Abstract
We investigated the effect of curcumin on liver injury in diabetic rats induced by streptozotocin (STZ) through modulation of endoplasmic reticulum stress (ERS) and unfolded protein response (UPR). Experimental diabetes was induced by a single intraperitoneal injection of STZ (55 mg/kg), and curcumin was given at 100 mg/kg by gavage for 56 days. We observed that curcumin improved the morphological and histopathological changes, significantly decreased hepatic ERS marker protein: glucose-regulated protein 78, and improved liver function in diabetic rats. Moreover, treatment with curcumin markedly decreased the sub-arm of the UPR signaling protein such as phospho–double-stranded RNA-dependent protein kinase-like ER kinase, CCAAT/enhancer-binding protein homologous protein, tumor necrosis factor receptor-associated factor 2, and inositol-requiring enzyme1α; and inhibited tumor necrosis factor α, interleukin 1β, phospho-p38 mitogen-activated protein kinase, and apoptosis signal-regulating kinase 1 in liver tissues of diabetic rats. Apoptotic and anti-apoptotic signaling proteins, such as cleaved caspase-3 and B-cell lymphoma 2, were significantly increased and decreased, respectively in diabetic rats; curcumin treatment prevented all of these alterations. In summary, our results indicate that curcumin has the potential to protect the diabetic liver by modulating hepatic ERS-mediated apoptosis, and provides a novel therapeutic strategy for the diabetic liver damage.
Keywords: curcumin, diabetes mellitus, liver, endoplasmic reticulum stress, apoptosis
Abbreviations:
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- ASK1
apoptosis signal-regulating kinase 1
- ATF6
activating transcription factor 6
- bcl2
B-cell lymphoma 2
- CHOP
CCAAT/enhancer-binding protein homologous protein
- eIF2α
eukaryotic initiation factor 2 alpha
- ERS
endoplasmic reticulum stress
- GRP78
glucose-regulated protein 78
- IL-1β
interleukin 1β
- IRE1α
inositol-requiring enzyme 1 alpha
- JNK
c-jun N-terminal kinase
- PERK
double-stranded RNA-dependent protein kinase-like endoplasmic reticulum kinase
- p38MAPK
p38 mitogen-activated protein kinase
- SD
Sprague-Dawley
- STZ
streptozotocin
- TLR4
toll-like receptor 4
- TNFα
tumor necrosis factor alpha
- TRAF2
TNF receptor-associated factor 2
- UPR
unfolded protein response
Introduction
Diabetes mellitus is one of the most common endocrine metabolic disorders. Studies have shown that hepatobiliary disorders, such as the inflammation and necrosis or fibrosis of non-alcoholic fatty liver disease can follow diabetes [1,2]. It has been reported that the standardized mortality rate from end-stage liver disease (i.e., cirrhosis) in diabetic patients is higher than those with cardiovascular disease [3]. Several mechanisms can cause pancreatic β-cell dysfunction, including chronic inflammation, oxidative stress, excessive hyperglycemia and nutrient levels, lipotoxicity, endoplasmic reticulum (ER) stress (ERS), etc. [4–6]. A previous study has shown that hepatic fat accumulation and oxidative stress play a critical role in the development of diabetic liver injury [7] and a number of reports have shown that antioxidants could attenuate the complications of diabetes, including fatty changes in patients and in experimental models [8,9]. Nowadays, ERS has attracted significant attention and has been proposed to play a crucial role in the development of insulin resistance [5]. It is also reported that insulin resistance, a common underlying reason for the β-cell failure that occurs in type 2 diabetes, is associated with higher levels of ERS in β-cells in animal models of disease [10,11] and also in humans [12,13]. It is proposed that oxidative damage caused by ERS may be the fundamental in the etiology of the β-cell failure associated with both type 1 and 2 diabetes [14]. Liver, as the major target organ of insulin, plays important roles in the development of insulin resistance and diabetes mellitus.
The ER is a complex intracellular membranous network that regulates protein synthesis and folding. Alterations in ER homeostasis, due to increased protein synthesis, accumulation of misfolded proteins, alterations in the calcium, or the redox balance of the ER, lead to a condition called “ERS” [15]. To overcome the deleterious effects of ERS induction, cells have evolved various protective strategies, which are known as the “unfolded protein response (UPR)” [16]. Furthermore, the results from these reports have suggested that induction of ERS is closely associated with the energy metabolism, especially the lipid metabolism with the involvement of UPR signaling pathways. Rutkowski and Cols examined the contribution of each arm of the UPR pathway to the regulation of metabolic genes and development of hepatic dyslipidemia and concluded that all three arms of UPR signaling pathway: double-stranded RNA-dependent protein kinase-like ER kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6) are activated, leading to metabolic dysregulation [17].
When ERS is prolonged in the presence of hyperglycemia condition, the ER triggers the apoptotic pathway by activating the CCAAT/enhancer-binding protein homologous protein (CHOP) [18], the IRE1–tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2)–apoptosis signal-regulating kinase 1 (ASK1)–mitogen-activated protein kinase (MAPK) pathway [19], and/or the ER-localized cysteine protease caspase-12 [20]. Moreover, cell death signaling cascades including p38 MAPK and ASK1 are also activated by pro-inflammatory cytokine TNFα, interleukin 1β (IL-1β), and toll-like receptor 4 (TLR4) and by the activation of the UPR signaling marker IRE1α in diabetic liver. Diabetes involving both inflammation and ERS lead to hepatic apoptosis and liver damage.
Curcumin is the active ingredient of the traditional herbal remedy and dietary spice turmeric (Curcuma longa) and is the subject of clinical trials for various diseases such as cancer, Alzheimer's disease, and ulcerative colitis [21]. The polyphenol curcumin (diferuloylmethane) comprises 2–8% of most turmeric preparations and is generally regarded as its most active component, having potent antioxidant, anti-inflammatory, and anti-carcinogenic properties. Curcumin has been shown to modulate the activity of protein kinases [22], membrane ATPases [23], and transcription factors [24,25]. It is also reported that curcumin plays an important role in diminishing myocardial ERS signaling proteins and in decreasing the key regulator or inducer of apoptosis in experimental autoimmune myocarditis rats [26]. A previous study has pointed to the protective effect of curcumin on acute liver injury by inhibiting nuclear factor kappa B (NF-κB) and oxidative stress [27]. However, to the best of our knowledge, studies have not revealed the effect of curcumin on ERS in diabetic liver. Although many aspects of curcumin-induced cytoprotection are studied, the molecular mechanism by which curcumin protects liver tissues against streptozotocin (STZ)-induced liver injury is not clear. The present study was designed to shed light on this issue.
