Effective, curative chemotherapy has been a goal of modern cancer medicine for half a century. Many new agents have been developed, leading to modest improvements in survival of affected persons. However, few new curative treatments of advanced cancers have been developed over the past quarter century — perhaps because of the focus on how drugs kill cancer cells without a full consideration of how tumor-cell function and death influences the subsequent host response.1 It may be that the functional status of a tumor cell, when it dies, is as important as whether it dies.
The host immune system can regulate malignant progression, and immune cell and cytokine/chemokine expression within tumors are associated with clinical outcome in patients with diverse malignancies. However, chemotherapy has been thought to eliminate immune cells and thereby limit the effectiveness of immunotherapy. And the targets of emergent immune responses in patients treated with chemotherapy are not known and thus cannot be easily measured. Perhaps it is for these reasons that the intersection between effective cancer chemotherapy and the induction of host-protective immunity has received little attention.
A recent study by Michaud et al provides a welcome attempt to marry these two issues2. It shows that the process of autophagy is critical to the anti-tumor immune response elicited by dying transplantable tumor cells. It thus implicates the process of autophagy as a critical link between effective chemotherapy and the host-derived anti-cancer immune responses observed in preclinical models.
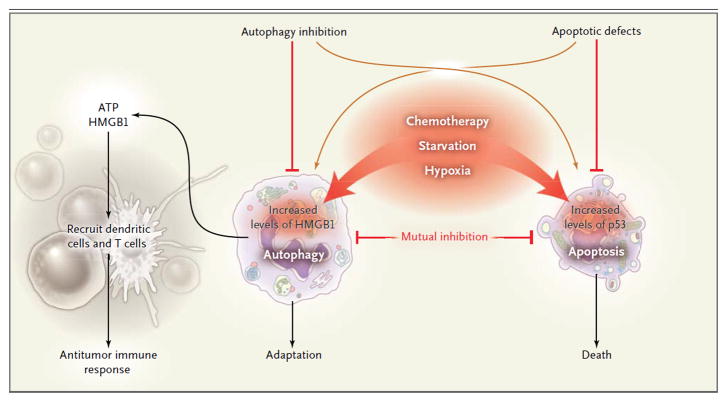
Autophagy, a form of programmed cell survival,3 means ‘self-eating’, and is one of two mutually antagonistic mechanisms by which cells respond to stress, the other being apoptosis, or programmed cell death (see Figure). While tumor cells upregulate anti-apoptotic proteins and lose the function of pro-apoptotic molecules such as p53, they maintain expression of the pro-autophagic nuclear protein, high mobility group B1 (HMGB1) as well as a capacity for enhanced autophagy. Hence, when autophagy-competent tumor cells die, immune clearance mechanisms are presented with a distinct constellation of signals to guide subsequent events.
Figure 1. Tumor Cell Autophagy and Immunity.
The induction of autophagy in cancer cells by chemotherapy can lead to induction of immunity in transplantable tumors. Heightened autophagy in tumor cells can be induced by chemotherapy, radiation therapy, or other cell stressors, including hypoxia and nutrient starvation. Autophagy involves the sequestration of intracellular contents, which are degraded after transport and fusion with a lysosome to release amino acids, nucleotides, adenosine triphosphate (ATP), and lipids that are important for anabolism and survival. The release of ATP into the extracellular milieu recruits dendritic cells, as well as CD4+ and CD8+ T-cells, resulting in antitumor immunity. Pharmacological induction of autophagy or ATP release in tumor cells during the initiation of the immune response could promote effective antitumor immunity. HMGB1 denotes high-mobility group box 1.
Study of autophagy over the past three decades has shown it to be a response to stress that includes hypoxia and starvation, which are often found in tumors.1,3 Indeed, autophagy is frequently observed in the setting of established cancers but its inhibition during early carcinogenesis actually promotes tumor progression, suggesting that an autophagic “switch” promotes a tumor’s transition to “autophagy addiction” to maintain viability in hypoxic, nutrient-limited microenvironments.
Tumors are currently perceived to use autophagy primarily as a self-protective mechanism, usually dying with rather than as a consequence of excessive self-eating. Michaud et al showed that the initial anti-tumor effects of chemotherapy of two distinct transplantable tumors, a colorectal cancer and a sarcoma, depend on the extent to which cells are capable of enhancing basal levels of autophagy2. Administration of the chemotherapeutic agents, mitoxantrone or oxaliplatin, led to tumor infiltration by antigen-presenting dendritic cells and cytotoxic T-cells in autophagy-competent tumor cells. The investigators went on to show that the release of adenosine triphosphate (ATP)2 by dying, autophagy-competent cells was critical to the induction of host-protective anti-tumor immunity. Cellular release of ATP has also been observed in the autophagic and immune responses to pathogens. ATP is an established DAMP – a damage-associated molecular pattern molecule, a “danger” signal that alerts the immune system to the presence of tissue damage and potentially dangerous microbial agents. In keeping with these concepts, Michaud et al found that chemotherapy-induced adaptive immunity against autophagy-deficient transplantable tumors was promoted by the introduction of exogenous ATP or the use of ATPase inhibitors.
These observations underscore the importance of understanding how tumors arise and are treated in the setting of active inflammatory and immune pathways. Much work remains to determine whether autophagy can be exploited so that its immunity-enhancing effects in the context of chemotherapy-induced apoptosis can modify clinical outcomes. Prior studies of autophagy and immunity have yielded conflicting results; the results described here, using transplantable tumors treated in the first few days following implantation, may not be applicable to the setting of established chemotherapy-resistant tumors found in patients with cancer that have interacted with the host immune system over several years in their development. Other well-described mechanisms, including the production of immunosuppressive transforming growth factor-β or other cytokines, or certain prostaglandins such as PGE2, that underlie tumor-derived effects on immunity must also be considered. Indeed, the very factors that help initiate the immune response, such as ATP and HMGB1, may also late in tumor progression, promote recruitment and sustenance of immunosuppressive myeloid derived suppressor cells and T regulatory cells1, thereby promoting tumor growth. Current strategies designed to inhibit autophagy3,4 in the setting of modern immunotherapy with antibodies5 or cytokines, chemotherapy, or radiation therapy should be extended to include measures of the antitumor immunologic response.
References
- 1.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011 Jul 13;475(7355):226–30. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 2.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, Vacchelli E, Galluzzi L, Ghiringhelli F, di Virgilio F, Zitvogel L, Kroemer G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011 Dec 16;334(6062):1573–7. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 3.Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT, White E. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011 Feb 15;17(4):654–66. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, Zeh HJ, Lotze MT. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010 Sep 23;29(38):5299–310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010 May;10(5):317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]