did not recover to pre-HEM levels. In contrast, the reductions in HR did recover to pre-HEM levels. The post-HEM increase in RR was diminished after injection of the first dose of I-saline and remained at these levels after the subsequent doses I-saline. The HEM-induced increases in HQR gradually and substantially increased after each injection of I-saline. The post-HEM-induced increases in MR remained relatively unchanged following each injection of I-saline.
Fig. 4.
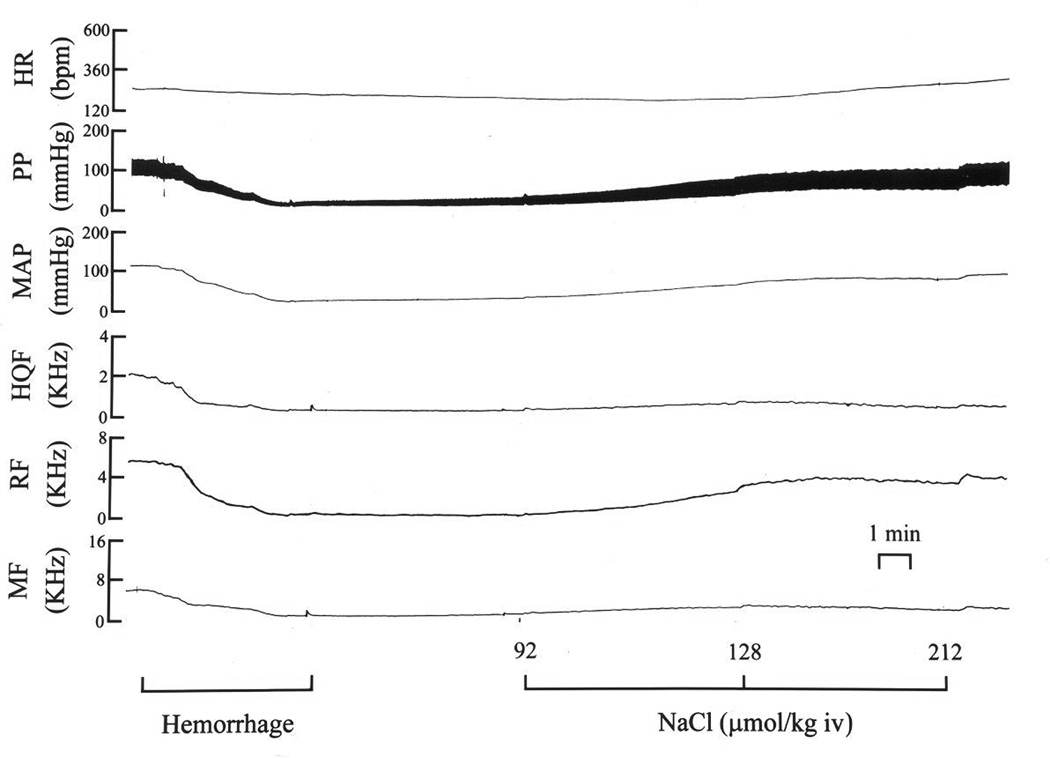
A typical example of the changes in (HR), pulsatile (PP) and mean (MAP) arterial blood pressures, and hindquarter (HQF), renal (RF) and mesenteric (MF) blood flow velocities produced by three bolus injections of hypertonic saline (750–3000 µmol/kg, i.v.) in a pentobarbital-anesthetized rat subjected to hemorrhage (5 ml at 1.5 ml/min).
Discussion
This study investigated the hemodynamic responses elicited by low-volume injections of I- or H-saline in pentobarbital-anesthetized rats subjected to HEM. The major novel findings were that (1) the injections I-saline promoted the gradual recovery of MAP toward (but still substantially below) pre-HEM levels via mechanisms involving diminished hindquarter vasodilation, (2) injections of H-saline elicited initial decreases in MAP and hindquarter vascular resistance but minimal changes in RR and MR, and (3) the injections of H-saline subsequently prompted recovery of MAP and HQR to pre-HEM levels but increases in RR and MR to levels above pre-HEM values (i.e., vasoconstriction). These findings demonstrate that the temporal changes in systemic vascular resistances following injections of I- and H-saline in HEM rats are substantially different from one another.