Materials and methods
Materials
Unless otherwise stated, all reagents were of analytical grade and purchased from Sigma (Tokyo, Japan).
Animals and experimental design
All animals were treated in accordance with the guidelines for animal experimentation of our institute [28]. Male Sprague-Dawley rats (weight: 250–300 g) were obtained from Charles River Japan Inc. (Kanagawa, Japan). Animals were housed in a temperature of 23 ± 2°C and humidity of 55 ± 15%, and were submitted to a 12-h light/dark cycle, and allowed free access to standard laboratory chow and tap water. Animals were allowed to fast for 4 h and then diabetes was induced by a single intraperitoneal (i.p.) injection of freshly prepared solution of STZ (Sigma-Aldrich Inc., Saint Louis, MO, USA) at a dose of 55 mg/kg, diluted in citrate buffer: 20 mM (pH: 4.5). Forty-eight hours later, blood glucose was measured by tail vein sampling using Medisafe chips (Terumo Inc., Tokyo, Japan). Diabetes was defined as a morning blood glucose reading of ≥ 300 mg/dL. Thirty rats were randomly divided into three groups (n = 10/group): non-diabetic normal control group (Normal); diabetic rats treated with vehicle, 1% gum Arabic (Control); and diabetic rats treated with curcumin 100 mg/kg/day [29] diluted in vehicle, 1% gum Arabic (Curcumin) (curcumin was purchased from Sigma-Aldrich, Tokyo, Japan). Curcumin treatment was started at 3 weeks after STZ injection and was administered via oral gavage for 8 weeks. All rats were sacrificed at 11 weeks after the induction of diabetes for analysis of liver tissues.
Biochemical analysis
Each week, rats were weighed and their blood glucose levels were measured. Urine samples were collected over a 24-h period in individual metabolic cages for the measurement of protein in urine at 1, 3, 7, and 11 weeks and were determined by the Bradford method. At the end of experimentation, heparinized whole blood was collected from anesthetized rats via heart puncture. Ethylenediaminetetraacetic acid–blood was centrifuged at 3000 g, for 15 min at 4°C for the separation of plasma. The plasma was used for the estimation of triglyceride (TG), total cholesterol (TC), and alanine aminotransferase (ALT).
Histopathological analysis
Formalin-fixed liver sections (4 μm) were stained with hematoxylin and eosin (H&E) or periodic acid Schiff (PAS). Morphological analysis was done by computerized image analysis system on ten microscopic fields per section examined in a 400-fold magnification (CIA-102; Olympus), with the observer blind to the study group [30,31].
Western blotting analysis
The liver tissue protein lysate was prepared using a method similar to that described by Soetikno [32]. The total protein concentration in the samples was measured by the bicinchoninic acid method. For the determination of protein levels, equal amounts of protein extracts (50 μg) were separated by 7.5–15% sodium dodecyl sulfate- polyacrylamide gel electrophoresis (Bio-Rad, CA, USA) and transferred electrophoretically to nitrocellulose membranes. Membranes were blocked with 5% non-fat dry milk or bovine serum albumin (BSA) in Tris-buffered saline Tween (20 mM of Tris, pH: 7.6, 137 mM of NaCl, and 0.1% Tween 20). Primary antibodies against glucose-regulated protein 78 (GRP78), IRE1α, p-IRE1α, TRAF2, ATF6, PERK, p-PERK, CHOP/growth arrest and DNA-damage-inducible protein (GADD153), TNFα, IL-1β, TLR4, and β-tubulin were obtained from Santa Cruz Biotechnology, Santa Cruz, CA, USA. Primary antibodies against bcl2 (B-cell lymphoma 2), cleaved caspase-3, phospho (p)-p38MAPK, and ASK1 were obtained from Cell Signaling Technology Inc., Beverly, MA, USA. The primary antibody, cleaved caspase-12 was obtained from Bio Vision Inc., Milpitas, CA, USA. All the antibodies were used at a dilution of 1:1000. The membrane was incubated overnight at 4°C with the primary antibody, and the bound antibody was visualized using the respective horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology Inc.) and chemiluminescence developing agents (Amersham Biosciences, Buckinghamshire, UK). The levels of β-tubulin, PERK (for p-PERK), and IRE1α (for p- IRE1α) were estimated in every sample to check for equal loading of samples. Films were scanned, and band densities were quantified by densitometric analysis using the Scion Image program (Epson GT-X700, Tokyo, Japan).
Immunohistochemistry
Formalin-fixed, paraffin-embedded liver tissue sections were used for immunohistochemical staining. After deparaffinization and hydration, the slides were washed in Tris-buffered saline (TBS; 10 mM/L of Tris HCl, 0.85% NaCl, pH: 7.2). Endogenous peroxidase activity was quenched by incubating the slides in methanol and 0.3% H2O2 in methanol. After overnight incubation with the primary antibody, that is, mouse monoclonal anti-ED1 antibody (diluted 1:50) (sc-59103; Santa Cruz Biotechnology Inc. CA, USA) at 4°C, the slides were washed in TBS, and then HRP-conjugated goat anti-mouse secondary antibody was added and the slides were further incubated at room temperature for 1 h. The slides were washed in TBS and incubated with diaminobenzidine tetrahydrochloride as the substrate, and counterstained with hematoxylin. A negative control without primary antibody was included in the experiment to verify the antibody specificity. ED1-positive hepatocytes were counted in 100 hepatocytes/group under 200-fold magnification and expressed as cells/hepatocyte cross section (gcs) [33].
Statistical analysis
All values are expressed as means ± standard error of mean (SEM) and were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's methods for post-hoc analysis or two-tailed t-test when appropriate. A value of p < 0.05 was considered statistically significant. For statistical analysis, GraphPad Prism 5 software (San Diego, CA, USA) was used.
