Abstract
TNF/CD80 mice, a CD8+ T cell-mediated model for type 1 diabetes, transgenically express tumor necrosis factor α (TNF-α) and the costimulatory molecule CD80 in their pancreatic islets. Here we show that these molecules bypass the need for CD40–CD154 costimulatory interactions in activation of CD8+ T cells, allowing us to determine the role of CD40–CD154 signals in regulation of autoaggressive CD8+ T cells after their in vivo priming. TNF/CD80 CD154-deficient mice rapidly develop diabetes, whereas CD154-sufficient mice do not. This finding correlates with the decreased numbers of CD4+CD25+ T regulatory (TR) cells in the islets and pancreatic lymph nodes, in comparison to disease-protected CD154-sufficient mice. Administration of a CD40 agonistic antibody induces a systemic and tissue-specific increase in TR cells. However, this increase fails to delay diabetes development in the absence of CD154. Adoptive transfer studies show that CD8+ T cells from TNF/CD80 CD154-deficient, but not CD154-sufficient, mice are resistant to regulation in vivo. This study provides evidence that CD40-transduced signals initiate TR cell increase in vivo and that CD154-transduced signals sensitize autoaggressive CD8+ T cells to suppression.
The deletion of self-reactive T cells by thymic negative selection is not complete, leading to release of cells with autoimmune potential into the periphery (1). Type 1 diabetes (T1D) is an autoimmune disease in which lymphocytic infiltration of the islets of Langerhans, a process termed insulitis, is followed by T cell-mediated destruction of the insulin-producing beta cells of the pancreas (2).
It has been demonstrated by in vivo depletion studies that beta cell destruction, in mouse models of T1D, is mediated by CD8+ T cells. However, both CD4+ and CD8+ T cells are required for diabetes development (3) because of the requirement for CD4+ T cell help in differentiation of CD8+ T cells into cytotoxic T lymphocytes (CTL). CD4+ T cell help is mediated, at least in part, by interaction between the costimulatory molecules CD40 expressed on antigen-presenting cells (APCs) and CD154 expressed on activated CD4+ T cells (4, 5). The CD40–CD154 interaction results in maturation of APCs characterized by up-regulation of costimulatory molecules (6) and transcription of survival genes (7). The mature APC is then capable of triggering activation and differentiation of CD8+ T cells. Ablation of CD40–CD154 signals via genetic manipulation or neutralizing antibodies (Ab) inhibits APC maturation, resulting in impaired humoral and cell-mediated immune responses (8). Because of this critical role for CD40–CD154 signals in the priming phase of immune responses, investigations have centered on ablation of this pathway for the prevention of autoimmune disease (9–11) and transplant rejection (12–14).
Inflammation resulting from viral infections has long been associated with development of autoimmunity (15). In particular, the proinflammatory cytokine tumor necrosis factor α (TNF-α) plays an important role in the pathogenesis of several autoimmune diseases, including T1D. CD40–CD154 blockade or CD154 deficiency in nonobese diabetic (NOD) mice prevents insulitis and T1D (10). However, this protection can be overcome by systemic administration, or constitutive islet-specific expression of TNF-α from a rat insulin promoter (RIP–TNF-α) (16, 17). The presence of TNF-α bypasses the requirement for CD40–CD154 interactions in priming and differentiation of islet-specific CD8+ T cells in CD154-deficient RIP–TNF-α NOD mice (18).
Recently, a unique population of thymic-derived T regulatory (TR) cells has been identified and shown to suppress activation of autoreactive T cells (19, 20). Phenotypic characterization of TR cells includes expression of CD4, CD25 (21), and CTL-associated antigen 4 (22). TR cell development and function depend on the transcription factor FoxP3 (23). In addition, costimulatory molecule interactions (24–26) and chemokines (27) play a crucial role in TR cell homeostasis, recruitment, and function. The mechanism of TR cell suppression of autoreactive CD4+ and CD8+ T cells requires cell–cell contact and is mediated, at least in part, by IL-10 (28) and transforming growth factor β (TGF-β) (29, 30).
We have previously described a C57BL/6 CD8+ T cell-mediated model for T1D, the TNF/CD80 mouse (31). TNF/CD80 mice possess dual transgenes directing islet-specific expression of TNF-α and the costimulatory molecule CD80. In addition, transgenic TNF-α expression is controlled by a doxycycline-responsive transcriptional on/off switch. TNF/CD80 mice display three diabetes phenotypes depending on the duration of TNF-α expression. These phenotypes are termed protected, delayed, and rapid and correspond to repression of TNF-α at 21, 25, and 28 days of age, respectively (31). Delay in T1D development correlates with an increase in potent TR cells in the islets and pancreatic lymph node (PLN) (26). Regulation of CD8+ T cells by these TR cells has been shown to depend on expression of functional TGF-β receptors on the CD8+ T cell (32). TNF/CD80 mice are an attractive model for determining the importance of CD40–CD154 signals in the regulation of anti-islet CD8+ T cell responses after their initial priming in vivo.
In this study, we use CD154-deficient TNF/CD80 mice to demonstrate a critical requirement for CD40–CD154 interactions in the regulation of anti-islet CD8+ T cells. TNF/CD80 mice display a rapid T1D phenotype independent of the duration of TNF-α expression. CD154 deficiency abrogates regulation of anti-islet CD8+ T cells in two ways. The first is a failure of TR cells to increase in number in the islets and PLN due to lack of CD40-transduced signals. The second is the presence of an anti-islet CD8+ T cell population resistant to regulatory mechanisms due to lack of CD154-transduced signals. Understanding the complex roles of CD40–CD154 signals may provide therapeutic strategies for autoimmune diseases such as T1D.