Hemodynamic responses produced by HEM in pentobarbital-anesthetized rats
The withdrawal of blood in rats, which did not subsequently receive injections of saline produced a reduction in HR and MAP that was sustained for at least 20 min (see below). It is likely that the depressor response involved a reduction in circulating blood volume and cardiac output. It could be expected that the reduction in MAP would trigger a baroreflex-mediated increases in HR. However, the HEM-induced fall in MAP was associated with a pronounced bradycardia. The precise mechanisms responsible for the bradycardia may be due to (i) cardiopulmonary afferent-mediated increase in cardiovagal activity, (ii) a withdrawal of sympathetic nerve activity, or (ii) diminished responsiveness of cardiac pacemakers to sympathetically-derived norepinephrine (Shires et al., 1995). HEM was also associated with a relatively pronounced reduction in HQR but no overall changes in RR or MR. We have not determined the mechanisms responsible for the HEM-induced vasodilation in the hindquarter bed of our pentobarbital-anesthetized rats. However, many mechanisms, including the desensitization of adrenoceptors (Ekelund et al., 1998; Liu et al., 2003), and the enhanced production/activity of nitric oxide (Thiemermann et al., 1993; Landín et al., 1994; Szabó and Thiemermann C, 1994; Li et al., 1996; Cuzzocrea et al., 1997; Hollenberg et al., 1999; Liu et al., 2003), S-nitrosothiols (Atkins et al., 2006), opioid peptides (Armstead, 1995), and inflammatory cytokines (Robert et al., 1992; Zingarelli et al., 1994; Simper et al., 1995), have been proposed to interfere with microvascular reactivity following HEM. It could be expected that the above mechanisms would elicit similar changes in vascular resistances in the hindquarter, renal and mesenteric beds. However, Liu et al (2003) demonstrated that whereas the vasoconstrictor responses to norepinephrine in various vascular beds of urethane-anesthetized rats were blunted following HEM, the loss of vasoconstriction was most pronounced in hindlimb bed. Accordingly, the vasodilation in the hindquarter bed of our pentobarbital-anesthetized rats may involve the relatively more pronounced down-regulation of α-adrenoreceptor-mediated vasoconstriction. It is also possible that the hindquarter vasodilation involves the activation of a unique neurogenic vasodilator pathway, which may release preformed pools of nitric oxide factors (Davisson et al., 1996a,b, 1997; Possas et al., 2006), with the capacity to directly dilate resistance arteries and blunt α-adrenoreceptor-mediated vasoconstriction (Nozik-Grayck et al., 2006).
Since the responses initiated by HEM in control rats remained constant over 20 min, it is evident that movement of fluid and proteins from interstitial spaces into the circulating volume as well as other compensatory mechanisms designed to recover hemodynamic status were not in effect. However it possible that a substantial amount of fluid did enter the circulation but that CO and MAP do not improve due to diminished cardiac contractility (Shires et al., 1995). As stated, the withdrawal of blood (4.3 ± 0.2 ml) in rats that did not subsequently receive injections of saline resulted in sustained decreases in MAP, HR and HQR without changes in RR or MR. The withdrawal of slightly larger amounts of blood (5.9 ± 0.5 ml) in rats that were to receive injections of H-saline resulted in similar hemodynamic responses. It should be noted that these HEM-induced hemodynamic responses were similar to those we have reported previously (Whalen et al., 2007) and that despite the relatively greater prior withdrawal of blood, the administration of H-saline elicited a marked improvement of resting MAP (see below). The withdrawal of blood (6.2 ± 0.5 ml) in rats that were to receive injections of I-saline, elicited similar initial falls in MAP and HR to the above groups. However, the falls in MAP were accompanied by increases in HQR, RR and MR. As such, it is evident that CO must have dropped more precipitously in the later group of rats. Since the body weights (and presumably blood volumes) and resting hemodynamic variables of the three groups were similar, and the rates of blood removal identical, we cannot explain why withdrawal of different volumes of blood were necessary to elicit similar reductions in MAP and why the later group displayed such markedly different changes in vascular resistances (and presumably CO). However, these data are consistent with the known complexity and variability of hemodynamic responses to HEM within human and animal subjects (see Drucker et al., 1981; Falk et al., 1983; Liu et al., 2003; Dubick et al., 2013; Thongrong et al., 2013).