Results
Biochemical parameters in experimental animals
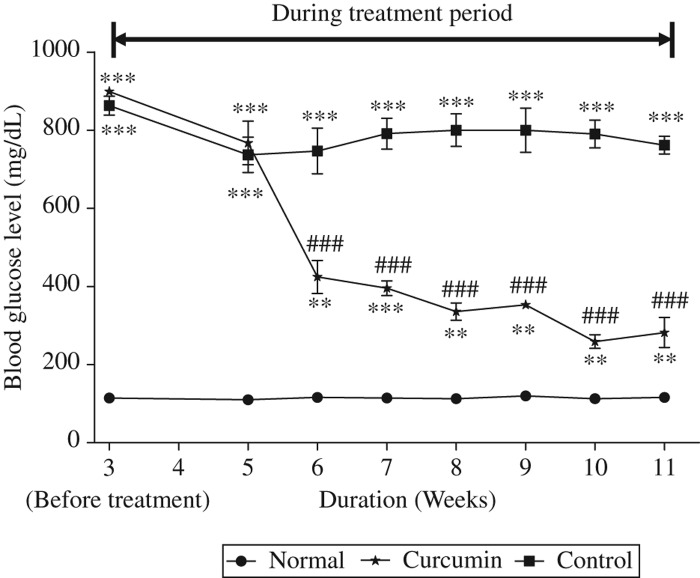
For the confirmation of diabetic model and the effect of treatment, the blood glucose level was checked periodically, which is shown in Figure 1; before treatment, the blood glucose level was significantly higher in diabetic rats than that of normal group but during the treatment period of 6 weeks curcumin treatment significantly decreased this blood glucose level. Moreover, it is also reported that curcumin has the capability to improve the pancreatic islets [34] and has been demonstrated in prevention of isolated β-cells death and dysfunction induced by STZ [34,35].
Figure 1.
Time-course changes in blood glucose. Blood glucose increased progressively in the untreated diabetic rats following induction of diabetes. Curcumin treatment significantly reduced blood glucose in the beginning of treatment and these were maintained throughout the study period until sacrifice. Values are means ± SEM. ∗∗p < 0.01, ∗∗∗p < 0.001 versus Normal, ###p < 0.001 versus Control.
As shown in Table I, body weight was significantly decreased in diabetic rats, but curcumin treatment could not prevent this declined body weight. The ratio of liver weight and body weight (g/kg) was significantly increased in untreated diabetic rats compared with that in normal rats—curcumin treatment reduced this ratio. Compared with the normal group, diabetic rats developed elevated mean plasma glucose level. Plasma TG, TC, and ALT levels were also elevated in the diabetic rats in comparison to the normal rats (p < 0.01, p < 0.001). All of these abnormalities were significantly attenuated by curcumin treatment (p < 0.05, p < 0.001). To evaluate the effect of curcumin on preventing hyperfiltration induced by STZ, we measured 24-h urine volume and urinary protein excretion. Although the control group showed a marked elevation in 24-h urine and urinary protein excretion, curcumin treatment could not reduce this elevated urinary excretion but significantly reduced the urinary protein excretion (p < 0.05).
Table I.
Changes in biochemical parameters after 8 weeks of treatment with curcumin in diabetic rats induced by STZ.
| Normal (n = 10) | Control (n = 10) | Curcumin (n = 10) | |
|---|---|---|---|
| Body weight (BW) (g) | 539.5 ± 38.48 | 316.33 ± 15.7∗∗∗ | 368.75 ± 24.62∗∗∗ |
| Liver weight/BW (g/kg) | 28.57 ± 1.47 | 43.23 ± 1.35∗∗∗ | 37.92 ± 4.64# ∗ |
| Plasma glucose (mg/dL) | 116.25 ± 22.1 | 761.8 ± 50.8∗∗∗ | 287.66 ± 78.4### ∗∗ |
| TG (mg/DL) | 94.75 ± 5.72 | 431.25 ± 118.92∗∗∗ | 109 ± 11.94### |
| TC (mg/dL) | 58.50 ± 3.8 | 105.5 ± 10.9∗∗∗ | 81.25 ± 13.72# |
| ALT (IU/L) | 39.75 ± 2.36 | 124 ± 28.82∗∗ | 97 ± 30.26∗ |
| Urine volume (mL/Kg/24 h) | 19 ± 1 | 618 ± 137∗∗∗ | 592 ± 109∗∗∗ |
| Protein in urine/24 h (g) | 13 ± 2 | 38 ± 15.63∗ | 15 ± 10# |
Normal, age-matched normal rats; Control, untreated diabetic rats; Curcumin, diabetic rats treated with curcumin 100 mg/kg/day, TG, triglyceride; TC, total cholesterol; ALT, alanine aminotransferase. Values are expressed as means ± SEM.
∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 versus normal, #p < 0.05, ###p < 0.001 versus control.
Effect of curcumin on ERS marker protein
Activation of GRP78 protein expression was used to demonstrate the ERS induction, whose expression is reported to be increased in the diabetic rats. ERS leads to the activation of caspase-12 and other markers involved in the hepatic changes associated with diabetes mellitus. Our study with diabetic rats also revealed the involvement of ERS as evidenced by a significant increase in the hepatic GRP78 expression but cleaved caspase-12 expression was not so significant. Prevention of ERS is one of the strategies involved in the reduction of liver complications of diabetes. In the present study, curcumin-treated animals had shown significant attenuation of GRP78, and cleaved caspase-12 was slightly attenuated when compared with the control group (Figure 2A and B).
Figure 2.
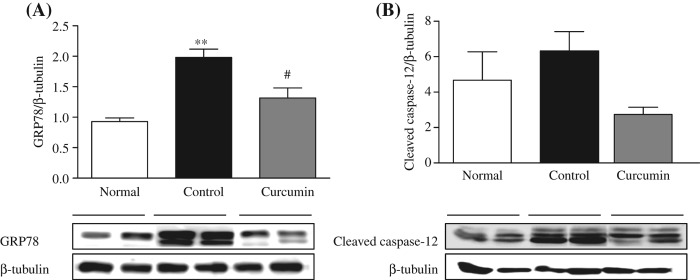
Expression of hepatic protein involved in ERS. Representative Western blots (lower panel) show specific bands for GRP78 (A) and Caspase-12 (B); representative histograms (upper panel) show the band densities with relative β-tubulin. The blots are representatives of five independent experiments. Each bar represents mean ± SE. Normal, age-matched normal rats; Control, untreated diabetic rats; Curcumin, diabetic rats treated with curcumin 100 mg/kg/day. ∗∗p < 0.01 versus Normal, #p < 0.05 versus Control based on one-way ANOVA followed by Tukey's test.
