Abstract
The hypothalamus is one of the master regulators of various physiological processes, including energy balance and nutrient metabolism. These regulatory functions are mediated by discrete hypothalamic regions that integrate metabolic sensing with neuroendocrine and neural controls of systemic physiology. Neurons and non-neuronal cells in these hypothalamic regions act supportively to execute metabolic regulations. Under conditions of brain and hypothalamic inflammation, which may result from overnutrition-induced intracellular stresses or disease-associated systemic inflammatory factors, extracellular and intracellular environments of hypothalamic cells are disrupted, leading to central metabolic dysregulations and various diseases. Recent research has begun to elucidate the effects of hypothalamic inflammation in causing diverse components of metabolic syndrome leading to diabetes and cardiovascular disease. These new understandings have provocatively expanded previous knowledge on the cachectic roles of brain inflammatory response in diseases, such as infections and cancers. This review describes the molecular and cellular characteristics of hypothalamic inflammation in metabolic syndrome and related diseases as opposed to cachectic diseases, and also discusses concepts and potential applications of inhibiting central/hypothalamic inflammation to treat nutritional diseases.
Keywords: Hypothalamus, brain, inflammation, energy balance, metabolism, disease
Introduction
The ability to properly maintain metabolic homeostasis is crucial for an organism’s survival and normal functioning. Unfortunately, due to various environmental and internal interfering factors, disruption of metabolic homeostasis is quite common and often leads to disease consequences. The prominent environmental changes in today’s post-industrial society are the easy availability to calorie-abundant food and sedentary lifestyles, which, in combination, have formed the most important etiological causes for obesity, type 2 diabetes (T2D) and cardiovascular disease (CVD).1–5 According to the latest statistics of the World Health Organization, 1.5 billion adults worldwide are overweight (defined by body mass index (BMI) greater than or equal to 25), and among these people, 200 million men and nearly 300 million women are obese (defined as BMI greater than or equal to 30).6 Being overweight or obese greatly increases the risk for developing a cluster of metabolic disorders, such as high blood pressure, hyperglycemia, insulin resistance, and hyperlipidemia, which, together with a few other pathophysiological abnormalities, are collectively referred to as metabolic syndrome or syndrome X.7–11 Metabolic syndrome is a serious medical condition because it greatly increases the risks for developing devastating diseases such as T2D, coronary artery disease, stroke, atherosclerosis, fatty liver disease, and aging-related degenerative diseases;11–19 however, effective interventions that target metabolic syndrome are still missing, largely due to our insufficient understanding of the root causes of these problems. Interestingly, recent research in the cross-disciplinary field of neurobiology and immunology has unexpectedly made quite significant contributions in this regard. Specifically, hypothalamic inflammation was revealed as a general yet multifaceted mediator for various components of metabolic syndrome. These understandings also fundamentally expanded earlier knowledge that had linked brain and hypothalamic inflammatory response to cachexia—a multifactorial wasting syndrome characterized by a paradoxical state of decreased voluntary food intake and increased metabolic rate despite pronounced negative energy balance.20–26 Strikingly differing from metabolic syndrome, cachectic syndrome is frequently seen in infectious diseases, cancers, and end-stage chronic diseases (e.g., congestive heart failure and chronic kidney disease), which are often accompanied by anorexia, physical inactivity, and sometimes fever.27–30 In the following chapters, we describe recent research advances that have revealed the role of hypothalamic inflammation in several elements of metabolic syndrome and related diseases, and also comparatively analyze the molecular/cellular and physiological characteristics of hypothalamic inflammation in metabolic syndrome-related diseases versus cachectic diseases.
Hypothalamic inflammation in nutritional disorders and diseases
The hypothalamus of the central nervous system (CNS) is a crucial brain structure functioning as the “headquarters” in the regulation of many fundamental physiological activities of the whole body. Among many physiological processes controlled by the hypothalamus, several are directly related to metabolic and energy homeostasis, including nutrient sensing, appetite control, energy expenditure, and carbohydrate and lipid metabolism.31–47 Through a broad network of hormonal and neural communications, the hypothalamus receives a variety of peripheral metabolic information, such as the amount of adiposity and nutrients indicated by circulating leptin and insulin, hunger versus satiety signals reported by gut neural input and gut hormones (e.g., ghrelin, peptide YY, cholecystokinin, gastric inhibitory polypeptide, and glucagon-like peptides), and nutritional conditions reported by circulating nutrients and their metabolites. Distinct groups of hypothalamic neurons are responsible for sensing these metabolic signals, which in turn employ an integrated neuroendocrine and neural network to direct peripheral metabolic activities. These metabolic sensing and regulatory neurons are located in several areas of the hypothalamus especially the mediobasal hypothalamus (MBH), paraventricular nucleus (PVN) and lateral hypothalamus, and the regulations are mediated by controlled synthesis, release and actions of hypothalamic neuropeptides and neurotransmitters.31–47 A well-characterized metabolic sensing center in MBH is the arcuate nucleus (ARC), which contains two types of functionally antagonizing neurons: anorexigenic pro-opiomelanocortin (POMC) neurons and orexigenic agouti-related peptide (AGRP) neurons. Hormonal or nutrient sensing by these neurons engages downstream neuroendocrine and neural activities to control energy intake, energy expenditure and body weight balance. Research has further established that the hypothalamus can employ body weight-independent mechanisms to regulate peripheral glucose metabolism and whole-body blood pressure balance.39,48–51 However, from the perspective of disease development, it remains a question whether and how disrupted hypothalamic functions may mediate different components of metabolic syndrome in either body weight-dependent or independent manners. Excitingly, research over the recent years has demonstrated the mechanistic involvement of central/hypothalamic inflammation across multiple components of metabolic syndrome and diseases, which represents a significant research development in delineating the central mechanisms of these diseases.
Initial interest in the relationship between metabolic syndrome and inflammation was drawn by the observations that metabolic diseases such as obesity and T2D are characterized by an atypical form of inflammation in the circulation and various metabolic tissues such as adipose tissues, liver, and muscle.52–65 Compared to classical (e.g., pathogen-induced) inflammation, overnutrition-induced inflammation is primarily triggered by nutrient (caloric) excess. Accordingly, a special term “metaflammation” or “metabolic inflammation” was used by some investigators to refer to this type of inflammation.60,61 Unlike inflammations following pathogen infection or tissue injury, which have appreciable symptoms, such as redness, tissue swelling, heat, and pain, metabolic inflammation is rather low-grade and usually discernible only at the molecular level. Further studies revealed that metabolic inflammation is frequently mediated by a subset of immune mediators that are produced downstream of activation of canonical proinflammatory pathways in metabolic tissues and cells, and such inflammation can generate prominent impacts on the metabolic homeostasis and regulations of peripheral tissues.52–65 Stemming from this background, recent research has found that in metabolic syndrome and related diseases like obesity and T2D, the hypothalamus is chronically presented with the similar type of low-grade metabolic inflammation.60–78 Research over past years has consistently shown that chronic high-fat diet (HFD) feeding in animals can employ proinflammatory pathways and related stress signaling in the hypothalamus to mediate overnutrition-induced metabolic diseases.66–77 Such hypothalamic inflammation can be acutely induced at the molecular level by nutritional excess. For example, an acute central over-supply of glucose66 or lipids66–69 were shown to induce hypothalamic inflammation within as short as a few hours to three days. These findings suggest that hypothalamic inflammation precedes and thus can be as a cause of overnutrition-induced diseases. On the chronic basis, prolonged inflammation can theoretically sustain and accumulate the deleterious effects of overnutrition on metabolic regulation and lead to overt disease outcomes. In terms of the underlying mechanism, hypothalamic inflammation was reported to affect hypothalamic hormonal (e.g., leptin and insulin) signaling to cause central dysregulation of energy balance leading to obesity development.66–68,74–77 Recently, the causal involvement of hypothalamic inflammation in metabolic disease has been expanded to body weight-independent metabolic and cardiovascular disorders. In this regard, hypothalamic inflammation was reported to disrupt peripheral insulin and glucose homeostasis in the development of systemic insulin resistance and pre-T2D70,77,79 and cardiovascular dysfunctions, such as hypertension.70,71,80 Overall, while standing out as an emerging new topic, this line of research is clearly consistent with two conceptual advancements of the recent decade: one is the increasing appreciation that the pathogenesis of metabolic syndrome-associated diseases is causally related to hypothalamic dysfunctions; the other is the recognition that inflammation is the not only a prominent feature but also a pathogenic basis of these diseases.52–78
In contrast to the above scenario related to metabolic syndrome, the effect of brain inflammation on energy homeostasis has also been related to the development of cachectic syndrome, a life-threatening condition seen in chronic infections, cancers, and heart failure, which features anorexia, decreased physical activity, increased metabolic rate, and fever. As generally understood,20–26 these cachectic diseases typically involve an intense degree of systemic classical (cachectic factors-induced) inflammation, which affects the whole body including the CNS and the comprised hypothalamus. There are many experimental systems to reproduce this disease model, including an aP2 promoter-driven p65 transgenic mouse line81 with a robust level of systemic inflammation, high metabolic rate, and catabolic outcomes. While cachectic inflammation can directly act on peripheral tissues to cause fat and muscle breakdown—which can be replicated with in vitro models,20–23 the CNS (particularly the hypothalamus) was also thought to be a necessary mediator for the cachectic effects.21–26 The latter possibility was suggested by pharmacologic studies showing that direct administration of inflammatory cytokines into the CNS can induce cachexia-like anorexia, fever, and tissue breakdown.82–90 Evidently, such cachectic inflammation has a different disease milieu compared to overnutrition-induced inflammation, which is often related to metabolic syndrome. Moreover, the inflammatory origin, duration, intensity, and mediators are different between cachectic inflammation versus overnutrition-inflammation as summarized in Table 1 and further discussed in following chapters.
Table 1.
Characteristics of metabolic inflammation versus cachexia-inducing inflammation of the hypothalamus
| Metabolic inflammation | Cachexia-inducing inflammation | |
|---|---|---|
| Disease association |
|
|
| Duration |
|
|
| Intensity |
|
|
| Nature of inflammatory stimuli |
|
|
| Source of inflammatory stimuli |
|
|
| Signaling activators |
|
|
| Signaling mediators |
|
|
| Cell types |
|
|
| Effects on energy balance |
|
|
| Effects on glucose homeostasis |
|
|
Types of inflammatory stimuli in the hypothalamus
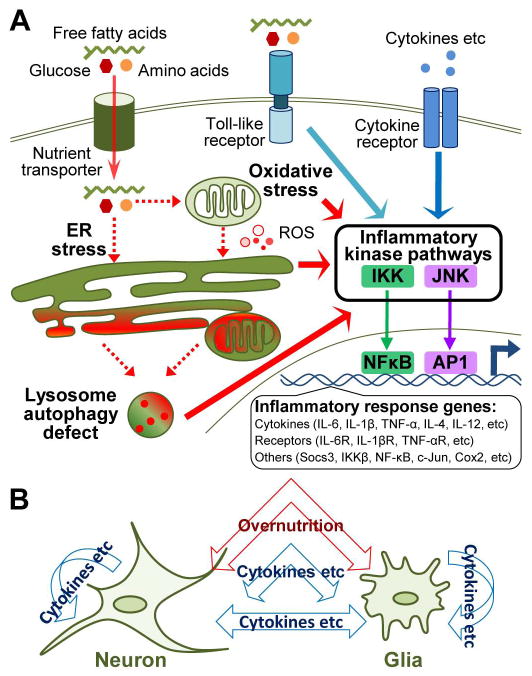
Several lines of evidences suggest that metabolic syndrome-related hypothalamic inflammation primarily results from local intracellular stresses caused by excessive nutrients presented to the hypothalamus. Animal studies have unequivocally shown that central administration of glucose or lipids to mimic overnutrition promptly activates proinflammatory pathways such as IκB kinase β (IKKβ) and downstream nuclear factor-κB (NF-κB) leading to expression of certain inflammatory genes in the hypothalamus.66–69 Moreover, inflammation in the hypothalamus induced by overnutrition can occur prior to the overt onset of obesity or other metabolic derangements,66–69 indicating that hypothalamic inflammation in obesity and related diseases can be a first-line intrabrain local response, which does not depend on peripheral inflammatory source. Using genetic and molecular approaches as discussed in detail below, recent research has appreciated, as also summarized in Figure 1, that overnutrition can induce hypothalamic inflammation at least via three avenues: membrane receptor-independent intracellular stresses, toll-like receptor (TLR) pathway, and cytokine/chemokine receptor pathways.
Figure 1.
Signaling cascades of metabolic inflammation in the hypothalamus. (A) Various cellular stresses such as oxidative stress and mitochondrial dysfunction, endoplasmic reticulum (ER) stress and late-stage lysosome autophagy defect are induced under the chronic condition of overnutrition. Stress of individual cellular organelle is spread through intracellular membrane network and mutually promoting, for instance, increased levels of reactive oxygen species (ROS) from oxidative stress exacerbate ER stress, whereas prolonged ER stress and oxidative stress lead to accumulation of damaged ER and mitochondria, resulting in increased lysosome stress and autophagy defect. All these stresses can lead to activation of proinflammatory regulators, such as IκB kinase (IKK) and c-Jun N-terminal kinase (JNK), which activates nuclear transcription factors NF-κB or AP1 to initiate gene expression of inflammatory response molecules. Certain nutrient species such as fatty acids can activate inflammatory pathways via activating toll-like receptors located at cellular surface. Low-grade excess of circulating cytokines during chronic overnutrition may cross the incomplete blood-brain barrier around the mediobasal hypothalamus to act on hypothalamic cells and thus additionally contribute to metabolic inflammation in the hypothalamus. (B) Metabolic inflammation in the hypothalamus involves neurons and glial cells and possibly their interactions through paracrine actions of inflammatory molecules.
Mitochondrial dysfunction and oxidative stress
Mitochondria are the primary organelles that generate energy in most eukaryotic cells and often referred to as powerhouse of the cell. Pathological mitochondrial dysfunction, on the other hand, cripples cellular energy supply and adversely affects cellular function. At the whole-organism level, mitochondrial dysfunction in metabolic tissues, such as skeletal muscle and liver has been linked to impaired glucose metabolism and the development of systemic insulin resistance and T2D.91,92 However, some studies also indicate a negligible causative role for impaired mitochondrial function in skeletal muscle in T2D.93–95 Mitochondrial function insufficiency in endothelial, vascular, and myocardial cells can directly lead to the development of cardiovascular abnormalities, such as atherosclerosis, hypertension, and heart disease.96–98 In contrast to its preponderant association with metabolic syndrome in peripheral tissues, mitochondrial dysfunction in the CNS was more intensively investigated in the context of neurological and neurodegenerative diseases.99,100 For instance, brain mitochondrial dysfunction was generally agreed to be a key factor in the development of aging-related cognitive dysfunction, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and other neurodegenerative disorders.99–104 Conversely, therapeutic interventions that improve mitochondrial function are neuroprotective and can ameliorate aging-related neurodegenerative symptoms.105 Albeit limited, recent literature has directly linked brain mitochondrial dysfunction to the central dysregulation of metabolic homeostasis. Experiments using genetic mouse models showed that overnutrition-induced mitochondrial dysfunction in hypothalamic POMC neurons impaired central glucose sensing,106 and defect of brain mitochondrial biogenesis induced by peroxisome proliferator-activated receptor coactivator 1α (PGC-1α) knockout impaired energy homeostasis.107 The role of brain mitochondrial defect in metabolic syndrome can be significantly mediated by neuronal accumulation of reactive oxygen species (ROS), a major intracellular change resulting from mitochondrial dysfunction.99,108–112 Indeed, brain neurons are highly susceptible to ROS-mediated oxidative stress,110–113 and oxidative stress has been tightly associated with brain inflammation in overnutrition-related diseases.102,108,114–116 Studies have further explored the mechanisms that mitochondrial dysfunction and oxidative stress cause inflammatory induction—accumulation of damaged mitochondria and ROS can activate NLRP3 inflammasome to promote inflammation.117 Reciprocally, an inflammatory state can enhance the cell’s susceptibility to oxidative stress through induction of enzymes that generate free radicals.118 From the interventional viewpoint, inhibition of oxidative stress by nutritional intervention such as caloric restriction (CR) can reduce inflammation and provide anti-disease and anti-aging benefits.119,120
ER dysfunction and ER stress
ER is the organelle responsible for protein folding, maturation, and trafficking, and thus can be viewed as a sentinel for cellular protein homeostasis. When excessive newly synthesized proteins are accumulated in ER, which exceeds the ER’s adaptive capacity, ER stress occurs through activation of unfolded protein response (UPR) pathways. There are three canonical cascades of UPR signaling—activating transcription factor 6 (ATF6), protein kinase RNA-like endoplasmic reticulum kinase (PERK) and downstream eukaryotic translation initiation factor 2α (eIF2α), and inositol requiring kinase 1 (IRE1). All of these signaling pathways can interact with and activate the proinflammatory network mediated by IKKβ/NF-κB and c-Jun N-terminal kinase (JNK).60,121 In fact, earlier studies in peripheral tissues have established ER stress signaling as a key proinflammatory mediator in the pathogenesis of overnutrition-related diseases.121 More recently, several lines of research have indicated that ER stress potently mediates the molecular and physiological effects of overnutrition-related inflammation in the hypothalamus. First, overnutrition including the associated inflammatory insults can clearly act in the hypothalamus to induce central ER stress,66,69,122 and inhibition of hypothalamic proinflammatory IKKβ/NF-κB pathway attenuated central ER stress and protected animals against HFD-induced obesity and glucose intolerance.66,70 Second, pharmacologic or genetic induction of ER stress in the hypothalamus or the brain can mimic metabolic inflammation to cause central leptin and insulin resistance, resulting in a broad range of metabolic disorders including overeating, glucose intolerance and hypertension, while pharmacologic or genetic reduction of ER stress significantly alleviated these metabolic derangements.66,70,74,123 In connection with other types of cellular stress and inflammation,121,124 chronic ER stress can elevate intracellular levels of ROS to promotes oxidative stress, and cellular accumulation of ROS can in turn reinforce ER stress to exacerbate inflammatory response.
Autophagy defect
Macroautophagy, or autophagy, is an evolutionarily conserved cellular degradation pathway that serves to maintain cellular homeostasis by counteracting stressors, such as infection, nutrient depletion, cellular oxidative stress, and intracellular accumulation of protein aggregate.125,126 Complete loss of autophagy is lethal,127 and tissue-specific ablation of autophagy in liver, skeletal muscle, pancreatic β cells, or fat cells causes various metabolic diseases, such as T2D, dyslipidemia and premature tissue aging.128–132 Neuron-specific loss of autophagy can lead to aging,126 neurodegenerative disease,133,134 obesity, and systemic insulin resistance,72 indicating that central autophagy defect is highly relevant for the development of metabolic syndrome. However, upstream and downstream mediators of autophagy defect in metabolic syndrome and related diseases are still unclear. Chronic and intense oxidative stress or ER stress can be inducers of autophagy defect,135,136 despite that these stresses at physiological levels can trigger adaptive upregulation of autophagy activity.137–139 Regarding downstream signaling, similar to oxidative stress and ER stress, hypothalamic autophagy defect was recently revealed to activate IKKβ/NF-κB pathway to disrupt central regulation of feeding and energy balance, leading to the progression of obesity and related glucose intolerance.72 Notably, the induction of autophagy dysfunction in the development of HFD-induced obesity and metabolic disorders represents a relatively late event; nonetheless, the late-involvement does not diminish its significant disease impacts.72 Assembling all these types of intracellular stress, a model underlying overnutrition-induced metabolic syndrome can be depicted as shown in Figure 1. In this model, excessive nutrients presented to the CNS and particularly the hypothalamus can disturb cellular homeostasis by inducing mitochondrial oxidative stress and ER stress—which can spread from one organelle to another via the well-connected intracellular membrane system, leading to central inflammation and dysregulation of metabolic physiology. The disease consequences of this model are particularly related to chronic challenges of these stresses, which induce autophagy defect to sustain and escalate central inflammation. Pertinent to the latter point, central inflammation-induced metabolic syndrome and diseases may be difficult to reverse in their late stages using conventional approaches.
Toll-like receptors
Toll-like receptors (TLRs) were originally identified as an important class of cellular pattern recognition receptors that mediate innate immune defense against a diverse range of pathogens.140,141 Recently, TLR signaling has been linked to overnutrition-induced metabolic inflammation and the associated obesity and T2D.68,69,142–153 Of all mammalian TLR members, TLR1, TLR2, TLR4, and TLR6 have been suggested as potential mediators for metabolic inflammation related to lipid overnutrition particularly, since these receptors are primarily activated by extracellular lipids and can respond to lipid stimulation to induce proinflammatory response in adipocytes, macrophages, and myocytes.143–146 In dietary obese mouse models, high levels of circulating fatty acids were shown to activate TLR2 and TLR4 signaling in adipocytes, macrophages, and muscle to mediate obesity-related inflammation,149,152 while whole-body or tissue-specific inhibition of TLR2 or TLR4 suppresses HFD-induced inflammation in fat, liver, and muscle,148–153 which accounts for protection against HFD feeding-induced insulin resistance and dyslipidemia147–153 and energy and body weight imbalance.150 TLR4, a TLR member widely expressed in the CNS,142 has recently received particular attention in overnutrition-induced brain inflammation and central metabolic dysregulation.68,69,142,143 Central over-supply of saturated fatty acids or HFD-feeding can induce hypothalamic inflammatory response via TLR4 activation,69 and brain-specific or whole-body inhibition of TLR4 signaling abrogated this induction of central inflammation, leading to attenuation of central leptin resistance, systemic insulin resistance, and weight gain.68,69 In terms of downstream mediators of lipid-activated TLR signaling, both IKKβ/NF-κB and JNK pathways have been shown to mediate the inflammatory response induced by TLR2 or TLR4 activation in adipose tissue, muscle and liver.145,146,148–150,152 In the hypothalamus, activation of IKKβ/NF-κB and JNK proinflammatory pathways coexists with lipid-induced TLR4 activation;68 however, brain-specific inhibition of TLR4 signaling only attenuated IKKβ but not JNK activation in the hypothalamus,68 indicating that TLR4 signaling is necessary for IKKβ/NF-κB but not JNK-mediated proinflammatory activation in the hypothalamus. Additionally, activation of TLR4 and hypothalamic inflammatory response by central lipid excess was associated with induction of brain ER stress,69 suggesting that ER stress may act downstream of TLR4 to promote IKKβ/NF-κB. Of note, lipid-induced TLR4 activation can also lead to apoptosis of affected neurons,154 suggesting that hypothalamic inflammation might employ neural degeneration in addition to signaling defects to mediate the development of HFD-induced diseases.
Cytokine/chemokine receptors
It is clear that cytokines and chemokines can participate in systemic inflammation under condition of chronic nutritional excess, especially in the late stage when peripheral tissues such as fat and liver are evidently altered leading to excessive release of inflammatory cytokines into the circulation.52–62 Among these cytokines, tumor necrosis factor-α (TNF-α) is a critical one that has been related to IKKβ/NF-κB–mediated hypothalamic inflammation in metabolic syndrome. For example, central administration of TNF-α at low dose mimics low-grade inflammation to cause obesity/diabetes-related molecular signaling changes in the hypothalamus, resulting in overeating and decreased energy expenditure82,155 or hypertension.71 Conversely, mice that are genetically deficient of either TNF-α156,157 or TNF-α receptor155,158 are protected against overnutrition-induced obesity and insulin resistance. Interleukin-4 (IL-4) was also reported to mediate hypothalamic inflammation and the central induction of obesity.159 Moreover, interleukin-10 (IL-10) and interleukin-6 (IL-6) were recently discovered as anti-inflammatory molecules that mediate the effects of exercise in reducing hypothalamic inflammation and consequent anti-disease benefits.160 In addition, resistin, an adipocyte-derived cytokine which has been implicated in insulin resistance,161 was studied for its central role in metabolic syndrome. Central injection of resistin in mice was found to increase hepatic glucose production and induce hepatic insulin resistance, which was associated with increased hepatic expression of inflammatory molecules TNF-α, IL-6, and SOCS3, but no effects on food intake or body weight were reported by these studies.162,163 Taken together, the mediators of hypothalamic inflammation in metabolic syndrome and related disease can include certain types of cytokines and chemokines in addition to intracellular stresses discussed above. In this context, it is necessary to point out a technical issue that the roles of individual cytokines and chemokines in metabolic syndrome are difficult to dissect by pharmacologic approaches—which often fail to replicate the low-grade characteristic of metabolic inflammation. In many cases, studies with central administration of cytokines or chemokines, such as IL-1β, IL-6, IL-18, leukemia inhibitory factor (LIF), brain-derived neurotrophic factor (BDNF), ciliary neutrophic factor (CNTF), granulocyte-macrophage colony stimulating factor (GM-CSF), and TNF-α,82–90 generated robust inflammatory reaction in the brain, which might resemble the features of cachectic diseases more. Physiological consequences in these conditions were often related to catabolic actions, such as anorexia, high metabolic rate, and fever in association with severe breakdown of lean body mass and weight loss. While some cytokines, such as IL-1β, IL-6, IL-18, LIF, BDNF, and GM-CSF predominantly have cachectic actions as suggested by genetic knockout or haploinsufficiency models86,90,164–167 and transgenic overexpression models,168,169 the central effects of these molecules remain to be established with brain-specific or neuron subtype-specific genetic models. These models can address whether robust central inflammatory reaction to cachectic cytokines, directly or indirectly via prostaglandin E2 released by blood-brain barrier (BBB),170,171 is responsible for cachectic outcome, while low-grade induction of these cytokines in the hypothalamus by overnutrition leads to metabolic syndrome.