Materials and Methods
Mice and Diabetes Detection. TNF/CD80 (31) and CD154-/- C57BL/6 mice (N11) (33) mice have been described elsewhere. For TNF-α repression, mice were fed a 2.3 g/kg doxycycline diet (Bio-Serv). All mice were maintained under specific pathogen-free conditions.
Diabetes was monitored by testing of urinary glucose using Diastyx (Bayer) and confirmed by blood glucose measurement using One-Touch strips (Lifescan). Mice with blood glucose >250 mg/dl on 2 consecutive days were deemed diabetic.
Cell Purification and in Vitro Assays. CD4+CD25+ T cells were isolated by using a MoFlo Cell Sorter (Dako Cytomation). CD8+ T cells were purified by negative selection using rat anti-mouse CD4 (RM4-4), Mac-1 (M1/70), B220 (RA3-6B2), and I-Ab (25-9-3) Abs (BD Pharmingen) followed by incubation with goat anti-rat IgG Biomag beads (Qiagen, Valencia, CA) according to the manufacturer's instructions.
Proliferation assays were carried out in triplicate by combining 2 × 104 CD8+ T cells and 2 × 104 TR cells, as indicated in 96-well, round-bottomed microtiter plates (Corning). Cells were cultured in RPMI medium 1640 (Sigma) supplemented with 10% FCS, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, and 100 units/ml each of penicillin and streptomycin (Invitrogen). Cells were stimulated with 5 × 105 irradiated splenocyte APCs and 10 μg/ml anti-CD3 for 72 h. During the last 6 h of culture, cells were pulsed with 1 μCi of [3H]thymidine. (Amersham Pharmacia; 1 Ci = 37 GBq) and proliferation was quantified as [3H] incorporation.
Flow Cytometry. The following fluorochrome-conjugated Abs were used for flow cytometry: anti-CD4 (GK1.5), anti-CD25 (PC61), and anti-CD8 (53.6) (BD Pharmingen). Hamster antimouse CD154 (39H5), Armenian hamster Ig isotype control, and anti-hamster FITC were obtained from (Serotec). Stained cells were acquired on a FACSCalibur and analyzed by using cellquest software (BD Biosciences).
In Vivo Assays. For in vivo CD154 blockade, mice received i.p. injections of 250 μg of anti-CD154 Ab (MR1) or Armenian hamster isotype control Ab. For in vivo stimulation of CD40, mice were injected i.p. with 250 μg of CD40 agonistic Ab (FGK45) or rat IgG isotype control Ab. For adoptive transfer, purified CD8+ T cells or CD4+CD25+ TR cells were washed twice in sterile tissue culture-grade PBS (Invitrogen) before injection into the lateral tail vein.
Real-Time PCR. CD4+CD25+, CD4+CD25-, and CD8+ T cells were isolated by cell sorting. RNA was prepared by using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized with random primers (Invitrogen). The expression of FoxP3 was measured by real-time RT-PCR with primers 5′-GGCCCTTCTCCAGGACAGA-3′ and 5′-ACAACCCAGCCATGATCAGC-3′ at a final concentration of 300 nM, and the internal TaqMan probe 5′-FAM-ACTTCATGCATCAGCTCTCCAC-TAM-1-3′ at a final concentration of 125 nM. β2 microglobulin (β2M) was used as an internal reference and was measured by using primers 5′-GCTATCCAGAAAACCCCTCAAA-3′ and 5′-CTGTGTTACGTAGCAGTTCAGTATGTTC-3′ at a final concentration of 300 nM, and the TaqMan probe 5′-FAM-AGTATACTCACGCCACCCACCGGAGAAT-TAM-1-3′ at a final concentration of 200 nM (all primers and probes were synthesized by Sigma, except the β2M probe, which was synthesized by Applied Biosystems). mRNA levels were quantified by using the ABI 7000 Sequence Detection System (Applied Biosystems). Samples were run in duplicate, and their relative expression of FoxP3 was determined by normalizing expression to β2M.
Results
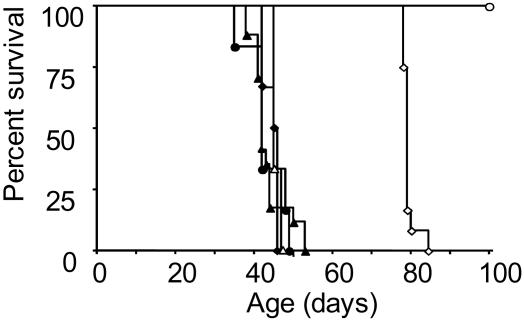
CD40–CD154 Signals Are Required for the Suppression of Anti-Islet CD8+ T Cells. Previous studies have shown that islet-specific expression of TNF-α can bypass the requirement for CD40–CD154 interactions in the initiation and effector phases of an autoimmune response (18). TNF/CD80 CD154-sufficient mice display three diabetes phenotypes termed protected, delayed, and rapid, depending on the duration of islet-specific TNF-α expression. To address the role of CD40–CD154 interactions in development of the delayed diabetes phenotype, we crossed the TNF/CD80 mouse onto a CD154-deficient background (TNF/CD80.KO) (33). TNF/CD80.WT and TNF/CD80.KO littermates developed T1D with identical rapid kinetics after repression of TNF-α at 28 days of age (Fig. 1). Thus, in agreement with previous reports, the presence of TNF-α bypasses the requirement for CD40–CD154 interactions in the priming and effector phases of the CD8+ T cell response. After repression of TNF-α at 25 days of age TNF/CD80.WT mice displayed delayed progression to T1D whereas TNF/CD80.KO littermates all developed T1D with rapid kinetics (Fig. 1). Finally, repression of TNF-α at 21 days of age induced a protected phenotype in TNF/CD80.WT mice, whereas TNF/CD80.KO littermates again progressed to T1D with rapid kinetics. The inability of TNF/CD80.KO mice to display the protected or delayed diabetes phenotypes suggests a critical role for CD40–CD154 interactions in controlling the effector phase of the autoaggressive CD8+ T cell response.
