Abstract
The intestinal epithelial barrier plays an important role in maintaining host health. Breakdown of intestinal barrier function is known to play a role in many diseases such as infectious enteritis, idiopathic inflammatory bowel disease, and neonatal inflammatory bowel diseases. Recently, increasing research has demonstrated the importance of understanding how intestinal epithelial barrier function develops in the premature neonate in order to develop strategies to promote its maturation. Optimizing intestinal barrier function is thought to be key to preventing neonatal inflammatory bowel diseases such as necrotizing enterocolitis. In this review, we will first summarize the key components of the intestinal epithelial barrier, what is known about its development, and how this may explain NEC pathogenesis. Finally, we will review what therapeutic strategies may be used to promote optimal development of neonatal intestinal barrier function in order to reduce the incidence and severity of NEC.
Keywords: apical junctional complex, commensal bacteria, intestinal epithelial barrier, Necrotizing enterocolitis (NEC), prematurity, probiotics, tight junctions
Abbreviations: AJ, adherens junctions; AJC, apical junction complex; Bb, Bifidobacterium bifidum; Bi, Bifidobacterium infantis; BAs, bile acids; EGF, epidermal growth factor; EPO, erythropoietin; IFNγ, interferon gamma; IEL, intestinal epithelial lymphocytes; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; TCRγδ, T-cell receptor gamma-delta; TJ, tight junctions; TPN, total parenteral nutrition; TGF-β, transforming growth factor-beta; TNFα, tumor necrosis factor alpha
Introduction
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency in the neonatal intensive care unit (NICU), primarily affecting premature infants.1,2 Severe NEC is characterized by hemorrhagic inflammatory necrosis of the distal ileum3 with clinical presentation ranging from abdominal distension, pneumatosis intestinalis, occult or frank blood in stools, intestinal gangrene, bowel perforation, sepsis and shock.4 Approximately 9,000 infants develop NEC in the United States per year and mortality rates range from 20–40%.5-8 Disease-associated costs are significant; premature infants diagnosed with NEC remain, on average, hospitalized for an additional 43.1 days9 with total estimated yearly costs of up to 5 billion dollars.10 NEC patients that require surgery to remove necrotic bowel can also develop short bowel syndrome with prolonged medical expenses and chronic gastrointestinal difficulties. Further, surgical NEC is a significant predictor of neurodevelopmental morbidity in preterm infants independent of other factors.11 Current strategies to reduce the risk of NEC include breastmilk feeds12,13 and probiotic therapy.14 While the major risk factors for NEC (prematurity and enteral formula feeding) are known, the pathophysiology of this disease remains poorly understood and treatment strategies are mainly supportive.
While the etiology of NEC remains unclear, immature gut host defenses and abnormal bacterial colonization are thought to play a critical role.15,16 The neonatal intestinal barrier is immature in the preterm neonate and has been shown to mature postnatally.17-21 Multiple factors can induce postnatal intestinal maturation of this barrier including diet,22-24 epidermal growth factor,25 endogenous glucocorticoids,26 and commensal bacteria.17,27 Commensal bacteria, in particular, are known to induce expression of tight junction proteins that can tighten the barrier.17,28 Thus, neonates with abnormal or delayed bacterial colonization of the gut may be at increased risk for intestinal inflammation and injury due to an immature or defective intestinal barrier that allows systemic entry of microbes, their products, or toxins from the gut lumen.29 This may explain why preterm infants with prolonged antibiotic exposure are at increased risk for NEC30 whereas infants treated with probiotics are protected against the disease.31 In this manuscript, we will review the role of the immature gut barrier as a predisposing factor in the pathogenesis of NEC and how active research is currently targeting the immature gut barrier in order to develop preventive therapeutics against this devastating disease.
After birth, intestinal colonization with microbes from both the birth canal and the environment occurs within 24 hours. Premature infants are often delivered via caesarean section and do not always receive breast milk immediately after birth. In the absence of these major sources of microbial colonization, detrimental microorganisms can proliferate. In addition, the intestinal barrier in neonates is more permeable, in part to allow movement of colostrum-derived antibodies into the infant's circulation. The intracellular structures that regulate intestinal permeability, tight junctions (TJ) and adherens junctions (AJ), as well as other barrier components that play a role in intestinal integrity have been shown to be altered in NEC. Further, inflammation, a hallmark of NEC, has been shown to adversely affect the intestinal barrier.
A comprehensive review of NEC epidemiology, clinical presentation, diagnosis, management, and pathogenesis is beyond the scope of this review. Interested readers are directed toward recent reviews on this topic.1,2 In this manuscript, we will review the role of the immature gut barrier as a predisposing factor in the pathogenesis of NEC and how active research is currently targeting the immature gut barrier in order to develop preventive therapeutics against this devastating disease.
Structure of the Intestinal Epithelial Barrier
The intestinal epithelium is formed by a single layer of cells which separates the host (submucosal side) from the intestinal lumen (luminal side) (Fig. 1). This epithelial layer regulates transport of nutrients, ions and bidirectional fluid flow.32 The luminal surface of the intestinal mucosa exists in a symbiotic eukaryotic-prokaryotic relationship with the commensal flora, which consists of a diverse ecosystem of up to 1011 organisms per gram of intestinal tissue. These bacteria benefit the host by metabolizing vitamins and degrading bile acids while thriving in the nutrient rich, temperature-controlled, anaerobic luminal environment.33 Host-flora interactions are also important for appropriate development of intestinal epithelial structure and barrier function.
Figure 1.
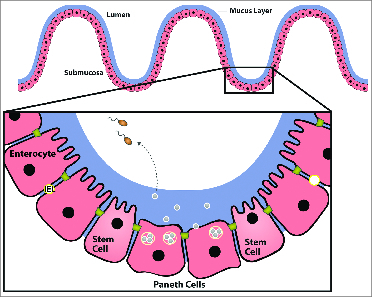
The intestinal epithelial barrier. A single layer of epithelia (including enterocytes, paneth cells and stem cells) separate the intestinal lumen from the submucosa (as labeled). Crypt Paneth cells secrete antimicrobial peptides which regulate microbial populations and protect neighboring stem cells. IEL are innate immune T-cells located between epithelial cells. Specialized IEL bearing the T-cell receptor (TCR) γδ (γδ IEL) have been shown to promote epithelial barrier function by preventing bacterial translocation, producing antimicrobial factors, and through interactions with the tight junction protein, occludin. Adapted with permission from Journal NeoReview, Vol. 10, Page(s) e180-e190, Copyright © 2009 by the AAP.
Epithelia are organized into crypts (invaginations) and villi (evaginations). At the base of these crypts are stem cells, which proliferate, differentiate into enterocytes, migrate to the villus tip, and eventually slough into the lumen via anoikis (a physiologic form of apoptosis). This entire process results in total reconstitution of the epithelium every 5 d.34 Thus, one form of intestinal defense against injury is this remarkable proliferative and self-regenerating capacity.
