Abstract
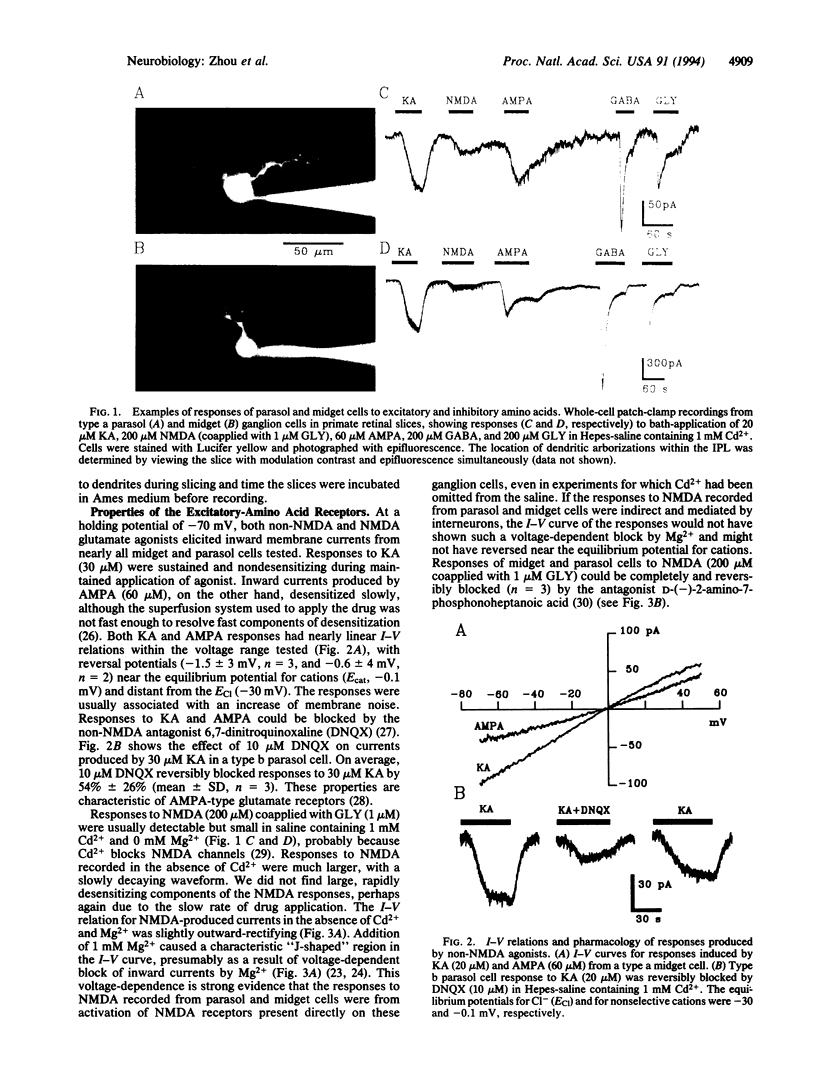
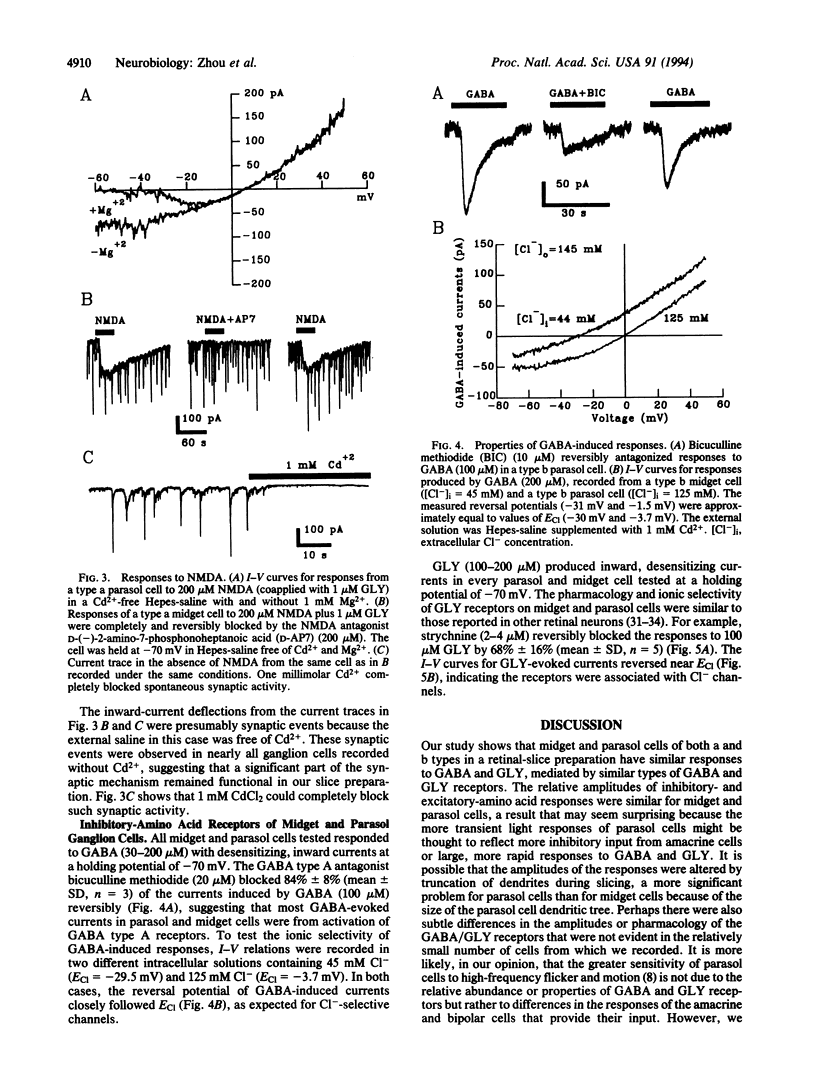
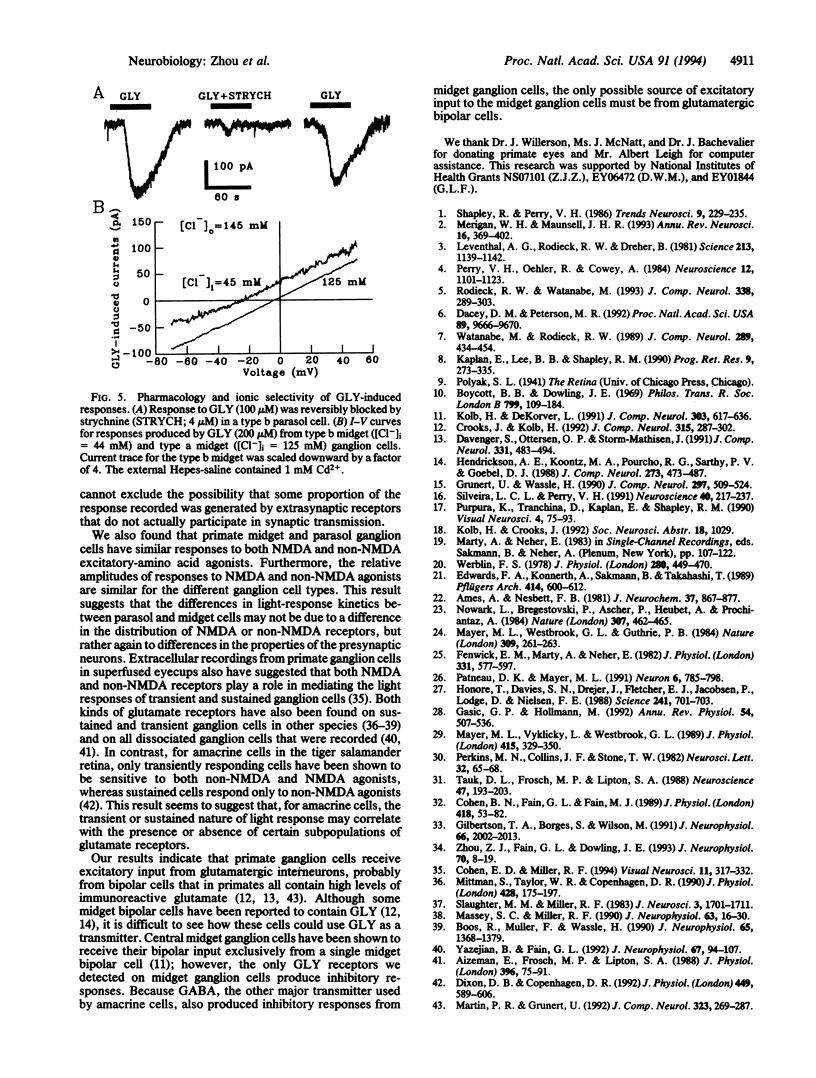
Primate retinas contain two major ganglion cell types. Midget (or P type) cells have relatively sustained responses to light; the amplitude and polarity of these responses vary with stimulus wavelength. Parasol (or M type) cells are more sensitive to stimulus contrast and respond more transiently but are not selective for color. Both types can be further subdivided into a and b subtypes, according to the level of their dendritic stratification in the inner plexiform layer. To determine whether differences in receptors for amino acid transmitters are the basis for any differences in ganglion cell light responses, we made whole-cell, patch-clamp recordings from identified ganglion cells in slice preparations of macaque and baboon retinas. We found that midget and parasol cells of both a and b types had similar responses to excitatory amino acids, including kainate, alpha-amino-3-hydroxy-5-methylisoxalzole-4-propionic acid, and N-methyl-D-aspartate, with reversal potentials near the equilibrium potential for cations. Kainate responses were blocked by 6,7-dinitroquinoxaline, and N-methyl-D-aspartate responses were blocked by D-(-)-2-amino-7-phosphonoheptanoic acid. The four types of ganglion cells also had similar responses to bath-applied inhibitory amino acids. All cells had both gamma-aminobutyric acid and glycine receptors with reversal potentials near the equilibrium potential for Cl-, and the relative amplitudes of the responses to excitatory and inhibitory amino acids were similar among the various cell types. These results suggest that the differences in response properties of the different classes of ganglion cells in primate retina may be determined, to a significant degree, by the properties of the amacrine and bipolar cells that provide their input rather than by the nature of their postsynaptic receptors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizenman E., Frosch M. P., Lipton S. A. Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. J Physiol. 1988 Feb;396:75–91. doi: 10.1113/jphysiol.1988.sp016951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A., 3rd, Nesbett F. B. In vitro retina as an experimental model of the central nervous system. J Neurochem. 1981 Oct;37(4):867–877. doi: 10.1111/j.1471-4159.1981.tb04473.x. [DOI] [PubMed] [Google Scholar]