Initial and delayed hemodynamic responses elicited by isotonic saline in HEM rats
The sequential injections of 250, 350 and 550 µl of I-saline (96, 135 and 212 µmol/kg, respectively) did not elicit immediate hemodynamic responses but prompted a relatively slow recovery of MAP to values that remained well below pre-HEM levels whereas HR recovered fully. This partial recovery in MAP possibly involved an improvement in CO due to partial restoration of circulating blood volume. The changes in vascular resistances following the injections of I-saline were bed specific. More specifically, (1) RR values (that were markedly elevated immediately following blood withdrawal) fell abruptly (i.e., the vasoconstriction abated) following the first injection of I-saline and remained at these lower levels following subsequent injections, (2) MR values (elevated immediately upon blood withdrawal) remained unchanged following the injections of I-saline (i.e., no change in vasoconstrictor status), and (3) HQR (elevated upon blood withdrawal) gradually increased following each injection of I-saline (i.e., the vasoconstriction increased). As will be discussed below, the changes in systemic vascular resistances accompanying the changes in MAP were markedly different to those elicited by H-saline.
Initial responses produced hypertonic saline
The sequential injection of 100, 200 and 400 µl volumes of H-saline (750, 1500 and 3000 µmol/kg, respectively) elicited immediate and substantial falls in MAP, which were accompanied by vasodilation in the renal and mesenteric beds and especially pronounced vasodilation in the hindquarter bed. The mechanisms underlying the initial responses to H-saline may involve (i) central or direct changes in sympathetic vasoconstrictor tone, or (ii) diminished vasoconstrictor responsiveness to neurogenic- or adrenal-derived catecholamines (Liu et al., 2004) via enhanced release of endothelium-derived relaxing factors (Liu et al., 2004) such as nitric oxide and S-nitrosothiols (Hashmi-Hill et al., 2007). The pronounced hindquarter vasodilation may also involve activation of the neurogenic vasodilator system described above (Davisson et al., 1996a,b, 1997; Possas et al., 2006). Interestingly, the administration of H-saline is also known to dilate resistance arteries in the skin (Holcroft, 2011), another organ in which the tone of the microvasculature of humans and animals that is controlled by neurogenic vasodilator systems involving nitric oxide factors (Holzer, 1998; Kellogg et al., 2011).