To summarize, induction of hypothalamic inflammation involves multiple levels of responses consisting of membrane and cytosolic changes. Regarding overnutrition-associated hypothalamic inflammation, intracellular stress responses, such as oxidative stress and ER stress most likely represent early changes that lead to molecular inflammation in involved cells. These changes possibly take place as adaptive responses to overnutrition-associated microenvironmental changes, but when prolonged they become harmful. For example, increased mitochondrial oxidation under overnutrition helps process excessive nutrients uptaken by the cell, but chronically it leads to elevated cellular oxidative stress from increased mitochondrial respiration. Such increased mitochondrial activity calls for increased synthesis of cytosolic proteins, which leads to heightened UPR of ER; however, prolonged and intense UPR can result in pathologic ER stress. As a result of sustained oxidative stress and ER stress, inflammatory products are produced to help cellular survival but at the expense of certain normal functions such as metabolic homeostasis. Also, under prolonged oxidative stress and ER stress, accumulation of defective mitochondria and ER demands increased workload of autophagy machinery, and this late-stage organelle adaptation, when beyond the physiological capacity, can cause autophagic stress and defect, which further contributes to hypothalamic inflammation. In parallel, extracellular nutrients in the form of fatty acids can directly activate the proinflammatory TLR pathway. In addition, hypothalamic inflammation can be promoted by cytokines, which are either produced locally by hypothalamic cells due to proinflammtory activation or transferred from the periphery due to increased circulating levels under chronic overnutrition. Overall, hypothalamic inflammation under chronic conditions, such as overnutrition, is a dynamic and integrated process; while the general goal is set to resolve environmental interruption of cellular biology, its chronic setup can be extremely detrimental to metabolic physiology which leads to nutritional diseases.
Types of inflammatory signaling and modulators in the hypothalamus
Metabolic inflammation represents a subtype of inflammatory changes and has many unique features in terms of the context of inflammatory signaling. Based on current knowledge, central inflammation in metabolic syndrome is significantly mediated by proinflammatory IKKβ/NF-κB and JNK pathways. Although both pathways are also implicated in cachectic inflammatory reaction, the molecular and intracellular events upstream and downstream of these kinase pathways have distinct characteristics in metabolic versus cachectic inflammation as summarized in Table 1 and discussed below:
IKKβ/NF-κB
Nuclear transcription factor NF-κB is the central mediator of immune response. A wide range of stimuli can stimulate NF-κB, and activated NF-κB induces the expression of a broad range of immune response genes.118 NF-κB is normally inactive in the cytoplasm bound by the inhibitory protein IκBα.172 Upon immune stimulation, IKKβ is activated and phosphorylates IκBα, leading to IκBα degradation and release of NF-κB activity. The liberated NF-κB translocates into the nucleus and induces transcription of a myriad of genes that promote inflammation. NF-κB–mediated inflammation in metabolic tissues has been recognized as a prominent feature of various metabolic disorders.55–62 Under various disease conditions such as stroke and brain aging, NF-κB in the CNS was shown to be activated by oxidative stress173,174 and ER stress.175,176 More recently, NF-κB–induced inflammation in the hypothalamus was identified as a central cause for multiple components of metabolic syndrome and related T2D and CVD.66–72,77 Hypothalamic IKKβ/NF-κB activation in obesity and obesity-related disease has been demonstrated to be induced by overnutrition-induced ER stress66,70 and autophagy defect72 to cause eating disorder and obesity66 or body weight-independent induction of systemic glucose intolerance70 and hypertension.70,71 Stress and inflammation can form a vicious cycle to sustain and prolong the disease effects, as hypothalamic inflammation by IKKβ/NF-κB was found to promote ER stress.66 In addition to being a stress response mediator, hypothalamic IKKβ/NF-κB can transduce the activation of membrane receptors (TLRs and cytokine receptors) into central inflammation in overnutrition-related diseases. Recent literature showed that hypothalamic IKKβ/NF-κB can mediate the effects of TLR4 signaling in the central mechanism of obesity.68 Also, activation of IKKβ/NF-κB in hypothalamic POMC neurons specifically via TNF-α receptor 2 was shown to play a major role in obesity-related hypertension.71 Additionally, IL-4 was shown to stimulate hypothalamic IKKβ/NF-κB activation leading to overeating and weight gain.159 The terminal effectors of hypothalamic IKKβ/NF-κB activation in causing and promoting metabolic syndrome are largely unclear, which may include intracellular stress-inducing enzymes (e.g., cytosolic phospholipase A2, cyclooxygenase-2, lipoxygenase, and nitric oxide synthase) and inflammatory molecules, such as suppressor of cytokine signaling 3 (SOCS3), cytokines, chemokines, and their receptors.66,118,177
JNKs
JNKs belong to the mitogen-activated protein kinase (MAPK) family, and as a major class of stress-activated protein kinases, JNKs play important roles in metabolic homeostasis control and the development of metabolic diseases.42,73,75,178–187 There are three mammalian JNK isoforms, with JNK1 and JNK2 being ubiquitously expressed, and JNK3 expression confined to a few tissues such as brain, pancreatic islet cells and heart.178 Due to their high degree of similarity and overlapping tissue expression patterns, the three isoforms may have partially redundant functions.179 JNKs are immediately activated through phosphorylation by MAPK kinases, and upstream mediators of JNKs predominantly involve inflammatory cytokines and environmental stress. In the context of metabolic regulation, lipid exposure, intracellular ER stress and oxidative stress are found to induce JNK activation.178–180 Activated JNKs modulate the function of a complex set of nuclear transcription factors (e.g., c-Jun, AP-1, ATF2 and FOXO4), nuclear hormone receptors (e.g., peroxisome proliferator-activated receptor-γ, glucocorticoid receptor, and retinoic acid receptor), and non-nuclear signaling molecules (e.g., insulin receptor substrates, mitochondrial Bcl family proteins, and 14-3-3 family of cytosolic signaling adaptors), thus affecting a broad range of biological processes. Various global or tissue-specific JNK1 genetic knockout models demonstrated that JNK activation in metabolic tissues (fat, liver and skeletal muscle) or non-metabolic myeloid cells mediates HFD-induced insulin resistance and related metabolic complications such as dyslipidemia.181–186 Recently, overnutrition-induced JNK activation was extended to include the CNS and the hypothalamus in particular.75,76,187 This interest was stimulated by the unresolved question that peripheral JNK1 knockouts could not replicate the anti-obesity effect of global JNK1 knockout against HFD overnutrition,181–186 which suggested a central site for JNK signaling in energy homeostasis control. Also, JNK activation was increased in the brain of rats and mice with dietary obesity.73,76 Using mice with brain-specific JNK1 knockout, Sabio et al. showed that central inhibition of JNK1 significantly prevented HFD-induced obesity, demonstrating that JNK-mediated inflammation acts in the brain to control energy homeostasis.75 HFD-induced suppression of peripheral insulin signaling in adipose tissue, muscle, and liver was prevented by brain-specific JNK1 deficiency in these mice.75 Also using a brain-specific JNK1 knockout model, Belgardt et al. observed similar metabolic benefits against HFD-feeding.76 Interestingly, both studies found that brain JNK1 deficiency increased the hypothalamic-pituitary-thyroid axis activity, and one study also observed reduction of growth hormone.75,76 Unger et al. also demonstrated that central inhibition of JNK1 in mice improved metabolic response to central insulin administration as evidenced by more pronounced appetite decrease and weight loss.187 However, the same study found central inhibition of JNK1 additionally potentiated the hyperphagic effect of central glucocorticoid administration.187 Taken together, these studies demonstrated that central JNK1 signaling regulates multiple endocrine axes and modules involving insulin, thyroid hormone, growth hormone and adrenal hormone. By contrast, JNK2 and JNK3 have not been directly studied pertaining to their possible roles in the central pathogenesis of metabolic syndrome, but both isoforms have been implicated in the central pathogenesis of neurodegenerative diseases and ischemic neuronal cell death.188–192 Studies have shown that JNK2 and JNK3 are required for the development of oxidative stress-related neuronal degeneration in mouse models of Parkinson’s disease, while JNK2 or JNK3 knockout protects mice against cellular oxidative stress and apoptosis and ameliorates the symptoms of neurodegenerative disease.189–191 Since intracellular stress is involved in both neurodegenerative diseases and obesity-related diseases, it is very likely that these JNK isoforms participate in the central mechanism of metabolic syndrome and related diseases, which calls for future investigations.
MyD88
Myeloid differentiation factor 88 (MyD88) is a central signaling adaptor for TLRs and IL-1 signaling to trigger downstream activation of proinflammatory kinase pathway mediated by IKKβ/NF-κB and JNKs etc.193,194 MyD88 is brought into attention in metabolic inflammation because proinflammatory activation induced by TLR4 signaling is implicated in central or peripheral lipid sensing and metabolic regulation.68,69,142–151 For example, fatty acids can induce inflammation through TLR4 activation in adipocytes, macrophages, muscle, and liver,143–146,149 while inhibition of TLR4 signaling substantially suppressed tissue inflammation and systemic insulin resistance against HFD overnutrition.148–151 In this background, brain-specific ablation of MyD88 was found to abolish TLR-mediated central inflammatory signaling through IKKβ/NF-κB in HFD-fed mice, resulting in metabolic protections against HFD-induced central leptin resistance and the development of obesity or central glucose dysregulation.68 In the same study, overnutrition-induced brain JNK activation was found unaffected by MyD88 deficiency, indicating that JNK-mediated metabolic inflammation in the CNS may not depend on MyD88. These findings were in line with another work that showed that LPS-induced TLR4 activation led to an early phase NF-κB activation in a MyD88-dependent manner and a late phase MAPK/JNK pathway activation in a MyD88-independent manner in astrocytes.195 This study provoked a question regarding the neuronal versus non-neuronal source for MyD88-induced central inflammation in metabolic syndrome and related diseases.
SOCS3
While MyD88 can act upstream of IKKβ/NF-κB–mediated metabolic inflammation, SOCS3 can be a key downstream player. SOCS family proteins were identified based on their abilities to inhibit JAK2–STAT3 signaling, which forms the mechanistic basis for SOCS proteins to inhibit leptin signaling.31,47,65,196 SOCS3 is particularly important for central metabolic dysregulation because HFD feeding specifically increases SOCS3 expression in the hypothalamus.66,197 Accordingly, the deleterious molecular and physiological effects of metabolic inflammation significantly depend on SOCS3 expression,198 particularly in brain neurons199 or hypothalamic neurons.77,79,200 Indeed, SOCS3 was demonstrated by multiple groups to negatively affect central insulin and leptin signaling through interrupting comediators, such as insulin receptor substrates, JAK2/STAT3 and FOXO1.31,47,65,196 Brain-specific SOCS3 knockout results in increased hypothalamic STAT3 phosphorylation and POMC induction.199 Conversely, SOCS3 overexpression in POMC neurons impairs STAT3 signaling.200 Interestingly, upregulation of hypothalamic SOCS3 by HFD feeding was shown to depend on IKKβ/NF-κB signaling in a cell autonomous manner;66 moreover, this NF-κB–dependent SOCS3 induction partially mediated the physiological effects of hypothalamic IKKβ/NF-κB activation in causing energy imbalance and obesity development.66
PTP1B and inflammation
In addition to SOCS3, protein-tyrosine phosphatase 1B (PTP1B) is another molecule that can inhibit insulin and leptin signaling.201–207 PTP1B belongs to the general family of protein-tyrosine phosphatases, which reduce the phosphorylation of many important signaling molecules.201,202 PTP1B is of particular interest in metabolic regulation because PTP1B is widely expressed in multiple insulin-responsive tissues such as skeletal muscle, liver, adipose tissue, and brain,208,209 and PTP1B mediates inhibition of leptin signaling in cultured cells.203 Whole-body204,205 or muscle-specific206 PTP1B deficiency improves insulin signaling in major metabolic sites such as liver and skeletal muscle. With regard to central metabolic regulation, brain-specific PTP1B knockout improves central leptin signaling in mice, resulting in a host of beneficial metabolic changes against overnutrition, including reduction of body weight and adiposity, increase of energy expenditure and improvement of glucose homeostasis, indicating that brain PTP1B is a key player in overnutrition-induced central metabolic dysregulation.207 HFD increases PTP1B expression in the hypothalamus,210 and factors that link HFD feeding to PTP1B upregulation can include excessive circulating glucose, lipids, and hormones (e.g., insulin and leptin).211–215 Interestingly, IKKβ/NF-κB activator TNF-α was recently demonstrated to induce PTP1B overexpression not only in adipocyte and hepatocyte cultures but also in liver, skeletal muscle, adipose tissue, and hypothalamus of animals.210 Considering that HFD feeding can activate hypothalamic IKKβ/NF-κB—a proinflammatory pathway pivotally responsible for the induction of central inflammation,66 PTP1B may represent another link in addition to SOCS3 between overnutrition and inflammatory reaction in metabolic syndrome and related diseases. Further bolstering this hypothesis, Loh et al. recently showed that hypothalamic levels of another tyrosine phosphatase TCPTP are also elevated in diet-induced obesity which additionally accounts for the development of central leptin resistance.216 Based on the observation that hypothalamic neuronal double-knockout of PTP1B and TCPTP has additive anti-obesity effects against overnutrition compared to PTP1B or TCPTP single knockout, the authors proposed a model where PTP1B and TCPTP, respectively, promote primary versus secondary hypothalamic inflammation associated with overnutrition-induced obesity.216
AMPK and inflammation
AMP-activated protein kinase (AMPK) is an evolutionarily conserved serine/threonine kinase and functions as an energy sensor at both cellular and whole-organism levels.40,217–226 AMPK is activated by physiological or pathological states of energy deficiency, such as exercise and muscle contraction, fasting, hypoxia, ischemia, glucose deprivation or hypoglycemia, and uncoupling of oxidative phosphorylation. Multiple tissue-specific knockout or overexpression models showed that AMPK activation in individual peripheral tissue counteracts the negative impact of energy deficiency on that specific tissue. For example, AMPK stimulates glucose and fatty acid uptake and oxidation in muscle, inhibits gluconeogenesis (an ATP-consuming process) of the liver, and stimulates lipolysis in adipose tissue. The net outcomes are decreased circulating levels of glucose and lipid, reduction of fat accumulation and enhanced insulin sensitivity, leading to protections against obesity and insulin resistance.217–220 In the CNS, AMPK acts as a hypothalamic neuronal fuel sensor and counteracts energy deficit by stimulating food intake and hepatic glucose production and by inhibiting energy expenditure and fatty acid oxidation.40,221–226 Consistently, anorexic hormone leptin or insulin, which signals energy sufficiency, inhibits hypothalamic AMPK activity; whereas orexigenic peptide ghrelin or AGRP increases hypothalamic AMPK activity.222,223 Thus, activation of hypothalamic AMPK is orexigenic, and excessive hypothalamic AMPK activity can be obesogenic. Indeed, leptin failed to inhibit AMPK2α activity in the arcuate and medial hypothalamus of mice with diet-induced obesity, which was associated with central leptin resistance.224 In agreement with this understanding, AGRP neuron-specific AMPK2α deletion was found to prevent obesity,225 although discrepancy exists that POMC neuron-specific AMPK2α deletion also somehow promotes obesity.225 Interestingly, hypothalamic AMPK inhibition was found to underlie the anti-obesity effect of central administration of anti-inflammatory α-lipoic acid.226 Thus, it is quite possible that excessive activation of hypothalamic AMPK participates in central inflammation and the resulting positive energy balance. Future research is needed to determine whether and how AMPK can be involved in HFD-induced hypothalamic inflammation, particularly in AGRP neurons versus POMC neurons.
mTOR and inflammation
The mammalian target of rapamycin (mTOR) is an evolutionarily conserved serine-threonine kinase that controls cell metabolism, growth, proliferation, and survival in response to nutrient availability.227–230 mTOR pathway responds to nutrients, cellular ATP levels, stresses, and growth factors, and activated mTOR controls a number of signaling cascades involved in transcription, translation, ribosome biogenesis, autophagy, and metabolism. Well-characterized mTOR targets include ribosomal S6 kinases (S6K1 and S6K2) and eukaryotic initiation factor 4E-binding protein 1, which function sequentially to activate translation of specific genes. Due to its pivotal role in cellular physiology, complete loss of mTOR is lethal.231 An interesting observation is although mTOR and S6K1 are ubiquitously expressed in brain, their phosphorylated forms are largely limited to the arcuate nucleus and the PVN in rats,232,233 indicating their function importance in these hypothalamic metabolic centers. mTOR is activated downstream of PI3K/Akt signaling,234 which is an important mediator of insulin and leptin signaling in metabolic control,50 suggesting that mTOR could act as an effector of central insulin or leptin sensing. Indeed, central administration of leptin leads to activation of mTOR/S6K1 pathway, while central inhibition of mTOR abrogates the anorexic effect of leptin.232 Similarly, hyperactivation of mTOR was shown to downregulate insulin signaling through S6K1-mediated negative feedback inhibition of insulin receptor substrates to cause insulin resistance in many types of peripheral tissues.235,236 This pathological action of mTOR/S6K may also apply to the hypothalamic insulin signaling, as suggested by a study showing that hypothalamic ablation of mTOR signaling inhibitor tumor suppressor complex 1 (TSC1) leads to uninhibited mTOR activity and the development of hyperphagia and obesity due to central insulin resistance.237 Along this line, constitutive activation of S6K in the hypothalamus was found to mimic HFD feeding to impair central insulin signaling with disease consequences, yet suppression of S6K in MBH restored central insulin signaling.238 Thus, hyperactivation of mTOR pathway may contribute to the pathogenesis of overnutrition-induced central metabolic dysregulation. In connection with inflammation, mTOR pathway has been shown to be activated by inflammatory cytokines, such as TNF-α and IL-6,239 and IKKβ activation can mediate TNF-α–induced mTOR activation via suppressing TSC1.240 Reciprocally, activated mTOR pathway can promote cellular inflammation by inducing inflammatory cytokines through activating NF-κB and ER stress.241,242 Overall, there still miss compelling evidences for mTOR hyperactivation being the downstream mediator of central inflammation, and an intriguing future research topic would be to investigate the connection between mTOR and IKKβ- or stress-induced hypothalamic inflammation and its disease significance.
Sirtuins and inflammation
Sirtuins refer to a family of nicotinamide adenine dinucleotide (NAD)-dependent protein deacetylases.243,244 Sirtuins are originally known for the anti-aging effects in yeast and metazoan species,245 although a recent study by Burnett et al.246 challenged the pro-longevity role of C. elegans and Drosophila Sir2 (ortholog of mammalian SIRT1). In mammals, the sirtuin family has seven functionally non-redundant homologues, namely SIRT1–7.247 Protein deacetylation by nuclear sirtuins (SIRT1, 2, 6, and 7) is associated with modulations of chromatins and transcription factors, which results in transcription activation of genes that control key metabolic pathways, and the overall physiologic effects of sirtuins have been related to enhanced stress tolerance and protections against aging and metabolic diseases.245 In fact, such anti-stress actions form the mechanistic basis for sirtuins to mediate the anti-aging effect of caloric restriction (CR).245 Protein deacetylation by mitochondrial sirtuins (SIRT3, 4 and 5), on the other hand, directly modulates the functions of mitochondrial proteins that govern metabolic pathways, and their roles in promoting cellular adaptation to metabolic stresses are more prominent.247 As overnutrition can induce metabolic stress, which mirrors the effect of CR in reducing metabolic stress, there might exist some molecular pathways that are oppositely targeted by overnutrition and CR. Sirtuins may represent such candidates which generate anti-inflammatory actions against overnutrition, as activation of sirtuins (e.g., SIRT1, 3, 4, and 7) reduces cellular oxidative stress9 and renders cells more resistant to stress-induced deleterious outcomes such as inflammation. This notion can be suggested by a recent study by Pflunger et al. showing that transgenic overexpression of SIRT1 in endogenous SIRT1-expressing cells improved energy expenditure and glucose tolerance and protected against hepatic steatosis in mice; more importantly, these metabolic benefits of SIRT1 overexpression are mediated by induction of antioxidants and reduction of proinflammatory cytokines via NF-κB downregulation.248 The working model of sirtuins in suppressing inflammation may similarly apply to the CNS and the hypothalamic neurons. Studies have shown that POMC neuron-specific SIRT1 deficiency rendered hypothalamic neurons susceptible to HFD-induced leptin resistance,249 and mice with either POMC neuron-specific or steroidogenic factor 1 (SF1) neuron-specific SIRT1 deletion were highly susceptible to the development of dietary obesity and T2D.249,250 However, there is one caveat with the POMC neuron-specific SIRT1 knockout model, since studies with rats have shown that hypothalamic inhibition of SIRT1 decreases food intake through its direct effects in NPY/AGRP neurons and indirect effects on POMC neurons.251,252 Independently of these genetic approaches, pharmacologic studies revealed that resveratrol activates SIRT1 to reduce HFD-induced hypothalamic inflammation and improve diabetic symptoms in mice, suggesting the central anti-inflammatory role of SIRT1 against overnutrition.253,254 Regarding other SIRT isoforms, neuronal deletion of SIRT6 resulted in striking histone hyperacetylation in neuroendocrine regions of the brain and the development of obesity, indicating that SIRT6 normally functions as a central anti-obesity molecule.255 Recently, mice deficient in SIRT3 were shown to have accelerated development of metabolic syndrome including obesity, insulin resistance, hyperlipidemia, and steatohepatitis when fed a HFD.256 In summary, the anti-inflammatory potential of sirtuins may account for their central actions in preventing overnutrition-related diseases, and it can be anticipated that significant amount of research interest and efforts will be attracted to test this hypothesis in the next few years.