The intestinal epithelial monolayer also protects and separates itself physically from exogenous stress by forming a thick protective layer of mucus over the intestinal mucosa. This mucus layer is composed of mucins, which are diverse, complex glycoproteins secreted by goblet cells (specialized secretory enterocytes). The mucus layer hampers direct microbial-epithelial binding, aggregates adherent bacteria, and enhances bacterial removal by reducing shear-forces of the luminal stream. Mucins also contain specific protein binding domains, which bind and stabilize critical trophic and reparative factors (e.g. epidermal growth factor (EGF) and intestinal trefoil factor) at the epithelial surface, potentially contributing to epithelial restitution.35,36
Chemical defenses secreted by both absorptive enterocytes and Paneth cells provide additional protection. Paneth cells are specialized secretory enterocytes located at the base of small intestinal crypts (Fig. 1). Adjacent to stem cells, Paneth cells protect by secreting lysozyme, phospholipase A2, and antimicrobial peptides (defensins (α and β) and cathelicidins37,38) that control microbial populations. Initially discovered in human neutrophils, defensins are small cationic peptides that play a key role in oxygen-independent killing of microbes.37 Defensins function by inserting into the membranes of a broad range of prokaryotic cells, including gram-positive and gram-negative bacteria, fungi, protozoa, spirochetes, and enveloped viruses. Once inside the microbial cell membrane, they form pores allowing the passage of anions through the membrane, thus depolarizing and killing the organism.39 Paneth cells secrete α-defensins (human defensin, HD5 and HD6) in response to microbial or cholinergic stimuli, contributing to the relatively sterile and protected environment within intestinal crypts. Intestinal epithelial cells primarily secrete β-defensins (hBD1, 2, and 3) with specific tissue distribution varying along the intestinal axis for each member of the β-defensin family.39 In vitro studies suggest that these antimicrobial peptides may contribute to host defense indirectly (by inducing host responses) as well as directly (by killing microbes).40 Cathelicidins and defensins may have proinflammatory properties by activating chemokine release resulting in immune cell chemotaxis and differentiation. Defensins released into the intestinal crypt may stimulate chloride secretion from nearby enterocytes in order to flush pathogens and toxins away from sensitive stem cells.41 Future studies will be required to characterize the roles of these additional defensin and cathelicidin-induced immune modulatory activities, in vivo.
Epithelial integrity is further regulated by the apical junction complex (AJC), subapical intercellular contacts consisting of membrane proteins and cytoskeletal anchor proteins, which interact to form tight junctions and AJs (Fig. 2). Tight junctions (TJ), which seal the intercellular space between enterocytes while regulating paracellular permeability,42-44 consist of over 40 transmembrane proteins including occludin, claudins, and junctional adhesion molecules such as JAM-A.45 Major proteins comprising the AJ include E-cadherin, and α- and β-catenin and help to anchor cells to one another. The AJCs are linked to the cytoplasmic cytoskeleton to create an F-actin ring. AJCs “zipper” together intestinal epithelial cells and regulate the flow of ions and small molecules between cells.46 The AJCs and their cytoskeletal connections are dynamic structures regulated by physiologic or pathogenic signals. In addition to regulating barrier function, the AJC proteins play important roles in epithelial cell proliferation and differentiation. The epithelial layer also regulates transcellular permeability to ions and other small molecules through alterations in expression of selective membrane ion channels and pores. Enterocytes control Cl- and water secretion through these channels resulting in secretory diarrhea, another defense mechanism which can be used to flush unwanted pathogens or toxins from the intestinal lumen.
Figure 2.
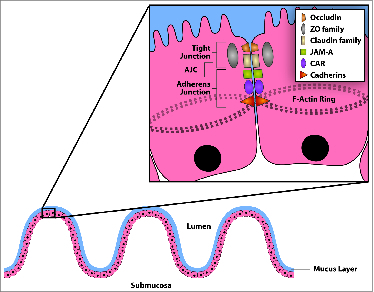
Structure of the apical junctional complex (AJC). Subapically located apical junctional complexes (AJC) protect the host by regulating paracellular flow. AJC is made up of tight junctions and adherens junctions. Major proteins included in the AJC are as labeled. Reproduced with permission from Journal NeoReview, Vol. 10, Page(s) e180-e190, © 2009 The AAP.
Innate immune cells have also recently been shown to contribute to intestinal barrier function. Intestinal epithelial lymphocytes (IEL) are innate immune T-cells located between epithelial cells that form the intestinal barrier. Specialized IEL bearing the T-cell receptor (TCR) γδ (γδ IEL) have been shown to promote epithelial barrier function by preventing bacterial translocation,47 producing antimicrobial factors48 and through interactions with the tight junction protein, occludin.49 TCR γδ IEL can also regulate inflammation and foster epithelial repair.50-53 Thus, the mature epithelium is exquisitely adapted to prevent infection and to maintain and restore barrier function in response to luminal insults using a variety of structures, secreted products and specialized cells.54
Development of the Intestinal Epithelial Barrier
Development of intestinal barrier function occurs both in utero and postnatally (Table 1).17-21 The initial structural barrier of the human intestinal epithelial monolayer forms in utero during the first trimester. As early as 8 weeks of gestation, enterocytes appear, eventually forming the crypt-villus architecture by 12 weeks. The AJC starts to form as early as 10 weeks gestation when intercellular tight junctions can be detected. Thus, by the end of the first trimester of human gestation, the early structural barrier of the intestine is formed. However, full secretory and absorptive capabilities of the intestinal mucosa require the presence of growth and trophic factors present in amniotic fluid to induce their maturation from 26 weeks to term.55
Table 1.
Immaturity of the intestinal barrier in the preterm infant
| Intestinal Barrier Component | Time of Maturation | Effect of Immaturity in the Preterm Infant |
|---|---|---|
| Epithelial Apical Junctional Complex (AJC) | Mature structure at 12 wks gestation (in utero)Mature function at term | Increased intestinal permeabilityImmature absorptive capability Immature secretory capability |
| Paneth Cells | Detectable at 12 wks gestation Secretory capability at 13–20 wks gestation | Decreased number Decreased secretory capability (Lack of antimicrobial peptides required to regulate intestinal colonization) |
| Mucin (Goblet Cells) | Term | Immature mucus layer allows bacteria to contact intestinal epithelia(Lack of physical protective barrier) |
| Intestinal epithelial lymphyocyte (IEL) | TCRγδ subset recruited early (24 wks gestation) | Early recruitment may be important to promote immature barrier function (Important to prevent bacterial translocation, promote TJ function, regulate inflammation, and promote epithelial repair) |
Soon after the initial formation of the AJC, the epithelial barrier begins to develop additional physical and chemical barriers with the production of defensins, lysozyme and mucin gel layers. Paneth cells can be detected by 12 weeks gestation and begin to produce antimicrobial defensins at 13 weeks and lysozyme at 20 weeks.56,57 Ontogeny studies have demonstrated that Paneth cells are developmentally deficient in number and function in the premature, 24-week gestation neonate.58 TCR γδ IEL have been shown to be recruited early to the premature gut (by 24 week gestation in humans and 1 week postnatally in mice), possibly to promote barrier function while the epithelial barrier is immature.59 Mucin expression can be detected as early as 6.5 weeks gestation, but expressional patterns continue to undergo maturation throughout gestation.60
While development of barrier function occurs in utero, there is ongoing postnatal maturation and multiple factors can induce postnatal intestinal barrier maturation including growth factors, hormones, nutrients and microbes.17,22-25,27 Given the benefit of a mature epithelial barrier in preventing injury and inflammation, encouraging growth of the appropriate complement of commensal bacteria may be of particular benefit in premature neonates who are deprived of the benefits of an in utero environment.
