Abstract
Lyconadins A–C are important members of the Lycopodium alkaloid family with challenging structural features and interesting biological profile. Herein, various synthetic strategies and methods for their preparation are summarized with the focus on constructive bond formation and our efficient and divergent synthesis based on functional group pairing (FGP) strategy.
Keywords: alkaloid, total synthesis, natural product, cyclization, neurodegeneration
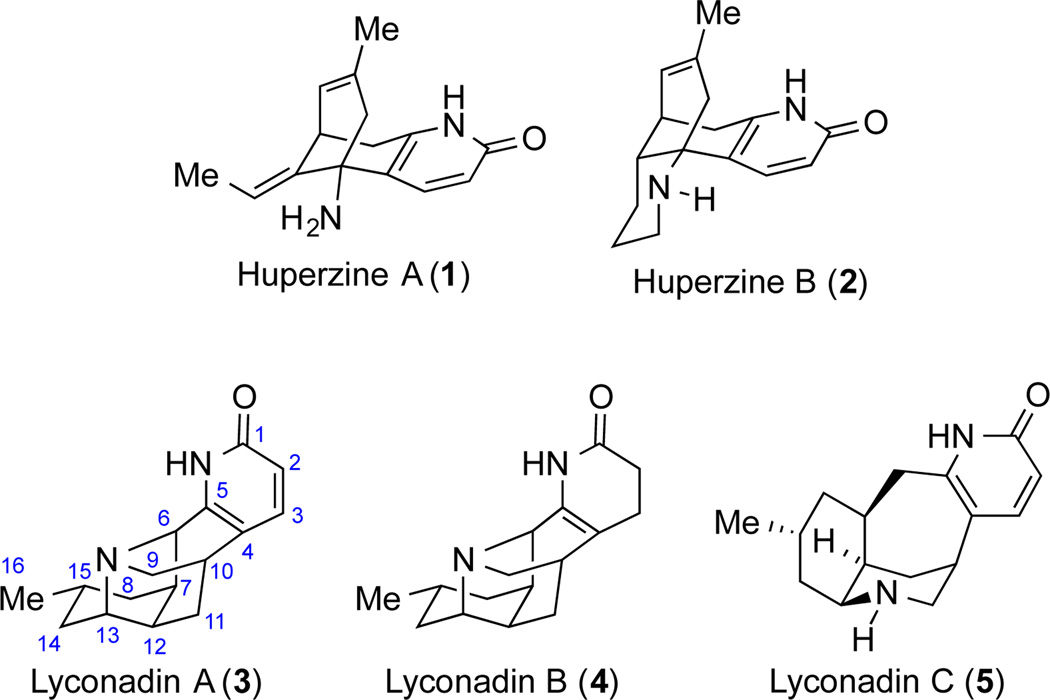
Nature has endowed us with an enormous amount of small molecules (small-molecule natural products, SMNPs) with diverse structural skeletons and important functions, some of which have played indispensable roles in discovering life-saving drug molecules1 and advancing synthetic chemistry in term of both synthetic tactic and strategy.2 Lycopodium alkaloids are one of the largest families of alkaloid natural products, which are enriched with molecules of potent neurotrophic activities.3 For example, huperzines A (1) and B (2) are both potent acetylcholine esterase inhibitors and are currently evaluated in clinical application for the treatment of Alzheimer’s disease.4 Effective therapeutic treatment for neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis and Huntington’s disease is still very limited. Therefore, natural products with neurotrophic activities are important lead compounds for anti-neurodegenerative drug development.5 Lyconadins A–C, members of the Lycopodium alkaloid family, were isolated by Kobayashi and co-workers from the club moss Lycopodium complanatum but in very low yield.6 Both lyconadins A and B have been shown to promote the biosynthesis of nerve growth factors (NGF),7 which are essential for maintaining neuronal balances. So far, their mechanisms of action remain unknown. Therefore, further evaluation of these molecules