Effect of curcumin on expression of UPR signaling proteins in liver
The signals from activated UPR molecules to the relevant effector proteins are conveyed by three different proteins: PERK, IRE1α, and ATF6α. As expected, here we have found that the phosphorylation of PERK protein was significantly increased in the diabetic rats compared with that in the normal Sprague-Dawley (SD) rats (Figure 3A). Diabetic rats have displayed a significant upregulation in the hepatocyte expression levels of p-IRE1α protein compared with the normal SD rats (Figure 3B). Curcumin treatment significantly attenuated the increased liver p-PERK and p-IRE1α levels. But interestingly, we did not find any significant difference in the level of ATF6α protein expression in the diabetic rats compared with that in the normal SD rats (Figure 3C).
Figure 3.
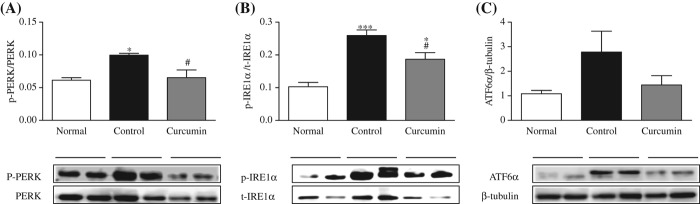
Expression of hepatic proteins involved in UPR signaling pathway. Representative Western blots (lower panel) show specific bands for p-PERK (A), p-IRE1α (B), and ATF6α (C); representative histograms (upper panel) show the band densities with relative β-tubulin, PERK (for p-PERK), and IRE1α (p-IRE1a). The blots are representatives of five independent experiments. Each bar represents mean ± SE. Normal, age-matched normal rats; Control, untreated diabetic rats; Curcumin, diabetic rats treated with curcumin 100 mg/kg/day. ∗p < 0.05, ∗∗∗p < 0.001 versus Normal, #p < 0.05 versus Control based on one-way ANOVA followed by Tukey's test.
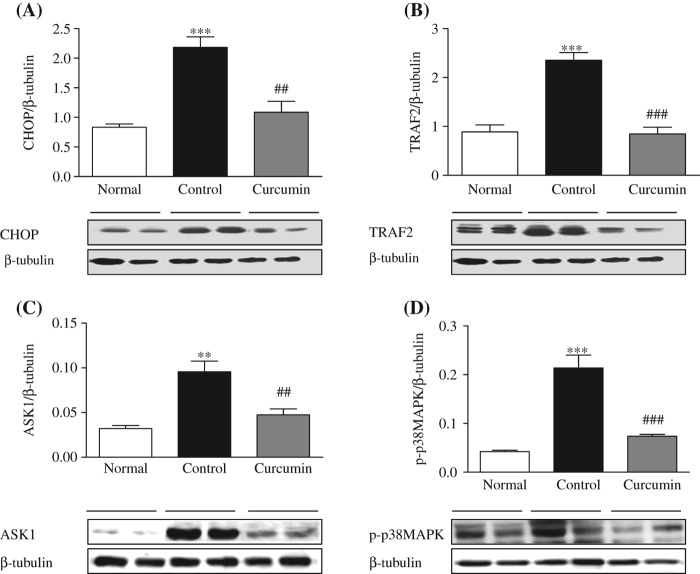
Effect of curcumin on hepatocyte protein expression levels of CHOP/GADD153 in the diabetic rats
It has been proposed that enhancement of the hepatocyte protein expression of CHOP/GADD153 gene could eventually induce apoptosis. Immunoblot analysis revealed that the diabetic rats have demonstrated significant elevation of CHOP/GADD153 protein expression compared with the normal rats (Figure 4A) and curcumin treatment significantly decreased the increased liver CHOP expression.
Figure 4.
Hepatic expressions of CHOP, TRAF2, ASK1, and p-p38MAPK. Representative Western blots (lower panel) show specific bands for CHOP/GADD153 (A), TRAF2 (B), ASK1 (C), and p-p38MAPK (D); representative histograms (upper panel) show the band densities with relative β-tubulin. The blots are representatives of five independent experiments. Each bar represents mean ± SE. Normal, age-matched normal rats; Control, untreated diabetic rats; Curcumin, diabetic rats treated with curcumin 100 mg/kg/day. ∗∗p < 0.01, ∗∗∗p < 0.001 versus Normal, ##p < 0.01, ###p < 0.001 versus Control.
Effect of curcumin on liver expression of TRAF2, ASK1, and p-p38MAPK
The TRAF2 protein expression was significantly higher in the diabetic rats than the normal rats, which was significantly attenuated by curcumin treatment (Figure 4B). There was a significant increase in the expression of p-p38MAPK and ASK1 in the diabetic control rats. This result suggests that activation of IRE1α recruits TRAF2, and then ASK1 directly binds to TRAF2 and gets activated. Curcumin treatment modified these changes in the liver of diabetic rats. Protein expressions of these markers were attenuated significantly in the curcumin-treated rats (Figure 4B–D).
Effect of curcumin on hepatocyte protein expression levels of cleaved caspase-3 and bcl2 in diabetic rats
Immunoblot analysis revealed that the diabetic rats have displayed an increase in the hepatocyte protein expression of cleaved caspase-3 compared with the normal rats (Figure 5A) and decreased protein expression level of bcl2. This increase in liver of cleaved caspase-3 protein expression was markedly suppressed and significantly increased the bcl2 protein expression by curcumin treatment in the liver of diabetic rats. This clearly indicates that curcumin attenuated the hepatocytes' apoptosis.
Figure 5.