Hypothalamic cell types affected by inflammation
The hypothalamus is a highly heterogeneous brain structure consisting of different types of neurons and non-neuronal cells. Hypothalamic neurons that regulate metabolic homeostasis are clustered into distinct groups located in different hypothalamic regions, and those in the MBH, such as POMC neurons and AGRP neurons, are primarily responsible for converting systemic metabolic signals to brain control of metabolic physiology. In this process, these neurons employ various neuropeptides and neurotransmitters to direct downstream neuroendocrine and neural events in concert with hunger, satiety, energy expenditure, thermogenesis, and peripheral nutrient metabolism. The hypothalamus contains different non-neuronal cell types including astrocytes, oligodendrocytes, microglial cells, ependymal cells, and endothelial cells, which maintain a homeostatic environment for neurons by providing nutrients, oxygen, physical support, and protection to neurons. Major neuronal and non-neuronal cell types in the brain express membrane-bound pattern recognition receptors (PPRs), which are a form of secreted soluble proteins displayed on cell surface that bind, phagocytose, and transduce extracellular signals based on their molecular patterns.257 Two major functional classes of membrane PPRs are endocytotic PPRs (e.g., mannose receptors and scavenger receptors) that promote phagocytosis of non-self molecules and signaling PPRs (e.g., TLRs and CD14) that promote synthesis and secretion of immune response molecules.141,258 Thus, at least via PPRs, both neurons and various glial cell types can mediate innate immune response to local and systemic inflammatory stimuli.259–261
Hypothalamic inflammation that underlies the development of obesity and related disease was initially demonstrated to occur in hypothalamic neurons, which can be induced through HFD-induced IKKβ/NF-κB activation.66 Several subsequent studies supportively demonstrated that overnutrition induces metabolic inflammation in the hypothalamus or the arcuate nucleus region in particular.67–69 Using mouse models of neuron-specific gene knockout, IKKβ inhibition in AGRP neurons and POMC neurons were respectively demonstrated to protect against HFD-induced obesity and obesity-associated disorders (e.g., glucose intolerance and blood pressure elevation).66,71,72 The underlying mechanisms include direct remedies of central leptin and insulin resistance66 and sympathetic nervous system alteration70,71 at neuronal levels. Neuron-specific knockouts of SOCS3 using synapsin 1 promoter-driven Cre-loxP system was shown to increase hypothalamic leptin sensitivity and prevent diet-induced obesity, indicating that SOCS3-mediated neuronal inflammatory signaling contributes to the development of central metabolic deregulation under overnutrition.199 Overall, because neurons mediate hypothalamic mechanism of metabolic syndrome and diseases, neuronal inflammation induced factors—particularly intracellular oxidative stress and ER stress, are most likely to be pertinent to the central inflammatory mechanism of these diseases. Based on this understanding, neuronal inhibition of stress and inflammatory signaling can significantly mediate the anti-obesity/T2D phenotypes in a variety of brain-specific knockout mouse models that targeted ER stress signaling enhancer X box binding protein-1,74 MyD88,68 or JNK1,75,76 although gene ablations (via nestin promoter-directed Cre-loxP recombination) in these models occurred in not only neurons but also glial cells.
Along with recent advances on hypothalamic inflammation in overnutrition-induced diseases, research also began to revisit the role of inflammatory reaction in hypothalamic mechanism of negative energy balance and cachexia, facilitated by recently available brain- or neuron-specific genetic models. Two recent studies have linked POMC neurons to the effect of central cachectic inflammation: one showed that upregulation of inflammatory cytokine LIF in POMC neurons accounts for LIF’s central cachectic action;87 the other showed that activation of IKKβ in POMC neurons is required for anorexia and weight loss in sickness response.262 In addition to POMC neurons which are localized in the MBH, some studies examined the lateral hypothalamus that contains orexin neurons and melanocortin concentrating hormone (MCH)-expressing neurons, and found that these neurons were critical for the attenuation of lipopolysaccharide (LPS)-induced cachectic effect induced by central administration of anti-inflammatory cytokine IL-10.263 Thus, neurons in the lateral hypothalamus may have an important role in terms of cachectic hypothalamic inflammation and disease consequence. Comparatively, a question can be asked whether neurons in lateral hypothalamus can be relevant to metabolic inflammation in obesity and obesity-related diseases.
Apart from neuronal inflammation, recent studies have alluded to the role of non-neuronal cells in hypothalamic inflammation with regard to overnutrition-induced diseases. Neonatal exposure to overnutrition (HFD feeding) was shown to cause microglial activation and increased local levels of IL-6 in the ventromedial hypothalamus at 60 days postnatal, leading to elevated circulating levels of leptin and moderate weight gain on a normal diet in rats.264 Also, another study reported that central IL-4 administration was able to induce microglial activation which promoted hypothalamic inflammation and weight gain, while blockade of proinflammatory kinase IKKβ abolished the obesogenic effect of IL-4.159 Based on this work, microglial cells may provide a permissive effect on neuronal inflammatory dysfunction. This idea is also suggested by an in vitro study showing that cultured pure population of hypothalamic neurons are not sensitive to lipid-induced inflammatory activation.265 Another intriguing question regarding the cellular basis of hypothalamic inflammation is whether metabolic versus cachectic inflammation may have cell type-dependent basis between neurons versus glial cells. Given the highly heterogeneous nature of hypothalamic cells, such a question seems quite legitimate; however, current research in this regard is scarce. Regardless, among non-neuronal glial cells, microglial cell-mediated hypothalamic inflammation in response to overnutrition has different characteristics from the classical inflammation mediated microglial cells in brain injuries and infections266–268 or neural autoimmune diseases.269 Astrocytes could be another glial cell type relevant to central induction of inflammation by overnutrition—especially lipid excess, given that astrocytes rely on free fatty acids as energy source and express plenty lipid regulators, such as PPARα, lipoprotein apoE, and brain fatty acid-binding proteins.45 Overall, the role of various glial cells in hypothalamic inflammation and nutritional diseases, whether being obesogenic or cachectic, remains an under-investigated topic.
Impacts of hypothalamic inflammation on neuroendocrine and neural systems
The hypothalamus functions as endocrine headquarters in regulating whole-body energy and metabolic homeostasis.31–47 Metabolic regulatory centers of the hypothalamus, such as those located in the MBH and the lateral hypothalamus, critically integrate metabolic sensing with central neuroendocrine and neural control of metabolic physiology. A crucial component that couples metabolic sensing with downstream physiological regulation is the regulatory network directed by various neuropeptides.270–272 Some well-studied neuropeptides in hypothalamic metabolic regulation include α-melanocyte stimulating hormone (α-MSH, a peptidyl product of POMC), NPY and AGRP—which are produced and released by POMC neurons or AGRP neurons in response to metabolic signals. These neuropeptides subsequently act on downstream hypothalamic neurons which use peptidyl hormones, such as thyrotropin-releasing hormone (TRH),273 corticotropin-releasing hormone (CRH), oxytocin, and MCH274 to control metabolic physiology. Two well-studied examples of such neuroendocrine systems are the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis.
Brain inflammation is known to have dramatic effects on hypothalamic endocrine systems (e.g., HPA and HPT axes) at multiple levels of. For example, brain inflammation following traumatic brain injury can result in hypothalamic-pituitary insufficiency, a cluster of disorders that affect many endocrine regulations leading to adrenal, thyroid, or growth hormone defects.275,276 Overnutrition-induced central inflammation is milder in comparison, but the chronic nature still results in (selective) disruption of hypothalamic neuroendocrine pathways to negatively impact metabolic physiology, as summarized in Table 2. Under obesogenic conditions, such as chronic HFD feeding, excessive nutrients can induce inflammation in the hypothalamus to impair neuronal sensitivity to central leptin and insulin, leading to feeding and energy imbalance and obesity.66 At the molecular level, such inflammation can be significantly mediated by IKKβ/NF-κB activation and SOCS3 upregulation.66 Hypothalamic inflammation can also inhibit the HPT axis to cause obesity, as demonstrated by two recent studies that inhibition of brain inflammation via brain-specific JNK1 knockout was associated with increased HPT axis activity,75,76 which accounted for the protection against HFD-induced obesity.75 Also, it was shown that hypothalamic inflammation induced by neuropsychiatric stress or brain L-glutamate–induced damage was associated with activation of HPA axis which led to visceral obesity.277–279 Furthermore, hypothalamic inflammation may activate the endocannabinoid system to promote overeating and obesity,280,281 since the appetite-suppressing and anti-obesity effects of cannabinoid-1 receptor antagonist rimonabant are related to a systemic decrease of inflammatory markers such as TNF-α and C-reactive protein.282
Table 2.
Impacts of hypothalamic inflammation on central neuroendocrine versus neural regulation
| Neuroendocrine regulation | Neural regulation | |
| Primary responsive sites (neurons) |
|
|
| Target sites (neurons) within the CNS |
|
|
| Systems involved |
|
|
| Physiology affected |
|
|
| Diseases associated with dysregulation |
|
|
In addition to being a neuroendocrine regulator, the hypothalamus is an important player in the neural control of metabolism and related diseases. The underlying anatomic basis is the extensive neural projections emanating from several hypothalamic nuclei to hindbrain autonomic sites such as rostral ventrolateral medulla (RVLM), nucleus tractus solitarius (NTS), and dorsal motor nucleus of the vagus nerve (DMX).31,37,49 In fact, such neural regulatory pathways have been speculated to mediate body weight-independent regulation of glucose homeostasis by the arcuate nucleus and comprised POMC neurons or AGRP neurons and by the ventromedial nucleus and comprised SF1 neurons.49,283–286 Under disease conditions, such as overnutrition-induced inflammation, normal hypothalamic neural regulation of energy homeostasis and metabolism can be disrupted, leading to various disease consequences. This model is suggested by the observations that acute overnutrition is associated with elevation of sympathetic nervous system activity,287,288 and inflammation also leads to sympathetic excitation in various experimental systems.70,71 Indeed, studies using mice with neuronal deletion of inflammatory mediator SOCS3 showed that neuronal inflammation impairs central regulation of glucose homeostasis in a body weight-dissociable manner,77,79 though these studies did not examine whether an alteration of neural output was accountable. However, two recent studies unequivocally demonstrated that NF-κB–mediated inflammation in hypothalamic arcuate nucleus causes peripheral insulin resistance70 and hypertension71 via sympathetic upregulation, and one study pinpointed that NF-κB–mediated inflammation in POMC neurons rather than AGRP neurons was required for overnutrition-induced sympathoexcitation.71 Thus, these two types of hypothalamic neurons can employ neuroendocrine versus neural programs differentially to decode overnutrition-related inflammation. In conjunction with the literature that NF-κB–mediated inflammation in AGRP neurons causes obesity,66 while NF-κB–mediated inflammation in POMC neurons underlies the cachectic effects of sickness response,262 it can be speculated that obesogenic versus cachectic effects of hypothalamic inflammation might be preferentially diverted to neuroendocrine versus neural changes by different types of hypothalamic neurons. In addition to sympathetic activation, brain inflammation has been recognized to downregulate parasympathetic pathway289 to cause glucose dysregulation49 and cardiac damage,290 yet restoring parasympathetic activity can antagonize the pathogenesis of inflammation-related stroke.291 However, it has been barely studied whether the parasympathetic pathway can be modulated by overnutrition-related hypothalamic inflammation to mediate the development of metabolic syndrome and diseases.
Impacts of hypothalamic inflammation on body weight
The hypothalamus is one central regulator of energy balance and body weight at whole-organism level (Fig. 2).31–46 The regulation is crucially mediated by multiple hypothalamic regions and locally released neuropeptides such as α-MSH, CART, AGRP, NPY, orexin, and MCH.292–301 The well-defined MBH region contains first-order neurons that sense metabolic cues (e.g., circulating leptin, insulin and nutrients) and instructs downstream neuroendocrine and neural systems to adjust energy intake (feeding) and energy expenditure.302–305 When energy intake exceeds expenditure, increased amount of leptin is produced by fat cells and acts on MBH neurons via leptin signaling consisted of JAK2 and STAT3. As a result, POMC neurons produce and release anorexigenic neuropeptides α-MSH and CART, while AGRP neurons stop producing orexigenic neuropeptides AGRP and NPY. Insulin generates similar effects on these neurons via insulin signaling, and some insulin signaling components such as PI3K and FOXO also participate in leptin signaling.31,37,42 In addition to neuropeptides, these neurons can use neurotransmitters to control downstream regulatory systems.44,306–311 Downstream effectors of these neuropeptides comprise both neuroendocrine pathways and neural routes that are linked to brain autonomic centers. However, under pathological conditions, such as overnutrition-induced inflammation or cachectic systemic inflammation, the regulatory functions of hypothalamic neurons and effectors169,312–318 are disrupted, leading to various types of energy and body weight imbalance as summarized in Table 3 and discussed below.
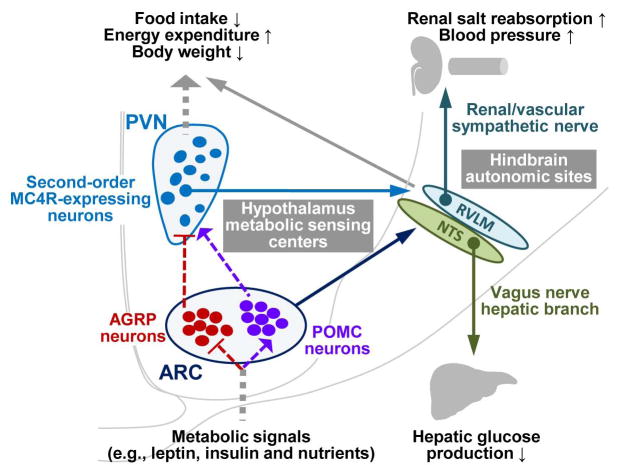
Figure 2.
Hypothalamic regulation of body weight, glucose and cardiovascular homeostasis. The arcuate nucleus (ARC) of mediobasal hypothalamus is a well-characterized hypothalamic metabolic sensing center, containing first-order nutrient sensing AGRP neurons and POMC neurons. Through activating POMC neurons but inhibiting ARGP neurons, metabolic signals activate MC4R-expressing neurons in paraventricular nucleus (PVN) and other hypothalamic areas, leading to the hypothalamic control of feeding, energy expenditure, and body weight. The ARC can also convey nutritional signals, directly or indirectly via the PVN neurons, to hindbrain autonomic sites such as the nucleus of solitary tract (NTS) and the rostral ventrolateral medulla (RVLM), and mediate the hepatic control of glucose homeostasis and the renal and cardiovascular control of blood pressure balance. Dashed lines represent endocrine or neuroendocrine regulation; solid lines represent neural projections; lines with an arrow end denote activation; lines with a bar end denote inhibition.
Table 3.
Effects of inflammation-related signaling mediators on energy balance and body weight
| Gene | Models | Site applied | Food intake | Energy expenditure | Body weight | Neuropeptide mRNA | Ref | |
|---|---|---|---|---|---|---|---|---|
| Proinflammatory cytokines | Tumor necrosis factor-α (TNF-α) | KO | Whole body | ↓ | Anti-obesity | 156,157 | ||
| icv injection (low-dose) | Brain | ↑ | ↓ | Pomc↓, TRH↓, CRH↓ | 82,155 | |||
| icv injection (high-dose) | Brain | ↓ | ↑ | Pomc↑, Agrp↓ | 82,312 | |||
| TNF-α receptor 1 (TNFR1) | KO | Whole body | ↑ | ↑ | Anti-obesity | Pomc↓, Agrp↑, TRH↑ | 155,158 | |
| Interleukin-1 (IL-1) | KO | Whole body | Mature-onset obesity | 164 | ||||
| icv injection (high-dose) | Brain | ↓ | ↑ | ↓ | 83,84,313,314 | |||
| Interleukin-1 receptor I (IL-1RI) | KO | Whole body | ↑ | ↓ | Mature-onset obesity | 165 | ||
| IL-1 receptor antagonist (IL-1Ra) | KO | Whole body | ↓ or no change | ↑ or no change | ↓ | No changes | 341,342 | |
| Interleukin-6 (IL-6) | KO | Whole body | ↑ | Mature-onset obesity | 166 | |||
| icv injection (high-dose) or overexpression | Brain | ↓ | ↑ | ↓ | Pomc↑, Npy↓, Agrp↓ | 85,166, 169 | ||
| Interleukin-18 (IL-18) | KO | Whole body | ↑ | ↓ | Mature-onset obesity | No changes | 86,315 | |
| icv injection (high-dose) | Brain | ↓ | ↓ | 86 | ||||
| Leukemia inhibitory factor (LIF) | icv injection or overexpression | Brain | ↓ | No change | ↓ | 87,168 | ||
| Brain-derived neurotrophic factor (BDNF) | KO (+/−) | Whole body | ↑ | ↑ | ↑ | No changes | 167 | |
| icv injection (high-dose) | Brain | ↓ | ↑ | ↓ | Pomc↑, Agrp↑, TRH↑ | 88,316 | ||
| Ciliary neurotrophic factor (CNTF) | icv injection (high-dose) | Brain | ↓ | ↓ | Npy↓ | 89,317 | ||
| GM-CSF | KO | Whole body | ↓ | Mature-onset obesity | No changes | 90 | ||
| icv injection (high-dose) | Brain | ↓ | ↓ | Npy↓, Agrp↓ | ||||
| Resistin | KO (+/−) | Whole body | ↓ | ↑ | 363 | |||
| icv injection (high-dose) | Brain | ↓ | ↓ | CART↑, Npy↓ or ↑, Agrp↓ | 163,318 | |||
| Proinflammatory factors | X box binding protein 1 (XBP1) | KO | Brain | ↑ | ↓ | ↑ | Npy↑, Agrp↑ | 74 |
| IKKβ | KO | Brain | ↓ | Anti-obesity | 66 | |||
| c-Jun N-terminal kinase 1 (JNK1) | KO | Whole body | Anti-obesity | 181 | ||||
| KO | Brain | ↓ | ↑ | Anti-obesity | Agrp↑ | 75,76,187 | ||
| Toll-like receptor 2 (TLR2) | KO | Whole body | Anti-obesity | 153 | ||||
| Toll-like receptor 4 (TLR4) | KO | Whole body | 149 | |||||
| MyD88 | KO | Brain | ↓ | ↑ | Anti-obesity | 68 | ||
| PTP1B | KO | Whole body | ↑ | Anti-obesity | 204,205 | |||
| KO | Brain | ↑ | Anti-obesity | 207 | ||||
| SOCS3 | KO (+/−) | Whole body | ↓ | Anti-obesity | Npy↓, Agrp↓ | 198 | ||
| KO | Brain | ↓ | Anti-obesity | leptin-induced Pomc↑ | 199 | |||
| KO | POMC neuron | ↓ | ↑ | Anti-obesity | Pomc↑, Npy↓ | 77 | ||
| KO | SF1 neuron | ↓ | ↓ | No change | 79 | |||
| α-lipoic acid | icv injection | Brain | ↓ | ↑ | Anti-obesity | 226 | ||
| AMPK 2α | KO | AGRP neuron | ↓ | No change | ↓ | 225 | ||
| POMC neuron | ↑ | ↓ | ↑ | |||||
| TSC1 (mTOR inhibitor) | KO | POMC | ↑ | ↑ | Pomc↓, Npy↑ | 237 | ||
| Sirtuin 1 (SIRT1) | Chemical inhibitor or siRHA | Brain or arcuate | ↓ | ↓ | Pomc↑, Agrp↓ | 251 | ||
| KO | AGRP neuron | ↓ | No change | ↓ | 252 | |||
| KO | POMC neuron | ↓ | ↑ | 249 | ||||
| KO | SF1 neuron | ↓ | ↑ | 250 | ||||
| Sirtuin 3 (SIRT3) | KO | Whole body | ↓ | ↑ | 256 | |||
| Sirtuin 6 (SIRT6) | KO | Brain | ↑ | Pomc↓ | 255 | |||
| Lipoprotein receptor LRP1 | KO | Brain | ↑ | ↓ | ↑ | Npy↑, Agrp↑ | 335 | |
| Lipoprotein lipase (LPL) | KO | Brain | ↑ | ↓ | ↑ | Npy↑, Agrp↑ | 336 | |
| Fatty acid synthase (FAS) | KO | via RIP Cre | ↓ | ↑ | ↓ | 334 | ||
| Long-chain fatty acyl-CoA | icv injection | Brain | ↓ | ↓ | Npy↓, Agrp↓ | 333 |
“High-dose” reflects the injected doses in ng or μg, while “low-dose” reflects the injected doses in pg. Abbreviations: KO, knockout; icv, intracerebroventricular; ↓ decrease; ↑ increase.
IKKβ/NF-κB and body weight
Various genetic models demonstrated that central leptin and insulin signaling are indispensible for proper feeding and body weight regulation, while impairment of central leptin or insulin signaling underlies the development of obesity.304,305,319–324 Activation of hypothalamic IKKβ/NF-κB inflammatory pathway is a major pathogenic mediator for overnutrition-induced obesity.66–69,72 Using HFD feeding or central administration of saturated fatty acids, lipid excess has been related to induction of NF-κB–related inflammation in the hypothalamus, resulting in impairment of central leptin and insulin signaling, increased food intake, decreased energy expenditure and the development of obesity.66–69 Inhibition of IKKβ/NF-κB via pharmacologic inhibition of hypothalamic IKKβ,67 gene knockout of TLR469,150 or NF-κB subunit p50,325 or brain-specific ablation of IKKβ or MyD8866,68 unanimously had protective effects against these metabolic disorders. Mechanistically, overnutrition was shown to activate neuronal IKKβ/NF-κB via cellular metabolic stress such as ER stress.66 Central induction of ER stress can mimic overnutrition to cause NF-κB activation in the hypothalamus of mice, while central inhibition of ER stress can reduce the effect of HFD feeding in inducing hypothalamic NF-κB activation.66 Furthermore, mice with genetic induction of brain ER stress were susceptible to HFD-induced leptin resistance and obesity.74 Taken together, an early-onset cascade induced by overnutrition can be inferred, i.e., cellular ER stress → hypothalamic IKKβ/NF-κB activation → central leptin and insulin resistance → energy imbalance and obesity. Besides ER stress, autophagy defect, which represents another type of cellular abnormality, can induce feeding and energy imbalance and obesity development by promoting hypothalamic IKKβ/NF-κB activation, since brain-specific IKKβ knockout abrogated the obesogenic effects of autophagy defect.72 Of note, the development of autophagy defect in hypothalamic arcuate nucleus is late-onset during overnutrition (HFD)-induced obesity,72 implying that ER stress and autophagy defect respectively represent early versus late mediator of IKKβ/NF-κB–mediated hypothalamic inflammation under obesogenic conditions.
Regarding the cellular basis for the obesogenic effect of hypothalamic IKKβ/NF-κB, AGRP neurons represent an important neuronal subtype, because AGRP neuron-specific IKKβ knockout partially protected mice against HFD feeding-induced obesity.66 The potential effect of IKKβ/NF-κB in POMC neurons cannot be excluded, although IKKβ inhibition in POMC neurons by itself was insufficient to reverse HFD-induced obesity.71 IKKβ/NF-κB in brain and hypothalamic glial cells may also mediate obesity development. Oh et al. reported that central administration of IL-4 promotes weight gain under HFD feeding condition via microglia-mediated activation of IKKβ signaling, yet pretreatment with IKKβ inhibitor in the brain prevents the effect of IL-4 in promoting obesity.159 Thus, mirroring the proinflammatory effect of macrophage activation in promoting peripheral metabolic disorders, activation of microglia also promotes a central inflammatory milieu which contributes to the development of central metabolic dysregulations. In addition to microglia, HFD was shown to stimulate astrocyte activities, indicating that astrocyte-mediated immune response may play a role in metabolic dysregulation associated with dietary obesity.45 In summary, these studies highlight the multiple cellular substrates and their potential cross-talk for central IKKβ proinflammatory pathway to cause obesity and related metabolic diseases.
IKKβ/NF-κB also mediates cachectic inflammation, as suggested by a study262 showing that in LPS-induced bacterial infection and in HIV-1 transactivator protein (Tat)-induced viral infection, NF-κB activation in POMC neurons was essential for hypothalamic expression of inflammatory cytokines and increased POMC expression, which accounted for the anorexic effects of LPS and Tat. In another cachexia model induced by central administration of high dose TNF-α, activation of hypothalamic NF-κB was observed and accompanied by a 25% reduction of 12-hr food intake as well as increased body temperature and respiratory quotient.82 Therefore, targeting central IKKβ/NF-κB may be significant not only for the control of metabolic syndrome, but also for the intervention of cachectic syndrome associated with AIDS, cancer, and end-stage heart and kidney diseases.