is expected to provide a clear understanding of their biological profile, identify new drug candidates and elucidate critical signaling pathways/cellular targets for neurodegenerative diseases. But their natural scarcity and structural complexity has limited their biomedical development. Therefore, de novo chemical syntheses of these molecules and their analogs become important. Lyconadins A–C share common structural motifs: a 2-pyridone moiety and a polycyclic carbon skeleton. These intriguing molecules haven’t escaped the radar of synthetic chemists. Significant efforts have been made toward their chemical synthesis,8 culminating in the total syntheses by the Smith (A and B),9 Sarpong (A),10 Fukuyama (A–C),11 Waters (C)12 groups and our research group (A and C).13 Herein, these syntheses were summarized chronologically with the focus on constructive bond formation and our divergent synthesis using functional group pairing (FGP) strategy.14
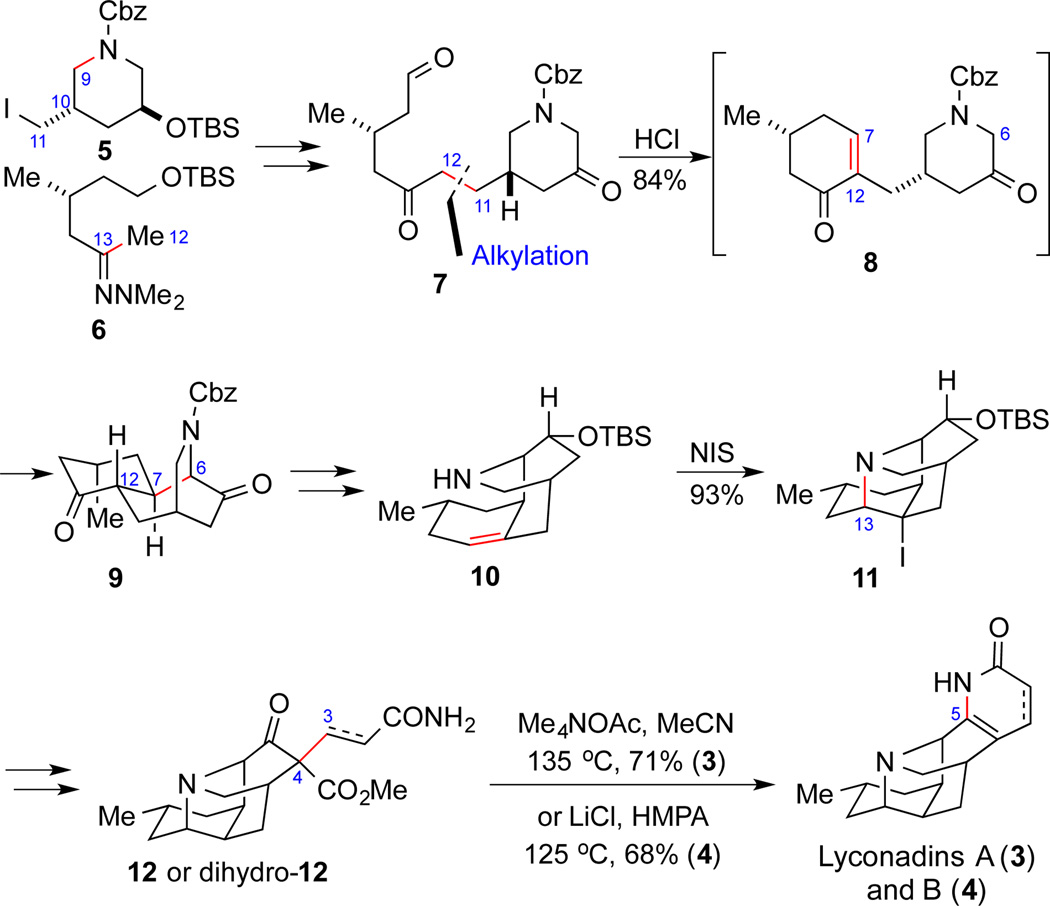
The first total synthesis of lyconadins A and B was accomplished by Smith and Beshore in 2007 (Scheme 1). Their synthesis features an α-alkylation to unite two chiral building blocks, 5 and 6, together to form the C11-C12 bond, an efficient one-pot aldol condensation to form the six-membered carbocycle (cf. 7→8) and an intramolecular conjugate addition to form the C6–C7 bond and furnish tricyclic compound 9, but with undesired stereochemistry at C12. After 9 was converted to amine 10, aminoiodination with N-iodosuccinimide (NIS) built the key C13-N bond and gave the cage-like compound 11. Compound 11 was then converted to 12 or dihydro-12 with the requisite carbons for the 2-(dihydro)pyridone synthesis, which was then converted to lyconadins A (3) and B (4) respectively in one-pot via cyclization of the amide and ketone after decarboxylation.15
Scheme 1.