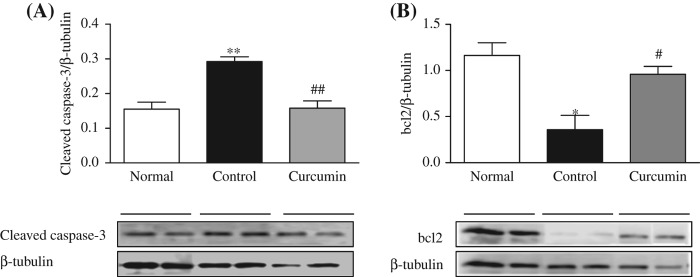
Hepatic expressions of cleaved caspae-3 and bcl2. Representative Western blots (lower panel) show specific bands for cleaved caspase-3 (A) and bcl2 (B); representative histograms (upper panel) show the band densities with relative β-tubulin. The blots are representatives of five independent experiments. Each bar represents mean ± SE. Normal, age-matched normal rats; Control, untreated diabetic rats; Curcumin, diabetic rats treated with curcumin 100 mg/kg/day. ∗p < 0.05, ∗∗p < 0.01 versus Normal, #p < 0.05, ##p < 0.01 versus Control.
Effect of curcumin on inflammatory marker proteins in the diabetic liver
Inflammation is involved in the mechanism of metabolism related diseases such as diabetes mellitus and obesity [36,37]. Inflammatory cytokines produced by liver infiltrated autoreactive immune cells are the major factors causing cell death in type 1 diabetes [38]. It has been proposed that ERS pathways play crucial roles in the inflammatory response. Therefore, we performed Western blot analysis for measuring the hepatocyte protein expression levels of TNFα, IL-1β, and TLR4 in diabetic rats. The diabetic rats displayed significantly upregulated protein expression levels of TNFα, IL-1β, and TLR4 compared with the normal rats (p < 0.05 and p < 0.001), and these increases were significantly attenuated by curcumin treatment (Figure 6A–C).
Figure 6.
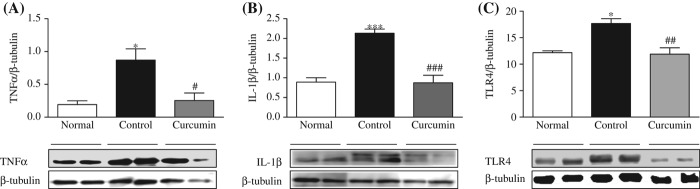
Hepatic expressions of TNFα, IL-1β, and TLR4. Representative Western blots (lower panel) show specific bands for TNFα (A), IL-1β (B), and TLR4 (C); representative histograms (upper panel) show the band densities with relative β-tubulin. The blots are representatives of five independent experiments. Each bar represents mean ± SE. Normal, age-matched normal rats; Control, untreated diabetic rats; Curcumin, diabetic rats treated with curcumin 100 mg/kg/day. ∗p < 0.05, ∗∗∗p < 0.001 versus Normal, #p < 0.05, ##p < 0.01 and ###p < 0.001 versus Control.
Histopathological findings
Normal histology was seen in the normal control rats (Figure 7). The normal liver contains a large amount of glycogen. This, therefore, leads to the intense pink staining of hepatocytes with a PAS stain. In the PAS staining, examined liver sections of normal control rats showed the normal pattern distribution of glycogen granules and a liver section of diabetic rats showed marked depletion in the glycogen granules; meanwhile this glycogen level became increased in curcumin-treated animals (Figure 7A). Fatty liver was shown by H&E staining as an unstained area in liver parenchymal cells (Figure 7B). In the untreated diabetic rats, microvascular vacuolization, focal necrosis, and inflammation in the portal area were significantly apparent compared with the normal rats and curcumin-treated diabetic rats (Figure 7B).
Figure 7.
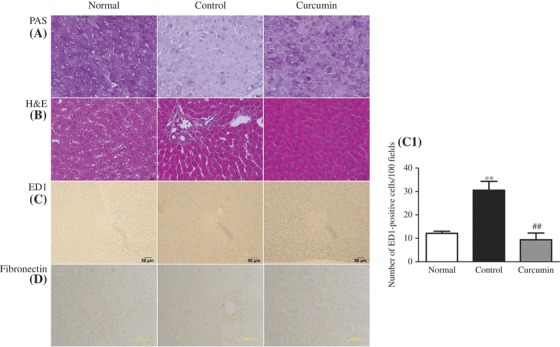
Effect of curcumin on histopathological changes. Histological staining with PAS in liver (A) shows that glycogen contents of rat liver decreased in diabetic animals when compared with those of normal control animals, but these levels increased to near normal after treatment with curcumin. In H&E, (B) light microscopic photographs of livers of experimental animal showed the liver of normal control group, lipid accumulation indicated by the unstained area in liver tissues, microvascular fattening and focal necrosis, and portal inflammation in the untreated diabetic group; in curcumin-treated diabetic group, the severity of these changes was less than those in the untreated diabetic group. (C and C1) Immunohistochemical staining for macrophage (ED1-positive cells) and its quantification graph in each group. (D) Immunohistochemical staining for fibronectin in liver section. Each bar represents mean ± SE. Normal, age-matched normal rats; Control, untreated diabetic rats; Curcumin, diabetic rats treated with curcumin 100 mg/kg/day. ∗∗p < 0.01 versus Normal, ##p < 0.01 versus Control.
Effect of curcumin on macrophage (Kupffer cells) infiltration and fibronectin accumulation
Macrophages are a heterogeneous population of myeloid-derived mononuclear cells that are a critical component of innate immune response [39,40]. We investigated that livers from normal rats did not show any significant macrophage infiltration (Figure 7C). On the other hand, diabetic rats demonstrated prominent macrophage (ED1-positive cells) recruitment in the liver (Figure 7C and C1), whereas diabetic rats treated with curcumin showed marked reduction in macrophage activation (p < 0.01) (Figure 7C and C1). Fibronectin accumulation in diabetic rat liver was also significantly higher compared with that of normal control group (Figure 7D). When curcumin was given to STZ diabetic groups, the sections of liver showed reduced fibronectin accumulation (Figure 7D).