An immature epithelial barrier causes increased intestinal permeability. This, in turn, predisposes the gut to invasion of toxins and bacteria located within the gut lumen resulting in both inflammation and injury.61 An important characteristic of the premature gut in humans and other mammals is a leaky epithelial barrier. Over time, the intestinal barrier tightens and becomes more selective in paracellular permeability to both large and small molecules18,19,62 Commensal colonization may be an important driving influence by which the developing intestine reduces intestinal permeability and probiotic bacteria may replicate these effects in premature infants with inappropriate intestinal microbial colonization. Both in vitro studies modeling the adult human intestinal epithelial barrier and in vivo animal studies modeling the premature intestinal epithelial barrier indicate that commensal and probiotic bacteria can improve intestinal epithelial barrier function.17,63 A small clinical trial also confirmed that probiotics can also improve intestinal barrier function by reducing intestinal permeability in premature infants64 and this may be a mechanism by which probiotics can reduce NEC in premature infants.65,66 Animal models of intestinal epithelial barrier disruption during both inflammation and injury have also noted beneficial effects of probiotic bacteria on intestinal epithelial barrier function and TJ expression and localization.67–69
Breakdown of the Intestinal Epithelial Barrier in NEC
We propose that disruption of the intestinal epithelial barrier can predispose premature infants to NEC (Table 2). Premature infants are known to have diminished intestinal barrier function compared to term infants.18,19 If an immature barrier allows invasion of microbes or toxins, this could lead to injury and inflammation in the immature gut which could further damage the already defective epithelial barrier. In fact, many studies have demonstrated how cytokines induced during intestinal inflammation, including NEC, can further weaken intestinal barrier function. For example, Prasad et. al, found the proinflammatory cytokines interferon gamma (IFNγ) and tumor necrosis factor α (TNFα) decreased claudin-2 and claudin-3, and caused redistribution of claudin 4 in T84 cells.70 Using the same human colonic cell line, another group found redistribution of occludin, claudins 1 and 4 and ZO-1 after exposure to IFNγ.71 In Caco 2 cells, TNFα increased permeability with down regulation and redistribution of ZO-1 protein, and interleukin-18 has been shown to disrupt occludin.72 In animal models, injection of mice with TNFα resulted in redistribution of intestinal occludin and ZO-1 73 and TNFα was found to play a role in the disappearance of occludin and ZO-1 during induction of experimental colitis in TNFα knockout mice.74 Further, when neonatal rats with NEC were injected with anti-TNFα, disease was reduced and ileal paracellular permeability was significantly decreased.75
Table 2.
Breakdown of the intestinal epithelial barrier in NEC
| Intestinal Barrier Component | NEC | Effect of NEC in the Preterm Infant |
|---|---|---|
| Epithelial Apical Junctional Complex (AJC) | Dysfunction may predispose to NEC NEC onset causes further TJ protein perturbations (especially reduced expression and abnormal localization of occludin and claudin-3) in part caused by proinflammatory cytokines (TNFα, IFNγ, IL-18) | Further increased intestinal permeability |
| Paneth Cells | Deficiency may predispose to NECNEC onset causes upregulated numbers but these cells are dysfunctional (caused in part by TNFα) | Dysfunctional Paneth cells unable to secrete antimicrobial peptides to control intestinal microbial populations |
| Mucin (Goblet Cells) | Deficiency may predispose to NECNEC onset causes reduced number (caused in part by TNFα) & reduced production of mucins and trefoil factor | Compromised mucus layer allows bacteria to breach epithelial barrier |
| Intestinal epithelial lymphyocyte (IEL) | Deficiency may predispose to NECNEC onset causes reduced overall number (TCRγδ subset preferentially reduced) | TCRγδ IEL deficiency further compromises barrier function (Increased risk of bacterial translocation, TJ dysfunction, and inflammation) |
In both experimental and human NEC, changes in TJs have been reported, although with considerable differences between studies (Table 3). One of the first studies of TJs in NEC,76 found occludin and claudin-3 mRNA and protein elevated in ileum from neonatal rats with NEC. Histologic evaluation revealed these increased proteins were not localized at the TJ, but rather throughout the cytoplasm of the ileal enterocytes. Later studies using the same model showed increased protein levels of claudins-1 and -3, but not occludin,77 increased expression of occludin and claudin-8, but decreased expression of claudins-1, -14, and -15,78 and increased claudin-3 without increased claudin-1 protein with loss of histological ZO-1 in pups with NEC compared to dam-fed controls.79 Bergmann, et al., reported increased claudin-2 in both neonatal mice with NEC as well as in human NEC surgical samples. Changes in localization of occludin, claudins -2, -4 and -7 were also observed in animals with NEC, with these TJ components found primarily in the cytoplasm, rather than at the TJ. Importantly, the concomitant increased permeability preceded over signs of NEC.80 Further, Weitkamp et. al, reported that human NEC samples demonstrate markedly reduced occludin gene expression (compared to age-matched controls).59
Table 3.
Changes in TJs in NEC
| Gene Expression | Protein | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Model | Occludin | Claudin 1 | Claudin 2 | Claudin 3 | Claudin 4 | Claudin 7 | Claudin 8 | Claudin 14 | ZO-1 | Occludin | Claudin 1 | Claudin 2 | Claudin 3 | Claudin 4 | Claudin 7 | Claudin 8 | Claudin 14 | ZO-1 | Localization |
| Clark | Rat | ↑ | NR | NR | ↑ | NR | NR | NR | NR | NR | ↑ | NR | NR | ↑ | NR | NR | NR | NR | NR | Occ, Cl-3 to cytoplasm |
| Rentea | Rat | ↑ | ↓ | NR | NR | NR | NR | ↑ | ↓ | NR | ↔ | ↑ | NR | ↑ | NR | NR | NR | NR | NR | |
| Shiou | Rat | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ↔ | NR | ↑ | NR | NR | NR | NR | ↓ | ZO-1 loss |
| Bergmann | Mouse | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ↑ | NR | ↓ | ↓ | NR | NR | NR | Internalization of Occ, Cl-2,4,7 |
| Human | NR | NR | NR | NR | NR | NR | NR | NR | NR | ↔ | NR | ↑ | NR | NR | NR | NR | NR | ↔ | Changes in CL-2, 4 | |
| Hogberg | Rat | ↑ | ↓ | NR | NR | NR | NR | ↑ | ↓ | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
In all studies, gene expression accessed using RT-PCR except for Hogberg, in which it was assessed via microarray analysis.
NR = not reported.
↑ = statistically significant increase in NEC versus control.
↓ = statistically significant decrease in NEC vs. control.
↔ = no statistically significant change in NEC versus control.
These studies emphasize the disparities among studies with regard to which TJ components are altered. It is also difficult to fully assess these data as in most studies, it is unclear if the authors chose to report only those components where changes occurred. Taken together, however, it is apparent that during the development of NEC, changes in TJs occur and these alterations are associated with increased paracellular permeability, with occludin and claudin-3 the most likely candidates for pathophysiologic effects. While findings of increased permeability in conjunction with elevated TJ proteins may seem paradoxical, it is important to remember that the structure of the AJC is complex. Some proteins in the AJC are known to tighten the TJ (occludin, claudin 3) causing reduced permeability but others are known to cause increased permeability (claudin 2).81 Further, correct localization of each protein to the AJC is critical to maintaining its appropriate function. In many of these studies, the increased components were not associated with the TJ structure, and thus, increased expression did not contribute to the maintenance of a healthy intestinal barrier.