The Smith synthesis of lyconadins A and B
In 2008, Sarpong, Bisai and West accomplished their elegant total synthesis of lyconadin A (Scheme 2). Unlike the Smith synthesis, which built the 2-pyridone moiety at last, the Sarpong synthesis started with bromomethoxypicoline 13, a masked 2-pyridone. The Stork-Danheiser reaction united 13 and vinylogous ester 14 to form the C6–C7 bond. A sequence of cross-metathesis (CM) reaction and Heck reaction converted the Stork- Danheiser product to tricyclic compound 15 with the desired seven-membered ring, which was further elaborated to compound 16 with a carbamate group derived from a Curtius rearrangement. After reductive amination to form the C13-N bond, Sarpong and co-workers developed an elegant and powerful oxidative C–H amination to build the challenging C6-N bond.16 The methoxypyridine was then converted to the desired 2-pyridone to complete their total synthesis of lyconadin A.
Scheme 2.
The Sarpong synthesis of lyconadin A
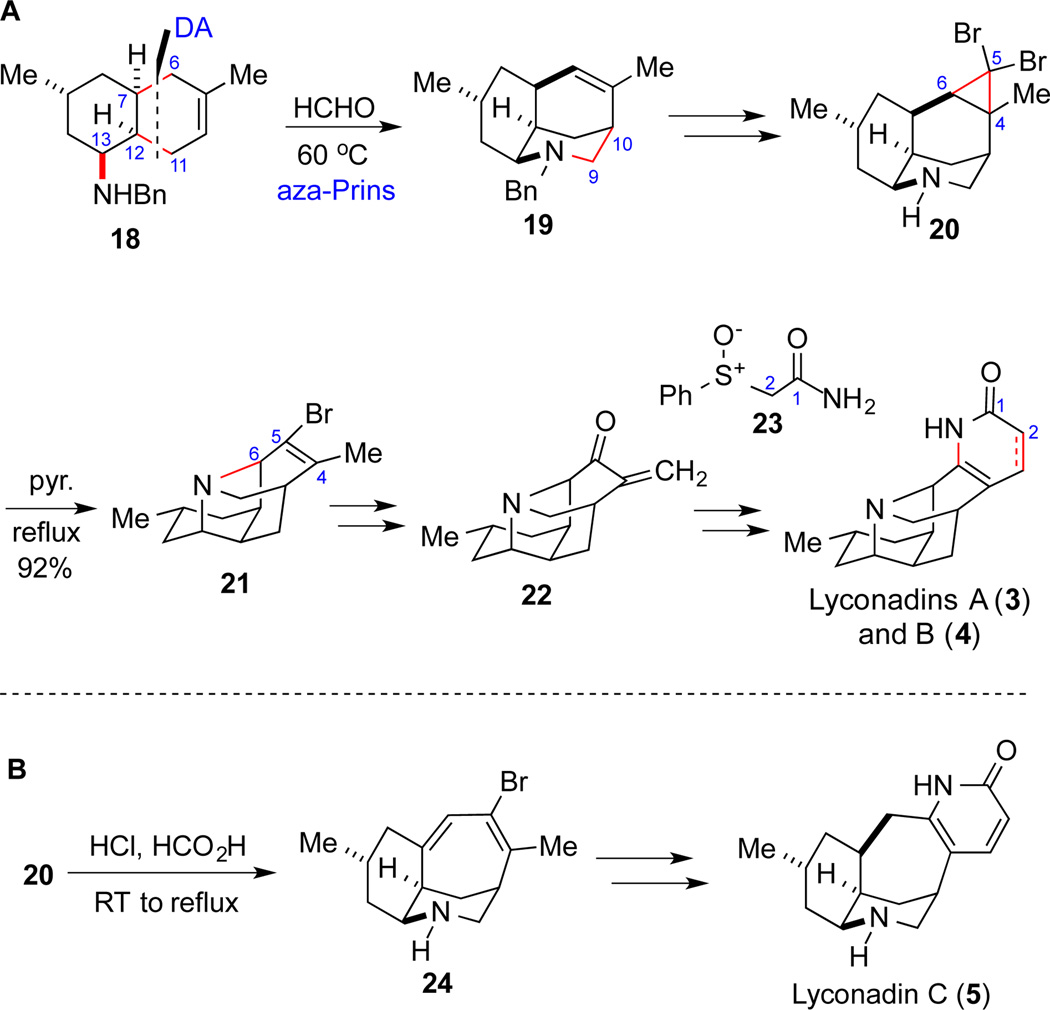
In 2011, the Fukuyama group completed a concise total synthesis of lyconadin A (Scheme 3A). Their synthetic approach was later adapted to the total synthesis of lyconadins B and C as well. Their synthesis commenced with a Diels-Alder reaction between isoprene and optically active 5-methylcyclohexenone followed by reductive amination to provide cis-decaline 18. Compound 18 then underwent an aza-Prins reaction to give tricyclic compound 19, which was quickly converted to dibromocyclopropane 20. The latter, upon heating in refluxing pyridine, was transformed to tetracyclic compound 21 presumably via electrocyclic ring opening of the dibromocyclopropane moiety followed by trapping the resulting allylic cation by the secondary amine. This cascade reaction efficiently expanded