Discussion
The ER is a membranous network that provides a specialized environment for processing and folding newly synthesized proteins. The ER regulates protein folding, calcium storage, and the biosynthesis of macromolecules such as steroids, lipids, and carbohydrates. Disruption of ER homeostasis, often termed ERS, has been observed in liver and adipose tissue of humans with non-alcoholic fatty liver disease and/or obesity [1,41–44]. When the metabolic demands increase, which can perturb the protein folding in the ER, the workload of this protein factory is collectively called “ERS” [45]. Song and Scheuner indicated that nutrient fluctuations and insulin resistance increase pro-insulin synthesis in β-cells beyond the capacity of folding of nascent polypeptide within the ER lumen, thereby disrupting ER homeostasis and triggering the UPR and chronic ERS-promoted apoptosis [14]. Since hepatocytes have a well-developed ER structure, ERS is involved in liver-related disease [46]. As curcumin also has the ERS inhibitory effect [26], in light of the important role of the liver in glucose homeostasis and the pathogenesis of diabetes, we sought to examine the potential effect of curcumin on the ERS response signal in hepatocytes of diabetic animals. So, we question the beneficial function of curcumin on suppressing the ERS in the liver.
The ER lumen provides a specialized environment for protein folding and maturation, and a unique complement of molecular chaperones and folding enzymes [47]. Recent studies have suggested that ERS and UPR signaling are tightly associated with hepatic lipid metabolism [48,49]. In mammalian cells, UPR activation involves three ER-localized proteins: IRE1α, PERK, and ATF6α [50]. It is currently thought that in unstressed cells, all three proteins are maintained in an inactive state via their association with the ER protein chaperone, GRP78. GRP78 acts as a master regulator of the activation of UPR signaling pathways. Upon ERS, GRP78 is released and sequestered on unfolded proteins, allowing activation of PERK, IRE1α, and ATF6α [51]. In the present study, we investigated ERS signaling in diabetic liver and observed the elevated protein expression of GRP78, p-PERK, and IRE1α in the liver of diabetic rats, which were attenuated by curcumin treatment (Figure 2A, 3A, and B). However, the expression of ATF6α was not significant in the liver of diabetic rats compared with that in the liver of normal rats (Figure 3C).
Considering all these findings, the increase in GRP78 expression and activation of the UPR are often used as indicators of ERS due to the complexity of directly measuring the ER integrity or protein aggregate levels, we hypothesize that the increase in the protein expression of GRP78 and UPR signaling proteins might actually indicate the induction of ERS in the diabetic rats. Curcumin treatment attenuated the ERS in the liver of diabetic rats by reducing these marker proteins.
Prolonged or insufficient ERS may turn physiological mechanisms into pathological consequences. When ER-inducing stresses are too severe or prolonged to allow for recovery of ER function, the apoptosis pathway is activated to remove damaged cells [52–56]. It is proposed that at least three pathways are involved in the ERS-mediated apoptosis [52–57]. The first is transcriptional activation of the gene for CHOP. The second is activation of the IRE1–TRAF2–ASK1–MAPK pathway. The third is activation of ER-associated caspase-12.
CHOP is expressed at low levels under physiological conditions, but it is strongly induced at the transcriptional level in response to ER stress [14,52,53,58,59]. The transcription of the CHOP gene is activated by all three ER stress sensors (IRE1α, ATF6, and PERK) signaling pathways. The activation of PERK plays a dominant role in the induction of transcription factor: CHOP/GADD153 [60] over that of ATF6α and IRE1α signaling pathways, although the presence of all three signaling pathways is required to achieve the maximal induction of CHOP [53,59]. Moreover, the failure of the UPR to ameliorate ERS can lead to cell death via several mechanisms, and CHOP is among the best characterized of the UPR-regulated pro-apoptotic proteins [53].
Correlating with these results, the phosphorylation of hepatic PERK and CHOP gene expression was shown to be significantly increased in the diabetic rats when compared with that in normal rats. Treatment with curcumin significantly attenuated the changes in the hepatic expression of p-PERK and CHOP/GADD153 and provided evidence for the prevention of ERS, one of the possible mechanisms of hepatic apoptosis in diabetic rats (Figures 3A and 4A).
Meanwhile, IRE1α also appears to mediate rapid degradation of specific mRNAs, presumably in an effort to reduce production of proteins that require folding in the ER lumen [61,62]. Under ERS conditions, activated IRE1α, one of the ERS sensors, recruits TRAF2; then ASK1 directly binds to TRAF2 and is activated. It is thought that the mitochondria pathway is involved in ASK1-mediated apoptosis. In this study, the liver of diabetic rats displayed a significant increase in TRAF2 and ASK1 protein expression (Figure 4B and C). Treatment with curcumin significantly downregulated the protein expression of TRAF2 and ASK1 in the liver of diabetic rats. IRE1α activation has also been linked to the activation of p38MAPK and c-jun N-terminal kinase (JNK) [63–65]. The p38MAPK and JNK belong to the subfamily of the MAPK superfamily and are classified as a stress response. In the present study, the hepatic levels of phosphorylated forms of p38MAPK are significantly lesser in the curcumin-treated rats when compared with those in the control group (Figure 4D), suggesting that curcumin treatment avoided the activation of MAPK signaling cascade with the prevention of stress in the liver of diabetic rats through which it has prevented the liver injury in STZ-induced diabetic rats. Interestingly, in this experiment we did not observe any significant difference in the phosphorylation of JNK among the three groups (data are not shown here).
Pro-caspase-12 is localized to the cytosolic side of ER membrane and is activated by ERS but the mechanism has not been confirmed. It is reported that caspase-12 knockout cells are partially resistant to ERS-induced apoptosis [54]. Interestingly, in this study, we did not observe any significant difference in the expression of cleaved caspase-12 among the normal, control, and treatment groups (Figure 2B). For further confirmation of apoptosis in the liver of diabetic rats and the effect of curcumin treatment on diabetic liver, we checked the apoptotic marker protein cleaved caspase-3 and anti-apoptotic protein bcl2. In this study, we found that the expression levels of activated caspase-3 were significantly increased and those of the anti-apoptotic proteins bcl2 were reduced in the diabetic liver. Curcumin treatment prevented all of these alterations (Figure 5A and B).
Furthermore, it is reported that the ERS pathway plays crucial roles in the inflammatory response [64,66,67]. IRE1α–TRAF2 complex also involved in the transcriptional induction of inflammation-related genes. Nanji et al. (2003) have demonstrated the protective effect of curcumin in rat liver injury induced by alcohol, where curcumin administration prevented increase in ALT level; and blocked the activation of NF-κB and the expression of proinflammatory cytokines (TNF-α) and inducible nitric oxide synthase [68]. In this experiment, we found that activation of TLR4 as well as proinflammatory cytokine expression levels of TNFα and IL-1β was increased in diabetic rats compared with that of the levels in normal rats. Curcumin prevented all of these alterations (Figure 6A–C). We showed that the significant elevation of ALT in diabetic rats was reduced slightly by curcumin treatment.