Delayed hemodynamic responses elicited by hypertonic saline
The administration of H-saline prompted full recovery of MAP in the HEM rats. The restoration of MAP is probably due to an increase in CO, which in turn is due to increased blood volume resulting from the enhanced movement of interstitial fluid and albumin into the circulation (see Drucker et al., 1981). It is also possible that the restoration of CO is due to the direct H-saline-induced improvement of cardiac contractility (Ogino et al., 1998). As with I-saline, the changes in vascular resistances following the injections of H-saline were bed specific. More specifically, (1) HQR values (that were decreased immediately following blood withdrawal) gradually recovered to pre-HEM values following the injections of H-saline (i.e., the vasodilation abated), (2) RR values (not changed immediately upon blood withdrawal) fell initially (vasodilation following injection 2 of H-saline) and then rose after injection 3 (relatively minor vasoconstriction), and (3) MR (not changed immediately upon blood withdrawal) gradually increased following each injection of H-saline to display frank vasoconstriction after injections 2 and 3. It would seem feasible that the administration of H-saline leads to exaggerated vasoconstrictor efficacy of neurogenic-derived catecholamines and relevant circulating factors. Lesions of the anteroventral region of the third ventricle (AV3V) impair the recovery of MAP induced by H-saline (7.5% NaCl, 4 ml/kg) in HEM rats (Barbosa et al., 1990, 1992). As such, it is likely that the beneficial actions of H-saline in our study also involved changes in the activities of AV3V neurons that control neurogenic outflow and the release of hormones regulating vascular tone and fluid/salt balance such as angiotensin II and arginine vasopressin (see Barbosa et al., 1990, 1992). It should be mentioned that systemic injections of γ2-melanocyte-stimulating hormone (γ2-MSH) elicits equivalent increases in resistance in the renal, mesenteric and hindquarter vascular beds of pentobarbital-anesthetized rats (Whalen et al., 2007). Since, systemic injections of γ2-MSH elicits pressor responses primarily via centrally-mediated increases in sympathetic drive and direct vasoconstriction in the renal microcirculation (see Whalen et al., 2007). it is evident that sympathetic effector mechanisms can be effectively stimulated in HEM rats. Based on these and the present findings, it is evident that H-saline does not elicit hemodynamic responses that can simply be explained by the activation of the sympathetic nervous system.
Summary
This study demonstrates that small-volume injections of H-saline elicit pronounced hemodynamic responses in pentobarbital-anesthetized rats but subsequently fully restore HEM-induced hypotension. Our findings are consistent with evidence that small volumes of H-saline alone (De Felippe and Timoner, 1980; Nakayama et al., 1984; Traveso et al., 1987; Baue et al., 1991; Coimbra et al., 1997; Dontigny, 1992; Shackford et al., 1998) or together with plasma expanders (Maningas et al., 1986; Kramer et al., 1986; Hannon et al., 1989; Mazzoni et al., 1990; Moore, 1991; Wade et al., 1997; Ogino et al., 1998;) restore MAP in patients in hemorrhagic shock by increasing circulating volume via movement of intracellular fluid into the vascular space (see Drucker et al., 1981). The use of reduced volume H-saline therapy reduces the potential development of cerebral edema in patients with head injury, which may occur with large volume therapy (Drucker et al., 1981; Falk et al., 1983; Shackford et al., 1998). In addition, the administration of H-saline directly increases cardiac contractility in patients with moderate to marked HEM (Ogino et al., 1998).
Our data also demonstrate that the recovery of MAP is due to the amelioration of HEM-induced vasodilation in the hindquarter bed and the expression of vasoconstriction in the renal and especially the mesenteric vascular beds. These findings support the results of clinical studies, which suggest that small-volume injections of H-saline are beneficial in hemorrhagic shock (De Felippe and Timoner, 1980; Nakayama et al., 1984; Traveso et al., 1987; Baue et al., 1991; Coimbra et al., 1997; Dontigny, 1992; Shackford et al., 1998). However, our finding that injections of H-saline produce immediate reductions in MAP via vasodilation of peripheral vascular beds would be an unwanted effect in patients with HEM-induced hypotension. Moreover, recent clinical data suggest that H-saline is not beneficial as a general therapy for hypovolemic shock or traumatic head injury, and may only benefit a yet to be defined subset of these patients (Dubick et al., 2013).
Acknowledgments
This work was supported in part by NHLBI HL 14388 and HL57472, NASA NAG5-6171, and the Office of Naval Research N00014-97-1-0145.
Footnotes
Disclosures
No conflicts of interest, financial or otherwise are declared by the authors.