JNK1 and body weight
As another class of canonical cellular proinflammatory kinases, JNKs are activated by inflammatory cytokine TNF-α and free fatty acids in cultured cells326–328 and by HFD feeding in metabolic tissues of animals.181 Global JNK1 knockout mice were protected against HFD-induced obesity, and reduction of weight gain was specifically caused by decreased adipocyte size and total adiposity, rather than changes of food intake or lipid metabolism.181 Consistently, JNK1 ablation significantly attenuated the genetic obesity of ob/ob mice.181 Tissue-specific JNK1 knockout studies showed that JNK1 deficiency in peripheral metabolic sites, such as fat, liver or muscle could not recapitulate the anti-obesity effect of global JNK1 knockout.182–184 However, brain-specific JNK1 deletion is sufficient to prevent HFD-induced weight gain as global JNK1 knockout.75 Sabio et al. found that brain-specific JNK1 knockout significantly improved central insulin sensitivity, leading to decreased food intake, increased physical activity, increased energy expenditure and reduced adiposity.75 Belgardt et al. similarly reported that mice with brain-specific JNK1 knockout mice showed improved central insulin sensitivity and glucose metabolism together with reduced body weight and epididymal fat mass, although the total body fat composition was not different from wild-type controls under the same HFD-feeding condition.76 Both studies found that HPT axis activity was increased by brain JNK1 ablation,75,76 yet such connection to HPT axis has not been reported in IKKβ/NF-κB–mediated brain inflammation. With regard to other types of metabolic inflammation stimuli, there have been no studies that directly linked central JNK activation to ER stress, oxidative stress or autophagy defect in the context of obesity. Regardless, ER stress was shown to induce JNK activation in adipose tissues,329 and mitochondrial oxidative stress was shown to activate JNK pathway in pancreatic β cells and liver cells.330 Finally, similar to IKKβ/NF-κB pathway, JNK activation may be involved in central cachectic inflammation, as suggested by a study showing that central administration of TNF-α at high dose activated canonical inflammatory pathways including JNK.82
SOCS3 and body weight
As an important negative regulator of leptin and insulin signaling, SOCS3 is closely related to central body weight dysregulation via central leptin and insulin resistance in hypothalamic nutrient-sensing neurons.31,65,66,77,198–200 Mice with haploinsufficiency of SOCS3 exhibit increased feeding suppression and weight loss in response to leptin administration, and are resistant to HFD-induced leptin resistance and associated metabolic complications.198 Further, brain neuron-specific SOCS3 deficiency similarly results in enhanced leptin sensitivity and protections against HFD-induced leptin or insulin resistance and weight gain, indicating that SOCS3 controls body weight and energy balance through brain neurons.199 Zhang et al. mechanistically demonstrated that overnutrition induces SOCS3 expression in the hypothalamus in a IKKβ/NF-κB–dependent manner, and this SOCS3 upregulation mediates the effects of IKKβ/NF-κB activation in promoting feeding and weight gain.66 Since hypothalamic IKKβ/NF-κB can be activated by TLR signaling,67–69 cytokine signaling,159 intracellular ER stress66 and autophagy defect,72 SOCS3 could act as a universal downstream player—an hypothesis that remains to be experimentally tested. Hypothalamic neurons that mediate SOCS3 action in body weight dysregulation should include AGRP neurons, since IKKβ inhibition in AGRP neurons can reduce the extent of overnutrition-induced obesity.66 POMC neurons have been experimentally targeted by two studies, which showed that SOCS3 upregulation in POMC neurons induces central leptin resistance and obesity,200 and SOCS3 deletion in POMC neurons leads to enhanced leptin sensitivity and reduced weight gain on a HFD.77 Interestingly, POMC neuron-specific IKKβ knockout was insufficient to reduce HFD-induced obesity,71 indicating that the upstream induction of SOCS3 in POMC neurons may involve synergistic actions between IKKβ/NF-κB and other pathway(s).
PTP1B and body weight
PTP1B is also a negative regulator of insulin and leptin signaling,204–207 and hitherto plays a role in central body weight dysregulation. Whole body PTP1B-deficient mice showed increased energy expenditure and decreased adiposity, and were protected from HFD-induced obesity.204,205 Moreover, brain207 or hypothalamic331,332 PTP1B knockout recapitulates the anti-obesity effects of whole-body PTP1B knockout, indicating that PTP1B acts in the hypothalamus to mediate overnutrition-induced central obesity. In support of this model, HFD feeding was shown to increase PTP1B expression in the hypothalamus in mice.210 In exploring the potential inflammatory basis of overnutrition-induced PTP1B expression, inflammatory cytokine treatment was shown to induce PTP1B expression in hypothalamic arcuate nucleus as well as metabolic tissues in vivo.210 Taken together, these studies suggest a pathway where overnutrition-induced hypothalamic inflammation upregulates PTP1B expression to promote obesity development.
TLRs and body weight
Known to be activated by lipid ligands, TLR2 and TLR4 are particularly implicated in metabolic inflammation and body weight dysregulation induced by lipid excess.68,69,142–144,146–153 TLR2 inhibition in skeletal muscle or fat tissue was found to improve tissue insulin sensitivity in association with a reduction of NF-κB activity in these tissues.152 More recently, whole-body TLR2 knockout mice were reported to exhibit strong protection against HFD-induced obesity, and this effect was associated with inflammatory reduction.152,153 However, the role of central TLR2 signaling in inducing body weight dysregulation or obesity has not been studied. TLR4 is more intensively studied with regard to overnutrition-related body weight control.68,69,144,146–151 Central knockout of TLR4 signaling adaptor MyD88 in mice increases central leptin sensitivity, decreases food intake, increases energy expenditure, and prevents weight gain or systemic insulin resistance against lipid overnutrition.68 Although MyD88 also facilitates signaling of other membrane receptors such as IL-1,193,194 this study highly indicates that central activation of TLR4 may be an important pathogenic link in metabolic syndrome related to lipid overnutrition. The anti-obesity effect of TLR4 inhibition was also supported by studies using a mouse line with a loss-of-function mutation (proline712 to histidine) in TLR4.150,151 Under HFD-feeding condition, these mutant mice showed decreased adiposity and increased basal metabolic rate, and were protected from dietary obesity,150 though another group only observed decreased food intake without significant anti-obesity effect.151 Whole-body TLR4 knockout was also reported to have no protection against HFD-induced obesity in CB57BL/6 mice.149 To clarify these inconsistencies, mouse models with brain-specific TLR4 inhibition need to be developed and analyzed for energy metabolic phenotypes. On the other hand, appropriate levels of TLR-mediated lipid signaling under physiological conditions may be required to maintain normal body weight and energy homeostasis.46,333–336 Evidence has shown that normalizing the levels of long-chain fatty acyl-CoA in the hypothalamus can correct energy imbalance in overfed rats.333 Also, hypothalamic inactivation of fatty acid synthase protects against diet-induced obesity,334 possibly through increasing cellular levels of malonyl-CoA which signals energy surfeit to the brain, leading to feeding reduction.46 Recently, brain-specific knockouts of lipoprotein receptor-related protein 1 (LRP1), which mediates cell surface lipid signaling335 or lipoprotein lipase, which releases free fatty acids from circulating triglyceride-rich lipoproteins,336 were both shown to cause obesity. While these studies suggest that normal central lipid sensing can help control overfeeding and weight gain, the physiological and pathological connections between central lipid sensing, TLR activation and downstream inflammatory pathways remain to be investigated.
Cytokine signaling and body weight
The effects of cytokine receptor-mediated hypothalamic inflammation on body weight are complicated and could have opposite directions depending on obesogenic or cachectic disease contexts. In overnutrition-induced hypothalamic inflammation, activation of proinflammatory pathways, such as IKKβ/NF-κB can induce expression of proinflammatory cytokines in both affected neurons and nearby glial cells via paracrine mechanisms. Besides, overnutrition-related diseases like obesity and T2D are accompanied by a low-grade elevation of circulating cytokines, which may be transported to MBH (the BBB is relatively leaky in this hypothalamic region) and activate low-grade inflammation via cytokine receptor signaling. By contrast, cachectic diseases are often associated with abundant circulating cytokines,20,22–25 which are likely to induce robust inflammatory reactions in the hypothalamus, via direct transfer of excessive cytokines across BBB or via secondary triggering of inflammation amplifier PGE2 in BBB.170,171 In support of the notion that the intensity of inflammatory cytokines determines the physiological outcome of positive versus negative energy balance, central administration of low-dose TNF-α reproduced the obesogenic effects of metabolic inflammation,82,155 while high-dose of central TNF-α administration caused cachectic effects.82 This disease model is also supported by mouse models with genetic knockout of TNF-α156,157 or TNF-α receptor 1 (TNFR1),155,158 both of which showed counteraction against diet-induced obesity. Central administration of a few other cytokines at high doses each induced anorexia, increased respiratory quotient and fever, resembling the pathophysiological changes in cachectic diseases.83–90
It also seems that overnutrition-induced inflammation versus cachectic inflammation can differentially impact energy intake versus expenditure to affect body weight. Increased energy intake (overfeeding) is an immediate and predominant factor for positive energy balance in overnutrition-induced brain inflammation.66–69 Yet reduction of energy expenditure becomes apparent only after prolonged overnutrition as seen in HFD-induced obesity or genetically obese ob/ob mice,337 which presumably reflects a secondary effect of increased adiposity. By sharp contrast, cachectic inflammation can potently increase basal energy expenditure (mainly in the forms of basal metabolic rate and thermogenesis) to induce negative energy balance. Cachectic changes induced by systemic inflammation due to NF-κB or JNK activation can occur without food intake reduction.81,338,339 In fact, overeating was observed in some cachexia mouse models, which may reflect an adaptive change in response to drastically elevated energy expenditure, or it could be that a subset of inflammatory factors induced isolated overnutrition-like metabolic effects. Further suggested by recent literature, anabolic versus catabolic effects of inflammation might differentially engage hypothalamic neurons. Ablation of IKKβ in AGRP neurons was shown to protect mice from HFD-induced hyperphagia and obesity,66 yet ablation of IKKβ in POMC neurons in mice did not prevent HFD-induced obesity.71 An additional study further linked IKKβ/NF-κB in POMC neurons to the cachectic effects of LPS-induced infection, and ablation of IKKβ in POMC neurons reduced the cachectic effects.262 Notably, sympathetic upregulation underlies both the cachectic action of POMC derived neuropeptide α-MSH.24 Taken together, AGRP neurons are prone to mediate overnutrition-induced inflammation and ensuing neuroendocrine dysregulation to cause obesity, while inflammation in POMC neurons is likely to induce sympathetic excitation to cause cachectic effects.
Regarding genetic knockout models that targeted cytokines or their receptors, global knockout of IL-1, IL-1, receptor or IL-6 can result in mature-onset obesity,164–166 clearly pointing to the catabolic actions of these cytokines. These studies were in line with the pharmacologic experiments demonstrating the central cachectic actions of IL-1β83,84 and IL-6.85 The late development of obesity in IL-1 and IL-6 signaling deficient mice may be caused by cumulative small positive energy balance that resulted from reduced energy expenditure, since increase of food intake was not evident until 10–13 months of age, which was several months later than the onset of obesity.165,166 These observations in general support the view that cytokine-related inflammation primarily increases energy expenditure to affect body weight. As discussed above, sympathetic activation represents an underlying basis, which was suggested by a study showing that increased sympathetic tone mediated the weight loss effect of IL-1 receptor antagonist knockout (an IL-1 signaling gain-of-function model).340 In this context, anti-inflammatory approaches targeting cytokine signaling can be valuable for the treatment of cachectic syndrome. On the other hand, although cachectic cytokine signaling can decrease adiposity,341,342 taking advantage of this pathological action of these cytokines to treat obesity seems dangerous, as the overall unhealthy impacts on whole body can be substantial and outweigh the benefit of obesity control.
Impact of hypothalamic inflammation on glucose homeostasis
The body’s ability to maintain stable circulating glucose levels is crucial for the normal functioning of organs and tissues. Glucose homeostasis is achieved through coordinated actions between multiple organs including brain, liver, pancreas, adipocytes, and skeletal muscle.343 For instance, in response to an increase of glucose level, pancreatic β-cells secrete insulin to promote tissue glucose uptake, while inhibit hepatic glucose production, whereas a decrease in glucose level stimulates counter-regulatory hormones, such as glucagon, corticosteroids, and catecholamines to promote hepatic glucose production. Research during the recent decade has appreciated that these activities are also monitored by regulatory neurons in the hypothalamus, which can sense metabolic signals to regulate glucose metabolism in peripheral tissues39,49,50,344–346 (Fig. 2). Such metabolic signals include circulating leptin, insulin, gut hormones, and nutrients, all of which can act on hypothalamus neurons to control peripheral glucose homeostasis. For example, insulin has central hypoglycemic actions via suppressing hepatic glucose production and promoting peripheral glucose uptake;347 while central administration of leptin rescues the insulin resistance and diabetic phenotype of lipodystrophic mice.348 Neurons in the hypothalamus, such as AGRP neurons, POMC neurons, and SF1 neurons are all responsive to leptin and insulin in exerting central regulation of peripheral glucose homeostasis.79,284,285,349 These neurons can direct downstream neurons to regulate several autonomic sites in other brain regions (e.g., DMX and NTS of the brain stem), which directly control glucose metabolism and related insulin signaling in peripheral organs.
Recent research has elucidated that hypothalamic regulation of systemic glucose homeostasis can be independent of hypothalamic control of feeding and body weight.39,49,50 For instance, leptin has been shown to act in hypothalamic arcuate nucleus to improve glucose homeostasis independently of its effects on body weight or food intake in mice350 and rats.351 A recent study further pinpointed this body weight- and food intake-independent regulation of glucose homeostasis by leptin (and insulin as well) to POMC neurons of the arcuate nucleus.349 Similarly, hypothalamic dysregulations that underlie weight gain versus glucose intolerance can be separated, as these two disorders are not necessarily linked together in patients and animal models.352–356 Systemic insulin resistance and glucose intolerance are core components of metabolic syndrome which form a critical pathophysiological basis for T2D. Recently, body weight-independent hypothalamic regulation of glucose homeostasis has become an emerging research topic. A critical understanding is that, since central leptin and insulin resistance can use body weight-independent mechanism to affect systemic glucose metabolism, hypothalamic inflammation may have similar effects through induction of central leptin and insulin resistance. However, it is often a technical challenge to mechanistically separate glucose metabolic abnormalities from weight changes. Nonetheless, clinical disease or animal research models have shown that IKKβ and JNK can dampen leptin or insulin signaling by antagonizing PI3K pathway357–360 to cause body weight-independent glucose dysregulation.186,361,362 Upon intracerebroventricular injection of low-dose TNF-α to mimic overnutrition-induced brain inflammation, mice showed activation of hypothalamic inflammatory signaling mediated by JNK, p38, and NF-κB, and developed insulin insensitivity and reduced thermogenesis without significant body weight changes.82,155 Moreover, acute induction of brain ER stress in mice can cause glucose intolerance and systemic and hepatic insulin resistance in the absence of body weight change, and these effects are reversible by hypothalamic NF-κB inhibition.70 As an NF-κB target in metabolic inflammation, SOCS3 was also studied in this regard. Chow-fed mice with POMC neuron-specific SOCS3 knockout exhibited improvement of glucose homeostasis at 23–26 weeks of age despite no effect on body weight.77 Adipose tissue-derived cytokine resistin was also found to act in the hypothalamus to impair glucose homeostasis independent of obesity.162,163 Central administration of resistin induced hepatic inflammatory response and hepatic insulin resistance independent of body weight, although it promoted expression of orexigenic NPY.162,163 The body weight-dissociable role of resistin in glucose metabolism is further supported by genetic ob/ob mice that simultaneously bear resistin haploinsufficiency. These mice showed improved glucose homeostasis in association with reduced inflammatory response in liver and muscle despite profound obesity.363
Central dysregulation of systemic glucose metabolism via hypothalamic leptin/insulin resistance should critically involve changes in various hypothalamic neuropeptides, since these neuropeptides work as effectors of hypothalamic leptin and insulin signaling. In addition, hypothalamic inflammation may impair glucose homeostasis via neurotransmitter signaling, as a recent study showed that hypothalamic leptin signaling is integrated with neurotransmitter GABA signaling in energy homeostasis control.309 Clearly the hypothalamus is critical for mediating hypoglycemia counteraction in response to hypoglycemia-induced glucagon and epinephrine release,364 and the underlying basis is essentially directed by hypothalamic glutamate and GABA signaling.285,364,365 Accordingly, defects of hypothalamic glutamatergic or GABAergic neurotransmission impair the counter-hypoglycemic function of the hypothalamus.285,366 Such hypothalamic regulation/dysregulation of glucose homeostasis is dissociable from hypothalamic control of body weight.79,286 Brain inflammation has been shown to alter glutamate and GABA signaling to affect the development of neurodegenerative disease and neuropsychiatric disorders.367–370 Prompted by the observation that the electrophysiology of hypothalamic glucose-sensing neurons is changed in obesity,365 an interesting question to test is whether obesity-induced hypothalamic inflammation mediates systemic glucose dysregulation via altering hypothalamic neurotransmitter signaling independently of body weight. If proved, such a mechanism can be applied to treat obesity-related T2D via targeting hypothalamic neurotransmitter signaling despite the obese condition.
Hypothalamic neuropeptide and neurotransmitter signaling can both relay metabolic sensing to downstream brain autonomic system which directly controls peripheral insulin actions and glucose metabolism. On this basis, brain inflammation may employ autonomic regulation to directly influence glucose homeostasis in a body weight-independent manner. For instance, brain inflammation is associated with upregulation of the sympathetic nervous system activity70–72 and downregulation of the parasympathetic nervous system activity.289 Both changes can increase hepatic glucose production and blood glucose levels while decrease systemic insulin sensitivity.343 Indeed, hypothalamic inflammation induced by brain ER stress causes glucose intolerance and systemic insulin resistance, and these effects can be reversed by sympathetic suppression.70 Furthermore, inhibition of NF-κB pathway in the hypothalamus can prevent sympathoactivation-induced hepatic insulin resistance and glucose intolerance in response to brain ER stress.70 Compared to overnutrition-induced central inflammation, classical inflammation can affect systemic glucose levels bidirectionally via the CNS-liver neural pathway, as seen in the inductions of a “flow phase” of hypoglycemia followed by an “ebb phase” of hyperglycemia in brain inflammation in response to acute injury.371,372
Impact of hypothalamic inflammation on blood pressure regulation
Obesity-related metabolic inflammation has been conclusively associated with the development of cardiovascular disorders including hypertension. As a key element of metabolic syndrome, hypertension has been extensively studied for inflammatory mechanism in the vasculature including endothelial cells, smooth muscles and resident macrophages.373–375 Meanwhile, the control of blood pressure homeostasis importantly involves neurological components, which tightly regulate many physiological factors such as cardiac output, vascular compliance and body fluid and salt balance to determine blood pressure level (Fig. 2). Research over the past decade has increasingly recognized that alteration of hypothalamic pathways, such as enhanced activation of melanocortin system due to selective leptin resistance, can contribute to the pathogenesis of obesity-related hypertension.376,377 More recently, it is revealed that obesity-related hypothalamic inflammation can induce sympathetic upregulation of blood pressure to cause hypertension,70,71,80 and IKKβ/NF-κB in hypothalamic POMC neurons was identified responsible for this central mechanism of obesity-related hypertension.71 In addition to obesity-related hypertension, other forms of hypertension could be related to NF-κB activities in the brain. For instance, brain NF-κB upregulation was reported to impair vasodilation and therefore contribute to spontaneously hypertension in rats, and indeed, central inhibition of NF-κB activity using pyrrolidine dithiocarbamate results in significant decrease of systolic blood pressure possibly via suppressing matrix metalloproteinase activities.378 In animal models with angiotensin II (ANG II)-induced hypertension, ANG II action via brain ANG II receptors was shown important.379–382 More recently, the central site for ANG II-induced is related to the hypothalamus, as binding of ANG II to ANG II receptors in hypothalamic paraventricular nucleus was found to cause cellular oxidative stress and NF-κB activation leading to sympathoexcitation and hypertension.383 Evidences that link hypothalamic inflammation to hypertension can also include the model of intracerebroventricular LPS injection, which causes both central inflammation and hypertension.384 In defining the underlying mechanism, p38 mitogen-activated protein kinase, an upstream mediator of IKKβ/NF-κB pathway, was found being activated in hypothalamic paraventricular nucleus, which promoted PGE2 production to account for sympathoexcitation and hypertension.384 Besides, hypothalamic inflammation is also found to activate central kallikrein-kinin system to induce essential hypertension.385 In summary, multiple experimental models support that central inflammation plays an important role in the pathogenesis of cardiovascular dysfunctions such as hypertension, and elucidating their underlying central molecular and cellular mechanisms represents a new and highly valuable direction to study metabolic syndrome associated cardiovascular disorders.
Clinical applications
Research in the past decades has rapidly expanded in elucidating the inflammatory mechanisms of metabolic syndrome and related diseases. In this background, significant progresses have been made in terms of applying anti-inflammatory medicines to treat clinical metabolic syndrome-related diseases such as T2D and CVD. One strategy in this endeavor relies on pharmacologic blockade of core pro-inflammatory signaling kinases using salicylate and its derivatives (e.g., aspirin and salsalate), which have inhibitory actions on cyclooxygenase-1/2 (COX-1/2) and IKKβ/NF-κB. Hundal et al. showed that two weeks of treatment with aspirin significantly improved glucose and lipid metabolism in T2D patients, resulting in an approximately 25% reduction of fasting plasma glucose, 30% reduction of insulin clearance, 50% reduction of triglycerides, and 15% reduction of total cholesterol.386 Two other clinical trials showed that salsalate effectively improved both glucose and lipid homeostasis in T2D patients.387,388 Boaz et al. performed a retrospective case-control study showing that anti-inflammatory intervention with aspirin markedly promoted weight loss in patients with T2D.389 Pro-inflammatory JNK pathway has also been clinically targeted using inhibitory peptide or synthetic small-molecule inhibitor, which effectively improved insulin action and glucose metabolism in humans.390 In addition to inhibiting inflammatory signaling, blocking inflammatory cytokines have been clinically tested for T2D treatment. For example, clinical trials showed that antagonizing inflammatory IL-1391 or TNF-α392,393 has therapeutic effects against metabolic syndrome related T2D. In parallel, while cellular ER stress and oxidative stress can mediate metabolic inflammation, pharmacologic approaches designed to reduce cellular stress proved useful for treatment of T2D and related metabolic problems.394–396 It was shown that a 4-week treatment with ER stress inhibitor tauroursodeoxycholic acid (TUDCA) increased hepatic and muscle insulin sensitivity by approximately 30% in obese men and women.397 Oxidative stress suppressant stavudine offers another candidate for T2D treatment, as it was shown to increase muscle insulin sensitivity in correlation with mitochondrial function improvement in healthy human subjects.398 Other compounds (for example, gp91 ds-tat, PR39, VAS2870, and apocynin) have been developed to inhibit oxidative stress via targeting NADPH oxidase and were demonstrated effective in preventing vascular dysfunction.399 Besides, α-lipoic acid and α-L-carnitine can both reduce oxidative stress and improve mitochondrial function; treatment with these compounds was shown to decrease systolic blood pressure in patients with coronary artery disease.400 Overall, considering the recent establishment that the hypothalamus is critical for the control of body weight and systemic glucose metabolism, some therapeutic effects of these clinical applications may take place via inhibition of central inflammation, as many of the small molecule drugs can cross BBB via diffusion or active transport. This central mechanism can be exemplified by a clinical study showing that systemic administration of candesartan reduces brain inflammation and treats eating disorder and the associated mood disorder.401
Anti-inflammatory therapy can also be a clinical strategy for treating cachexia syndrome seen in cachectic diseases. Due to the high mortality rate resulting from cachectic conditions, finding effective treatment of cachexia represents a clinical area of great concern. With the increasing recognition of the inflammatory cause of cachexia, therapeutic designs to attenuate inflammation have shown promising potentials.402–404 Among clinical studies, COX-2 inhibitors, which have good anti-inflammatory and antioxidant properties, have become an attractive class of cancer cachexia drugs.405 COX-2 inhibitor celecoxib appeared particularly successful, and has recently moved from phase II to phase III clinical trials on patients with cancer cachexia.406,407 Blocking TNF-α signaling is another anti-inflammatory strategy to treat cachexia. For examples, etanercept (a synthetic TNF-blocking agent) was shown effective in clinical trials on patients with rheumatoid arthritis-related cachexia and has been approved for clinical use,408 and TNF-α converting enzyme (TACE) inhibitors have been proposed for treating cachexia related with rheumatoid arthritis.409 Interestingly, ghrelin, which has central orexigenic and anti-inflammatory effects, is emerging as a new promising therapeutic for cachexia syndrome, and has proven effective in human studies,410,411 which supported the notion that reduction of central inflammation can underlie the anti-cachectic effects of inflammatory inhibitors.
