Abstract
Although both naive and effector T lymphocytes interact with antigen-expressing cells, the functional outcome of these interactions is distinct. Naive CD8+ T cells are activated to proliferate and differentiate into effector cytolytic T lymphocytes (CTL), whereas CTL interact with specific targets, such as tumor cells, to induce apoptotic death. We recently observed that several molecules linked to actin cytoskeleton dynamics were up-regulated in effector vs. naive CD8+ T cells, leading us to investigate whether T cell differentiation is accompanied by changes in actin-dependent processes. We observed that both naive and effector CD8+ T cells underwent T cell receptor capping and formed stable conjugates with antigen-specific antigen-presenting cells. However, the characteristics of the immunological synapse were distinct. Whereas accumulation of signaling molecules at the T cell/antigen-presenting cell contact site was detectable in both naive and effector CD8+ T cells, only effector cells developed a central supramolecular activation cluster as defined by punctate focusing of PKCθ, phospho-PKCθ, and phospho-ZAP70. Extended kinetics, CD28 costimulation, and high-affinity antigenic peptide did not promote PKCθ focusing in naive cells. Nonetheless, naive CD8+ T cells polarized the microtubule organizing center, produced IL-2, proliferated, and differentiated into effector cells. Our results suggest that the formation of a central supramolecular activation cluster is not required for activation of naive CD8+ T cells and support the notion that one role of an organized immune synapse is directed delivery of effector function.
CD8+ T cells play a critical role in the clearance of viral infections and the eradication of tumors. However, the acquisition of lytic activity occurs only after the differentiation of naive CD8+ T cells to the effector state. Activation of naive CD8+ T cells requires direct ligation of cell surface receptors on the T cell by cognate ligands on an antigen-presenting cell (APC). Subsequent to T cell–APC contact, large-scale rearrangement of the cytoskeleton and reorganization of cell surface and cytoplasmic molecules result in the formation of an “immunological synapse” (1, 2). Spatial segregation of accumulated molecules has been reported to occur at the interface, resulting in the formation of a central and peripheral supramolecular activation cluster (cSMAC and pSMAC, respectively) (3). The cSMAC has been characterized by punctate localization of PKCθ, whereas the pSMAC can be defined by a ring of accumulated talin (3). A dynamic actin cytoskeleton is required, at least in part, for these molecular rearrangements to occur (4).
The functional significance of cSMAC/pSMAC segregation is not clear. Early studies indicated that agonist peptides induced cSMAC formation, whereas partial agonist or antagonist peptides, which failed to induce cytokine production, did not (1). These observations implied by correlation that cSMAC formation might be necessary for full T cell activation. However, kinetic studies have not supported this model, as T cell receptor (TCR)-mediated tyrosine kinase signaling seems to occur at the periphery of the immunological synapse and before cSMAC formation (5). An alternative hypothesis is that immune synapse formation is less important for T cell activation but rather facilitates directional release of cytokines and other effector molecules toward antigen-expressing targets (6, 7). This model might predict distinct properties of the immune synapse between naive T cells, which lack effector function, and primed effector T cells, which produce effector cytokines and can possess cytolytic activity.
Using Affymetrix (Santa Clara, CA) gene arrays, we recently observed that several molecules linked to actin cytoskeletal dynamics were up-regulated in effector T cells compared with naive CD8+ TCR transgenic T cells (8). This observation, coupled with the fact that most analyses of the immunologic synapse have been done with primed CD4+ T cells, prompted a careful comparison of the T cell/APC interface in naive vs. effector CD8+ TCR transgenic T cells. We observed that effector T cells, but not naive 2C TCR transgenic CD8+ T cells, formed a cSMAC, thus correlating this structure with the acquisition of effector function. Despite the lack of detectable cSMAC formation in naive CD8+ T cells, microtubule organizing center (MTOC) polarization, IL-2 production, and subsequent proliferation and differentiation occurred, suggesting that cSMAC formation is not required for naive CD8+ T cell activation.
Materials and Methods
T Cell Purification and Differentiation. All mice were housed in the University of Chicago Animal Facility under specific pathogen-free conditions. 2C/recombination activating gene (RAG) 2-/- mice have been described in ref. 9. Naive and effector CD8+ T cells were purified and generated as described in ref. 8. Briefly, naive T cells were purified by negative selection from the spleens of 2C/RAG2-/- mice. Effector cells were generated by coculture of naive cells in vitro with mitomycin C-treated P815.B71 cells over two 4-day stimulations.