- Boos R., Müller F., Wässle H. Actions of excitatory amino acids on brisk ganglion cells in the cat retina. J Neurophysiol. 1990 Nov;64(5):1368–1379. doi: 10.1152/jn.1990.64.5.1368. [DOI] [PubMed] [Google Scholar]
- Cohen B. N., Fain G. L., Fain M. J. GABA and glycine channels in isolated ganglion cells from the goldfish retina. J Physiol. 1989 Nov;418:53–82. doi: 10.1113/jphysiol.1989.sp017828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E. D., Miller R. F. The role of NMDA and non-NMDA excitatory amino acid receptors in the functional organization of primate retinal ganglion cells. Vis Neurosci. 1994 Mar-Apr;11(2):317–332. doi: 10.1017/s0952523800001668. [DOI] [PubMed] [Google Scholar]
- Crooks J., Kolb H. Localization of GABA, glycine, glutamate and tyrosine hydroxylase in the human retina. J Comp Neurol. 1992 Jan 15;315(3):287–302. doi: 10.1002/cne.903150305. [DOI] [PubMed] [Google Scholar]
- Dacey D. M., Petersen M. R. Dendritic field size and morphology of midget and parasol ganglion cells of the human retina. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9666–9670. doi: 10.1073/pnas.89.20.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D. B., Copenhagen D. R. Two types of glutamate receptors differentially excite amacrine cells in the tiger salamander retina. J Physiol. 1992 Apr;449:589–606. doi: 10.1113/jphysiol.1992.sp019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic G. P., Hollmann M. Molecular neurobiology of glutamate receptors. Annu Rev Physiol. 1992;54:507–536. doi: 10.1146/annurev.ph.54.030192.002451. [DOI] [PubMed] [Google Scholar]
- Gilbertson T. A., Borges S., Wilson M. The effects of glycine and GABA on isolated horizontal cells from the salamander retina. J Neurophysiol. 1991 Dec;66(6):2002–2013. doi: 10.1152/jn.1991.66.6.2002. [DOI] [PubMed] [Google Scholar]