Finally, we want to point out that there are many practical difficulties facing the application of anti-inflammatory therapies against metabolic diseases. For example, although some systemic anti-inflammatory drugs have shown positive results in clinical studies, there are other factors that prevent them from becoming widely adopted treatment options, such as serious adverse side effects associated with long-term or high-dose usage and poor BBB permeability. However, given many successful cases of CNS drug treatments in other neurological disorders, it can be expected that recruitment of new technologies will facilitate the development of new therapeutic avenues that can counteract central and hypothalamic inflammation to treat and prevent metabolic diseases.
Concluding remarks and future questions
Research over the past decade has begun to unveil the pivotal role of central neuroimmune dysregulation in the pathogenesis of metabolic diseases. Hypothalamic inflammation in particular is found to underlie the development of multiple nutritional diseases, ranging from metabolic syndrome-related diseases to chronic diseases with cachexia syndrome. A general pathogenesis model is that brain inflammation impairs the normal functioning of central metabolic regulators, leading to pathologic uncoupling of central nutrient sensing from central neural and neuroendocrine regulation of peripheral physiology. A philosophical question is, why does the hypothalamus respond to environmental changes to induce inflammation and affect energy metabolism? This question is probably better understood in terms of hypothalamic cachectic inflammation, which can raise metabolic rate and body temperature to fight against cachexia-inducing factors such as infections or cancers. Overnutrition can be regarded as another type of environmental stress and also elicits adaptive biological responses which particularly apply to the body’s nutrient sensing center—the hypothalamus. Such stress responses and related inflammatory changes might be induced in order to promote the survival of cells and organs to adverse environmental changes. For example, many gene products resulting from ER stress or NF-κB activation, such as antioxidants, chaperones and anti-apoptotic proteins, are pro-survival. From this perspective, induction of neural stress and inflammation in response to environmental challenges such as overnutrition is a cellular reaction which is conceived to be immediately protective. However, under chronic settings, these hypothalamic inflammatory changes are beyond the physiological adaptive range, leading to hypothalamic dysfunctions and whole-body metabolic derangements. The disease consequences can be cachectic or obesogenic, depending on the nature of inflammatory stimuli and hypothalamic pathways involved. To understand the complex physiological relevance of hypothalamic inflammation currently represents a burgeoning field, which is challenged by the fact that the known cellular/molecular basis of hypothalamic inflammation is limited compared to the more appreciated peripheral inflammation. For example, recent studies on hypothalamic inflammation were mostly restrained to a few types of well-characterized neurons such as POMC neurons and AGRP neurons in the arcuate nucleus and SF1 neurons in ventromedial nucleus. Even within the same neuronal subtype, such as POMC neurons or AGRP neurons, cellular heterogeneity still exists. Moreover, non-neuronal cells such as microglial cells and astrocytes can have important roles in inducing and integrating central inflammation, which increases the challenge to depict central neuroimmune mechanism of metabolic diseases. Adding to this complexity, hypothalamic inflammation by itself is rather heterogeneous in terms of inflammatory stimuli, characteristics of inflammation, signaling mediators, cellular milieu and effectors. One striking example is that central inflammation can affect a same physiological process bidirectionally, as seen in hypothalamic inflammation-induced positive or negative energy balance. Hence, although central inflammation has been causally linked to a variety of metabolic, neural, and cardiovascular diseases, it can be predicted that more remain to be discovered. Considering all these factors, current knowledge of hypothalamic inflammation in the pathogenesis of metabolic diseases may only represent a tunnel view. Regardless, aided with the conceptual and experimental frameworks that are already established, and taking advantage of the fast developing scientific technologies, the near future will likely witness significant progresses in understanding the hypothalamic inflammatory basis of metabolic diseases. At the same time, one certain endpoint is to make major breakthroughs in translating basic research findings into novel clinical treatment and prevention applications.
Acknowledgments
This article was supported by NIH grants RO1 078750 and RO1 AG031774.
References
- 1.LICHTENSTEIN AH, APPEL LJ, BRANDS M, ARNETHON MC, DANIELS S, FRANCH HA, FRANKLIN B, KRIS-ETHERTON P, HARRIS WS, HOWARD B, KARANJA N, LEFEVRE M, RUDEL L, SACKS F, VAN HL, WINSTON M, WYLIE-ROSETT J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 2.FRIEDMAN JM. Obesity: Causes and control of excess body fat. Nature. 2009;459:340–342. doi: 10.1038/459340a. [DOI] [PubMed] [Google Scholar]
- 3.ABELSON P, KENNEDY D. The obesity epidemic. Science. 2004;304:1413. doi: 10.1126/science.304.5676.1413. [DOI] [PubMed] [Google Scholar]
- 4.OGDEN CL, YANOVSKI SZ, CARROLL MD, FLEGAL KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 5.ZIMMET P, ALBERTI KG, SHAW J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Media Centre. Obesity and overweight. 2011. Ref Type: Internet Communication. [Google Scholar]
- 7.DESPRES JP, LEMIEUX I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 8.ECKEL RH, GRUNDY SM, ZIMMET PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 9.GUARENTE L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 10.DUVNJAK L, DUVNJAK M. The metabolic syndrome - an ongoing story. J Physiol Pharmacol. 2009;60(Suppl 7):19–24. [PubMed] [Google Scholar]
- 11.SEMENKOVICH CF. Insulin resistance and atherosclerosis. J Clin Invest. 2006;116:1813–1822. doi: 10.1172/JCI29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GADDAM KK, VENTURA HO, LAVIE CJ. Metabolic syndrome and heart failure--the risk, paradox, and treatment. Curr Hypertens Rep. 2011;13:142–148. doi: 10.1007/s11906-011-0179-x. [DOI] [PubMed] [Google Scholar]
- 13.RODRIGUEZ A, MULLER DC, METTER EJ, MAGGIO M, HARMAN SM, BLACKMAN MR, ANDRES R. Aging, androgens, and the metabolic syndrome in a longitudinal study of aging. J Clin Endocrinol Metab. 2007;92:3568–3572. doi: 10.1210/jc.2006-2764. [DOI] [PubMed] [Google Scholar]
- 14.MOULANA M, LIMA R, RECKELHOFF JF. Metabolic syndrome, androgens, and hypertension. Curr Hypertens Rep. 2011;13:158–162. doi: 10.1007/s11906-011-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.BAMBA V, RADER DJ. Obesity and atherogenic dyslipidemia. Gastroenterology. 2007;132:2181–2190. doi: 10.1053/j.gastro.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 16.HUMPHREYS MH. The brain splits obesity and hypertension. Nat Med. 2011;17:782–783. doi: 10.1038/nm0711-782. [DOI] [PubMed] [Google Scholar]
- 17.KAHN SE, HULL RL, UTZSCHNEIDER KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 18.PAREKH S, ANANIA FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132:2191–2207. doi: 10.1053/j.gastro.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 19.VAN GAAL LF, MERTENS IL, DE BLOCK CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 20.SPIEGELMAN BM, HOTAMISLIGIL GS. Through thick and thin: wasting, obesity, and TNF alpha. Cell. 1993;73:625–627. doi: 10.1016/0092-8674(93)90243-j. [DOI] [PubMed] [Google Scholar]
- 21.PLATA-SALAMAN CR, SONTI G, BORKOSKI JP, WILSON CD, FRENCH-MULLEN JM. Anorexia induced by chronic central administration of cytokines at estimated pathophysiological concentrations. Physiol Behav. 1996;60:867–875. [PubMed] [Google Scholar]
- 22.PLATA-SALAMAN CR. 1998 Curt P. Richter Award. Brain mechanisms in cytokine-induced anorexia. Psychoneuroendocrinology. 1999;24:25–41. doi: 10.1016/s0306-4530(98)00045-6. [DOI] [PubMed] [Google Scholar]
- 23.DANTZER R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 24.GROSSBERG AJ, SCARLETT JM, MARKS DL. Hypothalamic mechanisms in cachexia. Physiol Behav. 2010;100:478–489. doi: 10.1016/j.physbeh.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.THALER JP, CHOI SJ, SCHWARTZ MW, WISSE BE. Hypothalamic inflammation and energy homeostasis: resolving the paradox. Front Neuroendocrinol. 2010;31:79–84. doi: 10.1016/j.yfrne.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 26.INUI A. Cytokines and sickness behavior: implications from knockout animal models. Trends Immunol. 2001;22:469–473. doi: 10.1016/s1471-4906(01)01981-0. [DOI] [PubMed] [Google Scholar]
- 27.SHARPSTONE D, GAZZARD B. Gastrointestinal manifestations of HIV infection. Lancet. 1996;348:379–383. doi: 10.1016/S0140-6736(96)01034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GRUNFELD C, FEINGOLD KR. Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:329–337. doi: 10.1056/NEJM199207303270506. [DOI] [PubMed] [Google Scholar]
- 29.INUI A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J Clin. 2002;52:72–91. doi: 10.3322/canjclin.52.2.72. [DOI] [PubMed] [Google Scholar]
- 30.PITTMAN JG, COHEN P. The pathogenesis of cardiac cachexia. N Engl J Med. 1964;271:403–409. doi: 10.1056/NEJM196408202710807. [DOI] [PubMed] [Google Scholar]
- 31.MYERS MG, COWLEY MA, MUNZBERG H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 32.MURPHY KG, BLOOM SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 33.SANDOVAL D, COTA D, SEELEY RJ. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu Rev Physiol. 2008;70:513–535. doi: 10.1146/annurev.physiol.70.120806.095256. [DOI] [PubMed] [Google Scholar]
- 34.COLL AP, FAROOQI IS, O’RAHILLY S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FLIER JS. Neuroscience. Regulating energy balance: the substrate strikes back. Science. 2006;312:861–864. doi: 10.1126/science.1127971. [DOI] [PubMed] [Google Scholar]
- 36.FRIEDMAN JM. Modern science versus the stigma of obesity. Nat Med. 2004;10:563–569. doi: 10.1038/nm0604-563. [DOI] [PubMed] [Google Scholar]
- 37.MORTON GJ, CUMMINGS DE, BASKIN DG, BARSH GS, SCHWARTZ MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 38.CONE RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 39.ELMQUIST JK, COPPARI R, BALTHASAR N, ICHINOSE M, LOWELL BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. doi: 10.1002/cne.20786. [DOI] [PubMed] [Google Scholar]
- 40.KAHN BB, ALQUIER T, CARLING D, HARDIE DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 41.PISSIOS P, BRADLEY RL, MARATOS-FLIER E. Expanding the scales: The multiple roles of MCH in regulating energy balance and other biological functions. Endocr Rev. 2006;27:606–620. doi: 10.1210/er.2006-0021. [DOI] [PubMed] [Google Scholar]
- 42.BELGARDT BF, BRUNING JC. CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci. 2010;1212:97–113. doi: 10.1111/j.1749-6632.2010.05799.x. [DOI] [PubMed] [Google Scholar]
- 43.VIRTUE S, VIDAL-PUIG A. Nothing Iffy about HIF in the Hypothalamus. PLoS Biol. 2011;9:e1001116. doi: 10.1371/journal.pbio.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MEISTER B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav. 2007;92:263–271. doi: 10.1016/j.physbeh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 45.YI CX, HABEGGER KM, CHOWEN JA, STERN J, TSCHOP MH. A role for astrocytes in the central control of metabolism. Neuroendocrinology. 2011;93:143–149. doi: 10.1159/000324888. [DOI] [PubMed] [Google Scholar]
- 46.LAM TK, SCHWARTZ GJ, ROSSETTI L. Hypothalamic sensing of fatty acids. Nat Neurosci. 2005;8:579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- 47.MORRIS DL, RUI L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LAM TK, POCAI A, GUTIERREZ-JUAREZ R, OBICI S, BRYAN J, GUILAR-BRYAN L, SCHWARTZ GJ, ROSSETTI L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 49.MORTON GJ. Hypothalamic leptin regulation of energy homeostasis and glucose metabolism. J Physiol. 2007;583:437–443. doi: 10.1113/jphysiol.2007.135590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.PLUM L, BELGARDT BF, BRUNING JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006;116:1761–1766. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.HUMPHREYS MH. Cardiovascular and renal actions of melanocyte-stimulating hormone peptides. Curr Opin Nephrol Hypertens. 2007;16:32–38. doi: 10.1097/MNH.0b013e3280117fb5. [DOI] [PubMed] [Google Scholar]
- 52.PETERSEN KF, SHULMAN GI. Etiology of insulin resistance. Am J Med. 2006;119:S10–S16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LEHRKE M, LAZAR MA. Inflamed about obesity. Nat Med. 2004;10:126–127. doi: 10.1038/nm0204-126. [DOI] [PubMed] [Google Scholar]
- 54.BERG AH, SCHERER PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 55.SCHENK S, SABERI M, OLEFSKY JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.SHOELSON SE, GOLDFINE AB. Getting away from glucose: fanning the flames of obesity-induced inflammation. Nat Med. 2009;15:373–374. doi: 10.1038/nm0409-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.KAHN BB, FLIER JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.FERRANTE AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 59.SONODA J, PEI L, EVANS RM. Nuclear receptors: decoding metabolic disease. FEBS Lett. 2008;582:2–9. doi: 10.1016/j.febslet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.GREGOR MF, HOTAMISLIGIL GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 61.CAI D. NFkappaB-mediated metabolic inflammation in peripheral tissues versus central nervous system. Cell Cycle. 2009;8:2542–2548. doi: 10.4161/cc.8.16.9386. [DOI] [PubMed] [Google Scholar]
- 62.LUMENG CN, SALTIEL AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.SABIO G, DAVIS RJ. cJun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem Sci. 2010;35:490–496. doi: 10.1016/j.tibs.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.AHIMA RS, QI Y, SINGHAL NS, JACKSON MB, SCHERER PE. Brain adipocytokine action and metabolic regulation. Diabetes. 2006;55(Suppl 2):S145–S154. doi: 10.2337/db06-s018. [DOI] [PubMed] [Google Scholar]
- 65.HOWARD JK, FLIER JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab. 2006;17:365–371. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 66.ZHANG X, ZHANG G, ZHANG H, KARIN M, BAI H, CAI D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.POSEY KA, CLEGG DJ, PRINTZ RL, BYUN J, MORTON GJ, VIVEKANANDAN-GIRI A, PENNATHUR S, BASKIN DG, HEINECKE JW, WOODS SC, SCHWARTZ MW, NISWENDER KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.KLEINRIDDERS A, SCHENTEN D, KONNER AC, BELGARDT BF, MAUER J, OKAMURA T, WUNDERLICH FT, MEDZHITOV R, BRUNING JC. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MILANSKI M, DEGASPERI G, COOPE A, MORARI J, DENIS R, CINTRA DE, TSUKUMO DM, ANHE G, AMARAL ME, TAKAHASHI HK, CURI R, OLIVEIRA HC, CARVALHEIRA JB, BORDIN S, SAAD MJ, VELLOSO LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.PURKAYASTHA S, ZHANG H, ZHANG G, AHMED Z, WANG Y, CAI D. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc Natl Acad Sci U S A. 2011;108:2939–2944. doi: 10.1073/pnas.1006875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.PURKAYASTHA S, ZHANG G, CAI D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med. 2011;17:883–887. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MENG Q, CAI D. Defective Hypothalamic Autophagy Directs the Central Pathogenesis of Obesity via the IkappaB Kinase beta (IKKbeta)/NF-kappaB Pathway. J Biol Chem. 2011;286:32324–32332. doi: 10.1074/jbc.M111.254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DE SOUZA CT, ARAUJO EP, BORDIN S, ASHIMINE R, ZOLLNER RL, BOSCHERO AC, SAAD MJ, VELLOSO LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 74.OZCAN L, ERGIN AS, LU A, CHUNG J, SARKAR S, NIE D, MYERS MG, Jr, OZCAN U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 75.SABIO G, CAVANAGH-KYROS J, BARRETT T, JUNG DY, KO HJ, ONG H, MOREL C, MORA A, REILLY J, KIM JK, DAVIS RJ. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 2010;24:256–264. doi: 10.1101/gad.1878510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.BELGARDT BF, MAUER J, WUNDERLICH FT, ERNST MB, PAL M, SPOHN G, BRONNEKE HS, BRODESSER S, HAMPEL B, SCHAUSS AC, BRUNING JC. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc Natl Acad Sci U S A. 2010;107:6028–6033. doi: 10.1073/pnas.1001796107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.KIEVIT P, HOWARD JK, BADMAN MK, BALTHASAR N, COPPARI R, MORI H, LEE CE, ELMQUIST JK, YOSHIMURA A, FLIER JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4:123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 78.THALER JP, SCHWARTZ MW. Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology. 2010;151:4109–4115. doi: 10.1210/en.2010-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.ZHANG R, DHILLON H, YIN H, YOSHIMURA A, LOWELL BB, MARATOS-FLIER E, FLIER JS. Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight. Endocrinology. 2008;149:5654–5661. doi: 10.1210/en.2008-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.RAHMOUNI K, DAVISSON RL, SIGMUND CD. Inflaming hypothalamic neurons raises blood pressure. Cell Metab. 2011;14:3–4. doi: 10.1016/j.cmet.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.TANG T, ZHANG J, YIN J, STASZKIEWICZ J, GAWRONSKA-KOZAK B, JUNG DY, KO HJ, ONG H, KIM JK, MYNATT R, MARTIN RJ, KEENAN M, GAO Z, YE J. Uncoupling of inflammation and insulin resistance by NF-kappaB in transgenic mice through elevated energy expenditure. J Biol Chem. 2010;285:4637–4644. doi: 10.1074/jbc.M109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.ROMANATTO T, CESQUINI M, AMARAL ME, ROMAN EA, MORAES JC, TORSONI MA, CRUZ-NETO AP, VELLOSO LA. TNF-alpha acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient--effects on leptin and insulin signaling pathways. Peptides. 2007;28:1050–1058. doi: 10.1016/j.peptides.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 83.FINCK BN, JOHNSON RW. Anorexia, weight loss and increased plasma interleukin-6 caused by chronic intracerebroventricular infusion of interleukin-1beta in the rat. Brain Res. 1997;761:333–337. doi: 10.1016/s0006-8993(97)00451-4. [DOI] [PubMed] [Google Scholar]
- 84.LAWRENCE CB, ROTHWELL NJ. Anorexic but not pyrogenic actions of interleukin-1 are modulated by central melanocortin-3/4 receptors in the rat. J Neuroendocrinol. 2001;13:490–495. doi: 10.1046/j.1365-2826.2001.00660.x. [DOI] [PubMed] [Google Scholar]
- 85.WALLENIUS K, WALLENIUS V, SUNTER D, DICKSON SL, JANSSON JO. Intracerebroventricular interleukin-6 treatment decreases body fat in rats. Biochem Biophys Res Commun. 2002;293:560–565. doi: 10.1016/S0006-291X(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 86.ZORRILLA EP, SANCHEZ-ALAVEZ M, SUGAMA S, BRENNAN M, FERNANDEZ R, BARTFAI T, CONTI B. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci U S A. 2007;104:11097–11102. doi: 10.1073/pnas.0611523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.GROSSBERG AJ, SCARLETT JM, ZHU X, BOWE DD, BATRA AK, BRAUN TP, MARKS DL. Arcuate nucleus proopiomelanocortin neurons mediate the acute anorectic actions of leukemia inhibitory factor via gp130. Endocrinology. 2010;151:606–616. doi: 10.1210/en.2009-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.XU B, GOULDING EH, ZANG K, CEPOI D, CONE RD, JONES KR, TECOTT LH, REICHARDT LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.KOKOEVA MV, YIN H, FLIER JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 90.REED JA, CLEGG DJ, SMITH KB, TOLOD-RICHER EG, MATTER EK, PICARD LS, SEELEY RJ. GM-CSF action in the CNS decreases food intake and body weight. J Clin Invest. 2005;115:3035–3044. doi: 10.1172/JCI25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.PETERSEN KF, BEFROY D, DUFOUR S, DZIURA J, ARIYAN C, ROTHMAN DL, DIPIETRO L, CLINE GW, SHULMAN GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.LOWELL BB, SHULMAN GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 93.BONNARD C, DURAND A, PEYROL S, CHANSEAUME E, CHAUVIN MA, MORIO B, VIDAL H, RIEUSSET J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.POSPISILIK JA, KNAUF C, JOZA N, BENIT P, ORTHOFER M, CANI PD, EBERSBERGER I, NAKASHIMA T, SARAO R, NEELY G, ESTERBAUER H, KOZLOV A, KAHN CR, KROEMER G, RUSTIN P, BURCELIN R, PENNINGER JM. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell. 2007;131:476–491. doi: 10.1016/j.cell.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 95.VIANNA CR, HUNTGEBURTH M, COPPARI R, CHOI CS, LIN J, KRAUSS S, BARBATELLI G, TZAMELI I, KIM YB, CINTI S, SHULMAN GI, SPIEGELMAN BM, LOWELL BB. Hypomorphic mutation of PGC-1beta causes mitochondrial dysfunction and liver insulin resistance. Cell Metab. 2006;4:453–464. doi: 10.1016/j.cmet.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.BERNAL-MIZRACHI C, GATES AC, WENG S, IMAMURA T, KNUTSEN RH, DESANTIS P, COLEMAN T, TOWNSEND RR, MUGLIA LJ, SEMENKOVICH CF. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature. 2005;435:502–506. doi: 10.1038/nature03527. [DOI] [PubMed] [Google Scholar]
- 97.WISLOFF U, NAJJAR SM, ELLINGSEN O, HARAM PM, SWOAP S, AL-SHARE Q, FERNSTROM M, REZAEI K, LEE SJ, KOCH LG, BRITTON SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 98.REN J, PULAKAT L, WHALEY-CONNELL A, SOWERS JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl) 2010;88:993–1001. doi: 10.1007/s00109-010-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.LIN MT, BEAL MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 100.DIMAURO S, SCHON EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- 101.KELLY DP. Cell biology: Ageing theories unified. Nature. 2011;470:342–343. doi: 10.1038/nature09896. [DOI] [PubMed] [Google Scholar]