Immunofluorescence Antibodies. The following reagents were used: polyclonal rabbit anti-PKCθ, rabbit antiphospho-Zap-70, and goat antitalin from Santa Cruz Biotechnology; mAb anti-CD3 (2C11, hamster IgG) from Pharmingen; mAb antiphosphotyrosine (4G10, murine IgG2b) from Upstate Biotechnology (Lake Placid, NY); mAb tubulin DM1A from NeoMarkers (Lab Vision, Fremont, CA); polyclonal rabbit antiphospho-PKCθ from Cell Signaling Technology (Beverly, MA); FITC-conjugated donkey anti-rabbit IgG or donkey anti-mouse IgG; and Texas red-conjugated donkey anti-goat IgG from Jackson ImmunoResearch.
TCR Capping. T cells (1 × 106) were incubated with FITC-anti-CD3 Ab (2C11, Pharmingen) at 4°C for 30 min. Prewarmed goat anti-hamster Ab (Cappel) was added, and cells were incubated at 37°C for the indicated duration. Cold PBS was added to stop the reaction, and cells were fixed and analyzed by confocal microscopy.
Conjugate Formation. This assay was performed similarly to the description in ref. 10. T cells were labeled with calcein AM (Molecular Probes), and either EL4 cells or P815 cells were labeled with PKH (Sigma). T cells (2.5 × 105) and targets (5 × 105) were mixed, centrifuged, lightly vortexed, and incubated at 37°C for the indicated times. Cells were then vortexed vigorously for 30 sec and immediately fixed. Two-color flow cytometric analysis was performed, and percent conjugates were determined by calculating the ratio of double-positive conjugates to the total number of T cells. Blocking of the class I MHC molecule Ld was carried out in the presence of either the anti-Ld Ab 30-5-7s or isotype control (final concentration of 50 μg/ml).
Immunofluorescence. To distinguish APCs from T cells, APCs were loaded with the vital dye 7-amino-4-chloromethylcoumarin (CMAC) Cell-Tracker Blue (Molecular Probes) as described in ref. 11. APCs (1.5 × 105) were mixed with an equal number of Ficoll/Hypaque purified T cells in DMEM with 10% FCS, centrifuged at 5,000 rpm for 30 sec, and incubated for the indicated time (minus 2 min) at 37°C. Supernatant was aspirated, and conjugates were gently resuspended in serum-free DMEM by using a 1,000-μl pipettor and plated onto poly(l)-lysine-coated (molecular weight 30,000–70,000, Sigma) slides for 2 min before fixation. Slides were fixed in 3% (wt/vol) paraformaldehyde in PBS for 15 min. Samples were permeabilized in 0.3% (vol/vol) Triton X-100 (Sigma) in PBS for 10 min, rinsed in PBS, and blocked in DMEM containing 10% FCS for 5 min. All subsequent Ab incubations were performed in calcium/magnesium-free DPBS containing 2% FCS. Primary and secondary Abs were applied sequentially for 60 min at room temperature and washed five times after each incubation with DPBS. After fluorochrome labeling, specimens were mounted in Mowiol 4-88 (Hoechst Celanese, Charlotte, NC), with 10% 1,4-diazobicyclo[2.2.2]octane (Sigma) added as an antifading agent. Samples were analyzed by using a Zeiss Axiovert 100 microscope. Image capture and deconvolution analysis, where appropriate, was performed by using slidebook software(Intelligent Imaging Innovations, Denver).
T2-Ld Peptide Loading. T2-Ld cells were incubated for 3 h either in the presence or absence of 100 μM QL9 peptide (QLSPFPFDL). During the final hour of peptide loading, CMAC labeling was performed as described in ref. 11, either in the presence or absence of 100 μM QL9 peptide.
IL-2 Assay. IL-2 concentrations were measured by ELISA with Ab pairs obtained from BD Pharmingen. Concentrations are expressed in units/ml, as determined by using recombinant cytokine as a standard.