- Grünert U., Wässle H. GABA-like immunoreactivity in the macaque monkey retina: a light and electron microscopic study. J Comp Neurol. 1990 Jul 22;297(4):509–524. doi: 10.1002/cne.902970405. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. E., Koontz M. A., Pourcho R. G., Sarthy P. V., Goebel D. J. Localization of glycine-containing neurons in the Macaca monkey retina. J Comp Neurol. 1988 Jul 22;273(4):473–487. doi: 10.1002/cne.902730404. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Kolb H., Dekorver L. Midget ganglion cells of the parafovea of the human retina: a study by electron microscopy and serial section reconstructions. J Comp Neurol. 1991 Jan 22;303(4):617–636. doi: 10.1002/cne.903030408. [DOI] [PubMed] [Google Scholar]
- Leventhal A. G., Rodieck R. W., Dreher B. Retinal ganglion cell classes in the Old World monkey: morphology and central projections. Science. 1981 Sep 4;213(4512):1139–1142. doi: 10.1126/science.7268423. [DOI] [PubMed] [Google Scholar]
- Martin P. R., Grünert U. Spatial density and immunoreactivity of bipolar cells in the macaque monkey retina. J Comp Neurol. 1992 Sep 8;323(2):269–287. doi: 10.1002/cne.903230210. [DOI] [PubMed] [Google Scholar]
- Massey S. C., Miller R. F. N-methyl-D-aspartate receptors of ganglion cells in rabbit retina. J Neurophysiol. 1990 Jan;63(1):16–30. doi: 10.1152/jn.1990.63.1.16. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Westbrook G. L. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol. 1989 Aug;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Merigan W. H., Maunsell J. H. How parallel are the primate visual pathways? Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Mittman S., Taylor W. R., Copenhagen D. R. Concomitant activation of two types of glutamate receptor mediates excitation of salamander retinal ganglion cells. J Physiol. 1990 Sep;428:175–197. doi: 10.1113/jphysiol.1990.sp018206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Patneau D. K., Mayer M. L. Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron. 1991 May;6(5):785–798. doi: 10.1016/0896-6273(91)90175-y. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Collins J. F., Stone T. W. Isomers of 2-amino-7-phosphonoheptanoic acid as antagonists of neuronal excitants. Neurosci Lett. 1982 Sep 20;32(1):65–68. doi: 10.1016/0304-3940(82)90230-0. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Oehler R., Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984 Aug;12(4):1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Purpura K., Tranchina D., Kaplan E., Shapley R. M. Light adaptation in the primate retina: analysis of changes in gain and dynamics of monkey retinal ganglion cells. Vis Neurosci. 1990 Jan;4(1):75–93. doi: 10.1017/s0952523800002789. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Watanabe M. Survey of the morphology of macaque retinal ganglion cells that project to the pretectum, superior colliculus, and parvicellular laminae of the lateral geniculate nucleus. J Comp Neurol. 1993 Dec 8;338(2):289–303. doi: 10.1002/cne.903380211. [DOI] [PubMed] [Google Scholar]
- Silveira L. C., Perry V. H. The topography of magnocellular projecting ganglion cells (M-ganglion cells) in the primate retina. Neuroscience. 1991;40(1):217–237. doi: 10.1016/0306-4522(91)90186-r. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J Neurosci. 1983 Aug;3(8):1701–1711. doi: 10.1523/JNEUROSCI.03-08-01701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauck D. L., Frosch M. P., Lipton S. A. Characterization of GABA- and glycine-induced currents of solitary rodent retinal ganglion cells in culture. Neuroscience. 1988 Oct;27(1):193–203. doi: 10.1016/0306-4522(88)90230-8. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Rodieck R. W. Parasol and midget ganglion cells of the primate retina. J Comp Neurol. 1989 Nov 15;289(3):434–454. doi: 10.1002/cne.902890308. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Transmission along and between rods in the tiger salamander retina. J Physiol. 1978 Jul;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazejian B., Fain G. L. Excitatory amino acid receptors on isolated retinal ganglion cells from the goldfish. J Neurophysiol. 1992 Jan;67(1):94–107. doi: 10.1152/jn.1992.67.1.94. [DOI] [PubMed] [Google Scholar]
- Zhou Z. J., Fain G. L., Dowling J. E. The excitatory and inhibitory amino acid receptors on horizontal cells isolated from the white perch retina. J Neurophysiol. 1993 Jul;70(1):8–19. doi: 10.1152/jn.1993.70.1.8. [DOI] [PubMed] [Google Scholar]