the 3,6-fused ring to the desired seven-membered ring and constructed the challenging C6-N bond. Compound 21 was then converted to enone 22 by several different protocols. The Fukuyama group then developed an efficient 2-pyridone synthesis method17 by reacting enone 22 with compound 23 to complete the total synthesis of lyconadin A. A similar approach was developed to synthesize lyconadin B as well.
Scheme 3.
The Fukuyama synthesis of lyconadins A–C
In their lyconadin C synthesis, acidic conditions were developed to convert 20 to tricyclic compound 24 (Scheme 3B). Since the secondary amine was protected as an ammonium salt under acidic conditions, a deprotonation, instead of C6-N bond formation, took place after generating the allylic cation from the cyclopropane ring opening to give 24, which was then converted to lyconadin C using a similar approach developed in their lyconadin A synthesis.
In continuation of their pursuit of Lycopodium alkaloid syntheses,18 the Waters group developed an elegant total synthesis of lyconadin C in 2013 (Scheme 4). Their synthesis also commenced with a Diels-Alder reaction to construct a cis-decaline intermediate (cf. 25), which was converted to tricyclic ketone 26 via a Mannich reaction. After 26 was elaborated to 27, a rhodium-catalyzed Tiffeneau-Demjanov homologation was developed to expand the six-membered ring to the required seven-membered ring. The resulting ring expansion product was further furnished to 28 via a triflation and a Stille cross coupling. Compound 28 was then converted to lyconadin C (5) using an interrupted Curtius rearrangement as the key transformation. In this efficient transformation, the resulting vinyl isocyanate intermediate underwent a 6π-electrocyclization to provide the 2-pyridone system in one-pot.
Scheme 4.