RT-PCR. Twenty micrograms of total RNA was used for first-strand cDNA synthesis. Samples were normalized based on equivalent β-actin expression by RT-PCR. For each PCR amplification, 1 μl of cDNA was used in a 50-μl reaction. To ensure that amplification remained within linear range, 1:5 serial dilutions were made. RT-PCR for β-actin (25 cycles) was used as a control for mRNA abundance. For IL-2 expression analysis, primers were purchased from BioSource International (Camarillo, CA), and 60 thermal cycles were performed. The annealing temperature was 55°C.
Results
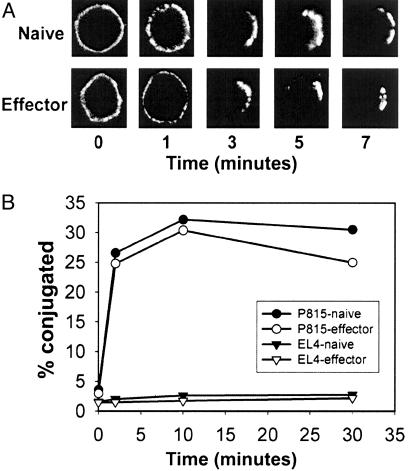
Naive and Effector CD8+ T Cells Are Capable of Redistributing TCR and Forming Conjugates with APCs with Similar Kinetics. To study homogeneous populations of CD8+ T cells in defined differentiation states, we used naive and effector T cells from 2C TCR transgenic/RAG2-/- mice (8, 9). To verify that both cell subsets possessed the machinery for receptor clustering, we examined TCR capping in response to secondary Ab crosslinking. As shown in Fig. 1A, both naive and effector CD8+ T cells underwent TCR capping with comparable kinetics. Because the ultimate aim of our study was to examine the nature of the immunologic synapse at the T cell/APC interface, naive and effector 2C cells were analyzed for the ability to form specific conjugates with antigen-expressing APCs. Conjugation assays were performed with P815 cells, a mouse mastocytoma cell that expresses the peptide P2Ca (LSPFPFDL), which is derived from the ubiquitous α-ketoglutarate dehydrogenase, in the context of the allogeneic class I molecule Ld (12). As shown in Fig. 1B, both naive and effector CD8+ T cells formed conjugates to similar extents and with similar kinetics. This conjugation was antigen-specific because neither naive nor effector CD8+ T cells conjugated with syngeneic EL4 cells (Fig. 1B), and because blocking of Ld significantly reduced conjugate formation (data not shown). Actin remodeling was required for both TCR capping and conjugate formation because these processes were blocked by the actin inhibitor cytochalasin D (data not shown).
Fig. 1.
TCR capping and conjugate formation are comparable in naive and effector CD8+ T cells. (A) Naive or effector T cells were preincubated with anti-CD3ε-FITC. Prewarmed goat anti-hamster Ab was added, and cells were incubated at 37°C for indicated durations of time, fixed, and analyzed by confocal microscopy. (B) Naive and effector CD8+ T cells and either EL4 cells or P815 cells were conjugated for the indicated times. Two-color flow cytometric analysis was performed, and percent conjugates were determined by calculating the ratio of double-positive cells to the total T cell population. Data are representative of three independent experiments.
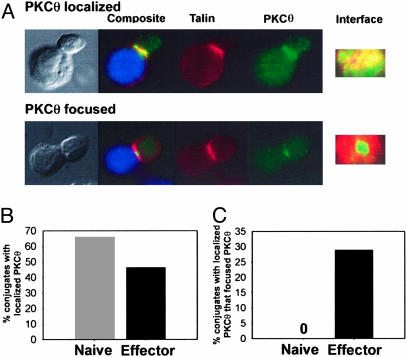
Focusing of PKCθ to the cSMAC Occurs in Effector but Not Naive CD8+ T Cells. Having established that both naive and effector CD8+ T cells formed conjugates with P815 cells, the molecular features of the T cell–APC contact site were examined. It has been shown previously that PKCθ, a member of the novel family of PKCs, is the only isoform of PKC that is recruited to the immunological synapse after TCR stimulation (13) and localized to the cSMAC (3). Therefore, it has been used as a marker for cSMAC/pSMAC segregation. Immunofluorescence microscopy was used to identify T cells that had only localized PKCθ to the interface vs. those that showed punctate focusing of PKCθ to the cSMAC (Fig. 2A). Conjugates were defined as CMAC-labeled APCs in contact with a T cell displaying talin localized to the T cell–APC interface. After 8 min of incubation with P815 cells, the majority of both naive and effector CD8+ T cells localized PKCθ to the interface (Fig. 2B). However, only effector CD8+ T cells focused PKCθ to the central one-third of the contact site, defining the cSMAC (Fig. 2C). It is important to note that the contact surface area between P815 cells and either naive (17.99 ± 2.97 μM) or effector CD8+ T cells (15.62 ± 2.51 μM) was comparable, as determined by direct measurement in two dimensions. Furthermore, PKCθ protein expression was comparable in naive and effector CD8+ T cells (data not shown). Therefore, the lack of visible PKCθ focusing in naive cells was not because of a smaller contact size or discordant PKCθ protein expression.