The majority of studies studying the role of TJs in NEC focus on the ileum, the site of injury in this disorder. However, liver-derived proinflammatory mediators play an important role in NEC pathogenesis by increasing the levels of these potentially damaging mediators in the small intestine.82 Changes in localization and abundance of hepatic occludin, claudins-2 and -3, and ZO-1 have been reported in neonatal rats with NEC.83 These alterations allow leakage of TNF-α into the intestinal lumen, which exacerbates intestinal injury, and treatment of rat pups with EGF, a growth factor that can influence epithelial cells to mature, normalizes these changes.83 In addition, bile acids (BAs) have been shown to modulate the structure of TJs and barrier functions in Caco-2 cells.84 BAs, produced in the liver and transported into the ileal lumen, are elevated in the ileum of neonatal rats and mice with NEC,85 and may influence intestinal TJ formation during disease development. Adherens junctions in NEC have been scantily reported, but there appears to be redistribution of ileal e-cadherin, and α and β catenin in NEC, which can be normalized with probiotic treatment86 and changes in hepatic adherens junction proteins observed in NEC are normalized with EGF.83
In addition to changes in the AJC, the goblet cell products mucin and trefoil factors have been investigated in NEC. In both humans87,88 and rats76 with NEC, the number of mucin 2 and trefoil factor 3 goblet cells is significantly reduced and mice with genetically aberrant mucin 2 develop more severe disease than those with normal mucin.89 In addition, ileal BAs, increased in NEC and associated with disease severity, decrease ileal mucin levels in neonatal but not adult ileum,89 data which indicate a possible mechanism for development of NEC in premature infants. Zhang et. al, showed administration of recombinant trefoil factor 3 decreased the incidence and severity of experimental NEC.90 TNFα injected into immature mice resulted in decreased mucin-producing goblet cells. Interestingly, much like BA's effects on mucin, adult animals showed no such effect.87
As early as 1998, decreased Paneth cell products were reported in human NEC. Coutinho, et. al, found decreased numbers of lysozyme positive Paneth cells in babies with NEC.91 More recently, McElroy, et. al, published Paneth cell numbers were decreased in human NEC87 and Puiman, et. al, found Paneth cell hyperplasia with elevated defensin levels after recovery from NEC, but not at diagnosis.92 McElroy, et. al, have also developed a novel model of NEC-like injury that specifically tests the role that Paneth cells may play in NEC pathogenesis and have discovered that TNFα may play a key role in Paneth cell defects seen in NEC.93-95 While Salzman, et. al, showed increased Paneth cell numbers, defensin production from these cells was deficient.58 TCRγδ IEL have also been shown to be reduced in human NEC tissue (compared to age-matched controls) and murine models of NEC-like injury confirm that deficiency of γδ IEL increases disease severity. This study also showed that reduced intestinal occludin could cause decreased intestinal barrier function through indirect effects on reducing γδ IEL recruitment to the epithelial barrier.59
How to Promote Barrier Function to Prevent or Treat NEC (Table 4)
In addition to examination of expression and localization of TJs in NEC, means to counteract or prevent these changes have been investigated. Human breast milk, which has long been known to decrease the risk of NEC,13,96 improves intestinal barrier function (when compared to formula feeding) in both humans and animal models.24 Multiple components in human milk may be responsible for these protective effects.97 Lactoferrin has been shown to reduce increased epithelial permeability caused by lipopolysaccharide in vitro,98 and whey protein and transforming growth factor-β (TGF-β) decrease intestinal permeability by upregulating claudin-4 expression.99 In addition, casein improves intestinal barrier function by upregulating claudin-1 expression while decreasing claudin-2 expression.100 Human milk also contains other growth factors such as EGF, which will be discussed in greater detail below. It is also important to note that lack of enteral feeds can adversely influence intestinal barrier function, in part through negative effects on IEL.101,102 Interestingly, glutamine supplementation in parenteral nutrition may reverse these effects.103
Table 4.
Interventions that may promote barrier function in the preterm infant
| Intestinal Barrier Component | Interventions that Negatively Influence Barrier Function | Interventions to Improve Barrier Function |
|---|---|---|
| Epithelial Apical Junctional Complex (AJC) | Bacterial LPS can increase intestinal permeability TPN can increase proinflammatory cytokines known to negatively affect TJ protein expression and epithelial permeability TPN can also directly reduce TJ protein expression | Breastmilk reduces intestinal permeability. Components (lactoferrin, whey, TGFβ, EGF) have positive effects on TJ protein expression.Probiotics promote TJ and AJ protein expression and reduce intestinal permeabilityAnti-TNF therapy may reverse effects of cytokine-induced increase in epithelial permeability |
| Paneth Cells | More research needed | More research needed |
| Mucin (Goblet Cells) | Cytokines negatively affect goblet cell number and mucin production | Probiotics can reverse these effects |
| Intestinal epithelial lymphyocyte (IEL) | TPN and lack of enteral feeds negatively influences IEL number and function | Trophic feeds and glutamine may promote IEL recruitment and function |
Premature infants are often given total parenteral nutrition (TPN) at birth. While TPN supplies basic nutritional needs for an infant that cannot tolerate enteral feeding, it also can have adverse effects on intestinal barrier function, in part through changes in IEL. In addition, increased expression of proinflammatory cytokines known to alter the epithelial barrier are increased with TPN administration. TPN has also been shown to be associated with decreases in expression of a number of TJ components, including occludin and claudins.101,102
A recent meta-analysis indicate that probiotics are a promising preventive therapy against NEC.31 The potential mechanisms by which probiotics may prevent NEC have been extensively studied in experimental animal models.86,104-116 Specifically, their effect on TJs has been studied by a number of laboratories. In 2009, Khailova, at al., published that oral administration of Bifidobacterium bifidum (Bb) decreased elevated protein levels of ileal occludin and claudin-3 observed in neonatal rats with NEC. In addition, based on localization of these proteins, Bb treatment seemed to enhance formation of more functional TJs compared to untreated pups with NEC.69 This group also demonstrated that oral therapy with Bb normalizes adherens junctions abnormalities and the number of mucin 2 and trefoil factor 3 positive cells in the distal ileum of rats with NEC.69 A separate group showed Bifidobacterium infantis (Bi) preserved the intestinal barrier during experimental NEC in mice by allowing occludin and claudin-4 to localize appropriately at the TJ.80 In a particularly elegant study, Shiou, et al., 106, found neonatal rats with NEC given conditioned media from combinations of Lactobacillus plantarum, Lactobacillus acidophilus and Bifidobacterium infantis cultures were protected from intestinal barrier dysfunction and maintained ZO-1 at the TJ. A small clinical trial also confirmed that probiotics can also improve intestinal barrier function by reducing intestinal permeability in premature infants64 and this may be a mechanism by which probiotics can reduce NEC in premature infants.65,66
Eukaryotic organisms rely on an effective intestinal barrier to protect against pathogenic prokaryotes while appropriately housing beneficial commensal symbiotes. In the developing immature host, an ineffective barrier can predispose to aberrant inflammatory and/or apoptotic responses to bacteria. When considering administration of probiotic therapy to premature infants who are developmentally immunodeficient, immature intestinal barrier function is particularly relevant because it potentially allows these beneficial bacteria access to the submucosa where it may exert pathologic effects. An immature intestinal epithelial barrier may contribute to the development of probiotic-associated sepsis, which has been reported in premature neonates and remains a significant concern mitigating its widespread clinical use.117 However, probiotic and commensal bacteria can also contribute to promoting maturation of this epithelial barrier.17 Thus timing and dosing of these probiotics may be critical to obtaining beneficial effects without unwanted side effects.