- 102.BISHOP NA, LU T, YANKNER BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.CUI L, JEONG H, BOROVECKI F, PARKHURST CN, TANESE N, KRAINC D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 104.POLYMENIDOU M, CLEVELAND DW. Motor neuron disease: The curious ways of ALS. Nature. 2008;454:284–285. doi: 10.1038/454284a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.HOEKSTRA JG, MONTINE KS, ZHANG J, MONTINE TJ. Mitochondrial therapeutics in Alzheimer’s disease and Parkinson’s disease. Alzheimers Res Ther. 2011;3:21. doi: 10.1186/alzrt83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.PARTON LE, YE CP, COPPARI R, ENRIORI PJ, CHOI B, ZHANG CY, XU C, VIANNA CR, BALTHASAR N, LEE CE, ELMQUIST JK, COWLEY MA, LOWELL BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 107.LIN J, WU PH, TARR PT, LINDENBERG KS, ST-PIERRE J, ZHANG CY, MOOTHA VK, JAGER S, VIANNA CR, REZNICK RM, CUI L, MANIERI M, DONOVAN MX, WU Z, COOPER MP, FAN MC, ROHAS LM, ZAVACKI AM, CINTI S, SHULMAN GI, LOWELL BB, KRAINC D, SPIEGELMAN BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 108.BALABAN RS, NEMOTO S, FINKEL T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 109.BOSSY-WETZEL E, TALANTOVA MV, LEE WD, SCHOLZKE MN, HARROP A, MATHEWS E, GOTZ T, HAN J, ELLISMAN MH, PERKINS GA, LIPTON SA. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron. 2004;41:351–365. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- 110.GOLDEN TR, HINERFELD DA, MELOV S. Oxidative stress and aging: beyond correlation. Aging Cell. 2002;1:117–123. doi: 10.1046/j.1474-9728.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- 111.MELOV S. Mitochondrial oxidative stress. Physiologic consequences and potential for a role in aging. Ann N Y Acad Sci. 2000;908:219–225. doi: 10.1111/j.1749-6632.2000.tb06649.x. [DOI] [PubMed] [Google Scholar]
- 112.MELOV S. Modeling mitochondrial function in aging neurons. Trends Neurosci. 2004;27:601–606. doi: 10.1016/j.tins.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 113.GOLDEN TR, MELOV S. Mitochondrial DNA mutations, oxidative stress, and aging. Mech Ageing Dev. 2001;122:1577–1589. doi: 10.1016/s0047-6374(01)00288-3. [DOI] [PubMed] [Google Scholar]
- 114.MATTSON MP, MAGNUS T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.GODBOUT JP, JOHNSON RW. Interleukin-6 in the aging brain. J Neuroimmunol. 2004;147:141–144. doi: 10.1016/j.jneuroim.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 116.COYLE JT, PUTTFARCKEN P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 117.ZHOU R, YAZDI AS, MENU P, TSCHOPP J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 118.PAHL HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 119.BARGER JL, WALFORD RL, WEINDRUCH R. The retardation of aging by caloric restriction: its significance in the transgenic era. Exp Gerontol. 2003;38:1343–1351. doi: 10.1016/j.exger.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 120.ZHENG J, MUTCHERSON R, HELFAND SL. Calorie restriction delays lipid oxidative damage in Drosophila melanogaster. Aging Cell. 2005;4:209–216. doi: 10.1111/j.1474-9726.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 121.HOTAMISLIGIL GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.DENIS RG, ARRUDA AP, ROMANATTO T, MILANSKI M, COOPE A, SOLON C, RAZOLLI DS, VELLOSO LA. TNF-alpha transiently induces endoplasmic reticulum stress and an incomplete unfolded protein response in the hypothalamus. Neuroscience. 2010;170:1035–1044. doi: 10.1016/j.neuroscience.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 123.WON JC, JANG PG, NAMKOONG C, KOH EH, KIM SK, PARK JY, LEE KU, KIM MS. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring) 2009;17:1861–1865. doi: 10.1038/oby.2009.194. [DOI] [PubMed] [Google Scholar]
- 124.CULLINAN SB, DIEHL JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol. 2006;38:317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 125.YORIMITSU T, KLIONSKY DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.LEVINE B, KROEMER G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.KOMATSU M, WAGURI S, UENO T, IWATA J, MURATA S, TANIDA I, EZAKI J, MIZUSHIMA N, OHSUMI Y, UCHIYAMA Y, KOMINAMI E, TANAKA K, CHIBA T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.YANG L, LI P, FU S, CALAY ES, HOTAMISLIGIL GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.MASIERO E, AGATEA L, MAMMUCARI C, BLAAUW B, LORO E, KOMATSU M, METZGER D, REGGIANI C, SCHIAFFINO S, SANDRI M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 130.FUJITANI Y, KAWAMORI R, WATADA H. The role of autophagy in pancreatic beta-cell and diabetes. Autophagy. 2009;5:280–282. doi: 10.4161/auto.5.2.7656. [DOI] [PubMed] [Google Scholar]
- 131.EBATO C, UCHIDA T, ARAKAWA M, KOMATSU M, UENO T, KOMIYA K, AZUMA K, HIROSE T, TANAKA K, KOMINAMI E, KAWAMORI R, FUJITANI Y, WATADA H. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8:325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 132.KIM JS, NITTA T, MOHUCZY D, O’MALLEY KA, MOLDAWER LL, DUNN WA, Jr, BEHRNS KE. Impaired autophagy: A mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology. 2008;47:1725–1736. doi: 10.1002/hep.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.KOMATSU M, WAGURI S, CHIBA T, MURATA S, IWATA J, TANIDA I, UENO T, KOIKE M, UCHIYAMA Y, KOMINAMI E, TANAKA K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 134.HARA T, NAKAMURA K, MATSUI M, YAMAMOTO A, NAKAHARA Y, SUZUKI-MIGISHIMA R, YOKOYAMA M, MISHIMA K, SAITO I, OKANO H, MIZUSHIMA N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 135.JIN S, WHITE E. Tumor suppression by autophagy through the management of metabolic stress. Autophagy. 2008;4:563–566. [PMC free article] [PubMed] [Google Scholar]
- 136.MARTINEZ-VICENTE M, SOVAK G, CUERVO AM. Protein degradation and aging. Exp Gerontol. 2005;40:622–633. doi: 10.1016/j.exger.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 137.SZEGEZDI E, MACDONALD DC, NI CT, GUPTA S, SAMALI A. Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol. 2009;296:C941–C953. doi: 10.1152/ajpcell.00612.2008. [DOI] [PubMed] [Google Scholar]
- 138.MATUS S, LISBONA F, TORRES M, LEON C, THIELEN P, HETZ C. The stress rheostat: an interplay between the unfolded protein response (UPR) and autophagy in neurodegeneration. Curr Mol Med. 2008;8:157–172. doi: 10.2174/156652408784221324. [DOI] [PubMed] [Google Scholar]
- 139.BUTLER D, BAHR BA. Oxidative stress and lysosomes: CNS-related consequences and implications for lysosomal enhancement strategies and induction of autophagy. Antioxid Redox Signal. 2006;8:185–196. doi: 10.1089/ars.2006.8.185. [DOI] [PubMed] [Google Scholar]
- 140.BEUTLER B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 141.TAKEUCHI O, AKIRA S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 142.KONNER AC, BRUNING JC. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol Metab. 2011;22:16–23. doi: 10.1016/j.tem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 143.FESSLER MB, RUDEL LL, BROWN JM. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr Opin Lipidol. 2009;20:379–385. doi: 10.1097/MOL.0b013e32832fa5c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.POULAIN-GODEFROY O, LE BO, PLANCQ P, LECOEUR C, PATTOU F, FRUHBECK G, FROGUEL P. Inflammatory role of Toll-like receptors in human and murine adipose tissue. Mediators Inflamm. 2010;2010:823486. doi: 10.1155/2010/823486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.NGUYEN MT, FAVELYUKIS S, NGUYEN AK, REICHART D, SCOTT PA, JENN A, LIU-BRYAN R, GLASS CK, NEELS JG, OLEFSKY JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 146.REYNA SM, GHOSH S, TANTIWONG P, MEKA CS, EAGAN P, JENKINSON CP, CERSOSIMO E, DEFRONZO RA, COLETTA DK, SRIWIJITKAMOL A, MUSI N. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57:2595–2602. doi: 10.2337/db08-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.FRISARD MI, MCMILLAN RP, MARCHAND J, WAHLBERG KA, WU Y, VOELKER KA, HEILBRONN L, HAYNIE K, MUOIO B, LI L, HULVER MW. Toll-like receptor 4 modulates skeletal muscle substrate metabolism. Am J Physiol Endocrinol Metab. 2010;298:E988–E998. doi: 10.1152/ajpendo.00307.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.SABERI M, WOODS NB, DE LC, SCHENK S, LU JC, BANDYOPADHYAY G, VERMA IM, OLEFSKY JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.SHI H, KOKOEVA MV, INOUYE K, TZAMELI I, YIN H, FLIER JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.TSUKUMO DM, CARVALHO-FILHO MA, CARVALHEIRA JB, PRADA PO, HIRABARA SM, SCHENKA AA, ARAUJO EP, VASSALLO J, CURI R, VELLOSO LA, SAAD MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 151.POGGI M, BASTELICA D, GUAL P, IGLESIAS MA, GREMEAUX T, KNAUF C, PEIRETTI F, VERDIER M, JUHAN-VAGUE I, TANTI JF, BURCELIN R, ALESSI MC. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 2007;50:1267–1276. doi: 10.1007/s00125-007-0654-8. [DOI] [PubMed] [Google Scholar]
- 152.CARICILLI AM, NASCIMENTO PH, PAULI JR, TSUKUMO DM, VELLOSO LA, CARVALHEIRA JB, SAAD MJ. Inhibition of toll-like receptor 2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J Endocrinol. 2008;199:399–406. doi: 10.1677/JOE-08-0354. [DOI] [PubMed] [Google Scholar]
- 153.HIMES RW, SMITH CW. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 2010;24:731–739. doi: 10.1096/fj.09-141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.MORAES JC, COOPE A, MORARI J, CINTRA DE, ROMAN EA, PAULI JR, ROMANATTO T, CARVALHEIRA JB, OLIVEIRA AL, SAAD MJ, VELLOSO LA. High-fat diet induces apoptosis of hypothalamic neurons. PLoS One. 2009;4:e5045. doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.ARRUDA AP, MILANSKI M, COOPE A, TORSONI AS, ROPELLE E, CARVALHO DP, CARVALHEIRA JB, VELLOSO LA. Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion. Endocrinology. 2011;152:1314–1326. doi: 10.1210/en.2010-0659. [DOI] [PubMed] [Google Scholar]
- 156.VENTRE J, DOEBBER T, WU M, MACNAUL K, STEVENS K, PASPARAKIS M, KOLLIAS G, MOLLER DE. Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes. 1997;46:1526–1531. doi: 10.2337/diab.46.9.1526. [DOI] [PubMed] [Google Scholar]
- 157.UYSAL KT, WIESBROCK SM, MARINO MW, HOTAMISLIGIL GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 158.ROMANATTO T, ROMAN EA, ARRUDA AP, DENIS RG, SOLON C, MILANSKI M, MORAES JC, BONFLEUR ML, DEGASPERI GR, PICARDI PK, HIRABARA S, BOSCHERO AC, CURI R, VELLOSO LA. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J Biol Chem. 2009;284:36213–36222. doi: 10.1074/jbc.M109.030874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 159.OH I, THALER JP, OGIMOTO K, WISSE BE, MORTON GJ, SCHWARTZ MW. Central administration of interleukin-4 exacerbates hypothalamic inflammation and weight gain during high-fat feeding. Am J Physiol Endocrinol Metab. 2010;299:E47–E53. doi: 10.1152/ajpendo.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.ROPELLE ER, FLORES MB, CINTRA DE, ROCHA GZ, PAULI JR, MORARI J, DE SOUZA CT, MORAES JC, PRADA PO, GUADAGNINI D, MARIN RM, OLIVEIRA AG, AUGUSTO TM, CARVALHO HF, VELLOSO LA, SAAD MJ, CARVALHEIRA JB. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS Biol. 2010;8:e1000465. doi: 10.1371/journal.pbio.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 161.AHIMA RS, LAZAR MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol. 2008;22:1023–1031. doi: 10.1210/me.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.MUSE ED, LAM TK, SCHERER PE, ROSSETTI L. Hypothalamic resistin induces hepatic insulin resistance. J Clin Invest. 2007;117:1670–1678. doi: 10.1172/JCI30440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.SINGHAL NS, LAZAR MA, AHIMA RS. Central resistin induces hepatic insulin resistance via neuropeptide Y. J Neurosci. 2007;27:12924–12932. doi: 10.1523/JNEUROSCI.2443-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.CHIDA D, OSAKA T, HASHIMOTO O, IWAKURA Y. Combined interleukin-6 and interleukin-1 deficiency causes obesity in young mice. Diabetes. 2006;55:971–977. doi: 10.2337/diabetes.55.04.06.db05-1250. [DOI] [PubMed] [Google Scholar]
- 165.GARCIA MC, WERNSTEDT I, BERNDTSSON A, ENGE M, BELL M, HULTGREN O, HORN M, AHREN B, ENERBACK S, OHLSSON C, WALLENIUS V, JANSSON JO. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55:1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- 166.WALLENIUS V, WALLENIUS K, AHREN B, RUDLING M, CARLSTEN H, DICKSON SL, OHLSSON C, JANSSON JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 167.KERNIE SG, LIEBL DJ, PARADA LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.BERETTA E, DHILLON H, KALRA PS, KALRA SP. Central LIF gene therapy suppresses food intake, body weight, serum leptin and insulin for extended periods. Peptides. 2002;23:975–984. doi: 10.1016/s0196-9781(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 169.SENARIS RM, TRUJILLO ML, NAVIA B, COMES G, FERRER B, GIRALT M, HIDALGO J. Interleukin-6 regulates the expression of hypothalamic neuropeptides involved in body weight in a gender-dependent way. J Neuroendocrinol. 2011;23:675–686. doi: 10.1111/j.1365-2826.2011.02158.x. [DOI] [PubMed] [Google Scholar]
- 170.EK M, ENGBLOM D, SAHA S, BLOMQVIST A, JAKOBSSON PJ, ERICSSON-DAHLSTRAND A. Inflammatory response: pathway across the blood-brain barrier. Nature. 2001;410:430–431. doi: 10.1038/35068632. [DOI] [PubMed] [Google Scholar]
- 171.BARTFAI T. Immunology. Telling the brain about pain. Nature. 2001;410:425, 427. doi: 10.1038/35068663. [DOI] [PubMed] [Google Scholar]
- 172.BAEUERLE PA, BALTIMORE D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 173.SONG YS, KIM MS, KIM HA, JUNG BI, YANG J, NARASIMHAN P, KIM GS, JUNG JE, PARK EH, CHAN PH. Oxidative stress increases phosphorylation of IkappaB kinase-alpha by enhancing NF-kappaB-inducing kinase after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1265–1274. doi: 10.1038/jcbfm.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.KORHONEN P, HELENIUS M, SALMINEN A. Age-related changes in the regulation of transcription factor NF-kappa B in rat brain. Neurosci Lett. 1997;225:61–64. doi: 10.1016/s0304-3940(97)00190-0. [DOI] [PubMed] [Google Scholar]
- 175.PASCHEN W. Dependence of vital cell function on endoplasmic reticulum calcium levels: implications for the mechanisms underlying neuronal cell injury in different pathological states. Cell Calcium. 2001;29:1–11. doi: 10.1054/ceca.2000.0162. [DOI] [PubMed] [Google Scholar]
- 176.KRIETE A, MAYO KL. Atypical pathways of NF-kappaB activation and aging. Exp Gerontol. 2009;44:250–255. doi: 10.1016/j.exger.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 177.FAROOQUI T, FAROOQUI AA. Lipid-mediated oxidative stress and inflammation in the pathogenesis of Parkinson’s disease. Parkinsons Dis. 2011;2011:247467. doi: 10.4061/2011/247467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.WESTON CR, DAVIS RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 179.VALLERIE SN, HOTAMISLIGIL GS. The role of JNK proteins in metabolism. Sci Transl Med. 2010;2:60rv5. doi: 10.1126/scitranslmed.3001007. [DOI] [PubMed] [Google Scholar]
- 180.ZHANG K, KAUFMAN RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.HIROSUMI J, TUNCMAN G, CHANG L, GORGUN CZ, UYSAL KT, MAEDA K, KARIN M, HOTAMISLIGIL GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 182.SABIO G, DAS M, MORA A, ZHANG Z, JUN JY, KO HJ, BARRETT T, KIM JK, DAVIS RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.SABIO G, CAVANAGH-KYROS J, KO HJ, JUNG DY, GRAY S, JUN JY, BARRETT T, MORA A, KIM JK, DAVIS RJ. Prevention of steatosis by hepatic JNK1. Cell Metab. 2009;10:491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.SABIO G, KENNEDY NJ, CAVANAGH-KYROS J, JUNG DY, KO HJ, ONG H, BARRETT T, KIM JK, DAVIS RJ. Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance. Mol Cell Biol. 2010;30:106–115. doi: 10.1128/MCB.01162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.VALLERIE SN, FURUHASHI M, FUCHO R, HOTAMISLIGIL GS. A predominant role for parenchymal c-Jun amino terminal kinase (JNK) in the regulation of systemic insulin sensitivity. PLoS One. 2008;3:e3151. doi: 10.1371/journal.pone.0003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.SOLINAS G, VILCU C, NEELS JG, BANDYOPADHYAY GK, LUO JL, NAUGLER W, GRIVENNIKOV S, WYNSHAW-BORIS A, SCADENG M, OLEFSKY JM, KARIN M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 187.UNGER EK, PIPER ML, OLOFSSON LE, XU AW. Functional role of c-Jun-N-terminal kinase in feeding regulation. Endocrinology. 2010;151:671–682. doi: 10.1210/en.2009-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.BOGOYEVITCH MA. The isoform-specific functions of the c-Jun N-terminal Kinases (JNKs): differences revealed by gene targeting. Bioessays. 2006;28:923–934. doi: 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- 189.BRUCKNER SR, TAMMARIELLO SP, KUAN CY, FLAVELL RA, RAKIC P, ESTUS S. JNK3 contributes to c-Jun activation and apoptosis but not oxidative stress in nerve growth factor-deprived sympathetic neurons. J Neurochem. 2001;78:298–303. doi: 10.1046/j.1471-4159.2001.00400.x. [DOI] [PubMed] [Google Scholar]
- 190.BRUCKNER SR, ESTUS S. JNK3 contributes to c-jun induction and apoptosis in 4-hydroxynonenal-treated sympathetic neurons. J Neurosci Res. 2002;70:665–670. doi: 10.1002/jnr.10437. [DOI] [PubMed] [Google Scholar]
- 191.HUNOT S, VILA M, TEISMANN P, DAVIS RJ, HIRSCH EC, PRZEDBORSKI S, RAKIC P, FLAVELL RA. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2004;101:665–670. doi: 10.1073/pnas.0307453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.KUAN CY, WHITMARSH AJ, YANG DD, LIAO G, SCHLOEMER AJ, DONG C, BAO J, BANASIAK KJ, HADDAD GG, FLAVELL RA, DAVIS RJ, RAKIC P. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci U S A. 2003;100:15184–15189. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.KAWAI T, AKIRA S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 194.BARTON GM, MEDZHITOV R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 195.GORINA R, FONT-NIEVES M, MARQUEZ-KISINOUSKY L, SANTALUCIA T, PLANAS AM. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFkappaB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59:242–255. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- 196.RUI L, YUAN M, FRANTZ D, SHOELSON S, WHITE MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 197.MUNZBERG H, FLIER JS, BJORBAEK C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 198.HOWARD JK, CAVE BJ, OKSANEN LJ, TZAMELI I, BJORBAEK C, FLIER JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004;10:734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- 199.MORI H, HANADA R, HANADA T, AKI D, MASHIMA R, NISHINAKAMURA H, TORISU T, CHIEN KR, YASUKAWA H, YOSHIMURA A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 200.REED AS, UNGER EK, OLOFSSON LE, PIPER ML, MYERS MG, Jr, XU AW. Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes. 2010;59:894–906. doi: 10.2337/db09-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.BYON JC, KUSARI AB, KUSARI J. Protein-tyrosine phosphatase-1B acts as a negative regulator of insulin signal transduction. Mol Cell Biochem. 1998;182:101–108. [PubMed] [Google Scholar]
- 202.GOLDSTEIN BJ, AHMAD F, DING W, LI PM, ZHANG WR. Regulation of the insulin signalling pathway by cellular protein-tyrosine phosphatases. Mol Cell Biochem. 1998;182:91–99. [PubMed] [Google Scholar]
- 203.REN D, LI M, DUAN C, RUI L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab. 2005;2:95–104. doi: 10.1016/j.cmet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 204.ELCHEBLY M, PAYETTE P, MICHALISZYN E, CROMLISH W, COLLINS S, LOY AL, NORMANDIN D, CHENG A, HIMMS-HAGEN J, CHAN CC, RAMACHANDRAN C, GRESSER MJ, TREMBLAY ML, KENNEDY BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 205.KLAMAN LD, BOSS O, PERONI OD, KIM JK, MARTINO JL, ZABOLOTNY JM, MOGHAL N, LUBKIN M, KIM YB, SHARPE AH, STRICKER-KRONGRAD A, SHULMAN GI, NEEL BG, KAHN BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.DELIBEGOVIC M, BENCE KK, MODY N, HONG EG, KO HJ, KIM JK, KAHN BB, NEEL BG. Improved glucose homeostasis in mice with muscle-specific deletion of protein-tyrosine phosphatase 1B. Mol Cell Biol. 2007;27:7727–7734. doi: 10.1128/MCB.00959-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.BENCE KK, DELIBEGOVIC M, XUE B, GORGUN CZ, HOTAMISLIGIL GS, NEEL BG, KAHN BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 208.DUBE N, TREMBLAY ML. Involvement of the small protein tyrosine phosphatases TC-PTP and PTP1B in signal transduction and diseases: from diabetes, obesity to cell cycle, and cancer. Biochim Biophys Acta. 2005;1754:108–117. doi: 10.1016/j.bbapap.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 209.BOURDEAU A, DUBE N, TREMBLAY ML. Cytoplasmic protein tyrosine phosphatases, regulation and function: the roles of PTP1B and TC-PTP. Curr Opin Cell Biol. 2005;17:203–209. doi: 10.1016/j.ceb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 210.ZABOLOTNY JM, KIM YB, WELSH LA, KERSHAW EE, NEEL BG, KAHN BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem. 2008;283:14230–14241. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.PICKUP JC, CROOK MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 212.MANTZOROS CS, FLIER JS. Insulin resistance: the clinical spectrum. Adv Endocrinol Metab. 1995;6:193–232. [PubMed] [Google Scholar]
- 213.LAM NT, LEWIS JT, CHEUNG AT, LUK CT, TSE J, WANG J, BRYER-ASH M, KOLLS JK, KIEFFER TJ. Leptin increases hepatic insulin sensitivity and protein tyrosine phosphatase 1B expression. Mol Endocrinol. 2004;18:1333–1345. doi: 10.1210/me.2002-0193. [DOI] [PubMed] [Google Scholar]
- 214.TAGHIBIGLOU C, RASHID-KOLVEAR F, VAN IDERSTINE SC, LE-TIEN H, FANTUS IG, LEWIS GF, ADELI K. Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J Biol Chem. 2002;277:793–803. doi: 10.1074/jbc.M106737200. [DOI] [PubMed] [Google Scholar]
- 215.OBATA T, MAEGAWA H, KASHIWAGI A, PILLAY TS, KIKKAWA R. High glucose-induced abnormal epidermal growth factor signaling. J Biochem. 1998;123:813–820. doi: 10.1093/oxfordjournals.jbchem.a022009. [DOI] [PubMed] [Google Scholar]