Fig. 2.
Effector, but not naive, CD8+ T cells detectably focus PKCθ. (A) Naive or effector CD8+ T cells were cocultured for 8 min with CMAC-labeled P815 (blue), fixed, stained for talin (red) and PKCθ (green), and imaged by immunofluorescence microscopy, and z axis image reconstructions of the interface were performed. Representative images of conjugates displaying only localized PKCθ (Upper) or focused PKCθ (Lower) are shown. (B) CD8+ T cells in contact with P815 cells with talin enrichment at the interface were scored as conjugates. The percentage of conjugates with localized PKCθ was calculated. (C) Conjugates that localized PKCθ were rescored for punctuate focusing of PKCθ, and this percentage was calculated. Results are representative of five similar experiments.
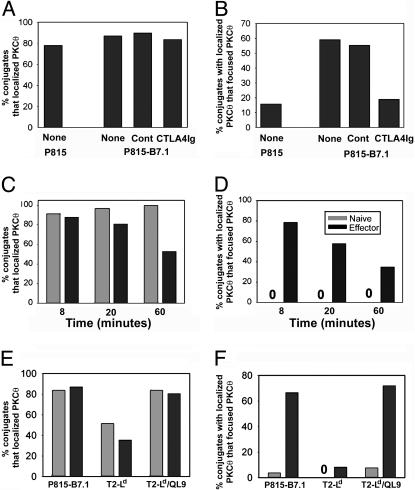
CD28 Costimulation, Prolonged Kinetics, and Superagonist Peptide Did Not Promote PKCθ Focusing in Naive CD8+ T Cells. Naive CD8+ T cells have been shown to display a higher threshold for TCR-mediated activation and to have a greater requirement for CD28 costimulation for induction of cytokine production (8, 9). Therefore, we investigated whether P815 cells transfected with B7-1 would induce PKCθ focusing in naive CD8+ T cells. As shown in Fig. 3A, expression of B7-1 on P815 cells augmented PKCθ focusing in effector CD8+ T cells, and this augmentation was decreased to the level observed with P815 cells in the presence of the B7-1-blocking fusion protein CTLA4Ig. Nonetheless, PKCθ focusing at 8 min remained undetectable in naive CD8+ T cells (Fig. 3B).
Fig. 3.
CD28 costimulation, extended kinetics, and high-affinity peptide fail to promote PKCθ focusing in naive CD8+ T cells. (A and B) Effector CD8+ T cells were cocultured with either CMAC-labeled P815 or P815-B7.1 cells for 8 min in the presence of medium (None), isotype control Ab (Cont), or CTLA4Ig and scored for PKCθ localization and focusing. (C–F) Either naive or effector CD8+ T cells were cocultured for the indicated time with CMAC-labeled P815.B71 or T2-Ld either in the presence or absence of the QL9 peptide. The percentage of conjugates with localized PKCθ (C and E), and of these conjugates the percentage of those displaying focused PKCθ (D and F) was calculated. Data are representative of two independent experiments.
It was conceivable that naive CD8+ T cells required more time to reorganize molecules at the immunological synapse. To address this question, we extended the time course of coincubation. However, coculture for as long as 60 min did not promote detectable PKCθ focusing in naive CD8+ T cells (Fig. 3B).