Stimuli that injure the liver can activate multiple intracellular stress responses, such as the inflammatory response. Macrophage or Kupffer cells have been implicated in the pathogenesis of various liver diseases [69]. In the present study, using the accumulation of ED1 as a marker of macrophage activation, we have demonstrated increased activation of macrophage/Kupffer cells in the hepatic tissue of diabetic animals (Figure 7C and C1) and increased fibronectin accumulation in diabetic liver (Figure 7D). In this study, we demonstrated that macrophage recruitment in diabetic liver is ameliorated by the administration of curcumin. Curcumin is a representative polyphenolic compound found in the dietary spice turmeric. From the morphological study, we found that long-term curcumin treatment improved many pathological changes, degenerated hepatocytes with polymorphic nuclei, and dilated sinusoids and mononuclear cell infiltrate extending through hepatic tissue in diabetic rats. In the present study, curcumin significantly reduced the blood glucose level. It is also reported that it has the capability to improve the activity of pancreas, thus curcumin is able to increase the insulin secretion [34]. Pancreatic islet cell death is the cause of deficient insulin production in diabetes. Approaches toward prevention of cell death are of prophylactic importance in control and management of hyperglycemia. Meghana et al. investigated and reported that islet viability and insulin secretion in curcumin- pretreated islets are significantly higher than those in islets exposed to STZ alone [35]. Recently, it is reported that curcumin enhances pancreatic β-cell function by inhibiting phosphodiesterase activity and regulated insulin secretion under glucose-stimulated condition [70]. From this experiment, we can conclude that curcumin may indirectly reduce the ERS by reducing hyperglycemia or it may directly reduce the ERS in diabetic liver and protect the liver from damage.
Conclusion
The ERS-related inflammation and apoptosis are involved in the pathogenesis of various diseases, including hepatopathy. Therefore, the ERS pathway can be a new target for the treatment of those diseases. The result presented here shows that the administration of curcumin inhibits ERS, and ERS-related apoptosis and inflammation in the liver of STZ-induced diabetic rats. All the above results suggest that the beneficial effect of curcumin occurs, at least in part, through modulation of the UPR signaling pathway. Given these promising preclinical findings, we believe that curcumin might be considered as a potential adjuvant entity for preventing diabetic liver damage.
Acknowledgements
This research was supported by Ministry of Education, Culture, Sports, Sciences and Technology, Japan and by a grant from the promotion and mutual aid corporation for private schools, Japan (23602012 and 26460239), respectively.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- [1].Das AV, Padayatti PS, Paulose CS. Effect of leaf extract of Aegle marmelose (L.) Correa ex Roxb. on histological and ultrastructural changes in tissues of streptozotocin induced diabetic rats. Indian J Exp Biol. 1996;34:341–345. [PubMed] [Google Scholar]
- [2].Latry P, Bioulac-Sage P, Echinard E, Gin H, Boussarie L, Grimaud JA, Balabaud C. Perisinusoidal fibrosis and basement membrane-like material in the livers of diabetic patients. Hum Pathol. 1987;18:775–780. doi: 10.1016/s0046-8177(87)80050-3. [DOI] [PubMed] [Google Scholar]
- [3].Harrison SA. Liver disease in patients with diabetes mellitus. J Clin Gastroenterol. 2006;40:68–76. doi: 10.1097/01.mcg.0000190774.91875.d2. [DOI] [PubMed] [Google Scholar]
- [4].Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- [5].Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- [6].Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17:171–180. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- [8].Shams ME, Al-Gayyar MM, Barakat EA. Type 2 diabetes mellitus induced hyperglycemia in patients with NAFLD and normal LFTs: relationship to lipid profile, oxidative stress and pro-inflammatory cytokines. Sci Pharm. 2011;79:623–634. doi: 10.3797/scipharm.1104-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de Cavanagh EM, Inserra F, Toblli J, Stella I, Fraga CG, Ferder L. Enalapril attenuates oxidative stress in diabetic rats. Hypertension. 2001;38:1130–1136. doi: 10.1161/hy1101.092845. [DOI] [PubMed] [Google Scholar]
- [10].Scheuner D, Vander MD, Song B, Flamez D, Creemers JW, Tsukamoto K, et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11:757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- [11].Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4:391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- [12].Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, Butler PC. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- [13].Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- [14].Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves β cell function and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein 5response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- [16].Lakshmanan AP, Harima M, Suzuki K, Soetikno V, Nagata M, Nakamura T, et al. The hyperglycemia stimulated myocardial endoplasmic reticulum (ER) stress contributes to diabetic cardiomyopathy in the transgenic non-obese type 2 diabetic rats: a differential role of unfolded protein response (UPR) signaling proteins. Int J Biochem Cell Biol. 2013;45:438–447. doi: 10.1016/j.biocel.2012.09.017. [DOI] [PubMed] [Google Scholar]
- [17].Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang XZ, Lawson B, Brewer JW, Zinszner H, Sanjay A, Mi LJ, et al. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol Cell Biol. 2006;16:4273–4280. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54:S73–S78. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- [20].Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- [21].Hatcher H, Planalp R, Cho J, Torti SV. Curcumin from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu JY, Lin SJ, Lin JK. Inhibitory effects of curcumin on protein kinase C activity induced by 12-O-tetradecanoyl- phorbol-13-acetate in NIH 3T3 cells. Carcinogenesis. 1993;14:857–861. doi: 10.1093/carcin/14.5.857. [DOI] [PubMed] [Google Scholar]
- [23].Mahmmoud YA. Curcumin modulation of Na,K-ATPase: phosphoenzyme accumulation, decreased K+ occlusion, and inhibition of hydrolytic activity. Br J Pharmacol. 2005;145:236–245. doi: 10.1038/sj.bjp.0706185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Choi H, Chun YS, Kim SW, Kim MS, Park JW. Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: a mechanism of tumor growth inhibition. Mol Pharmacol. 2006;70:1664–1671. doi: 10.1124/mol.106.025817. [DOI] [PubMed] [Google Scholar]