Other factors previously shown to decrease incidence and severity of experimental NEC have been investigated for their ability to normalize components of TJs altered in this disease. These include EGF,76 and erythropoietin (EPO).79 Much like the results observed in the use of probiotics in NEC, treatment with EGF and EPO normalized TJ components. Specifically, EGF treatment normalized expression of occludin and claudin-3, and EPO treatment normalized expression of ZO-1, when compared to untreated, NEC controls. As mentioned previously, EGF has also been shown to normalize changes in adherens junctions caused by NEC.83
Summary
Intestinal epithelial barrier dysfunction plays an important role not only in predisposing premature infants to NEC, but also in propagating further intestinal injury and inflammation that can increase the severity of NEC. Many studies have already confirmed the importance of optimizing expression and localization of TJ proteins in the immature intestine in order to reduce the incidence and severity of experimental NEC. Promising therapies include promoting human milk feeds, encouraging growth of commensal bacteria, probiotics, and growth factor supplementation (EGF, TGF-β). Small clinical studies indicate that improvement in premature intestinal barrier function can be achieved with these therapies. Animal studies also indicate that anti-TNFα therapy may also be promising, but clinical trials are needed. Future research to identify changes in key components of the immature intestinal barrier that predispose to or worsen NEC may lead to the development of targeted preventive and treatment strategies as well as to the development of biomarkers to guide optimal timing of clinical interventions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 2006; 368(9543):1271-83; PMID:; http://dx.doi.org/ 10.1016/S0140-6736(06)69525-1 [DOI] [PubMed] [Google Scholar]
- 2.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011; 364(3):255-64; PMID:; http://dx.doi.org/ 10.1056/NEJMra1005408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Israel EJ. Neonatal necrotizing enterocolitis, a disease of the immature intestinal mucosal barrier. Acta Paediatr Suppl 1994; 396:27-32; PMID:; http://dx.doi.org/ 10.1111/j.1651-2227.1994.tb13238.x [DOI] [PubMed] [Google Scholar]
- 4.Neu J. Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr Clin North Am 1996; 43(2):409-32; PMID:; http://dx.doi.org/ 10.1016/S0031-3955(05)70413-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, Verter J, Temprosa M, Wright LL, Ehrenkranz RA, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 2001; 107(1):E1; PMID:; http://dx.doi.org/ 10.1542/peds.107.1.e1 [DOI] [PubMed] [Google Scholar]
- 6.de Souza JC, da Motta UI, Ketzer CR, Prognostic factors of mortality in newborns with necrotizing enterocolitis submitted to exploratory laparotomy. J Pediatr Surg 2001; 36(3):482-6; PMID:; http://dx.doi.org/ 10.1053/jpsu.2001.21603 [DOI] [PubMed] [Google Scholar]
- 7.Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, Lillehei C, Valim C, Horbar JD, Jaksic T. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg 2009; 44(6):1072-5; discussion 1075-6; PMID:; http://dx.doi.org/ 10.1016/j.jpedsurg.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 8.Thyoka M, de Coppi P, Eaton S, Khoo K, Hall NJ, Curry J, Kiely E, Drake D, Cross K, Pierro A. Advanced necrotizing enterocolitis part 1: mortality. Eur J Pediatr Surg 2012; 22(1):8-12; PMID:; http://dx.doi.org/ 10.1055/s-0032-1306263 [DOI] [PubMed] [Google Scholar]
- 9.Ganapathy V.,Hay JW, Kim JH. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed Med 2011; 7(1):29-37; PMID: [DOI] [PubMed] [Google Scholar]
- 10.Bisquera JA, Cooper TR, Berseth CL, Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics 2002; 109(3):423-8; PMID:; http://dx.doi.org/ 10.1542/peds.109.3.423 [DOI] [PubMed] [Google Scholar]
- 11.Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, Simon NP, Wilson DC, Broyles S, Bauer CR, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993-1994. Pediatrics 2000; 105(6):1216-26; PMID: ; http://dx.doi.org/ 10.1542/peds.105.6.1216 [DOI] [PubMed] [Google Scholar]
- 12.McGuire W, Anthony MY. Donor human milk versus formula for preventing necrotising enterocolitis in preterm infants: systematic review. Arch Dis Child Fetal Neonatal Ed 2003; 88(1):F11-4; PMID: ; http://dx.doi.org/ 10.1136/fn.88.1.F11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet 1990; 336(8730):1519-23; PMID:; http://dx.doi.org/ 10.1016/0140-6736(90)93304-8 [DOI] [PubMed] [Google Scholar]
- 14.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid Based Child Health 2014; 9(3):584-671; PMID:; http://dx.doi.org/10.1002/ebch.1976 [DOI] [PubMed] [Google Scholar]
- 15.Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, Hamilton FA, Higgins R, Raju TN. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res 2007; 62(4):510-4; PMID:; http://dx.doi.org/ 10.1203/PDR.0b013e318142580a [DOI] [PubMed] [Google Scholar]
- 16.Patel RM, Lin PW. Developmental biology of gut-probiotic interaction. Gut Microbes 2010; 1(3):186-95; PMID:; http://dx.doi.org/ 10.4161/gmic.1.3.12484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW., Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol 2012; 180(2):626-35; PMID: ; http://dx.doi.org/ 10.1016/j.ajpath.2011.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver LT, Laker MF, Nelson R. Intestinal permeability in the newborn. Arch Dis Child 1984; 59(3):236-41; PMID:; http://dx.doi.org/ 10.1136/adc.59.3.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Elburg RM, Fetter WP, Bunkers CM, Heymans HS. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch Dis Child Fetal Neonatal Ed 2003; 88(1):F52-5; PMID: ; http://dx.doi.org/ 10.1136/fn.88.1.F52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bezerra JA, Thompson SH, Morse M, Koldovský O, Udall JN, Jr. Intestinal permeability to intact lactose in newborns and adults. Biol Neonate 1990; 58(6):334-42; PMID:; http://dx.doi.org/ 10.1159/000243288 [DOI] [PubMed] [Google Scholar]
- 21.Kuvaeva IB. Permeability of the gastronintestinal tract for macromolecules in health and disease. Hum Physiol 1979; 4(2):272-83; PMID: [PubMed] [Google Scholar]
- 22.Goldman AS. Modulation of the gastrointestinal tract of infants by human milk. Interfaces and interactions. An evolutionary perspective. J Nutr 2000; 130(2S Suppl):426S-431S; PMID: [DOI] [PubMed] [Google Scholar]
- 23.Colome G, Sierra C, Blasco J, García MV, Valverde E, Sánchez E. Intestinal permeability in different feedings in infancy. Acta Paediatr 2007; 96(1):69-72; PMID:; http://dx.doi.org/ 10.1111/j.1651-2227.2007.00030.x [DOI] [PubMed] [Google Scholar]
- 24.Weaver LT, Laker MF, Nelson R, Lucas A. Milk feeding and changes in intestinal permeability and morphology in the newborn. J Pediatr Gastroenterol Nutr 1987; 6(3):351-8; PMID:; http://dx.doi.org/ 10.1097/00005176-198705000-00008 [DOI] [PubMed] [Google Scholar]
- 25.Dvorak B.Milk epidermal growth factor and gut protection. J Pediatr 2010; 156(2 Suppl):S31-5; PMID: ; http://dx.doi.org/ 10.1016/j.jpeds.2009.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henning SJ. Development of the gastrointestinal tract. Proc Nutr Soc 1986; 45(1):39-44; PMID: ; http://dx.doi.org/ 10.1079/PNS19860033 [DOI] [PubMed] [Google Scholar]
- 27.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004; 118(2):229-41; PMID:; http://dx.doi.org/ 10.1016/j.cell.2004.07.002 [DOI] [PubMed] [Google Scholar]
- 28.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001; 291(5505):881-884; PMID:; http://dx.doi.org/ 10.1126/science.291.5505.881 [DOI] [PubMed] [Google Scholar]