Fig. 1.
CD40–CD154 signals are required for the suppression of anti-islet CD8+ T cells. TNF-α was expressed from birth and repressed at 21 (circles), 25 (diamonds), or 28 (triangles) days of age in TNF/CD80.WT (open symbols) and TNF/CD80.KO (filled symbols) mice, and diabetes development was monitored. These data are representative of six mice per group.
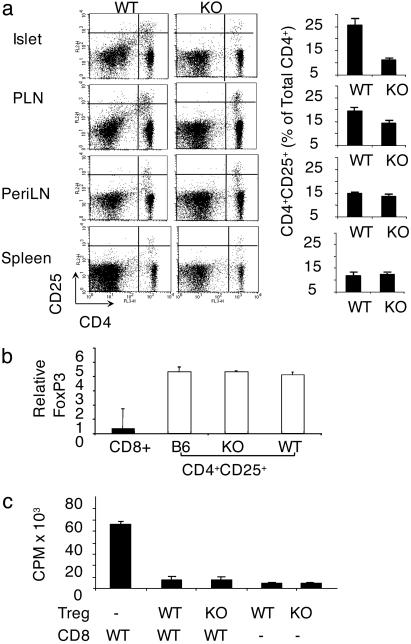
CD154 Deficiency Prevents Increase in TR Cell Numbers in Inflamed Tissue. The delayed diabetes phenotype in TNF/CD80.WT mice is associated with high numbers of TR cells in the islets and PLN, termed the regulatory phase (26). Therefore, we compared CD4+CD25+ T cell numbers in the islets, PLN, peripheral lymph nodes (PeriLNs), and spleen of TNF/CD80.KO and TNF/CD80.WT mice after repression of TNF-α at 25 days of age. TNF/CD80.KO mice showed decreased numbers of CD4+CD25+ T cells compared with TNF/CD80.WT mice in the PLN and islet (Fig. 2a). The number of CD4+CD25+ T cells in the spleen and PeriLNs did not differ between the two strains of mice. To determine whether this CD4+CD25+ T cell pool represented a pure TR cell population or a mixed population of effector CD4+ T cells and TR cells, we assessed the mRNA levels of the TR-specific transcription factor FoxP3. CD4+CD25+ T cells were isolated from the PLN of TNF/CD80.WT, TNF/CD80.KO mice and control B6 mice. B6-derived CD8+ T cells were included as a negative control. In all mice examined, FoxP3 mRNA expression in CD4+CD25+ T cells was similar (Fig. 2b). This provides evidence that the CD4+CD25+ T cell population isolated from the PLN of TNF/CD80.WT and TNF/CD80.KO mice represents TR cells and not activated CD4+ T cells. In addition, CD4+CD25+ T cells from TNF/CD80.KO and TNF/CD80.WT mice expressed similar levels of the TR markers glucocorticoid-induced TNF receptor and CTL-associated antigen 4, as assessed by flow cytometry (data not shown).
Fig. 2.
CD154 deficiency blocks increase in TR cells in inflamed tissue. (a) Lymphocytes were isolated from the tissues shown. The percentage of CD4+ T cells that expressed CD25 was determined by fluorescence-activated cell sorting (FACS). The data presented are gated on the small, live, CD45+ population. The results shown are representative of six individual animals analyzed for each group of mice. (b) CD4+CD25+ T cells were isolated from the pooled PLNs of three mice per group by cell sorting. FoxP3 levels from CD8+ and CD4+CD25+ T cells isolated from control B6 mice are included as negative and positive controls, respectively. The expression of FoxP3 relative to β2M was assessed by real-time PCR. The results are representative of two independent experiments. (c) CD4+CD25+ T cells were isolated from the pooled periLN and PLN of TNF/CD80.KO and TNF/CD80.WT mice. CD8+ responder T cells and APCs were isolated from TNF/CD80.WT mice. Values represent the mean ± standard deviation of triplicate cultures. The data are representative of three independent experiments.
Finally, in vitro TR cell suppressor assays demonstrated that CD154-deficient CD4+CD25+ T cells were as capable of suppressing WT CD8+ T cell proliferation as CD154-sufficient CD4+CD25+ T cells (Fig. 2c). These findings are in agreement with other reports that TR cell homeostasis is independent of CD154–CD40 signals (34). However, in the absence of CD40–CD154 signals, TR cells cannot increase at the site of inflammation.
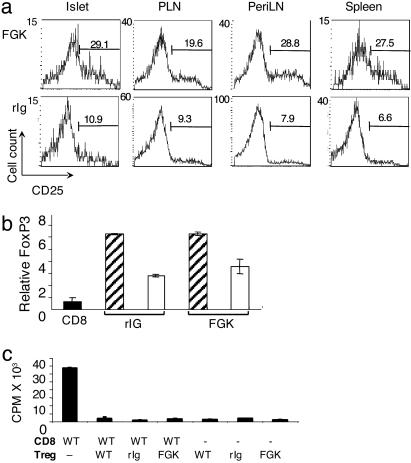
CD40 Is an Important Costimulator for TR Cell Increase in Inflamed Tissue. Recent reports have documented the importance of increased TR cell numbers in inflamed tissue for regulation of autoreactive T cells. This increase can be mediated by TGF-β–TGF-β receptor (TGF-βR) (30) and TNF-related activation-induced cytokine (TRANCE)-receptor activator of NFκB (RANK) interactions (26). Our data showing decreased numbers on TR cells in the PLN and islets of TNF/CD80.KO mice suggested a role for CD40–CD154 signals in TR cell homeostasis in inflamed tissue. To address whether CD40-transduced signals play a role in increasing TR cells at the site of inflammation, we used a CD40 agonistic Ab, FGK45 (FGK) (4). TNF/CD80.KO mice were injected with FGK or rat isotype control (rIg). Within 48 h of CD40 stimulation, the percentage of CD4+ T cells expressing CD25 increased from 11% to 29% in the islet, 9% to 19% in the PLN, 8% to 29% in the periLNs, and 7% to 28% in the spleen compared with rIg-treated mice (Fig. 3a). Interestingly, CD40 stimulation of TNF/CD80.WT mice did not alter CD4+CD25+ T cell numbers compared with rIg-treated mice (data not shown).