The Waters synthesis of lyconadin C
Our interest in developing natural product based therapeutic treatment for neurodegenerative diseases prompted us to initiate the synthesis of lyconadins in October 2012. We have two synthetic goals in this specific project: (i) achieve total syntheses of these challenging lyconadin natural molecules to quickly provide sufficient materials for biological evaluations; (ii) create a focused small-molecule library based on these privileged structures in order to explore the related chemical space and identify new agents for anti-neurodegenerative development. To accomplish these two goals, we need to find both efficiency and diversity in our synthetic approaches. Inspired by the build-couple-pair (BCP) strategy in diversity-oriented synthesis (DOS) of small molecule libraries,19 we envisioned a divergent synthesis of lyconadins by using a functional group pairing (FGP) strategy (Scheme 5).20 The central notion of this strategy is to quickly synthesize a pivotal intermediate with the requisite functional groups followed by tuning these functional groups into different pairing modes to build diverse structural skeletons. These skeletons would serve as platforms for the synthesis of the natural products of interest and more importantly a focused small molecule library to allow the investigation of the related chemical space. In the lyconadin case, compound 29 containing the cis 6,7-fused carbon bicycle of lyconadins, an amine and a masked enone was designed as our pivotal intermediate, which could be converted to compounds 30, 31, and 32 through a formal aza-[4+2] cyclization, [20] double bond reduction/Mannich reaction, and aza-Michael addition, respectively. These new products would then lead to natural lyconadins as well as a library of unnatural analogs. Obviously, the success of such strategy also requires an efficient and practical approach to prepare the pivotal intermediate (cf. 29) in large scale.
Scheme 5.
Divergent total synthesis of lyconadins A and C by Dai and co-workers
Our synthesis commenced with conjugate addition of vinylcuprate to enone 33 followed by trapping the resulting enolate with aldehyde 34 to form the C6–C7 and C11–C12 bonds. After converting alcohol 35 to triene 36, ring closing metathesis catalyzed by the Grubbs second-generation catalyst was employed to close the seven-membered ring. Chemo- and stereoselective reduction of 37 provided the cis 6,7-fused bicyclic compound 38. In addition to its quick production from 32, each step to compound 38 can be conducted in gram scale as well. For the synthesis of lyconadin A, compound 38 was converted to 29 with the challenging cage-like polycyclic system via a reductive amination followed by a formal aza- [4+2] cyclization. In this aza-[4+2] cyclization, C6-N and C9-C10 bonds, whose construction had required multiple steps in the previous synthesis, were formed in one step and formaldehyde served well as a one-carbon stitching unit. Compound 30 was quickly advanced to known enone 22 with a sequence of Lebel olefination21 and allylic oxidations. Using the Fukuyama 2-pyridone synthesis protocol, we were able to complete a short total synthesis of lyconadin A.
We then synthesized lyconadin C (5) from the same intermediate 38. A sequence of reductive amination, diimide reduction and removal of the benzyl group converted 38 to 41. The latter underwent a Mannich reaction to form the tricyclic compound 42, which was elaborated to lyconadin C (5) using a similar approach employed in our lyconadin A (3) synthesis.
Retrospectively, protection of the C4 ketone of 38 as a ketal group turned out to be critical for the success of our lyconadin A synthesis because it allows an in situ release of the ketone or its enol derivative for the formal aza- [4+2] cyclization (Scheme 7, 44→45→30). When the enone was released first, an aza-Michael reaction took place to convert 38 to 32. Efforts to convert 32 to 30 via a Mannich reaction were fruitless presumably due to the structural rigidity of the tricyclic system of 32. This result rules out the possibility of an aza- Michael-Mannich process for the conversion of 44 to 30. The possibility still exists for a concerted Diels-Alder cycloaddition or a stepwise Mannich-aza-Michael reaction for this conversion.
In summary, a brief analysis of the total synthesis of lyconadins A-C has been described in this Synpacts article. New strategies and methods have been developed. These syntheses enable further biological evaluations of these neurotrophically active molecules in the context of anti-neurodegeneration.
Figure 1.
Selected Lycopodium alkaloids
Figure 2.
Our FGP strategy to natural lyconadins and unnatural analogs
Scheme 6.
Synthesis of 32 and investigation of the formal aza-[4+2] cyclization
Acknowledgments
Acknowledgment
We thank Purdue University, the Purdue Center for Cancer Research and Purdue Research Foundation for financial support. M. Dai is a recipient of the 2013 ORAU Ralph E. Powe Junior Faculty Enhancement Award.