References
- Armstead WM. Opioids and nitric oxide contribute to hypoxia-induced pial arterial vasodilation in newborn pigs. Am. J. Physiol. 1995;268:H226–H232. doi: 10.1152/ajpheart.1995.268.1.H226. [DOI] [PubMed] [Google Scholar]
- Atkins JL, Day BW, Handrigan MT, Zhang Z, Pamnani MB, Gorbunov NV. Brisk production of nitric oxide and associated formation of S-nitrosothiols in early hemorrhage. J. Appl. Physiol. 2006;100:1267–1277. doi: 10.1152/japplphysiol.01059.2005. [DOI] [PubMed] [Google Scholar]
- Baue AE, Tragus ET, Parkins WM. Effects of increased osmolality and correction of acidosis on blood flow and oxygen consumption in hemorrhagic shock. J. Surg. Res. 1967;7:349–356. [Google Scholar]
- Baue AE, Tragus ET, Parkins WM. A comparison of isotonic and hypertonic solutions and blood on blood flow and oxygen consumptions in the initial treatment of hemorrhagic shock. J. Trauma. 1991;7:743–756. doi: 10.1097/00005373-196709000-00012. [DOI] [PubMed] [Google Scholar]
- Barbosa SP, Camargo LA, Saad WA, Renzi A, De Luca LA, Jr, Menani JV. Lesion of the anteroventral third ventricle region impairs the recovery of arterial pressure induced by hypertonic saline in rats submitted to hemorrhagic shock. Brain Res. 1992;587:109–114. doi: 10.1016/0006-8993(92)91434-g. [DOI] [PubMed] [Google Scholar]
- Barbosa SP, Saad WA, Camargo LA, Renzi A, De Luca LA, Jr, Fracasso JF, Menani JV. Lesion of the anteroventral third ventricle region abolishes the beneficial effects of hypertonic saline on hemorrhagic shock in rats. Brain Res. 1990;530:342–344. doi: 10.1016/0006-8993(90)91308-4. [DOI] [PubMed] [Google Scholar]
- Boyd DR, Mansberger AR. Serum water and osmolal changes in hemorrhagic shock: An experimental and clinical study. Am. Surg. 1968;34:744–749. [PubMed] [Google Scholar]
- Brooks CM. The reaction of chronic spinal animals to hemorrhage. Am. J. Physiol. 1935;114:30–39. [Google Scholar]
- Coimbra R, Hoyt DB, Junger WG, Angle N, Wolf P, Loomis W, Evers MF. Hypertonic saline resuscitation decreases susceptibility to sepsis after hemorrhagic shock. J. Trauma: Injury, Infection and Critical Care. 1997;42:602–606. doi: 10.1097/00005373-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Filippelli A, Zingarelli B, Caputi AP, Rossi F. Role of nitric oxide in a nonseptic shock model induced by zymosan in the rat. Shock. 1997;7:351–357. doi: 10.1097/00024382-199705000-00007. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Shaffer RA, Johnson AK, Lewis SJ. Stimulation of lumbar sympathetic nerves may produce hindlimb vasodilation via the release of pre-formed stores of nitrosyl factors. Neuroscience. 1996a;72:881–887. doi: 10.1016/0306-4522(96)00090-5. 1996. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Shaffer RA, Johnson AK, Lewis SJ. Use-dependent loss of active sympathetic neurogenic vasodilation after nitric oxide synthase inhibition in conscious rats. Evidence for the presence of preformed stores of nitric oxide-containing factors. Hypertension. 1996b;28:347–353. doi: 10.1161/01.hyp.28.3.347. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Possas OS, Murphy SP, Lewis SJ. Neurogenically derived nitrosyl factors mediate sympathetic vasodilation in the hindlimb of the rat. Am. J. Physiol. 1997;272:H2369–H2376. doi: 10.1152/ajpheart.1997.272.5.H2369. [DOI] [PubMed] [Google Scholar]
- De Felippe J, Timoner J. Treatment of refractory hypovolaemic shock by 7.5% sodium chloride injections. Lancet. 1980;ii:1002–1004. doi: 10.1016/s0140-6736(80)92157-1. [DOI] [PubMed] [Google Scholar]