Fig. 3.
Stimulation of CD40 in vivo increases TR cell numbers. TNF-α was expressed from birth until 25 days of age in TNF/CD80.WT (WT) and TNF/CD80.KO (KO). On days 28 and 30, animals were injected with FGK45 Ab or control rat Ig. Cells were isolated 48 h later by cell sorting. (a) Lymphocytes were isolated from the tissues named. The percentage of CD4+ T cells that expressed CD25 was determined by fluorescence-activated cell sorting (FACS). The data presented are gated on the small, live, CD45+CD4+ population. The results shown are representative of three individual animals analyzed for each group of mice. (b) FoxP3 mRNA expression in CD4+CD25+ (hatched bars) and CD4+CD25- (open bars) T cells. T cells were isolated by cell sorting from the pooled PLNs of two mice per group. The expression of FoxP3 relative to β2M was assessed by real-time PCR. CD8+ T cells were included in the assay as a negative control (filled bar). The results are representative of two independent experiments. (c) CD4+CD25+ TR cells were isolated from the pooled periLN and PLN of untreated TNF/CD80.WT and FGK- and rIg-treated TNF/CD80.KO mice. CD8+ responder T cells and APCs were isolated from TNF/CD80.WT mice. Values represent the mean ± standard deviation of triplicate cultures. The data are representative of four independent experiments.
FoxP3 mRNA expression was used to determine whether CD40 stimulation triggered an increase in TR cells or activated CD4+ T cells. CD4+CD25+ T cells were isolated from FGK- and rIg-treated TNF/CD80.KO mice. CD4+CD25+ T cells and CD8+ T cells from unmanipulated B6 mice were included as positive and negative controls (23). CD4+CD25+ T cell populations isolated from the PLN (Fig. 3b) and PeriLNs (data not shown) of all groups of mice all expressed equal levels of FoxP3 mRNA, providing evidence that the CD40-stimulation-induced CD4+CD25+ T cell population consisted of TR cells.
To further validate that CD4+CD25+ T cells in CD40-stimulated TNF/CD80.KO mice were TR cells we performed an in vitro suppressor assay. CD4+CD25+ T cells were isolated from pooled PLN and PeriLNs of TNF/CD80.KO mice after administration of FGK or rIg, and their ability to suppress CD8+ T cell proliferation was assessed. As a positive control, CD4+CD25+ T cells were isolated from the same tissues of TNF/CD80.WT mice. CD4+CD25+ T cells from all groups of mice were anergic to anti-CD3 Ab stimulation and, at a 1:1 ratio of effector CD8+ T cell/TR cell, suppressed the proliferation of effector CD8+ T cells by ≈95% (Fig. 3c).
Together, these data provide evidence that CD40 stimulation is capable of triggering a systemic and tissue-specific increase in TR cells numbers.
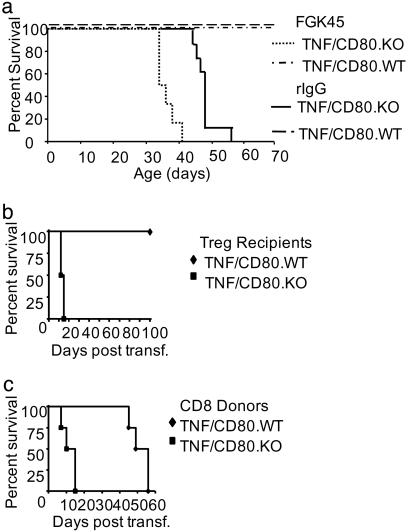
CD40-Signals Do Not Delay Diabetes Development in TNF/CD80.KO Mice. The capacity for CD40 stimulation to increase TR cell numbers in TNF/CD80.KO mice led us to investigate whether FGK administration could rescue the rapid development of T1D in TNF/CD80.KO mice. TNF/CD80.KO and TNF/CD80.WT mice were injected with either FGK or recombinant Ig (rIg) during induction of regulation, and disease progression was monitored. FGK or rIg administration to TNF/CD80.WT mice had no effect on TR cell-mediated suppression of anti-islet CD8+ T cells. All mice remained diabetes-free until the end of the observation period, at 70 days of age (Fig. 4a). In contrast, not only did administration of FGK45 Ab fail to delay diabetes progression in TNF/CD80.KO mice, but CD40 stimulation accelerated disease progression compared to rIg-treated mice (P < 0.05, using the rank log test). In two further groups of mice, FGK or rIg was administered when the regulatory phase is normally initiated in TNF/CD80.WT mice (between 21 and 25 days of age) or throughout the regulatory phase (between 21 and 35 days of age). Both FGK treatment groups displayed rapid disease development compared with rIg-treated animals (data not shown), despite the presence of increased numbers of TR cells in the islets and PLN in the FGK-treatment groups (data not shown). Thus, failure to delay disease was independent of both the time and duration of CD40 stimulation. This inability of CD40 stimulation to rescue regulation in TNF/CD80.KO mice suggests a critical role for CD154 signals in regulating anti-islet CD8+ T cells.