- 29.Piena-Spoel M, Albers MJ, ten Kate J, Tibboel D. Intestinal permeability in newborns with necrotizing enterocolitis and controls: does the sugar absorption test provide guidelines for the time to (re-)introduce enteral nutrition? J Pediatr Surg 2001; 36(4):587-92; PMID:; http://dx.doi.org/ 10.1053/jpsu.2001.22288 [DOI] [PubMed] [Google Scholar]
- 30.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sánchez PJ, Ambalavanan N, Benjamin DK, Jr; NICHD Neonatal Research Network. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009; 123(1):58-66; PMID:; http://dx.doi.org/ 10.1542/peds.2007-3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2014; 4:CD005496; PMID: [DOI] [PubMed] [Google Scholar]
- 32.Madara JL. Warner-Lambert/Parke-Davis Award lecture. Pathobiology of the intestinal epithelial barrier. Am J Pathol 1990; 137(6):1273-81; PMID: [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Gordon JI. Inaugural Article: Honor thy symbionts. Proc Natl Acad Sci U S A 2003; 100(18):10452-9; PMID:; http://dx.doi.org/ 10.1073/pnas.1734063100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res 2005; 306(2):357-63; PMID:; http://dx.doi.org/ 10.1016/j.yexcr.2005.02.022 [DOI] [PubMed] [Google Scholar]
- 35.Corfield AP, Carroll D, Myerscough N, Probert CS. Mucins in the gastrointestinal tract in health and disease. Front Biosci 2001; 6:D1321-57; PMID: ; http://dx.doi.org/ 10.2741/Corfield [DOI] [PubMed] [Google Scholar]
- 36.Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem 2006; 99(6):1616-27; PMID:; http://dx.doi.org/ 10.1002/jcb.20947 [DOI] [PubMed] [Google Scholar]
- 37.Otte JM, Kiehne K, Herzig KH. Antimicrobial peptides in innate immunity of the human intestine. J Gastroenterol 2003; 38(8):717-26; PMID:; http://dx.doi.org/ 10.1007/s00535-003-1136-5 [DOI] [PubMed] [Google Scholar]
- 38.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 2003; 3(9):710-20; PMID:; http://dx.doi.org/ 10.1038/nri1180 [DOI] [PubMed] [Google Scholar]
- 39.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol 2007; 19(2):70-83; PMID: ; http://dx.doi.org/ 10.1016/j.smim.2007.04.002 [DOI] [PubMed] [Google Scholar]
- 40.Lin PW, A.N., Andrew S. Innate immunity and epithelial biology: special considerations in the neonatal gut, in gastroenterology and nutrition: neonatal questions and controversies, J. Neu, Editor. 2008, Saunders: Philadelphia. p 51-72 [Google Scholar]
- 41.Lin PW, Simon PO, Jr, Gewirtz AT, Neish AS, Ouellette AJ, Madara JL, Lencer WI. Paneth cell cryptdins act in vitro as apical paracrine regulators of the innate inflammatory response. J Biol Chem 2004; 279(19):19902-7; PMID:; http://dx.doi.org/ 10.1074/jbc.M311821200 [DOI] [PubMed] [Google Scholar]
- 42.Balda MS, Fallon MB, Van Itallie CM, Anderson JM. Structure, Regulation, and Pathophysiology of Tight Junctions in the Gastrointestinal-Tract. Yale Journal of Biology and Medicine 1992; 65(6):725-735; PMID: [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson JM, Van Itallie CM. Tight junctions. Curr Biol 2008; 18(20):R941-3; PMID:; http://dx.doi.org/ 10.1016/j.cub.2008.07.083 [DOI] [PubMed] [Google Scholar]
- 44.Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol, 2014; 36C:157-165; PMID:; http://dx.doi.org/ 10.1016/j.semcdb.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol 2006; 22(2):85-9; PMID:; http://dx.doi.org/ 10.1097/01.mog.0000203864.48255.4f [DOI] [PubMed] [Google Scholar]
- 46.Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol 2000; 279(5):G851-7; PMID: [DOI] [PubMed] [Google Scholar]
- 47.Dalton JE, Cruickshank SM, Egan CE, Mears R, Newton DJ, Andrew EM, Lawrence B, Howell G, Else KJ, Gubbels MJ. et al. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology 2006; 131(3):818-29; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 48.Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, Ruhn KA, Hou B, DeFranco AL, Yarovinsky F, et al. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci U S A 2011; 108(21):8743-8; PMID:; http://dx.doi.org/ 10.1073/pnas.1019574108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edelblum KL, Shen L, Weber CR, Marchiando AM, Clay BS, Wang Y, Prinz I, Malissen B, Sperling AI, Turner JR. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci U S A 2012; 109(18):7097-102; PMID: ; http://dx.doi.org/ 10.1073/pnas.1112519109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A 2002; 99(22):14338-43; PMID:; http://dx.doi.org/ 10.1073/pnas.212290499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, Hayday AC. T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci U S A 1996; 93(21):11774-9; PMID:; http://dx.doi.org/ 10.1073/pnas.93.21.11774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gamma delta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol 2004; 172(7):4151-8; PMID:; http://dx.doi.org/ 10.4049/jimmunol.172.7.4151 [DOI] [PubMed] [Google Scholar]
- 53.Komano H, Fujiura Y, Kawaguchi M, Matsumoto S, Hashimoto Y, Obana S, Mombaerts P, Tonegawa S, Yamamoto H, Itohara S. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A 1995; 92(13):6147-51; PMID: ; http://dx.doi.org/ 10.1073/pnas.92.13.6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hecht G. Innate mechanisms of epithelial host defense: spotlight on intestine. Am J Physiol 1999; 277(3 Pt 1):C351-8; PMID: [DOI] [PubMed] [Google Scholar]
- 55.Lebenthal A, Lebenthal E. The ontogeny of the small intestinal epithelium. JPEN J Parenter Enteral Nutr 1999; 23(5 Suppl):S3-6; PMID:; http://dx.doi.org/ 10.1177/014860719902300502 [DOI] [PubMed] [Google Scholar]
- 56.Mallow EB, Harris A, Salzman N, Russell JP, DeBerardinis RJ, Ruchelli E, Bevins CL. Human enteric defensins. Gene structure and developmental expression. J Biol Chem 1996; 271(8):4038-45; PMID:; http://dx.doi.org/ 10.1074/jbc.271.8.4038 [DOI] [PubMed] [Google Scholar]
- 57.Rumbo M, Schiffrin EJ. Ontogeny of intestinal epithelium immune functions: developmental and environmental regulation. Cell Mol Life Sci 2005; 62(12):1288-96; PMID:; http://dx.doi.org/ 10.1007/s00018-005-5033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salzman NH, Polin RA, Harris MC, Ruchelli E, Hebra A, Zirin-Butler S, Jawad A, Martin Porter E, Bevins CL. Enteric defensin expression in necrotizing enterocolitis. Pediatr Res 1998; 44(1):20-6; PMID:; http://dx.doi.org/ 10.1203/00006450-199807000-00003 [DOI] [PubMed] [Google Scholar]
- 59.Weitkamp JH, Rosen MJ, Zhao Z, Koyama T, Geem D, Denning TL, Rock MT, Moore DJ, Halpern MD, Matta P, et al. Small intestinal intraepithelial TCRgammadelta+ T lymphocytes are present in the premature intestine but selectively reduced in surgical necrotizing enterocolitis. PLoS One 2014; 9(6):e99042; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0099042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buisine MP, Devisme L, Savidge TC, Gespach C, Gosselin B, Porchet N, Aubert JP. Mucin gene expression in human embryonic and fetal intestine. Gut 1998; 43(4):519-24; PMID:; http://dx.doi.org/ 10.1136/gut.43.4.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bjarnason I. Intestinal permeability. Gut 1994; 35(1 Suppl):S18-22; PMID:; http://dx.doi.org/ 10.1136/gut.35.1_Suppl.S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Urao M, Okuyama H, Drongowski RA, Teitelbaum DH, Coran AG. Intestinal permeability to small- and large-molecular-weight substances in the newborn rabbit. J Pediatr Surg 1997; 32(10):1424-8; PMID:; http://dx.doi.org/ 10.1016/S0022-3468(97)90553-4 [DOI] [PubMed] [Google Scholar]
- 63.Madsen K. Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001; 121(3):580-91; PMID:; http://dx.doi.org/ 10.1053/gast.2001.27224 [DOI] [PubMed] [Google Scholar]