It remained possible that the density of the p2Ca peptide–Ld complex generated on the surface of P815 cells was not high enough to induce pSMAC/cSMAC segregation at the immunological synapse in naive CD8+ T cells. To increase the number and avidity of TCR ligands, we used the superagonist peptide QLSPFPFDL (QL9) (14) loaded onto T2 cells deficient in transporter associated with antigen processing transfected to express Ld (15). As with P815 cells, QL9 peptide-pulsed T2-Ld cells induced efficient PKCθ focusing in effector but not naive 2C T cells (Fig. 3C), suggesting that inadequate density or avidity of TCR ligands did not account for the lack of PKCθ focusing to the cSMAC in naive CD8+ T cells.
Focusing of Phospho-PKCθ and Phospho-Zap70 Is Readily Detectable in Effector but Not Naive CD8+ T Cells. We were interested in determining whether only total PKCθ failed to be recruited to the cSMAC in naive CD8+ T cells or whether other molecules followed the same pattern. The latter result would suggest that the cSMAC itself failed to be generated.
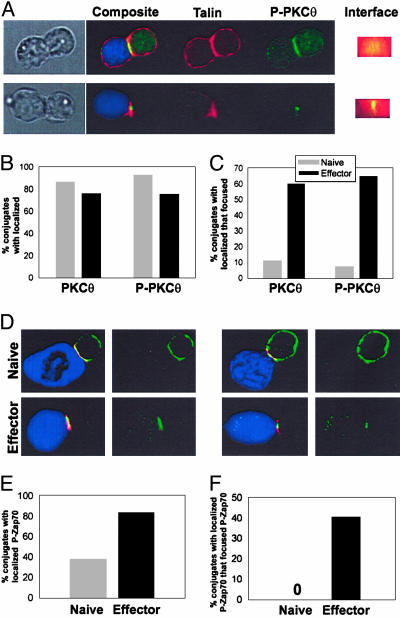
Despite lack of focusing of total PKCθ, it was possible that activated PKCθ might be found in the cSMAC naive CD8+ T cells, thus marking the presence of upstream activators. Phosphorylation of PKCθ has been proposed as critical for its activation (13, 16). Therefore, we used a phosphospecific PKCθ Ab to assess the location of activated PKCθ (Fig. 4A). However, like total PKCθ, phospho-PKCθ localized to the interface in both naive and effector cells (Fig. 4B), but focusing to the cSMAC was readily detectable only in effector cells (Fig. 4C).
Fig. 4.
Neither phospho-PKCθ nor phospho-Zap70 display cSMAC focusing in naive CD8+ T cells. Naive or effector CD8+ T cells were cocultured for 8 min with CMAC-labeled P815 (blue), fixed, stained for talin (red) and either phospho-PKCθ (A–C) or phospho-Zap70 (D–F) (green), and imaged by immunofluorescence microscopy. (A) Representative images of conjugates displaying localized (Upper) or focused (Lower) phospho-PKCθ are shown. The percentages of conjugates displaying localized and focused phospho-PKCθ are shown in B and C, respectively. (D) Representative images of naive and effector conjugates showing the predominant staining patterns observed. The percentages of conjugates displaying localized and focused phospho-Zap70 among naive and effector cells are shown in E and F. Data are representative of three independent experiments.
A proximal tyrosine kinase critical for early TCR signaling is ZAP-70, which is recruited to TCR complexes containing phosphorylated CD3 immunoreceptor tyrosine-based activation motifs (17, 18). We therefore performed immunofluorescence analysis to examine phospho-Zap70 localization to the APC contact site in naive and effector CD8+ T cells. As shown in Fig. 4D, we observed that localization of phospho-Zap-70 to the interface was inefficient in naive cells. Moreover, the staining patterns observed in naive and effector CD8+ T cells were qualitatively distinct. In contrast to either the localized or focused phospho-Zap70 distribution observed in effector CD8+ T cells, phospho-Zap70 was only partially localized to the contact interface in a subset of the naive cell conjugates with the predominant phospho-Zap70 staining pattern being an even distribution (Fig. 4E). In contrast, focusing of phospho-Zap70 to the cSMAC was readily detectable in effector but not naive 2C T cells (Fig. 4F). Collectively, these results suggest that the formation of the cSMAC itself is not efficiently generated in naive CD8+ T cells.