- Dontigny L. Small-volume resuscitation. Can. J. Surg. 1992;35:31–33. [PubMed] [Google Scholar]
- Drucker WR, Chadwick CDJ, Gann DS. Transcapillary refill in hemorrhage and shock. Arch. Surg. 1981;116:1344–1353. doi: 10.1001/archsurg.1981.01380220088014. [DOI] [PubMed] [Google Scholar]
- Dubick MA, Shek P, Wade CE. ROC trials update on prehospital hypertonic saline resuscitation in the aftermath of the US-Canadian trials. Clinics. 2013;68:883–886. doi: 10.6061/clinics/2013(06)25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekelund U, Björnberg J, Mellander S. α2 adrenoceptor activation may trigger the: increased production of endothelium-derived nitric oxide in skeletal muscle during acute hemorrhage. Acta Physiol. Scand. 1988;164:285–292. doi: 10.1046/j.1365-201X.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- Falk JL, Rackow EC, Weil MH. Colloid and crystalloid fluid resuscitation. Acute Care. 1983;84:59–94. [PubMed] [Google Scholar]
- Friedman SG, Pearce FJ, Drucker WR. Blood glucose in defense of plasma volume during hemorrhage. J. Trauma. 1982;22:86–91. doi: 10.1097/00005373-198202000-00002. [DOI] [PubMed] [Google Scholar]
- Guyton AC. Interstitial fluid pressure: II. Pressure-volume curves of interstitial space. Circ. Res. 1965;16:452–460. doi: 10.1161/01.res.16.5.452. [DOI] [PubMed] [Google Scholar]
- Hannon JP, Wade CE, Bossone CA, Hunt MM, Loveday JA. Oxygen delivery and demand to conscious pigs subjected to fixed volume hemorrhage and resuscitated with 7.5% NaCl in 6% dextran. Circ. Shock. 1989;29:205–217. [PubMed] [Google Scholar]
- Hashmi-Hill MP, Sandock K, Bates JN, Robertson TP, Lewis SJ. Flavin adenine dinucleotide may release preformed stores of nitrosyl factors from the vascular endothelium of conscious rats. J. Cardiovasc. Pharmacol. 2007;50:142–154. doi: 10.1097/FJC.0b013e31805c1646. [DOI] [PubMed] [Google Scholar]
- Holcroft JW. The hypertonic saline trial: a possible downside to the gold standard of double blinding. Ann. Surg. 2011;253:442–443. doi: 10.1097/SLA.0b013e31820d32d0. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Easington CR, Osman J, Broussard M, Parrillo JE. Effects of nitric oxide synthase inhibition on microvascular reactivity in septic mice. Shock. 1999;12:262–267. doi: 10.1097/00024382-199910000-00003. [DOI] [PubMed] [Google Scholar]
- Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- Jarhult J. Osmotic fluid transfer from tissue to blood during hemorrhagic hypotension. Acta Physiol. Scand. 1973;89:213–226. doi: 10.1111/j.1748-1716.1973.tb05514.x. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Zhao JL, Wu Y, Johnson JM. Antagonism of soluble guanylyl cyclase attenuates cutaneous vasodilation during whole body heat stress and local warming in humans. J. Appl. Physiol. 2011;110:1406–1413. doi: 10.1152/japplphysiol.00702.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer GC, Perron PR, Lindsey DC, Ho HS, Gunther RA, Boyle WA, Holcroft JW. Small-volume resuscitation with hypertonic saline dextran solution. Surgery. 1986;100:239–247. [PubMed] [Google Scholar]
- Landín L, Lorente JA, Renes E, Canas P, Jorge P, Liste D. Inhibition of nitric oxide synthesis improves the vasoconstrictive effect of noradrenaline in sepsis. Chest. 1994;106:250–256. doi: 10.1378/chest.106.1.250. [DOI] [PubMed] [Google Scholar]