Fig. 4.
CD154-/- CD8+ T cell are resistant to regulation in vivo. TNF-α was expressed from birth to 25 days of age. On days 28 and 30, animals were injected with FGK (n = 6) or rIg (n = 6), and diabetes development was assessed as before. The data presented are the pooled results of three independent experiments. (b) TNF/CD80.KO-derived CD8+ T cells are resistant to regulation in vivo. Donor TNF/CD80.WT mice expressed TNF-α from birth to 25 days of age. At 32 days of age, PLN-derived TR cells were isolated by cell sorting. Recipient TNF/CD80.WT (diamonds; n = 4) and TNF/CD80.KO (squares; n = 4) mice expressed TNF-α from birth to 28 days. On day 30, recipient mice were injected with 2,000 TR cells, and the progression to diabetes was monitored. The data presented are the pooled results of two independent experiments. (c) Donor TNF/CD80.WT and TNF/CD80.KO mice expressed TNF-α from birth to 28 days of age. At 32 days of age, PLN-derived CD8+ T cells were isolated by cell sorting. TNF/CD80.WT recipients expressed TNF-α from birth to 25 days of age. At 32 days of age, 3 × 104 TNF/CD80.WT (diamonds, n = 4) or TNF/CD80.KO (squares, n = 4) CD8+ T cells were transferred into TNF/CD80.WT recipient mice. Animals were monitored for diabetes development. The data presented are the pooled results of two independent experiments.
CD154-/- CD8+ T Cells Resist Regulation by TR Cells in Vivo. The surprising finding that restoration of high TR cell numbers in the PLN and islet of TNF/CD80.KO mice failed to delay diabetes development suggested that lack of regulation in the absence of CD154 may not be solely due to decreased numbers of TR cells at the site of autoimmune attack. An alternative explanation is that CD8+ T cells in TNF/CD80.KO mice may be resistant to suppression by TR cells. To address this possibility, we performed a series of adoptive transfers. Recipient TNF/CD80.KO or TNF/CD80.WT mice were induced to develop diabetes with rapid kinetics, associated with failure of TR cells to increase in the inflamed tissue. Transfer of 2,000 PLN-derived TR cells from TNF/CD80.WT mice prevented diabetes development over a 100-day observation period in TNF/CD80.WT mice (Fig. 4b), confirming our previous report (26). However, transfer of TR cells did not prevent or delay development of diabetes in TNF/CD80.KO mice, in which diabetes developed within 15 days of transfer.
The failure of transferred TNF/CD80.WT-derived TR cells to delay disease progression in TNF/CD80.KO mice may be explained by a failure of TR cell expansion in vivo (35) in the absence of CD40-transduced signals, or an inability of TR cells to function in TNF/CD80.KO mice. An alternative explanation is the presence of a CD8+ T cell population resistant to regulation in TNF/CD80.KO mice. To address this possibility, CD8+ T cells were isolated from the PLN of TNF/CD80.WT or TNF/CD80.KO mice and transferred into TNF/CD80.WT mice during the regulatory phase. Endogenous TR cells in recipient mice efficiently suppressed transferred CD8+ T cells from TNF/CD80.WT mice, in which diabetes developed by 56 days after transfer (Fig. 4c). In contrast, all animals receiving CD8+ T cells from TNF/CD80.KO mice rapidly progressed to diabetes between 7–15 days after transfer. This finding supports the hypothesis that islet-reactive CD8+ T cells in TNF/CD80.KO mice are resistant to regulation and suggests a previously undescribed role for CD154-transduced signals in the negative regulation of autoreactive CD8+ T cells.
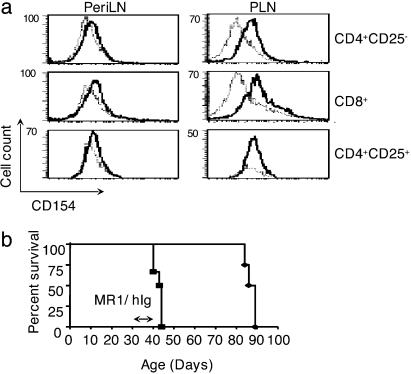
CD154–CD40 Signals Maintain Regulation of Anti-Islet CD8+ T Cells. Because CD154 deficiency results in a CD8+ T cell population resistant to regulation in vivo, suppression of anti-islet CD8+ T cells by TR cells may require expression of CD154 on the CD8+ T cell. To test this, we compared CD154 expression on subpopulations of T cells isolated from the PLN and PeriLNs of TNF/CD80.WT mice during the regulatory phase. CD154 expression was not detectable on CD8+ or CD4+CD25- T cells isolated from the PeriLNs (Fig. 5a). In contrast, CD154 was up-regulated on both CD4+CD25- T cells and CD8+ T cells isolated from the PLN. Interestingly, CD154 was not detectable on the surface of CD4+CD25+ T cells isolated from the PeriLNs and PLN, suggesting that CD154 expression on TR cells is not required for regulation of anti-islet CD8+ T cells.
Fig. 5.
CD40–CD154 signals maintain regulation of anti-islet CD8+ T cells. (a) TNF-α was expressed from birth to 25 days of age in TNF/CD80.WT mice, and on day 32 the PLN and PeriLNs were isolated. Cells were stained with CD4, CD8, CD25, and either CD154 or hamster isotype control. Expression of CD154 (bold lines) and isotype control Ab (broken lines) was determined on CD4+CD25-, CD4+CD25+, and CD8+ T cells. The data are representative of six individual mice examined. (b) TNF-α was expressed from birth to 25 days of age in TNF/CD80.WT mice. Between 33 and 37 days of age, mice were immunized four times with MR1 Ab (n = 6; squares) or control hamster Ig (n = 4; diamonds), and diabetes development was monitored.