Biography

Mingji Dai (left) received his B.S. degree from Peking University in 2002. After two years’ research with Professors Zhen Yang and Jiahua Chen in the same university, he went to New York in 2004 and pursued graduate study under the guidance of Professor Samuel J. Danishefsky. After earning his Ph.D. degree in 2009, he took a postdoctoral position in the laboratory of Professor Stuart L. Schreiber at Harvard University and the Broad Institute. In the August of 2012, Mingji began his independent career at the Chemistry Department of Purdue University.
Yang Yang (right) received his B.S. degree from the Huazhong University of Science and Technology, Wuhan, China in 2006. He then went to Shanghai and obtained his Ph.D. degree at the Shanghai Institute of Organic Chemistry under the guidance of Professor Hongbin Zhai. He is currently a postdoctoral fellow in the group of Professor Mingji Dai at Purdue University.
References
- 1.(a) Nicolaou KC, Montagnon T. Molecules that Changed the World. Wiley-VCH: Weinheim; 2008. [Google Scholar]; (b) Newman DJ, Cragg GM. J. Nat. Prod. 2012;75:311. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Corey EJ. The Logic of Chemical Synthesis. New York: Wiley-Interscience; 1989. [Google Scholar]; (b) Trost BM. Science. 1991;254:1471. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Sorensen EJ. Classics in Total Synthesis. Weinheim: Wiley-VCH; 1996. [Google Scholar]; (d) Nicolaou KC, Snyder SA. Classics in Total Synthesis II. Weinheim: Wiley-VCH; 2003. [Google Scholar]; (e) Nicolaou KC, Chen JS. Classics in Total Synthesis III. Wiley-VCH: Weinheim; 2011. [Google Scholar]; (f) Wender PA, Miller BL. Nature. 2009;460:197. doi: 10.1038/460197a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Ma X, Gang DR. Nat. Prod. Rep. 2004;21:752. doi: 10.1039/b409720n. [DOI] [PubMed] [Google Scholar]; (b) Hirasawa Y, Kobayashi J, Morita H. Heterocycles. 2009;77:679. [Google Scholar]
- 4.For a review: Olafsdóttir ES, Halldorsdottir ES, Pich NM, Omarsdottir S. In: Natural Products. Ramawat KG, Mérillon JM, editors. Berlin Heidelberg: Springer-Verlag; 2013. pp. 1239–1262.
- 5.(a) Wilson RW, Danishefsky SJ. Acc. Chem. Res. 2006;39:539. doi: 10.1021/ar068018n. [DOI] [PubMed] [Google Scholar]; (b) Xu J, Lacoske MH, Theodorakis EA. Angew. Chem. Int. Ed. 2014;53:956. doi: 10.1002/anie.201302268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Kobayashi J, Hirasawa Y, Yoshida N, Morita H. J. Org. Chem. 2001;66:5901. doi: 10.1021/jo0103874. [DOI] [PubMed] [Google Scholar]; (b) Ishiuchi K, Kubota T, Hoshino T, Obara Y, Nakahata N, Kobayashi J. Bioorg. Med. Chem. 2006;14:5995. doi: 10.1016/j.bmc.2006.05.028. [DOI] [PubMed] [Google Scholar]; (c) Ishiuchi K, Kubota T, Ishiyama H, Hayashi S, Shibata T, Kobayashi J. Tetrahedron Lett. 2011;52:289. [Google Scholar]; (d) Ishiuchi K, Kubota T, Ishiyama H, Hayashi S, Shibata T, Mori K, Obara Y, Nakahata N, Kobayashi J. Bioorg. Med. Chem. 2011;19:749. doi: 10.1016/j.bmc.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 7.(a) He X-L, Garcia KC. Science. 2004;304:870. doi: 10.1126/science.1095190. [DOI] [PubMed] [Google Scholar]; (b) Bartus RT. Neurobiol. Dis. 2012;48:153. doi: 10.1016/j.nbd.