- Li S, Fan SX, McKenna TM. Role of nitric oxide in sepsis-induced hyporeactivity in isolated rat lungs. Shock. 1996;5:122–129. doi: 10.1097/00024382-199602000-00007. [DOI] [PubMed] [Google Scholar]
- Liu LM, Ward JA, Dubick MA. Hemorrhage-induced vascular hyporeactivity to norepinephrine in select vasculatures of rats and the roles of nitric oxide and endothelin. Shock. 2004;19:208–214. doi: 10.1097/00024382-200303000-00003. [DOI] [PubMed] [Google Scholar]
- Mazzoni MC, Borgstrom P, Intaglietta M, Arfors K-E. Capillary narrowing in hemorrhagic shock is rectifies by hyperosmotic saline-dextran infusion. Circ. Shock. 1990;31:407–418. [PubMed] [Google Scholar]
- Maningas PA, DeGuzman LR, Tillman FJ, Hinson CS, Priegnitz KJ, Volk KA, Bellamy RF. Small-volume infusion of 7.5% NaCl in 6% dextran 70 for the treatment of severe hemorrhagic shock in swine. Ann. Emerg. Med. 1986;15:1131–1137. doi: 10.1016/s0196-0644(86)80852-6. [DOI] [PubMed] [Google Scholar]
- Mittman U, Schmidt HD, Schmier J, Wirth RH. Hemorrhagic shock with fixed hypotension and with spontaneous recovery of blood pressure. A comparison of two shock models. Basic Res. Cardiol. 1976;71:47–59. doi: 10.1007/BF01907782. 1976. [DOI] [PubMed] [Google Scholar]
- Moore EE. Hypertonic saline dextran for post-injury resuscitation: Experimental background and clinical evidence. Aust. N.Z. J. Surg. 1991;61:732–736. [PubMed] [Google Scholar]
- Moss GS, Gould SA. Plasma expanders: An update. Am. J. Surg. 1988;155:425–434. doi: 10.1016/s0002-9610(88)80106-5. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Sibley L, Gunther RA, Holcroft JW, Kramer GC. Small-volume resuscitation with hypertonic saline (2400 mOsm/liter) during hemorrhagic shock. Circ. Shock. 1984;13:149–159. [PubMed] [Google Scholar]
- Nozik-Grayck E, Whalen EJ, Stamler JS, McMahon TJ, Chitano P, Piantadosi CA. S-nitrosoglutathione inhibits alpha1-adrenergic receptor-mediated vasoconstriction and ligand binding in pulmonary artery. Am. J. Physiol. 2006;290:L136–L143. doi: 10.1152/ajplung.00230.2005. [DOI] [PubMed] [Google Scholar]
- Ogino R, Suzuki K, Kohno M, Nishina M, Kohama A. Effects of hypertonic saline and dextran 70 on cardiac contractility after hemorrhagic shock. J. Trauma. 1998;44:59–69. doi: 10.1097/00005373-199801000-00005. [DOI] [PubMed] [Google Scholar]
- Pirkle JC, Gann DS. Expansion of interstitial fluid is required for full restitution of blood volume after hemorrhage. J. Trauma. 1976;16:937–947. doi: 10.1097/00005373-197612000-00001. [DOI] [PubMed] [Google Scholar]
- Possas OS, Johnson AK, Lewis SJ. Role of nitrosyl factors in the hindlimb vasodilation elicited by baroreceptor afferent nerve stimulation. Am. J. Physiol. 2006;290:R741–R748. doi: 10.1152/ajpregu.00660.2005. [DOI] [PubMed] [Google Scholar]
- Robert R, Chapelain B, Jean T, Néliat G. Interleukin-1 impairs both vascular contraction and relaxation in rabbit isolated aorta. Biochem. Biophys. Res. Commun. 1992;182:733–739. doi: 10.1016/0006-291x(92)91793-p. [DOI] [PubMed] [Google Scholar]
- Shackford SR, Bourguignon PR, Wald SL, Rogers FB, Osler TM, Clark DE. Hypertonic saline resuscitation of patients with head injury: a prospective, randomized clinical trial. J. Trauma. 1998;44:50–58. doi: 10.1097/00005373-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Shires GT, Barber AE, Illner HP. Current status of resuscitation: solutions including hypertonic saline. Adv. Surg. 1995;28:133–170. [PubMed] [Google Scholar]