- 64.Stratiki Z, Costalos C, Sevastiadou S, Kastanidou O, Skouroliakou M, Giakoumatou A, Petrohilou V. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev 2007; 83(9):575-9; PMID:; http://dx.doi.org/ 10.1016/j.earlhumdev.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 65.Alfaleh K, Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2008; 23(1):CD005496. [DOI] [PubMed] [Google Scholar]
- 66.Martin CR, Walker WA. Probiotics: role in pathophysiology and prevention in necrotizing enterocolitis. Semin Perinatol 2008; 32(2):127-37; PMID: ; http://dx.doi.org/ 10.1053/j.semperi.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 67.Qin HL, Shen TY, Gao ZG, Fan XB, Hang XM, Jiang YQ, Zhang HZ. Effect of lactobacillus on the gut microflora and barrier function of the rats with abdominal infection. World J Gastroenterol 2005; 11(17):2591-6; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luyer MD, Buurman WA, Hadfoune M, Speelmans G, Knol J, Jacobs JA, Dejong CH, Vriesema AJ, Greve JW. Strain-specific effects of probiotics on gut barrier integrity following hemorrhagic shock. Infect Immun 2005; 73(6):3686-92; PMID:; http://dx.doi.org/ 10.1128/IAI.73.6.3686-3692.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, Dvorak B. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2009; 297(5):G940-9; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00141.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 2005; 85(9):1139-62; PMID:; http://dx.doi.org/ 10.1038/labinvest.3700316 [DOI] [PubMed] [Google Scholar]
- 71.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 2003; 171(11):6164-72; PMID:; http://dx.doi.org/ 10.4049/jimmunol.171.11.6164 [DOI] [PubMed] [Google Scholar]
- 72.Lapointe TK, Buret AG. Interleukin-18 facilitates neutrophil transmigration via myosin light chain kinase-dependent disruption of occludin, without altering epithelial permeability. Am J Physiol Gastrointest Liver Physiol 2011; 302(3):G343-51; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00202.2011 [DOI] [PubMed] [Google Scholar]
- 73.Marchiando AM, Shen L, Graham WV, Edelblum KL, Duckworth CA, Guan Y, Montrose MH, Turner JR, Watson AJ. The epithelial barrier is maintained by in vivo tight junction expansion during pathologic intestinal epithelial shedding. Gastroenterology 2011; 140(4):1208-1218 e1-2; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fries W, Muja C, Crisafulli C, Cuzzocrea S, Mazzon E. Dynamics of enterocyte tight junctions: effect of experimental colitis and two different anti-TNF strategies. Am J Physiol Gastrointest Liver Physiol 2008; 294(4):G938-47; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00469.2007 [DOI] [PubMed] [Google Scholar]
- 75.Halpern MD, Clark JA, Saunders TA, Doelle SM, Hosseini DM, Stagner AM, Dvorak B. Reduction of experimental necrotizing enterocolitis with anti-TNF-{alpha}. Am J Physiol Gastrointest Liver Physiol 2006; 290:G757-764; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00408.2005 [DOI] [PubMed] [Google Scholar]
- 76.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol 2006; 291(5):G938-49; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00090.2006 [DOI] [PubMed] [Google Scholar]
- 77.Rentea RM, Liedel JL, Welak SR, Cassidy LD, Mayer AN, Pritchard KA. Jr, Oldham KT, Gourlay DM. Intestinal alkaline phosphatase administration in newborns is protective of gut barrier function in a neonatal necrotizing enterocolitis rat model. J Pediatr Surg 2012; 47(6):1135-42; PMID:; http://dx.doi.org/ 10.1016/j.jpedsurg.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 78.Hogberg N, Stenbäck A, Carlsson PO, Wanders A, Lilja HE. Genes regulating tight junctions and cell adhesion are altered in early experimental necrotizing enterocolitis. J Pediatr Surg 2013; 48(11):2308-12; PMID:; http://dx.doi.org/ 10.1016/j.jpedsurg.2013.06.027 [DOI] [PubMed] [Google Scholar]
- 79.Shiou SR, Yu Y, Chen S, Ciancio MJ, Petrof EO, Sun J, Claud EC. Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. J Biol Chem 2011; 286(14):12123-32; PMID:; http://dx.doi.org/ 10.1074/jbc.M110.154625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bergmann KR, Liu SX, Tian R, Kushnir A, Turner JR, Li HL, Chou PM, Weber CR, De Plaen IG. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol 2013; 182(5):1595-606; PMID:; http://dx.doi.org/ 10.1016/j.ajpath.2013.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson JM, Van CM. Itallie, Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 2009; 1(2):a002584; PMID:; http://dx.doi.org/ 10.1101/cshperspect.a002584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Halpern MD, Holubec H, Dominguez JA, Meza YG, Williams CS, Ruth MC, McCuskey RS, Dvorak B. Hepatic inflammatory mediators contribute to intestinal damage in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2003; 284(4):G695-702; PMID: [DOI] [PubMed] [Google Scholar]
- 83.Khailova L, Dvorak K, Arganbright KM, Williams CS, Halpern MD, Dvorak B. Changes in hepatic cell junctions structure during experimental necrotizing enterocolitis: effect of EGF treatment. Pediatr Res 2009; 66(2):140-4; PMID:; http://dx.doi.org/ 10.1203/PDR.0b013e3181aa3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raimondi F, Santoro P, Barone MV, Pappacoda S, Barretta ML, Nanayakkara M, Apicella C, Capasso L, Paludetto R. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol 2008; 294(4):G906-13; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00043.2007 [DOI] [PubMed] [Google Scholar]
- 85.Halpern MD, Holubec H, Saunders TA, Dvorak K, Clark JA, Doelle SM, Ballatori N, Dvorak B. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology 2006; 130(2):359-72; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2005.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, Dvorak B. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol, 2009; 297(5):G940-9; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McElroy SJ, Prince LS, Weitkamp JH, Reese J, Slaughter JC, Polk DB. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: a potential role in neonatal necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2011; 301:G656-G666; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00550.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schaart MW, de Bruijn AC, Bouwman DM, de Krijger RR, van Goudoever JB, Tibboel D, Renes IB. Epithelial functions of the residual bowel after surgery for necrotising enterocolitis in human infants. J Pediatr Gastroenterol Nutr 2009; 49(1):31-41; PMID:; http://dx.doi.org/ 10.1097/MPG.0b013e318186d341 [DOI] [PubMed] [Google Scholar]
- 89.Martin NA, Mount Patrick SK, Estrada TE, Frisk HA, Rogan DT, Dvorak B, Halpern MD. Active transport of bile acids decreases mucin 2 in neonatal ileum: implications for development of necrotizing enterocolitis. PLoS One 2011; 6(12):e27191; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0027191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang BH, Yu HG, Sheng ZX, Luo HS, Yu JP. The therapeutic effect of recombinant human trefoil factor 3 on hypoxia-induced necrotizing enterocolitis in immature rat. Regul Pept 2003; 116(1-3):53-60; PMID:; http://dx.doi.org/ 10.1016/S0167-0115(03)00177-0 [DOI] [PubMed] [Google Scholar]
- 91.Coutinho HB, da Mota HC, Coutinho VB, Robalinho TI, Furtado AF, Walker E, King G, Mahida YR, Sewell HF, Wakelin D. Absence of lysozyme (muramidase) in the intestinal Paneth cells of newborn infants with necrotising enterocolitis. J Clin Pathol 1998; 51(7):512-4; PMID:; http://dx.doi.org/ 10.1136/jcp.51.7.512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Puiman PJ, Burger-Van Paassen N, Schaart MW, De Bruijn AC, De Krijger RR, Tibboel D, Van Goudoever JB, Renes IB. Paneth cell hyperplasia and metaplasia in necrotizing enterocolitis. Pediatr Res 2011; 69(3):217-23; PMID:; http://dx.doi.org/ 10.1203/PDR.0b013e3182092a9a [DOI] [PubMed] [Google Scholar]