To test the importance of the up-regulation of CD154 on T cell populations during TR cell-mediated regulation, we used the CD154 blocking Ab MR1. TNF/CD80.WT were injected with MR1 or an isotype control Ab during the regulatory phase, and T1D development was monitored. CD154 blockade rapidly released CD8+ T cells from regulation; diabetes developed in all animals 3–7 days after injection of MR1 (Fig. 5b). In contrast, CD8+ T cells in isotype control Ab-treated mice remained sensitive to TR cell suppression; diabetes developed 47–52 days after injection. Thus, CD154-transduced signals play a negative regulatory role in both sensitizing and maintaining CD8+ T cell regulation.
Discussion
Successful manipulation of CD40–CD154 signals for therapeutic treatment of autoimmune diseases such as T1D requires a detailed knowledge of the roles of these signals at all stages of the autoimmune process. Here, we provide evidence for previously undescribed roles of CD40 and CD154 in suppression of autoaggressive CD8+ T cells after in vivo priming. CD40-transduced signals trigger an increase in TR cells in inflamed tissue, and CD154-transduced signals sensitize anti-islet CD8+ T cells to suppression.
The importance of CD40–CD154 interactions in the regulation of anti-islet CD8+ T cells was demonstrated by finding that TNF/CD80.KO mice exhibit rapid diabetes development and a lack of regulation seen in TNF/CD80.WT littermates. The regulation that delays diabetes development in the TNF/CD80 model is characterized by increased numbers of TR cells in the islets and PLN, and these cells actively suppress islet-specific CD8+ T cells (26). Rapid disease progression in TNF/CD80.KO mice and TNF/CD80.WT mice after prolonged islet expression of TNF-α is associated with failure of TR cells to increase in number in the pancreas and associated lymphoid tissue, suggesting a possible association between TNF-α and CD40–CD154 signals. It is interesting to hypothesize that prolonged exposure to TNF-α may shut down the mechanism whereby CD40–CD154 interactions trigger a tissue-specific increase in TR cells.
Previous studies have shown that CD154 deficiency not only inhibits priming of anti-islet CD8+ T cells but also enhances TR cell responses in vivo. On a CD154-deficient nonobese diabetic background TR cells suppressed islet-specific T cell receptor transgenic CD8+ T cells indirectly by preventing maturation of dendritic cells (DC) (31). The ability of CD154-deficient TR cells to suppress CD8+ T cells was abrogated by CD40 activation of DCs. This interesting study supports our findings that TR cells from TNF/CD80.KO mice display a typical regulatory phenotype. However, in the TNF/CD80 model, the presence of APCs activated by TNF-α or CD40 stimulation does not compromise the ability of TR cells to suppress islet-specific CD8+ T cells in a CD154-sufficient environment. This finding suggests a requirement for CD40–CD154 interactions in suppression of the effector phase of the CD8+ T cell response beyond inhibition of APC maturation. In the absence of CD154, TNF-α-activated APCs fail to deliver the appropriate signals to trigger an increase in TR cells in inflamed tissue, and this is restored by in vivo stimulation of CD40. This observation demonstrates a dissociation of TNF-α and CD40 stimulation in activation and function of APCs. TRANCE–RANK (15) and TGF-β–TGF-βR (30) interactions have also been shown to play a role in increasing TR cell numbers in inflamed tissue. It will be interesting to see whether the CD40–CD154 pathway intersects with these pathways in active suppression of autoimmunity in vivo.
The classical biological effects of CD40–CD154 interactions are mediated by ligation of CD40 on B cells and APCs leading to their activation and maturation (8). Here we show that stimulation of CD40 in the absence of CD154 triggers a systemic and tissue-specific increase in TR cells. The mechanism of TR cell increase after CD40 stimulation is unknown. According to the classical APC-activation role of CD40 signals, it is likely that these activated APCs promote migration, expansion, and enhanced survival of TR cells in the PLN and islets. Alternatively, CD40-stimulated APCs may promote generation of TR cells from CD4+CD25- T cell progenitors. However, another explanation arises from recent reports documenting expression of CD40 on CD4+ and CD8+ T cells (36, 37). CD40-transduced signals may therefore act directly on TR cells, triggering their expansion, or on CD4+CD25- progenitors, triggering their differentiation into TR cells. It was interesting that CD40 stimulation did not increase TR cell numbers in TNF/CD80.WT mice (data not shown). This finding highlights the possibility that CD154 may play a role in systemic, as well as tissue-specific, TR cell homeostasis. An intact CD40–CD154 signaling pathway may prevent aberrant expansion of the TR population, protecting against inappropriate immune suppression after a strong inflammatory stimulus that may occur, for example, during viral infection. Identifying how CD40 stimulation induces an increase in TR cells may identify useful targets for the therapeutic manipulation of the TR cell population
Despite the ability of CD40 signals to increase TR cell numbers in inflamed tissue, this finding did not translate into protection from T1D. CD40-transduced signals therefore cannot substitute for CD154 deficiency in control of islet-specific CD8+ T cells. This finding suggested that CD154-transduced signals might negatively regulate autoaggressive CD8+ T cells. In support of this hypothesis, CD154 is up-regulated on CD4+CD25- and CD8+ T cells in the PLN of TNF/CD80.WT mice where TR cells suppress activation of islet-reactive CD8+ T cells. However, although there is evidence that CD154-transduced signals lead to CD4+ T cell activation in vitro (38) and in vivo (39), the ability of CD8+ T cells to receive CD154-transduced signals is less well defined. There are several possible cellular interactions that could result in CD154-dependent suppression of CD8+ T cells. TR cells may express CD40 and interact directly with CD154-expressing autoaggressive CD8+ T cells to deliver a suppressive signal. However, the distribution of CD40 expression on T cell subsets in this model remains to be defined. Alternatively, the CD154-transduced signal may be delivered to the CD8+ T cell from an APC after conditioning of the APC by interaction with a TR cell. Understanding the mechanism whereby self-reactive CD8+ T cells may resist regulation by TR cells may shed light on the etiology of autoimmune diseases mediated by CD8+ T cells.