- Simper D, Strobel WM, Linder L, Haefeli WE. Indirect evidence for stimulation of nitric oxide release by tumor necrosis factor-alpha in human veins in vivo. Cardiovasc. Res. 1995;30:960–964. [PubMed] [Google Scholar]
- Smith GJ, Kramer GC, Perron P, Nakayama S, Gunther RA, Holcroft JW. A comparison of several hypertonic solutions for resuscitation of bled sheep. J. Surg. Res. 1985;39:517–528. doi: 10.1016/0022-4804(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Soucy DM, Sindlinger JF, Greene SP, Barber AE, Illner HP, Shires GT. Isotonic saline resuscitation in uncontrolled hemorrhage under various anesthetic conditions. Ann. Surg. 1995a;222:87–93. doi: 10.1097/00000658-199507000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy DM, Sindlinger JF, Greene SP, Barber A, Illner H, Shires GT. Effects of anesthesia on a model of uncontrolled hemorrhage in rats. Crit. Care Med. 1995b;23:1528–1532. doi: 10.1097/00003246-199509000-00013. [DOI] [PubMed] [Google Scholar]
- Starke RM, Dumont AS. The role of hypertonic saline in neurosurgery. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.03.019. In press. [DOI] [PubMed] [Google Scholar]
- Starling EH. On the absorption of fluids from connective tissue spaces. J. Physiol. 1896;19:312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JP, Schutzer SF, McCoy S, Drucker WR. Contribution of glucose to the hyperosmolality of prolonged hypovolemia. Am. Surg. 1977;43:1–5. [PubMed] [Google Scholar]
- Szabó C, Thiemermann C. Invited opinion: role of nitric oxide in hemorrhagic, traumatic, and anaphylactic shock and thermal injury. Shock. 1994;2:145–155. [PubMed] [Google Scholar]
- Thiemermann C, Szabó C, Mitchell JA, Vane JR. Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide. Proc. Natl. Acad. Sci. USA. 1993;90:267–271. doi: 10.1073/pnas.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongrong C, Kong N, Govindarajan B, Allen D, Mendel E, Bergese SD. Current purpose and practice of hypertonic saline in neurosurgery: A review of the literature. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.02.027. In press. [DOI] [PubMed] [Google Scholar]
- Traveso LW, Bellamy RF, Hollenbach SJ, Wichter LD. Hypertonic sodium chloride solutions: Effects on hemorrhage in swine. J. Trauma. 1987;27:32–39. [PubMed] [Google Scholar]
- Wade CE, Kramer GC, Grady JJ, Fabian TC, Younes RN. Efficacy of hypertonic 7.5% saline and 6% dextran-70 in treating trauma: a meta-analysis of controlled clinical studies. Surgery. 1997;122:609–616. doi: 10.1016/s0039-6060(97)90135-5. [DOI] [PubMed] [Google Scholar]
- Whalen EJ, Johnson AK, Lewis SJ. Hemodynamic actions of systemically-injected pituitary adenylate cyclase activating polypeptide-27 (PACAP-27) in the rat. Eur. J. Pharmacol. 1999;365:205–215. doi: 10.1016/s0014-2999(98)00852-8. [DOI] [PubMed] [Google Scholar]
- Whalen EJ, Johnson AK, Lewis SJ. β-Adrenoceptor dysfunction after inhibition of NO synthesis. Hypertension. 2000;36:376–382. doi: 10.1161/01.hyp.36.3.376. [DOI] [PubMed] [Google Scholar]
- Whalen EJ, Lewis SJ, Johnson AK. Hemodynamic responses elicited by γ2-MSH or blood replacement in hemorrhaged rats. J. Surg. Res. 2007;139:121–127. doi: 10.1016/j.jss.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Zingarelli B, Squadrito F, Altavilla D, Calapai G, Di Rosa M, Caputi AP. Role of tumor necrosis factor-alpha in acute hypovolemic hemorrhagic shock in rats. Am. J. Physiol. 1994;266:H1512–H1515. doi: 10.1152/ajpheart.1994.266.4.H1512. [DOI] [PubMed] [Google Scholar]