- [25].Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) J Biol Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- [26].Mito S, Thandavarayan R, Ma M, Lakshman A, Suzuki K, Kodama M, Watanabe K. Inhibition of cardiac oxidative and endoplasmic reticulum stress-mediated apoptosis by curcumin treatment contributes to protection against acute myocarditis. Free Radic Res. 2011;45:1223–1231. doi: 10.3109/10715762.2011.607252. [DOI] [PubMed] [Google Scholar]
- [27].Reyes-Gordillo K, Segovia J, Shibayama M, Vergara P, Moreno MG, Muriel P. Curcumin protects against acute liver damage in the rat by inhibiting NF-kappa B, proinflammatory cytokines production and oxidative stress. Biochim Biophys. Acta. 2007;1770:989–996. doi: 10.1016/j.bbagen.2007.02.004. [DOI] [PubMed] [Google Scholar]
- [28].Watanabe K, Ohta Y, Nakazawa M, Higuchi H, Hasegawa G, Naito M, et al. Low dose carvedilol inhibits progression of heart failure in rats with dilated cardiomyopathy. Br J Pharmacol. 2000;130:1489–1495. doi: 10.1038/sj.bjp.0703450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jain SK, Rains J, Croad J, Larson B, Jones K. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal. 2009;11:241–249. doi: 10.1089/ars.2008.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, et al. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha dependent pathway. Diabetes. 2008;57:460–469. doi: 10.2337/db07-1119. [DOI] [PubMed] [Google Scholar]
- [31].Guven A, Yavuz O, Cam M, Ercan F, Bukan N, Comunoglu C, Gokce F. Effects of melatonin on streptozocin –induced diabetic liver injury in rats. Acta Histochem. 2006;108:85–93. doi: 10.1016/j.acthis.2006.03.005. [DOI] [PubMed] [Google Scholar]
- [32].Soetikno V, Harima M, Suzuki K, Watanabe K. Amelioration of Liver Injury by Curcumin in Streptozotocin –Induced Diabetic Rats. International Conference on Medical and Pharmaceutical Sciences;2012 June 16–17; Bangkok; [Google Scholar]
- [33].Soetikno V, Sari FR, Veeraveedu PT, Thandavarayan RA, Harima M, Sukumaran V, et al. Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab. 2011;8:35. doi: 10.1186/1743-7075-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chanpoo M, Petchpiboonthai H, Panyarachun B, Anupunpisit V. Effect of curcumin in the amelioration of pancreatic islets in streptozotocin-induced diabetic mice. J Med Assoc Thai. 2010;93:S152–S159. [PubMed] [Google Scholar]
- [35].Meghana K, Sanjeev G, Ramesh B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: a prophylactic and protective role. Eu J Pharmacol. 2007;577:183–191. doi: 10.1016/j.ejphar.2007.09.002. [DOI] [PubMed] [Google Scholar]
- [36].Chung HY, Sung B, Jung KJ, Zon Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- [37].Navarro-Gonzalez JF, Mora-Fernadez C, De Fuentes MM, Garcia-Perz J. Inflammatory molecules and pathways in pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- [38].Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and βcell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- [39].Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- [40].Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- [41].Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, et al. Increase in endoplasmic reticulum stress related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- [44].Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, et al. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab. 2008;93:4532–4541. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gu F, Ngusen DT, Stible M, Dube N, Tremblay ML, Chevet E. Protein- tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic stress. J Biol Chem. 2004;279:49689–49693. doi: 10.1074/jbc.C400261200. [DOI] [PubMed] [Google Scholar]
- [46].Ji C, Kaplowitz N. ER stress: Can the liver cope? J Hepatol. 2006;45:321–333. doi: 10.1016/j.jhep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- [47].Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- [48].Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yamamoto K, Takahara K, Oyadomari S, Okada T, Sato T, Harada A, More k. Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Biol Cell. 2010;21:2975–2986. doi: 10.1091/mbc.E09-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [51].Zhang K, Kaufman RJ. From endoplasmic reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gotoh T, Mori M. Nitric oxide and endoplasmic reticulum stress. Arterioscler Thromb Vasc Biol. 2006;26:1439–1446. doi: 10.1161/01.ATV.0000223900.67024.15. [DOI] [PubMed] [Google Scholar]
- [53].Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- [54].Nakagawa T, Yuan J. Cross-talk between two cysteine protease families: activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- [56].Homma K, Katagiri K, Nishitoh H, Ichijo H. Targeting ASK1 in ER stress-related neurodegenerative diseases. Expert Opin Ther Targets. 2009;13:653–664. doi: 10.1517/14728220902980249. [DOI] [PubMed] [Google Scholar]
- [57].Hattori K, Naguro I, Runchel C, Ichijo H. The roles of ASK family proteins in stress responses and diseases. Cell Commun Signal. 2009;7:1–10. doi: 10.1186/1478-811X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- [59].Harding HP, Ron D. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes. 2002;51:S455–S461. doi: 10.2337/diabetes.51.2007.s455. [DOI] [PubMed] [Google Scholar]
- [60].Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- [61].Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- [62].Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1 dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hetz C, Glimcher LH. Fine-tuning of the unfolded protein response: assembling the IRE1α interactome. Mol Cell. 2009;35:551–561. doi: 10.1016/j.molcel.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nguyen DT, Kebache S, Fazel A, Wong HN, Jenna S, et al. Nck-dependent activation of extracellular signal-regulated kinase-1 and regulation of cell survival during endoplasmic reticulum stress. Mol Biol Cell. 2004;15:4248–4260. doi: 10.1091/mbc.E03-11-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, et al. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-α and interleukin-6: model of NF- κB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- [67].Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- [68].Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Thomas P, Dannenberg AJ. Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B- dependent genes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G321–G327. doi: 10.1152/ajpgi.00230.2002. [DOI] [PubMed] [Google Scholar]
- [69].Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, et al. Kupffer Cells Promote Hepatic Steatosis Via Interleukin-1β –Dependent Suppression of Peroxisome Proliferator-Activated Receptor α Activity. Hepatology. 2010;51:511–522. doi: 10.1002/hep.23337. [DOI] [PubMed] [Google Scholar]
- [70].Rouse M, Younes A, Egan JM. Resveratrol and curcumin enhance pancreatic β-cell function by inhibiting phosphor-diesterase activity. J Endocrinol. 2014;223:107–117. doi: 10.1530/JOE-14-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]