Blockade of CD154 by using the MR1 Ab has been shown to prevent development of autoimmunity (10) and transplant rejection (14). Several mechanisms have been reported for the immune suppression induced by MR1. These mechanisms include inhibition of T cell priming (40), induction of novel regulatory cells (11) and deletion of allograft-reactive T cells (41). However, the latter result is controversial (42). Here we show that administration of MR1 Ab after priming of islet-specific CD8+ T cells in TNF/CD80.WT mice abrogates regulation of autoreactive CD8+ T cells, as assessed by rapid progression to T1D. This finding does not support a role for deletion of autoaggressive CD8+ T cells. However, it is possible that CD154 blockade may induce deletion of TR cells. In light of our previous data, an attractive explanation for our observations is that MR1, acting as a blocking Ab, inhibits CD154-dependent negative regulatory signals and releases CD8+ T cells from suppression. We have previously shown that TR suppression of CD8+ T cells requires expression of functional TGF-βR on the surface of the CD8+ T cell (32). It will be interesting to establish whether the negative regulatory role of CD154 in autoaggressive CD8+ T cells is mediated by intersection with the TGF-β–TGF-βR pathway.
This study demonstrates roles for CD40- and CD154-transduced signals in the regulation of autoreactive CD8+ T cells in inflamed tissue. By dual signaling through CD40 and CD154, TR cells receive the appropriate signals to increase in numbers at the site of autoimmunity, and islet-reactive CD8+ T cells are sensitized to suppression by regulatory mechanisms. Therapeutic manipulation of these signals may have important implications for treatment of T1D and possibly other autoimmune diseases.
Acknowledgments
We thank S. McCallum for cell-sorting expertise and L. S. Wicker and S. Carter for critical reading of the manuscript. This work was funded by the Juvenile Diabetes Research Foundation, the Wellcome Trust (E.A.G.), and the Medical Research Council (C.M.M.).
Abbreviations: T1D, type 1 diabetes; APC, antigen-presenting cell; CTL, cytotoxic T lymphocyte; TGF, transforming growth factor; TGF-βR, TGF-β receptor; periLN, peripheral lymph nodes; TR, T regulatory; PLN, pancreatic lymph node; TNF, tumor necrosis factor; β2M, β2 microglobulin.
References
- 1.Zauderer, M. & Natarajan, K. (1990) Immunol. Rev. 116, 159-170. [DOI] [PubMed] [Google Scholar]
- 2.Delovitch, T. L. & Singh, B. (1997) Immunity 7, 727-738. [DOI] [PubMed] [Google Scholar]
- 3.Mora, C., Wong, F. S., Chang, C. H. & Flavell, R. A. (1999) J. Immunol. 162, 4576-4588. [PubMed] [Google Scholar]
- 4.Schoenberger, S. P., Toes, R. E., van der Voort, E. I., Offringa, R. & Melief, C. J. (1998) Nature 393, 480-483. [DOI] [PubMed] [Google Scholar]
- 5.Bennett, S. R., Carbone, F. R., Karamalis, F., Flavell, R. A., Miller, J. F. & Heath, W. R. (1998) Nature 393, 478-480. [DOI] [PubMed] [Google Scholar]
- 6.Caux, C., Burdin, N., Galibert, L., Hermann, P., Renard, N., Servet-Delprat, C. & Banchereau, J. (1994) Res. Immunol. 145, 235-239; and discussion, 244-249. [DOI] [PubMed] [Google Scholar]
- 7.Wang, Z., Karras, J. G., Howard, R. G. & Rothstein, T. L. (1995) J. Immunol. 155, 3722-3725. [PubMed] [Google Scholar]
- 8.Grewal, I. S. & Flavell, R. A. (1998) Annu. Rev. Immunol. 16, 111-135. [DOI] [PubMed] [Google Scholar]
- 9.Grewal, I. S., Foellmer, H. G., Grewal, K. D., Xu, J., Hardardottir, F., Baron, J. L., Janeway, C. A., Jr., & Flavell, R. A. (1996) Science 273, 1864-1867. [DOI] [PubMed] [Google Scholar]
- 10.Balasa, B., Krahl, T., Patstone, G., Lee, J., Tisch, R., McDevitt, H. O. & Sarvetnick, N. (1997) J. Immunol. 159, 4620-4627. [PubMed] [Google Scholar]
- 11.Homann, D., Jahreis, A., Wolfe, T., Hughes, A., Coon, B., van Stipdonk, M. J., Prilliman, K. R., Schoenberger, S. P. & von Herrath, M. G. (2002) Immunity 16, 403-415. [DOI] [PubMed] [Google Scholar]
- 12.Gordon, E. J., Markees, T. G., Phillips, N. E., Noelle, R. J., Shultz, L. D., Mordes, J. P., Rossini, A. A. & Greiner, D. L. (1998) Diabetes 47, 1199-1206. [DOI] [PubMed] [Google Scholar]