- 93.Brown KS, Gong H, Frey MR, Pope B, Golden M, Martin K, Obey M, McElroy SJ. Tumor necrosis factor induces developmental stage-dependent structural changes in the immature small intestine. Mediators Inflamm 2014; 2014:852378; PMID:; http://dx.doi.org/ 10.1155/2014/852378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McElroy SJ, Underwood MA, Sherman MP. Paneth cells and necrotizing enterocolitis: a novel hypothesis for disease pathogenesis. Neonatology 2013; 103(1):10-20; PMID:; http://dx.doi.org/ 10.1159/000342340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang C, Sherman MP, Prince LS, Bader D, Weitkamp JH, Slaughter JC, McElroy SJ. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis Model Mech 2012; 5(4):522-32; PMID:; http://dx.doi.org/ 10.1242/dmm.009001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 2014; 4:CD002971; PMID: [DOI] [PubMed] [Google Scholar]
- 97.Kotler BM, Kerstetter JE, Insogna KL. Claudins, dietary milk proteins, and intestinal barrier regulation. Nutr Rev 2013; 71(1):60-5; PMID:; http://dx.doi.org/ 10.1111/j.1753-4887.2012.00549.x [DOI] [PubMed] [Google Scholar]
- 98.Hirotani Y, Ikeda K, Kato R, Myotoku M, Umeda T, Ijiri Y, Tanaka K. Protective effects of lactoferrin against intestinal mucosal damage induced by lipopolysaccharide in human intestinal Caco-2 cells. Yakugaku Zasshi 2008; 128(9):1363-8; PMID:; http://dx.doi.org/ 10.1248/yakushi.128.1363 [DOI] [PubMed] [Google Scholar]
- 99.Hering NA, Andres S, Fromm A, van Tol EA, Amasheh M, Mankertz J, Fromm M, Schulzke JD. Transforming growth factor-beta, a whey protein component, strengthens the intestinal barrier by upregulating claudin-4 in HT-29/B6 cells. J Nutr 2011; 141(5):783-9; PMID:; http://dx.doi.org/ 10.3945/jn.110.137588 [DOI] [PubMed] [Google Scholar]
- 100.Visser JT, Lammers K, Hoogendijk A, Boer MW, Brugman S, Beijer-Liefers S, Zandvoort A, Harmsen H, Welling G, Stellaard F, et al. Restoration of impaired intestinal barrier function by the hydrolysed casein diet contributes to the prevention of type 1 diabetes in the diabetes-prone BioBreeding rat. Diabetologia 2010; 53(12):2621-8; PMID:; http://dx.doi.org/ 10.1007/s00125-010-1903-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wildhaber BE, Yang H, Spencer AU, Drongowski RA, Teitelbaum DH. Lack of enteral nutrition–effects on the intestinal immune system. J Surg Res 2005; 123(1):8-16; PMID:; http://dx.doi.org/ 10.1016/j.jss.2004.06.015 [DOI] [PubMed] [Google Scholar]
- 102.Yang H, Feng Y, Sun X, Teitelbaum DH. Enteral versus parenteral nutrition: effect on intestinal barrier function. Ann N Y Acad Sci 2009; 1165:338-46; PMID:; http://dx.doi.org/ 10.1111/j.1749-6632.2009.04026.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nose K, Yang H, Sun X, Nose S, Koga H, Feng Y, Miyasaka E, Teitelbaum DH. Glutamine prevents total parenteral nutrition-associated changes to intraepithelial lymphocyte phenotype and function: a potential mechanism for the preservation of epithelial barrier function. J Interferon Cytokine Res 2010; 30(2):67-80; PMID:; http://dx.doi.org/ 10.1089/jir.2009.0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Y, Tran DQ, Fatheree NY, Marc Rhoads J. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2014; 307(2):G177-86; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00038.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Good M, Sodhi CP, Ozolek JA, Buck RH, Goehring KC, Thomas DL, Vikram A, Bibby K, Morowitz MJ, Firek B, et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am J Physiol Gastrointest Liver Physiol 2014; 306(11):G1021-32; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00452.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shiou SR, Yu Y, Guo Y, He SM, Mziray-Andrew CH, Hoenig J, Sun J, Petrof EO, Claud EC. Synergistic protection of combined probiotic conditioned media against neonatal necrotizing enterocolitis-like intestinal injury. PLoS One 2013; 8(5):e65108; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0065108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu SF, Chiu HY, Chen AC, Lin HY, Lin HC, Caplan M. Efficacy of different probiotic combinations on death and necrotizing enterocolitis in a premature rat model. J Pediatr Gastroenterol Nutr 2013; 57(1):23-8; PMID:; http://dx.doi.org/ 10.1097/MPG.0b013e3182929210 [DOI] [PubMed] [Google Scholar]
- 108.Mirpuri J, Sotnikov I, Myers L, Denning TL, Yarovinsky F, Parkos CA, Denning PW, Louis NA. Lactobacillus rhamnosus (LGG) regulates IL-10 signaling in the developing murine colon through upregulation of the IL-10R2 receptor subunit. PLoS One 2012; 7(12):e51955; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0051955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ganguli K, Meng D, Rautava S, Lu L, Walker WA, Nanthakumar N. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am J Physiol Gastrointest Liver Physiol 2013; 304(2):G132-41; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00142.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Underwood MA, Kananurak A, Coursodon CF, Adkins-Reick CK, Chu H, Bennett SH, Wehkamp J, Castillo PA, Leonard BC, Tancredi DJ, et al. Bifidobacterium bifidum in a rat model of necrotizing enterocolitis: antimicrobial peptide and protein responses. Pediatr Res 2012; 71(5):546-51; PMID:; http://dx.doi.org/ 10.1038/pr.2012.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cilieborg MS, Thymann T, Siggers R, Boye M, Bering SB, Jensen BB, Sangild PT. The incidence of necrotizing enterocolitis is increased following probiotic administration to preterm pigs. J Nutr 2011; 141(2):223-30; PMID:; http://dx.doi.org/ 10.3945/jn.110.128561 [DOI] [PubMed] [Google Scholar]
- 112.Khailova L, Mount Patrick SK, Arganbright KM, Halpern MD, Kinouchi T, Dvorak B. Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2010; 299(5):G1118-27; PMID:; http://dx.doi.org/ 10.1152/ajpgi.00131.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.D'Souza A, Fordjour L, Ahmad A, Cai C, Kumar D, Valencia G, Aranda JV, Beharry KD. Effects of probiotics, prebiotics, and synbiotics on messenger RNA expression of caveolin-1, NOS, and genes regulating oxidative stress in the terminal ileum of formula-fed neonatal rats. Pediatr Res 2010; 67(5):526-31; PMID:; http://dx.doi.org/ 10.1203/PDR.0b013e3181d4ff2b [DOI] [PubMed] [Google Scholar]
- 114.Siggers RH, Siggers J, Boye M, Thymann T, Mølbak L, Leser T, Jensen BB, Sangild PT. Early administration of probiotics alters bacterial colonization and limits diet-induced gut dysfunction and severity of necrotizing enterocolitis in preterm pigs. J Nutr 2008; 138(8):1437-44; PMID: [DOI] [PubMed] [Google Scholar]
- 115.Lin PW, Myers LE, Ray L, Song SC, Nasr TR, Berardinelli AJ, Kundu K, Murthy N, Hansen JM, Neish AS. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic Biol Med 2009; 47(8):1205-11; PMID:; http://dx.doi.org/ 10.1016/j.freeradbiomed.2009.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin PW, Nasr TR, Berardinelli AJ, Kumar A, Neish AS. The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatr Res 2008; 64(5):511-6; PMID:; http://dx.doi.org/ 10.1203/PDR.0b013e3181827c0f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Caplan MS. Probiotic and prebiotic supplementation for the prevention of neonatal necrotizing enterocolitis. J Perinatol 2009; 29Suppl 2:S2-6; PMID:; http://dx.doi.org/ 10.1038/jp.2009.21 [DOI] [PubMed] [Google Scholar]