- 216.LOH K, FUKUSHIMA A, ZHANG X, GALIC S, BRIGGS D, ENRIORI PJ, SIMONDS S, WIEDE F, REICHENBACH A, HAUSER C, SIMS NA, BENCE KK, ZHANG S, ZHANG ZY, KAHN BB, NEEL BG, ANDREWS ZB, COWLEY MA, TIGANIS T. Elevated Hypothalamic TCPTP in Obesity Contributes to Cellular Leptin Resistance. Cell Metab. 2011;14:684–699. doi: 10.1016/j.cmet.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.LONG YC, ZIERATH JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.MILLER EJ, LI J, LENG L, MCDONALD C, ATSUMI T, BUCALA R, YOUNG LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 219.WATT MJ, DZAMKO N, THOMAS WG, ROSE-JOHN S, ERNST M, CARLING D, KEMP BE, FEBBRAIO MA, STEINBERG GR. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med. 2006;12:541–548. doi: 10.1038/nm1383. [DOI] [PubMed] [Google Scholar]
- 220.YAMAUCHI T, NIO Y, MAKI T, KOBAYASHI M, TAKAZAWA T, IWABU M, OKADA-IWABU M, KAWAMOTO S, KUBOTA N, KUBOTA T, ITO Y, KAMON J, TSUCHIDA A, KUMAGAI K, KOZONO H, HADA Y, OGATA H, TOKUYAMA K, TSUNODA M, IDE T, MURAKAMI K, AWAZAWA M, TAKAMOTO I, FROGUEL P, HARA K, TOBE K, NAGAI R, UEKI K, KADOWAKI T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 221.XUE B, KAHN BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol. 2006;574:73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.MINOKOSHI Y, ALQUIER T, FURUKAWA N, KIM YB, LEE A, XUE B, MU J, FOUFELLE F, FERRE P, BIRNBAUM MJ, STUCK BJ, KAHN BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 223.ANDERSSON U, FILIPSSON K, ABBOTT CR, WOODS A, SMITH K, BLOOM SR, CARLING D, SMALL CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 224.MARTIN TL, ALQUIER T, ASAKURA K, FURUKAWA N, PREITNER F, KAHN BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem. 2006;281:18933–18941. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- 225.CLARET M, SMITH MA, BATTERHAM RL, SELMAN C, CHOUDHURY AI, FRYER LG, CLEMENTS M, AL-QASSAB H, HEFFRON H, XU AW, SPEAKMAN JR, BARSH GS, VIOLLET B, VAULONT S, ASHFORD ML, CARLING D, WITHERS DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.KIM MS, PARK JY, NAMKOONG C, JANG PG, RYU JW, SONG HS, YUN JY, NAMGOONG IS, HA J, PARK IS, LEE IK, VIOLLET B, YOUN JH, LEE HK, LEE KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- 227.ZONCU R, EFEYAN A, SABATINI DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.POLAK P, HALL MN. mTOR and the control of whole body metabolism. Curr Opin Cell Biol. 2009;21:209–218. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 229.STEFATER MA, SEELEY RJ. Central nervous system nutrient signaling: the regulation of energy balance and the future of dietary therapies. Annu Rev Nutr. 2010;30:219–235. doi: 10.1146/annurev.nutr.012809.104723. [DOI] [PubMed] [Google Scholar]
- 230.CATANIA C, BINDER E, COTA D. mTORC1 signaling in energy balance and metabolic disease. Int J Obes (Lond) 2011;35:751–761. doi: 10.1038/ijo.2010.208. [DOI] [PubMed] [Google Scholar]
- 231.GANGLOFF YG, MUELLER M, DANN SG, SVOBODA P, STICKER M, SPETZ JF, UM SH, BROWN EJ, CEREGHINI S, THOMAS G, KOZMA SC. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.COTA D, PROULX K, SMITH KA, KOZMA SC, THOMAS G, WOODS SC, SEELEY RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 233.INHOFF T, STENGEL A, PETER L, GOEBEL M, TACHE Y, BANNERT N, WIEDENMANN B, KLAPP BF, MONNIKES H, KOBELT P. Novel insight in distribution of nesfatin-1 and phospho-mTOR in the arcuate nucleus of the hypothalamus of rats. Peptides. 2010;31:257–262. doi: 10.1016/j.peptides.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.WULLSCHLEGER S, LOEWITH R, HALL MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 235.KHAMZINA L, VEILLEUX A, BERGERON S, MARETTE A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 236.UM SH, FRIGERIO F, WATANABE M, PICARD F, JOAQUIN M, STICKER M, FUMAGALLI S, ALLEGRINI PR, KOZMA SC, AUWERX J, THOMAS G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 237.MORI H, INOKI K, MUNZBERG H, OPLAND D, FAOUZI M, VILLANUEVA EC, IKENOUE T, KWIATKOWSKI D, MACDOUGALD OA, MYERS MG, Jr, GUAN KL. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 2009;9:362–374. doi: 10.1016/j.cmet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.ONO H, POCAI A, WANG Y, SAKODA H, ASANO T, BACKER JM, SCHWARTZ GJ, ROSSETTI L. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest. 2008;118:2959–2968. doi: 10.1172/JCI34277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.CHEN C, LIU Y, LIU Y, ZHENG P. Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice. J Clin Invest. 2010;120:4091–4101. doi: 10.1172/JCI43873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.LEE DF, KUO HP, CHEN CT, HSU JM, CHOU CK, WEI Y, SUN HL, LI LY, PING B, HUANG WC, HE X, HUNG JY, LAI CC, DING Q, SU JL, YANG JY, SAHIN AA, HORTOBAGYI GN, TSAI FJ, TSAI CH, HUNG MC. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 241.WEICHHART T, COSTANTINO G, POGLITSCH M, ROSNER M, ZEYDA M, STUHLMEIER KM, KOLBE T, STULNIG TM, HORL WH, HENGSTSCHLAGER M, MULLER M, SAEMANN MD. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 242.WOUTERS BG, KORITZINSKY M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 243.DONMEZ G, GUARENTE L. Aging and disease: connections to sirtuins. Aging Cell. 2010;9:285–290. doi: 10.1111/j.1474-9726.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- 244.HAIGIS MC, SINCLAIR DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.GUARENTE L. Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med. 2011;364:2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- 246.BURNETT C, VALENTINI S, CABREIRO F, GOSS M, SOMOGYVARI M, PIPER MD, HODDINOTT M, SUTPHIN GL, LEKO V, MCELWEE JJ, VAZQUEZ-MANRIQUE RP, ORFILA AM, ACKERMAN D, AU C, VINTI G, RIESEN M, HOWARD K, NERI C, BEDALOV A, KAEBERLEIN M, SOTI C, PARTRIDGE L, GEMS D. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.MILNE JC, DENU JM. The Sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol. 2008;12:11–17. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 248.PFLUGER PT, HERRANZ D, VELASCO-MIGUEL S, SERRANO M, TSCHOP MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.RAMADORI G, FUJIKAWA T, FUKUDA M, ANDERSON J, MORGAN DA, MOSTOSLAVSKY R, STUART RC, PERELLO M, VIANNA CR, NILLNI EA, RAHMOUNI K, COPPARI R. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.RAMADORI G, FUJIKAWA T, ANDERSON J, BERGLUND ED, FRAZAO R, MICHAN S, VIANNA CR, SINCLAIR DA, ELIAS CF, COPPARI R. SIRT1 Deacetylase in SF1 Neurons Protects against Metabolic Imbalance. Cell Metab. 2011;14:301–312. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.CAKIR I, PERELLO M, LANSARI O, MESSIER NJ, VASLET CA, NILLNI EA. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS One. 2009;4:e8322. doi: 10.1371/journal.pone.0008322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.DIETRICH MO, ANTUNES C, GELIANG G, LIU ZW, BOROK E, NIE Y, XU AW, SOUZA DO, GAO Q, DIANO S, GAO XB, HORVATH TL. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci. 2010;30:11815–11825. doi: 10.1523/JNEUROSCI.2234-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.RAMADORI G, GAUTRON L, FUJIKAWA T, VIANNA CR, ELMQUIST JK, COPPARI R. Central administration of resveratrol improves diet-induced diabetes. Endocrinology. 2009;150:5326–5333. doi: 10.1210/en.2009-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.KNIGHT CM, GUTIERREZ-JUAREZ R, LAM TK, RRIETA-CRUZ I, HUANG L, SCHWARTZ G, BARZILAI N, ROSSETTI L. Mediobasal Hypothalamic SIRT1 Is Essential for Resveratrol’s Effects on Insulin Action in Rats. Diabetes. 2011;60:2691–2700. doi: 10.2337/db10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.SCHWER B, SCHUMACHER B, LOMBARD DB, XIAO C, KURTEV MV, GAO J, SCHNEIDER JI, CHAI H, BRONSON RT, TSAI LH, DENG CX, ALT FW. Neural sirtuin 6 (Sirt6) ablation attenuates somatic growth and causes obesity. Proc Natl Acad Sci U S A. 2010;107:21790–21794. doi: 10.1073/pnas.1016306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.HIRSCHEY MD, SHIMAZU T, JING E, GRUETER CA, COLLINS AM, AOUIZERAT B, STANCAKOVA A, GOETZMAN E, LAM MM, SCHWER B, STEVENS RD, MUEHLBAUER MJ, KAKAR S, BASS NM, KUUSISTO J, LAAKSO M, ALT FW, NEWGARD CB, FARESE RV, Jr, KAHN CR, VERDIN E. SIRT3 Deficiency and Mitochondrial Protein Hyperacetylation Accelerate the Development of the Metabolic Syndrome. Mol Cell. 2011;44:177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.ELWARD K, GASQUE P. “Eat me” and “don’t eat me” signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Mol Immunol. 2003;40:85–94. doi: 10.1016/s0161-5890(03)00109-3. [DOI] [PubMed] [Google Scholar]
- 258.MEYLAN E, TSCHOPP J, KARIN M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 259.SCHWARTZ M, MOALEM G, LEIBOWITZ-AMIT R, COHEN IR. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci. 1999;22:295–299. doi: 10.1016/s0166-2236(99)01405-8. [DOI] [PubMed] [Google Scholar]
- 260.NGUYEN MD, JULIEN JP, RIVEST S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- 261.HAUWEL M, FURON E, CANOVA C, GRIFFITHS M, NEAL J, GASQUE P. Innate (inherent) control of brain infection, brain inflammation and brain repair: the role of microglia, astrocytes, “protective” glial stem cells and stromal ependymal cells. Brain Res Brain Res Rev. 2005;48:220–233. doi: 10.1016/j.brainresrev.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 262.JANG PG, NAMKOONG C, KANG GM, HUR MW, KIM SW, KIM GH, KANG Y, JEON MJ, KIM EH, LEE MS, KARIN M, BAIK JH, PARK JY, LEE KU, KIM YB, KIM MS. NF-kappaB activation in hypothalamic pro-opiomelanocortin neurons is essential in illness- and leptin-induced anorexia. J Biol Chem. 2010;285:9706–9715. doi: 10.1074/jbc.M109.070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.HOLLIS JH, LEMUS M, EVETTS MJ, OLDFIELD BJ. Central interleukin-10 attenuates lipopolysaccharide-induced changes in food intake, energy expenditure and hypothalamic Fos expression. Neuropharmacology. 2010;58:730–738. doi: 10.1016/j.neuropharm.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 264.TAPIA-GONZALEZ S, GARCIA-SEGURA LM, TENA-SEMPERE M, FRAGO LM, CASTELLANO JM, FUENTE-MARTIN E, GARCIA-CACERES C, ARGENTE J, CHOWEN JA. Activation of microglia in specific hypothalamic nuclei and the cerebellum of adult rats exposed to neonatal overnutrition. J Neuroendocrinol. 2011;23:365–370. doi: 10.1111/j.1365-2826.2011.02113.x. [DOI] [PubMed] [Google Scholar]
- 265.CHOI SJ, KIM F, SCHWARTZ MW, WISSE BE. Cultured hypothalamic neurons are resistant to inflammation and insulin resistance induced by saturated fatty acids. Am J Physiol Endocrinol Metab. 2010;298:E1122–E1130. doi: 10.1152/ajpendo.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.YADAV A, COLLMAN RG. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmune Pharmacol. 2009;4:430–447. doi: 10.1007/s11481-009-9174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.COLTON CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.GARDEN GA, MOLLER T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- 269.SAIJO K, COLLIER JG, LI AC, KATZENELLENBOGEN JA, GLASS CK. An ADIOL-ERbeta-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011;145:584–595. doi: 10.1016/j.cell.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.BALTHASAR N, DALGAARD LT, LEE CE, YU J, FUNAHASHI H, WILLIAMS T, FERREIRA M, TANG V, MCGOVERN RA, KENNY CD, CHRISTIANSEN LM, EDELSTEIN E, CHOI B, BOSS O, ASCHKENASI C, ZHANG CY, MOUNTJOY K, KISHI T, ELMQUIST JK, LOWELL BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 271.HUSZAR D, LYNCH CA, FAIRCHILD-HUNTRESS V, DUNMORE JH, FANG Q, BERKEMEIER LR, GU W, KESTERSON RA, BOSTON BA, CONE RD, SMITH FJ, CAMPFIELD LA, BURN P, LEE F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 272.STE ML, MIURA GI, MARSH DJ, YAGALOFF K, PALMITER RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci U S A. 2000;97:12339–12344. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.NILLNI EA. Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Front Neuroendocrinol. 2010;31:134–156. doi: 10.1016/j.yfrne.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.SPIEGELMAN BM, FLIER JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 275.SCHAEFER S, BOEGERSHAUSEN N, MEYER S, IVAN D, SCHEPELMANN K, KANN PH. Hypothalamic-pituitary insufficiency following infectious diseases of the central nervous system. Eur J Endocrinol. 2008;158:3–9. doi: 10.1530/EJE-07-0484. [DOI] [PubMed] [Google Scholar]
- 276.POWNER DJ, BOCCALANDRO C. Adrenal insufficiency following traumatic brain injury in adults. Curr Opin Crit Care. 2008;14:163–166. doi: 10.1097/MCC.0b013e3282f57528. [DOI] [PubMed] [Google Scholar]
- 277.CASTROGIOVANNI D, GAILLARD RC, GIOVAMBATTISTA A, SPINEDI E. Neuroendocrine, metabolic, and immune functions during the acute phase response of inflammatory stress in monosodium L-glutamate-damaged, hyperadipose male rat. Neuroendocrinology. 2008;88:227–234. doi: 10.1159/000124131. [DOI] [PubMed] [Google Scholar]
- 278.KYROU I, TSIGOS C. Stress hormones: physiological stress and regulation of metabolism. Curr Opin Pharmacol. 2009;9:787–793. doi: 10.1016/j.coph.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 279.ELENKOV IJ, IEZZONI DG, DALY A, HARRIS AG, CHROUSOS GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 280.VIVEROS MP, DE FONSECA FR, BERMUDEZ-SILVA FJ, MCPARTLAND JM. Critical role of the endocannabinoid system in the regulation of food intake and energy metabolism, with phylogenetic, developmental, and pathophysiological implications. Endocr Metab Immune Disord Drug Targets. 2008;8:220–230. doi: 10.2174/187153008785700082. [DOI] [PubMed] [Google Scholar]
- 281.COTA D, MARSICANO G, TSCHOP M, GRUBLER Y, FLACHSKAMM C, SCHUBERT M, AUER D, YASSOURIDIS A, THONE-REINEKE C, ORTMANN S, TOMASSONI F, CERVINO C, NISOLI E, LINTHORST AC, PASQUALI R, LUTZ B, STALLA GK, PAGOTTO U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.DUFFY D, RADER D. Endocannabinoid antagonism: blocking the excess in the treatment of high-risk abdominal obesity. Trends Cardiovasc Med. 2007;17:35–43. doi: 10.1016/j.tcm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 283.CLARET M, SMITH MA, KNAUF C, AL-QASSAB H, WOODS A, HESLEGRAVE A, PIIPARI K, EMMANUEL JJ, COLOM A, VALET P, CANI PD, BEGUM G, WHITE A, MUCKET P, PETERS M, MIZUNO K, BATTERHAM RL, GIESE KP, ASHWORTH A, BURCELIN R, ASHFORD ML, CARLING D, WITHERS DJ. Deletion of Lkb1 in pro-opiomelanocortin neurons impairs peripheral glucose homeostasis in mice. Diabetes. 2011;60:735–745. doi: 10.2337/db10-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.KONNER AC, JANOSCHEK R, PLUM L, JORDAN SD, ROTHER E, MA X, XU C, ENRIORI P, HAMPEL B, BARSH GS, KAHN CR, COWLEY MA, ASHCROFT FM, BRUNING JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 285.TONG Q, YE C, MCCRIMMON RJ, DHILLON H, CHOI B, KRAMER MD, YU J, YANG Z, CHRISTIANSEN LM, LEE CE, CHOI CS, ZIGMAN JM, SHULMAN GI, SHERWIN RS, ELMQUIST JK, LOWELL BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.PARANJAPE SA, CHAN O, ZHU W, HORBLITT AM, GRILLO C, WILSON S, REAGAN L, SHERWIN RS. Chronic Reduction of Insulin Receptors in the Ventromedial Hypothalamus Produces Glucose Intolerance and Islet Dysfunction in the Absence of Weight Gain. Am J Physiol Endocrinol Metab. 2011;301:E978–983. doi: 10.1152/ajpendo.00304.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.O’DEA K, ESLER M, LEONARD P, STOCKIGT JR, NESTEL P. Noradrenaline turnover during under- and over-eating in normal weight subjects. Metabolism. 1982;31:896–899. doi: 10.1016/0026-0495(82)90178-0. [DOI] [PubMed] [Google Scholar]
- 288.LANDSBERG L, YOUNG JB. Fasting, feeding and regulation of the sympathetic nervous system. N Engl J Med. 1978;298:1295–1301. doi: 10.1056/NEJM197806082982306. [DOI] [PubMed] [Google Scholar]
- 289.SHARSHAR T, HOPKINSON NS, ORLIKOWSKI D, ANNANE D. Science review: The brain in sepsis--culprit and victim. Crit Care. 2005;9:37–44. doi: 10.1186/cc2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.MASHALY HA, PROVENCIO JJ. Inflammation as a link between brain injury and heart damage: the model of subarachnoid hemorrhage. Cleve Clin J Med. 2008;75(Suppl 2):S26–S30. doi: 10.3949/ccjm.75.suppl_2.s26. [DOI] [PubMed] [Google Scholar]
- 291.CHEYUO C, JACOB A, WU R, ZHOU M, COPPA GF, WANG P. The parasympathetic nervous system in the quest for stroke therapeutics. J Cereb Blood Flow Metab. 2011;31:1187–1195. doi: 10.1038/jcbfm.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.CHALLIS BG, COLL AP, YEO GS, PINNOCK SB, DICKSON SL, THRESHER RR, DIXON J, ZAHN D, ROCHFORD JJ, WHITE A, OLIVER RL, MILLINGTON G, APARICIO SA, COLLEDGE WH, RUSS AP, CARLTON MB, O’RAHILLY S. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3–36) Proc Natl Acad Sci U S A. 2004;101:4695–4700. doi: 10.1073/pnas.0306931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.SAINSBURY A, SCHWARZER C, COUZENS M, FETISSOV S, FURTINGER S, JENKINS A, COX HM, SPERK G, HOKFELT T, HERZOG H. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc Natl Acad Sci U S A. 2002;99:8938–8943. doi: 10.1073/pnas.132043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.SHIMADA M, TRITOS NA, LOWELL BB, FLIER JS, MARATOS-FLIER E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 295.MARSH DJ, WEINGARTH DT, NOVI DE, CHEN HY, TRUMBAUER ME, CHEN AS, GUAN XM, JIANG MM, FENG Y, CAMACHO RE, SHEN Z, FRAZIER EG, YU H, METZGER JM, KUCA SJ, SHEARMAN LP, GOPAL-TRUTER S, MACNEIL DJ, STRACK AM, MACINTYRE DE, VAN DER PLOEG LH, QIAN S. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci U S A. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.CHEN AS, MARSH DJ, TRUMBAUER ME, FRAZIER EG, GUAN XM, YU H, ROSENBLUM CI, VONGS A, FENG Y, CAO L, METZGER JM, STRACK AM, CAMACHO RE, MELLIN TN, NUNES CN, MIN W, FISHER J, GOPAL-TRUTER S, MACINTYRE DE, CHEN HY, VAN DER PLOEG LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 297.HANADA R, TERANISHI H, PEARSON JT, KUROKAWA M, HOSODA H, FUKUSHIMA N, FUKUE Y, SERINO R, FUJIHARA H, UETA Y, IKAWA M, OKABE M, MURAKAMI N, SHIRAI M, YOSHIMATSU H, KANGAWA K, KOJIMA M. Neuromedin U has a novel anorexigenic effect independent of the leptin signaling pathway. Nat Med. 2004;10:1067–1073. doi: 10.1038/nm1106. [DOI] [PubMed] [Google Scholar]
- 298.ISHII M, FEI H, FRIEDMAN JM. Targeted disruption of GPR7, the endogenous receptor for neuropeptides B and W, leads to metabolic defects and adult-onset obesity. Proc Natl Acad Sci U S A. 2003;100:10540–10545. doi: 10.1073/pnas.1334189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.OH I, SHIMIZU H, SATOH T, OKADA S, ADACHI S, INOUE K, EGUCHI H, YAMAMOTO M, IMAKI T, HASHIMOTO K, TSUCHIYA T, MONDEN T, HORIGUCHI K, YAMADA M, MORI M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 300.BARTOLOMUCCI A, LA CG, POSSENTI R, LOCATELLI V, RIGAMONTI AE, TORSELLO A, BRESCIANI E, BULGARELLI I, RIZZI R, PAVONE F, D’AMATO FR, SEVERINI C, MIGNOGNA G, GIORGI A, SCHININA ME, ELIA G, BRANCIA C, FERRI GL, CONTI R, CIANI B, PASCUCCI T, DELL’OMO G, MULLER EE, LEVI A, MOLES A. TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity. Proc Natl Acad Sci U S A. 2006;103:14584–14589. doi: 10.1073/pnas.0606102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.KARAGIANNIDES I, TORRES D, TSENG YH, BOWE C, CARVALHO E, ESPINOZA D, POTHOULAKIS C, KOKKOTOU E. Substance P as a novel anti-obesity target. Gastroenterology. 2008;134:747–755. doi: 10.1053/j.gastro.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.DHILLON H, ZIGMAN JM, YE C, LEE CE, MCGOVERN RA, TANG V, KENNY CD, CHRISTIANSEN LM, WHITE RD, EDELSTEIN EA, COPPARI R, BALTHASAR N, COWLEY MA, CHUA S, JR, ELMQUIST JK, LOWELL BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 303.KLOCKENER T, HESS S, BELGARDT BF, PAEGER L, VERHAGEN LA, HUSCH A, SOHN JW, HAMPEL B, DHILLON H, ZIGMAN JM, LOWELL BB, WILLIAMS KW, ELMQUIST JK, HORVATH TL, KLOPPENBURG P, BRUNING JC. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat Neurosci. 2011;14:911–918. doi: 10.1038/nn.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.BRUNING JC, GAUTAM D, BURKS DJ, GILLETTE J, SCHUBERT M, ORBAN PC, KLEIN R, KRONE W, MULLER-WIELAND D, KAHN CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 305.CHOUDHURY AI, HEFFRON H, SMITH MA, AL-QASSAB H, XU AW, SELMAN C, SIMMGEN M, CLEMENTS M, CLARET M, MACCOLL G, BEDFORD DC, HISADOME K, DIAKONOV I, MOOSAJEE V, BELL JD, SPEAKMAN JR, BATTERHAM RL, BARSH GS, ASHFORD ML, WITHERS DJ. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J Clin Invest. 2005;115:940–950. doi: 10.1172/JCI24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.WILLIAMS KW, SCOTT MM, ELMQUIST JK. Modulation of the central melanocortin system by leptin, insulin, and serotonin: co-ordinated actions in a dispersed neuronal network. Eur J Pharmacol. 2011;660:2–12. doi: 10.1016/j.ejphar.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.TONG Q, YE CP, JONES JE, ELMQUIST JK, LOWELL BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.XU Y, JONES JE, KOHNO D, WILLIAMS KW, LEE CE, CHOI MJ, ANDERSON JG, HEISLER LK, ZIGMAN JM, LOWELL BB, ELMQUIST JK. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.VONG L, YE C, YANG Z, CHOI B, CHUA S, JR, LOWELL BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.HEISLER LK, JOBST EE, SUTTON GM, ZHOU L, BOROK E, THORNTON-JONES Z, LIU HY, ZIGMAN JM, BALTHASAR N, KISHI T, LEE CE, ASCHKENASI CJ, ZHANG CY, YU J, BOSS O, MOUNTJOY KG, CLIFTON PG, LOWELL BB, FRIEDMAN JM, HORVATH T, BUTLER AA, ELMQUIST JK, COWLEY MA. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 311.ROSSI J, BALTHASAR N, OLSON D, SCOTT M, BERGLUND E, LEE CE, CHOI MJ, LAUZON D, LOWELL BB, ELMQUIST JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.ENDO M, MASAKI T, SEIKE M, YOSHIMATSU H. Involvement of stomach ghrelin and hypothalamic neuropeptides in tumor necrosis factor-alpha-induced hypophagia in mice. Regul Pept. 2007;140:94–100. doi: 10.1016/j.regpep.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 313.SCARLETT JM, JOBST EE, ENRIORI PJ, BOWE DD, BATRA AK, GRANT WF, COWLEY MA, MARKS DL. Regulation of central melanocortin signaling by interleukin-1 beta. Endocrinology. 2007;148:4217–4225. doi: 10.1210/en.2007-0017. [DOI] [PubMed] [Google Scholar]