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 8.(a) Crich D, Neelamkavil S. Org. Lett. 2002;4:2573. doi: 10.1021/ol026204x. [DOI] [PubMed] [Google Scholar]; (b) Tracey MR, Hsung R. Abstract of Papers, Presented at the 226th National Meeting of the American Chemical Society; New York. 2003. Sep, paper ORGN-721. [Google Scholar]; (c) Castle SL. Presented at the 232nd Meeting of the American Chemical Society; San Francisco, CA. 2006. Sep, paper ORGN-064. [Google Scholar]; (d) Grant SW, Zhu K, Zhang Y, Castle SL. Org. Lett. 2006;8:1867. doi: 10.1021/ol0604264. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Loertscher BM, Zhang Y, Castle SL. Beilstein J. Org. Chem. 2013;9:1179. doi: 10.3762/bjoc.9.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Beshore DC, Smith AB. J. Am. Chem. Soc. 2007;129:4148. doi: 10.1021/ja070336+. [DOI] [PubMed] [Google Scholar]; (b) Beshore DC, Smith AB. J. Am. Chem. Soc. 2008;130:13778. doi: 10.1021/ja804939r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Bisai A, West SP, Sarpong R. J. Am. Chem. Soc. 2008;130:7222. doi: 10.1021/ja8028069. [DOI] [PubMed] [Google Scholar]; (b) West SP, Bisai A, Lim AD, Narayan RR, Sarpong R. J. Am. Chem. Soc. 2009;131:11187. doi: 10.1021/ja903868n. [DOI] [PubMed] [Google Scholar]; (c) Sarpong R. In: Strategies and Tactics in Organic Synthesis. Harmata M, editor. Vol. 8. Elsevier; 2012. pp. 291–315. [Google Scholar]
- 11.(a) Nishimura T, Unni AK, Yokoshima S, Fukuyama T. J. Am. Chem. Soc. 2011;133:418. doi: 10.1021/ja109516f. [DOI] [PubMed] [Google Scholar]; (b) Nishimura T, Unni AK, Yokoshima S, Fukuyama T. J. Am. Chem. Soc. 2013;135:3243. doi: 10.1021/ja312065m. [DOI] [PubMed] [Google Scholar]
- 12.Cheng X, Waters SP. Org. Lett. 2013;15:4226. doi: 10.1021/ol401954f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Haskins CW, Zhang W, Low PL, Dai MJ. Angew. Chem. Int. Ed. 2014;53:3922. doi: 10.1002/anie.201400416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Hendrickson JB. J. Am. Chem. Soc. 1975;97:5784. [Google Scholar]; (b) Baran PS, Maimone TJ, Richter JM. Nature. 2007;446:404. doi: 10.1038/nature05569. [DOI] [PubMed] [Google Scholar]
- 15.Smith AB, III, Atasoylu O, Beshore DC. Synlett. 2009:2643–2646. doi: 10.1055/s-0029-1217749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Jeffrey JL, Bartlett ES, Sarpong R. Angew. Chem. Int. Ed. 2013;52:2194. doi: 10.1002/anie.201209591. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jeffrey JL, Sarpong R. Chem. Sci. 2013;4:4092. [Google Scholar]
- 17.Fujii M, Nishimura T, Koshiba T, Yokoshima S, Fukuyama T. Org. Lett. 2013;15:232–234. doi: 10.1021/ol303320c. [DOI] [PubMed] [Google Scholar]
- 18.Cheng X, Waters SP. Org. Lett. 2010;12:205–207. doi: 10.1021/ol902455y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen TE, Schreiber SL. Angew. Chem. Int. Ed. 2008;47:48. doi: 10.1002/anie.200703073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comer E, Rohan E, Deng L, Porco JA., Jr Org. Lett. 2007;9:2123. doi: 10.1021/ol070606t. [DOI] [PubMed] [Google Scholar]
- 21.Lebel H, Guay D, Paquet V, Huard K. Org. Lett. 2004;6:3047. doi: 10.1021/ol049085p. [DOI] [PubMed] [Google Scholar]