- 13.Kenyon, N. S., Chatzipetrou, M., Masetti, M., Ranuncoli, A., Oliveira, M., Wagner, J. L., Kirk, A. D., Harlan, D. M., Burkly, L. C. & Ricordi, C. (1999) Proc. Natl. Acad. Sci. USA 96, 8132-8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada, A. & Sayegh, M. H. (2002) Transplantation 73, S36-S39. [DOI] [PubMed] [Google Scholar]
- 15.Ehl, S., Hombach, J., Aichele, P., Rulicke, T., Odermatt, B., Hengartner, H., Zinkernagel, R. & Pircher, H. (1998) J. Exp. Med. 187, 763-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green, E. A., Eynon, E. E. & Flavell, R. A. (1998) Immunity 9, 733-743. [DOI] [PubMed] [Google Scholar]
- 17.Yang, X. D. & McDevitt, H. O. (1994) Circ. Shock 43, 198-201. [PubMed] [Google Scholar]
- 18.Green, E. A., Wong, F. S., Eshima, K., Mora, C. & Flavell, R. A. (2000) J. Exp. Med. 191, 225-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 155, 1151-1164. [PubMed] [Google Scholar]
- 20.Suri-Payer, E., Amar, A. Z., Thornton, A. M. & Shevach, E. M. (1998) J. Immunol. 160, 1212-1218. [PubMed] [Google Scholar]
- 21.Takahashi, T., Kuniyasu, Y., Toda, M., Sakaguchi, N., Itoh, M., Iwata, M., Shimizu, J. & Sakaguchi, S. (1998) Int. Immunol. 10, 1969-1980. [DOI] [PubMed] [Google Scholar]
- 22.Read, S., Malmstrom, V. & Powrie, F. (2000) J. Exp. Med. 192, 295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hori, S., Nomura, T. & Sakaguchi, S. (2003) Science 299, 1057-1061.12522256 [Google Scholar]
- 24.Salomon, B., Lenschow, D. J., Rhee, L., Ashourian, N., Singh, B., Sharpe, A. & Bluestone, J. A. (2000) Immunity 12, 431-440. [DOI] [PubMed] [Google Scholar]
- 25.McHugh, R. S., Whitters, M. J., Piccirillo, C. A., Young, D. A., Shevach, E. M., Collins, M. & Byrne, M. C. (2002) Immunity 16, 311-323. [DOI] [PubMed] [Google Scholar]
- 26.Green, E. A., Choi, Y. & Flavell, R. A. (2002) Immunity 16, 183-191. [DOI] [PubMed] [Google Scholar]
- 27.Bystry, R. S., Aluvihare, V., Welch, K. A., Kallikourdis, M. & Betz, A. G. (2001) Nat. Immunol. 2, 1126-1132. [DOI] [PubMed] [Google Scholar]
- 28.Asseman, C., Mauze, S., Leach, M. W., Coffman, R. L. & Powrie, F. (1999) J. Exp. Med. 190, 995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powrie, F., Carlino, J., Leach, M. W., Mauze, S. & Coffman, R. L. (1996) J. Exp. Med. 183, 2669-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng, Y., Laouar, Y., Li, M. O., Green, E. A. & Flavell, R. A. (2004) Proc. Natl. Acad. Sci. USA 101, 4572-4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green, E. A. & Flavell, R. A. (2000) Immunity 12, 459-469. [DOI] [PubMed] [Google Scholar]
- 32.Green, E. A., Gorelik, L., McGregor, C. M., Tran, E. H. & Flavell, R. A. (2003) Proc. Natl. Acad. Sci. USA 100, 10878-10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu, J., Foy, T. M., Laman, J. D., Elliott, E. A., Dunn, J. J., Waldschmidt, T. J., Elsemore, J., Noelle, R. J. & Flavell, R. A. (1994) Immunity 1, 423-431. [DOI] [PubMed] [Google Scholar]
- 34.Serra, P., Amrani, A., Yamanouchi, J., Han, B., Thiessen, S., Utsugi, T., Verdaguer, J. & Santamaria, P. (2003) Immunity 19, 877-889. [DOI] [PubMed] [Google Scholar]
- 35.Walker, L. S., Chodos, A., Eggena, M., Dooms, H. & Abbas, A. K. (2003) J. Exp. Med. 198, 249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner, D. H., Jr., Vaitaitis, G., Sanderson, R., Poulin, M., Dobbs, C. & Haskins, K. (2002) Proc. Natl. Acad. Sci. USA 99, 3782-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner, D. H., Jr., Newell, E., Sanderson, R. J., Freed, J. H. & Newell, M. K. (1999) Int. J. Mol. Med. 4, 231-242. [DOI] [PubMed] [Google Scholar]
- 38.Brenner, B., Koppenhoefer, U., Grassme, H., Kun, J., Lang, F. & Gulbins, E. (1997) FEBS Lett. 417, 301-306. [DOI] [PubMed] [Google Scholar]
- 39.Amrani, A., Serra, P., Yamanouchi, J., Han, B., Thiessen, S., Verdaguer, J. & Santamaria, P. (2002) Immunity 16, 719-732. [DOI] [PubMed] [Google Scholar]
- 40.Im, S. H., Barchan, D., Maiti, P. K., Fuchs, S. & Souroujon, M. C. (2001) J. Immunol. 166, 6893-6898. [DOI] [PubMed] [Google Scholar]
- 41.Monk, N. J., Hargreaves, R. E., Marsh, J. E., Farrar, C. A., Sacks, S. H., Millrain, M., Simpson, E., Dyson, J. & Jurcevic, S. (2003) Nat. Med. 9, 1275-1280. [DOI] [PubMed] [Google Scholar]
- 42.Nathan, M. J., Yin, D., Eichwald, E. J. & Bishop, D. K. (2002) Am. J. Transplant. 2, 323-332. [DOI] [PubMed] [Google Scholar]