- 314.SCARLETT JM, ZHU X, ENRIORI PJ, BOWE DD, BATRA AK, LEVASSEUR PR, GRANT WF, MEGUID MM, COWLEY MA, MARKS DL. Regulation of agouti-related protein messenger ribonucleic acid transcription and peptide secretion by acute and chronic inflammation. Endocrinology. 2008;149:4837–4845. doi: 10.1210/en.2007-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.YAMAMOTO Y, TANAHASHI T, KATSUURA S, KUROKAWA K, NISHIDA K, KUWANO Y, KAWAI T, TESHIMA-KONDO S, CHIKAHISA S, TSURUO Y, SEI H, ROKUTAN K. Interleukin-18 deficiency reduces neuropeptide gene expressions in the mouse amygdala related with behavioral change. J Neuroimmunol. 2010;229:129–139. doi: 10.1016/j.jneuroim.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 316.BYERLY MS, SIMON J, LEBIHAN-DUVAL E, DUCLOS MJ, COGBURN LA, PORTER TE. Effects of BDNF, T3, and corticosterone on expression of the hypothalamic obesity gene network in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1180–R1189. doi: 10.1152/ajpregu.90813.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.PU S, DHILLON H, MOLDAWER LL, KALRA PS, KALRA SP. Neuropeptide Y counteracts the anorectic and weight reducing effects of ciliary neurotropic factor. J Neuroendocrinol. 2000;12:827–832. doi: 10.1046/j.1365-2826.2000.00526.x. [DOI] [PubMed] [Google Scholar]
- 318.VAZQUEZ MJ, GONZALEZ CR, VARELA L, LAGE R, TOVAR S, SANGIAO-ALVARELLOS S, WILLIAMS LM, VIDAL-PUIG A, NOGUEIRAS R, LOPEZ M, DIEGUEZ C. Central resistin regulates hypothalamic and peripheral lipid metabolism in a nutritional-dependent fashion. Endocrinology. 2008;149:4534–4543. doi: 10.1210/en.2007-1708. [DOI] [PubMed] [Google Scholar]
- 319.LEE GH, PROENCA R, MONTEZ JM, CARROLL KM, DARVISHZADEH JG, LEE JI, FRIEDMAN JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 320.BATES SH, STEARNS WH, DUNDON TA, SCHUBERT M, TSO AW, WANG Y, BANKS AS, LAVERY HJ, HAQ AK, MARATOS-FLIER E, NEEL BG, SCHWARTZ MW, MYERS MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 321.BALTHASAR N, COPPARI R, MCMINN J, LIU SM, LEE CE, TANG V, KENNY CD, MCGOVERN RA, CHUA SC, Jr, ELMQUIST JK, LOWELL BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 322.COHEN P, ZHAO C, CAI X, MONTEZ JM, ROHANI SC, FEINSTEIN P, MOMBAERTS P, FRIEDMAN JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.OBICI S, FENG Z, KARKANIAS G, BASKIN DG, ROSSETTI L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 324.BURKS DJ, FONT DE MJ, SCHUBERT M, WITHERS DJ, MYERS MG, TOWERY HH, ALTAMURO SL, FLINT CL, WHITE MF. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. 2000;407:377–382. doi: 10.1038/35030105. [DOI] [PubMed] [Google Scholar]
- 325.GAO Z, YIN J, ZHANG J, HE Q, MCGUINNESS OP, YE J. Inactivation of NF-kappaB p50 leads to insulin sensitization in liver through post-translational inhibition of p70S6K. J Biol Chem. 2009;284:18368–18376. doi: 10.1074/jbc.M109.007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.CHANG L, KARIN M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 327.SHIN EA, KIM KH, HAN SI, HA KS, KIM JH, KANG KI, KIM HD, KANG HS. Arachidonic acid induces the activation of the stress-activated protein kinase, membrane ruffling and H2O2 production via a small GTPase Rac1. FEBS Lett. 1999;452:355–359. doi: 10.1016/s0014-5793(99)00657-2. [DOI] [PubMed] [Google Scholar]
- 328.KYRIAKIS JM, AVRUCH J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 329.OZCAN U, CAO Q, YILMAZ E, LEE AH, IWAKOSHI NN, OZDELEN E, TUNCMAN G, GORGUN C, GLIMCHER LH, HOTAMISLIGIL GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 330.NISHIKAWA T, ARAKI E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2007;9:343–353. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 331.PICARDI PK, CALEGARI VC, PRADA PO, MORAES JC, ARAUJO E, MARCONDES MC, UENO M, CARVALHEIRA JB, VELLOSO LA, SAAD MJ. Reduction of hypothalamic protein tyrosine phosphatase improves insulin and leptin resistance in diet-induced obese rats. Endocrinology. 2008;149:3870–3880. doi: 10.1210/en.2007-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.BANNO R, ZIMMER D, DE JONGHE BC, ATIENZA M, RAK K, YANG W, BENCE KK. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest. 2010;120:720–734. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.POCAI A, LAM TK, OBICI S, GUTIERREZ-JUAREZ R, MUSE ED, ARDUINI A, ROSSETTI L. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest. 2006;116:1081–1091. doi: 10.1172/JCI26640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.CHAKRAVARTHY MV, ZHU Y, YIN L, COLEMAN T, PAPPAN KL, MARSHALL CA, MCDANIEL ML, SEMENKOVICH CF. Inactivation of hypothalamic FAS protects mice from diet-induced obesity and inflammation. J Lipid Res. 2009;50:630–640. doi: 10.1194/jlr.M800379-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.LIU Q, ZHANG J, ZERBINATTI C, ZHAN Y, KOLBER BJ, HERZ J, MUGLIA LJ, BU G. Lipoprotein receptor LRP1 regulates leptin signaling and energy homeostasis in the adult central nervous system. PLoS Biol. 2011;9:e1000575. doi: 10.1371/journal.pbio.1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.WANG H, ASTARITA G, TAUSSIG MD, BHARADWAJ KG, DIPATRIZIO NV, NAVE KA, PIOMELLI D, GOLDBERG IJ, ECKEL RH. Deficiency of lipoprotein lipase in neurons modifies the regulation of energy balance and leads to obesity. Cell Metab. 2011;13:105–113. doi: 10.1016/j.cmet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.BUTLER AA, KOZAK LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59:323–329. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.MCCLUNG JM, JUDGE AR, POWERS SK, YAN Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol. 2010;298:C542–C549. doi: 10.1152/ajpcell.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.LI H, MALHOTRA S, KUMAR A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med (Berl) 2008;86:1113–1126. doi: 10.1007/s00109-008-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.CHIDA D, HASHIMOTO O, KUWAHARA M, SAGARA H, OSAKA T, TSUBONE H, IWAKURA Y. Increased fat:carbohydrate oxidation ratio in Il1ra (−/−) mice on a high-fat diet is associated with increased sympathetic tone. Diabetologia. 2008;51:1698–1706. doi: 10.1007/s00125-008-1075-z. [DOI] [PubMed] [Google Scholar]
- 341.MATSUKI T, HORAI R, SUDO K, IWAKURA Y. IL-1 plays an important role in lipid metabolism by regulating insulin levels under physiological conditions. J Exp Med. 2003;198:877–888. doi: 10.1084/jem.20030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.SOMM E, HENRICHOT E, PERNIN A, JUGE-AUBRY CE, MUZZIN P, DAYER JM, NICKLIN MJ, MEIER CA. Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: impact on adipogenesis, food intake, and energy expenditure. Diabetes. 2005;54:3503–3509. doi: 10.2337/diabetes.54.12.3503. [DOI] [PubMed] [Google Scholar]
- 343.TIRONE TA, BRUNICARDI FC. Overview of glucose regulation. World J Surg. 2001;25:461–467. doi: 10.1007/s002680020338. [DOI] [PubMed] [Google Scholar]
- 344.HERMAN MA, KAHN BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J Clin Invest. 2006;116:1767–1775. doi: 10.1172/JCI29027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.ROSEN ED, SPIEGELMAN BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.DRUCKER DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.OBICI S, ZHANG BB, KARKANIAS G, ROSSETTI L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 348.ASILMAZ E, COHEN P, MIYAZAKI M, DOBRZYN P, UEKI K, FAYZIKHODJAEVA G, SOUKAS AA, KAHN CR, NTAMBI JM, SOCCI ND, FRIEDMAN JM. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest. 2004;113:414–424. doi: 10.1172/JCI19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.HILL JW, ELIAS CF, FUKUDA M, WILLIAMS KW, BERGLUND ED, HOLLAND WL, CHO YR, CHUANG JC, XU Y, CHOI M, LAUZON D, LEE CE, COPPARI R, RICHARDSON JA, ZIGMAN JM, CHUA S, SCHERER PE, LOWELL BB, BRUNING JC, ELMQUIST JK. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.COPPARI R, ICHINOSE M, LEE CE, PULLEN AE, KENNY CD, MCGOVERN RA, TANG V, LIU SM, LUDWIG T, CHUA SC, Jr, LOWELL BB, ELMQUIST JK. The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 351.MORTON GJ, NISWENDER KD, RHODES CJ, MYERS MG, Jr, BLEVINS JE, BASKIN DG, SCHWARTZ MW. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fa(k)/fa(k)) rats. Endocrinology. 2003;144:2016–2024. doi: 10.1210/en.2002-0115. [DOI] [PubMed] [Google Scholar]
- 352.KARCZEWSKA-KUPCZEWSKA M, STRACZKOWSKI M, ADAMSKA A, NIKOLAJUK A, OTZIOMEK E, GORSKA M, KOWALSKA I. Decreased serum brain-derived neurotrophic factor concentration in young nonobese subjects with low insulin sensitivity. Clin Biochem. 2011;44:817–820. doi: 10.1016/j.clinbiochem.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 353.VAAG A, LUND SS. Non-obese patients with type 2 diabetes and prediabetic subjects: distinct phenotypes requiring special diabetes treatment and (or) prevention? Appl Physiol Nutr Metab. 2007;32:912–920. doi: 10.1139/H07-100. [DOI] [PubMed] [Google Scholar]
- 354.PRANDO R, CHELI V, MELGA P, GIUSTI R, CIUCHI E, ODETTI P. Is type 2 diabetes a different disease in obese and nonobese patients? Diabetes Care. 1998;21:1680–1685. doi: 10.2337/diacare.21.10.1680. [DOI] [PubMed] [Google Scholar]
- 355.MORINAGA H, YAMAMOTO H, SAKATA K, FUKUDA S, ITO M, SASASE T, MIYAJIMA K, UEDA N, OHTA T, MATSUSHITA M. Characterization of hepatic glucose metabolism disorder with the progress of diabetes in male Spontaneously Diabetic Torii rats. J Vet Med Sci. 2008;70:1239–1245. doi: 10.1292/jvms.70.1239. [DOI] [PubMed] [Google Scholar]
- 356.YAGUCHI M, NAGASHIMA K, IZUMI T, OKAMOTO K. Neuropathological study of C57BL/6Akita mouse, type 2 diabetic model: enhanced expression of alphaB-crystallin in oligodendrocytes. Neuropathology. 2003;23:44–50. doi: 10.1046/j.1440-1789.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- 357.CHAPUIS N, PARK S, LEOTOING L, TAMBURINI J, VERDIER F, BARDET V, GREEN AS, WILLEMS L, AGOU F, IFRAH N, DREYFUS F, BISMUTH G, BAUD V, LACOMBE C, MAYEUX P, BOUSCARY D. IkappaB kinase overcomes PI3K/Akt and ERK/MAPK to control FOXO3a activity in acute myeloid leukemia. Blood. 2010;116:4240–4250. doi: 10.1182/blood-2009-12-260711. [DOI] [PubMed] [Google Scholar]
- 358.AIKIN R, MAYSINGER D, ROSENBERG L. Cross-talk between phosphatidylinositol 3-kinase/AKT and c-jun NH2-terminal kinase mediates survival of isolated human islets. Endocrinology. 2004;145:4522–4531. doi: 10.1210/en.2004-0488. [DOI] [PubMed] [Google Scholar]
- 359.ALLEN RT, KRUEGER KD, DHUME A, AGRAWAL DK. Sustained Akt/PKB activation and transient attenuation of c-jun N-terminal kinase in the inhibition of apoptosis by IGF-1 in vascular smooth muscle cells. Apoptosis. 2005;10:525–535. doi: 10.1007/s10495-005-1882-3. [DOI] [PubMed] [Google Scholar]
- 360.AUTRET A, MARTIN-LATIL S, BRISAC C, MOUSSON L, COLBERE-GARAPIN F, BLONDEL B. Early phosphatidylinositol 3-kinase/Akt pathway activation limits poliovirus-induced JNK-mediated cell death. J Virol. 2008;82:3796–3802. doi: 10.1128/JVI.02020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.JIANG S, MESSINA JL. Role of inhibitory {kappa}B kinase and c-Jun NH2-terminal kinase in the development of hepatic insulin resistance in critical illness diabetes. Am J Physiol Gastrointest Liver Physiol. 2011;301:G454–G463. doi: 10.1152/ajpgi.00148.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.ENJYOJI K, KOTANI K, THUKRAL C, BLUMEL B, SUN X, WU Y, IMAI M, FRIEDMAN D, CSIZMADIA E, BLEIBEL W, KAHN BB, ROBSON SC. Deletion of cd39/entpd1 results in hepatic insulin resistance. Diabetes. 2008;57:2311–2320. doi: 10.2337/db07-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.QI Y, NIE Z, LEE YS, SINGHAL NS, SCHERER PE, LAZAR MA, AHIMA RS. Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes. 2006;55:3083–3090. doi: 10.2337/db05-0615. [DOI] [PubMed] [Google Scholar]
- 364.CHAN O, ZHU W, DING Y, MCCRIMMON RJ, SHERWIN RS. Blockade of GABA(A) receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes. 2006;55:1080–1087. doi: 10.2337/diabetes.55.04.06.db05-0958. [DOI] [PubMed] [Google Scholar]
- 365.SONG Z, LEVIN BE, MCARDLE JJ, BAKHOS N, ROUTH VH. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2001;50:2673–2681. doi: 10.2337/diabetes.50.12.2673. [DOI] [PubMed] [Google Scholar]
- 366.CHAN O, PARANJAPE S, CZYZYK D, HORBLITT A, ZHU W, DING Y, FAN X, SEASHORE M, SHERWIN R. Increased GABAergic output in the ventromedial hypothalamus contributes to impaired hypoglycemic counterregulation in diabetic rats. Diabetes. 2011;60:1582–1589. doi: 10.2337/db10-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.SLUKA KA, WILLIS WD, WESTLUND KN. Inflammation-induced release of excitatory amino acids is prevented by spinal administration of a GABAA but not by a GABAB receptor antagonist in rats. J Pharmacol Exp Ther. 1994;271:76–82. [PubMed] [Google Scholar]
- 368.ANSELONI VC, GOLD MS. Inflammation-induced shift in the valence of spinal GABA-A receptor-mediated modulation of nociception in the adult rat. J Pain. 2008;9:732–738. doi: 10.1016/j.jpain.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.RINGHEIM GE, SZCZEPANIK AM. Brain inflammation, cholesterol, and glutamate as interconnected participants in the pathology of Alzheimer’s disease. Curr Pharm Des. 2006;12:719–738. doi: 10.2174/138161206775474215. [DOI] [PubMed] [Google Scholar]
- 370.MCNALLY L, BHAGWAGAR Z, HANNESTAD J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13:501–510. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- 371.BUCZKOWSKA EO. Alterations of blood glucose homeostasis during septic or injury stress--hyperglycemia. Wiad Lek. 2002;55:731–744. [PubMed] [Google Scholar]
- 372.PERSEGHIN G, REGALIA E, BATTEZZATI A, VERGANI S, PULVIRENTI A, TERRUZZI I, BARATTI D, BOZZETTI F, MAZZAFERRO V, LUZI L. Regulation of glucose homeostasis in humans with denervated livers. J Clin Invest. 1997;100:931–941. doi: 10.1172/JCI119609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.DUAN SZ, USHER MG, MORTENSEN RM. PPARs: the vasculature, inflammation and hypertension. Curr Opin Nephrol Hypertens. 2009;18:128–133. doi: 10.1097/MNH.0b013e328325803b. [DOI] [PubMed] [Google Scholar]
- 374.HARRISON DG, GUZIK TJ, GORONZY J, WEYAND C. Is hypertension an immunologic disease? Curr Cardiol Rep. 2008;10:464–469. doi: 10.1007/s11886-008-0073-6. [DOI] [PubMed] [Google Scholar]
- 375.SAVOIA C, SCHIFFRIN EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–158. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- 376.RAHMOUNI K, HAYNES WG, MARK AL. Cardiovascular and sympathetic effects of leptin. Curr Hypertens Rep. 2002;4:119–125. doi: 10.1007/s11906-002-0036-z. [DOI] [PubMed] [Google Scholar]
- 377.RAHMOUNI K, CORREIA ML, HAYNES WG, MARK AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 378.WU KI, SCHMID-SCHONBEIN GW. Nuclear factor kappa B and matrix metalloproteinase induced receptor cleavage in the spontaneously hypertensive rat. Hypertension. 2011;57:261–268. doi: 10.1161/HYPERTENSIONAHA.110.158709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.SAKAI K, SIGMUND CD. Molecular evidence of tissue renin-angiotensin systems: a focus on the brain. Curr Hypertens Rep. 2005;7:135–140. doi: 10.1007/s11906-005-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.MORIMOTO S, SIGMUND CD. Angiotensin mutant mice: a focus on the brain renin-angiotensin system. Neuropeptides. 2002;36:194–200. doi: 10.1054/npep.2002.0894. [DOI] [PubMed] [Google Scholar]
- 381.LAZARTIGUES E, DUNLAY SM, LOIHL AK, SINNAYAH P, LANG JA, ESPELUND JJ, SIGMUND CD, DAVISSON RL. Brain-selective overexpression of angiotensin (AT1) receptors causes enhanced cardiovascular sensitivity in transgenic mice. Circ Res. 2002;90:617–624. doi: 10.1161/01.res.0000012460.85923.f0. [DOI] [PubMed] [Google Scholar]
- 382.ZUCKER IH. Brain angiotensin II: new insights into its role in sympathetic regulation. Circ Res. 2002;90:503–505. doi: 10.1161/01.res.0000014287.96335.21. [DOI] [PubMed] [Google Scholar]
- 383.KANG YM, MA Y, ZHENG JP, ELKS C, SRIRAMULA S, YANG ZM, FRANCIS J. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82:503–512. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.ZHANG ZH, YU Y, WEI SG, FELDER RB. Centrally administered lipopolysaccharide elicits sympathetic excitation via NAD(P)H oxidase-dependent mitogen-activated protein kinase signaling. J Hypertens. 2010;28:806–816. doi: 10.1097/HJH.0b013e3283358b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.QADRI F, HAUSER W, JOHREN O, DOMINIAK P. Kinin B1 and B2 receptor mRNA expression in the hypothalamus of spontaneously hypertensive rats. Can J Physiol Pharmacol. 2002;80:258–263. doi: 10.1139/y02-051. [DOI] [PubMed] [Google Scholar]
- 386.HUNDAL RS, PETERSEN KF, MAYERSON AB, RANDHAWA PS, INZUCCHI S, SHOELSON SE, SHULMAN GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.GOLDFINE AB, SILVER R, ALDHAHI W, CAI D, TATRO E, LEE J, SHOELSON SE. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36–43. doi: 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.GOLDFINE AB, FONSECA V, JABLONSKI KA, PYLE L, STATEN MA, SHOELSON SE. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152:346–357. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.BOAZ M, LISY L, ZANDMAN-GODDARD G, WAINSTEIN J. The effect of anti-inflammatory (aspirin and/or statin) therapy on body weight in Type 2 diabetic individuals: EAT, a retrospective study. Diabet Med. 2009;26:708–713. doi: 10.1111/j.1464-5491.2009.02747.x. [DOI] [PubMed] [Google Scholar]
- 390.KANETO H, NAKATANI Y, MIYATSUKA T, KAWAMORI D, MATSUOKA TA, MATSUHISA M, KAJIMOTO Y, ICHIJO H, YAMASAKI Y, HORI M. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10:1128–1132. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- 391.LARSEN CM, FAULENBACH M, VAAG A, VOLUND A, EHSES JA, SEIFERT B, MANDRUP-POULSEN T, DONATH MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 392.STANLEY TL, ZANNI MV, JOHNSEN S, RASHEED S, MAKIMURA H, LEE H, KHOR VK, AHIMA RS, GRINSPOON SK. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E146–E150. doi: 10.1210/jc.2010-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.DOMINGUEZ H, STORGAARD H, RASK-MADSEN C, STEFFEN HT, IHLEMANN N, BAUNBJERG ND, SPOHR C, KOBER L, VAAG A, TORP-PEDERSEN C. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42:517–525. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- 394.LI X, ZHANG K, LI Z. Unfolded protein response in cancer: the physician’s perspective. J Hematol Oncol. 2011;4:8. doi: 10.1186/1756-8722-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.MCLAUGHLIN M, VANDENBROECK K. The endoplasmic reticulum protein folding factory and its chaperones: new targets for drug discovery? Br J Pharmacol. 2011;162:328–345. doi: 10.1111/j.1476-5381.2010.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.KIM I, XU W, REED JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 397.KARS M, YANG L, GREGOR MF, MOHAMMED BS, PIETKA TA, FINCK BN, PATTERSON BW, HORTON JD, MITTENDORFER B, HOTAMISLIGIL GS, KLEIN S. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59:1899–1905. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.FLEISCHMAN A, JOHNSEN S, SYSTROM DM, HROVAT M, FARRAR CT, FRONTERA W, FITCH K, THOMAS BJ, TORRIANI M, COTE HC, GRINSPOON SK. Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. Am J Physiol Endocrinol Metab. 2007;292:E1666–E1673. doi: 10.1152/ajpendo.00550.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.WESELER AR, BAST A. Oxidative stress and vascular function: implications for pharmacologic treatments. Curr Hypertens Rep. 2010;12:154–161. doi: 10.1007/s11906-010-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.MCMACKIN CJ, WIDLANSKY ME, HAMBURG NM, HUANG AL, WELLER S, HOLBROOK M, GOKCE N, HAGEN TM, KEANEY JF, Jr, VITA JA. Effect of combined treatment with alpha-Lipoic acid and acetyl-L-carnitine on vascular function and blood pressure in patients with coronary artery disease. J Clin Hypertens (Greenwich) 2007;9:249–255. doi: 10.1111/j.1524-6175.2007.06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.BENICKY J, SANCHEZ-LEMUS E, HONDA M, PANG T, ORECNA M, WANG J, LENG Y, CHUANG DM, SAAVEDRA JM. Angiotensin II AT(1) receptor blockade ameliorates brain inflammation. Neuropsychopharmacology. 2011;36:857–870. doi: 10.1038/npp.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.MURPHY KT, LYNCH GS. Update on emerging drugs for cancer cachexia. Expert Opin Emerg Drugs. 2009;14:619–632. doi: 10.1517/14728210903369351. [DOI] [PubMed] [Google Scholar]
- 403.MACDONALD N. Cancer cachexia and targeting chronic inflammation: a unified approach to cancer treatment and palliative/supportive care. J Support Oncol. 2007;5:157–162. [PubMed] [Google Scholar]
- 404.DEANS C, WIGMORE SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care. 2005;8:265–269. doi: 10.1097/01.mco.0000165004.93707.88. [DOI] [PubMed] [Google Scholar]
- 405.MANTOVANI G, MADEDDU C. Cyclooxygenase-2 inhibitors and antioxidants in the treatment of cachexia. Curr Opin Support Palliat Care. 2008;2:275–281. doi: 10.1097/spc.0b013e32830f47e4. [DOI] [PubMed] [Google Scholar]
- 406.MANTOVANI G, MACCIO A, MADEDDU C, SERPE R, ANTONI G, MASSA E, DESSI M, PANZONE F. Phase II nonrandomized study of the efficacy and safety of COX-2 inhibitor celecoxib on patients with cancer cachexia. J Mol Med (Berl) 2010;88:85–92. doi: 10.1007/s00109-009-0547-z. [DOI] [PubMed] [Google Scholar]
- 407.LAI V, GEORGE J, RICHEY L, KIM HJ, CANNON T, SHORES C, COUCH M. Results of a pilot study of the effects of celecoxib on cancer cachexia in patients with cancer of the head, neck, and gastrointestinal tract. Head Neck. 2008;30:67–74. doi: 10.1002/hed.20662. [DOI] [PubMed] [Google Scholar]
- 408.MARCORA SM, CHESTER KR, MITTAL G, LEMMEY AB, MADDISON PJ. Randomized phase 2 trial of anti-tumor necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. Am J Clin Nutr. 2006;84:1463–1472. doi: 10.1093/ajcn/84.6.1463. [DOI] [PubMed] [Google Scholar]
- 409.MURUMKAR PR, DASGUPTA S, CHANDANI SR, GIRIDHAR R, YADAV MR. Novel TACE inhibitors in drug discovery: a review of patented compounds. Expert Opin Ther Pat. 2010;20:31–57. doi: 10.1517/13543770903465157. [DOI] [PubMed] [Google Scholar]
- 410.ASHITANI J, MATSUMOTO N, NAKAZATO M. Ghrelin and its therapeutic potential for cachectic patients. Peptides. 2009;30:1951–1956. doi: 10.1016/j.peptides.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 411.DEBOER MD. Emergence of ghrelin as a treatment for cachexia syndromes. Nutrition. 2008;24:806–814. doi: 10.1016/j.nut.2008.06.013. [DOI] [PubMed] [Google Scholar]