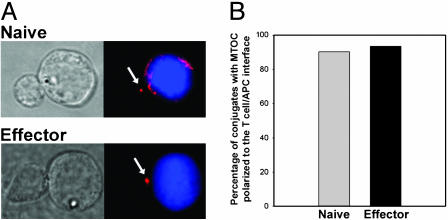
MTOC Polarization Is Readily Detectable in both Naive and Effector CD8+ T Cells. An early event in the directional release of cytokines and lytic granules is the orientation of the microtubule-organizing center to the T cell immunological synapse. To explore whether naive or effector CD8+ T cells were capable of reorienting the MTOC to the T cell/APC interface, we assessed MTOC localization by tubulin staining. As shown in Fig. 5, both naive and effector CD8+ T cells displayed comparable MTOC orientation to the T cell/APC interface.
Fig. 5.
MTOC polarization is comparable in naive and effector CD8+ T cells. Naive or effector CD8+ T cells were cocultured for 8 min with CMAC-labeled P815-B7.1 (blue), fixed, stained for tubulin, and imaged by immunofluorescence microscopy. (A) Representative images of conjugates displaying polarized MTOC are shown. (B) The percentages of conjugates displaying MTOC localized to the one-third of the T cells proximal to the interface were scored as polarized. Data are representative of two independent experiments.
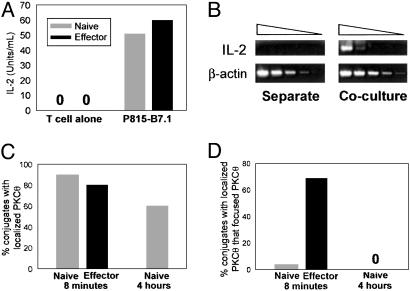
IL-2 mRNA Is Induced in Naive CD8+ T Cells Despite Lack of Detectable cSMAC Formation. To explore whether naive CD8+ T cells were productively activated even without generation of a detectable cSMAC, cytokine production was examined. Stimulation with P815.B7-1 cells induced robust IL-2 production by both naive and effector CD8+ T cells as detected at 16 h (Fig. 6A). To rule out the possibility that cSMAC formation occurred later and thus facilitated functional activation, we assessed IL-2 mRNA induction at 4 h. At this time point, IL-2 gene expression was readily detected in response to P815.B7-1 cells (Fig. 6B), yet focusing of PKCθ still had not detectably occurred (Fig. 6 C and D). These results suggest that cSMAC formation is not required for activation of naive CD8+ T cells.
Fig. 6.
Naive CD8+ T cells produce IL-2 despite lack of detectable cSMAC formation. (A) Either naive or effector CD8+ T cells were cultured in either the presence or absence of P815-B7.1 cells, and IL-2 protein secretion was assessed at 16 h. (B) Naive CD8+ T cells and P815-B7.1 were either cultured separately (Left) or cocultured (Right) for 4 h, and serial dilutions of cDNA were assayed for IL-2 mRNA (Upper) and β-action mRNA (Lower). C and D show, at 4 h, the percentage of conjugates with localized PKCθ and, of these conjugates, the percentage displaying focused PKCθ. Data are representative of three independent experiments.
Discussion
The importance of cSMAC formation in T cell activation and/or performance of effector function is controversial (19). It has been hypothesized that cSMAC formation is required for TCR-mediated T cell activation. This hypothesis is based on the observation that a subset of signaling molecules becomes localized to the cSMAC and that stimulation with antagonist peptides correlated the absence of cSMAC formation with the lack of T cell activation (1). However, recent studies have raised questions about the importance of cSMAC formation in TCR signaling. Specifically, Lee et al. (5) have demonstrated that proximal signaling events precede the formation of the cSMAC. In addition, we have observed that the actin polymerization inhibitor cytochalasin D augmented cytokine production despite disrupting stable conjugate formation and immunologic synapse organization (20). In our current study, we analyzed the segregation of molecules at the T cell/APC interface in naive and effector 2C/RAG2-/- TCR transgenic CD8+ T cells. We observed that formation of a cSMAC was not detectable in naive T cells in this model, yet IL-2 production and MTOC reorientation readily occurred. These results argue that cSMAC formation is not required for the full activation of naive CD8+ T cells. Because these stimulation conditions also led to proliferation and differentiation into lytic effector cells, our results imply that those processes also do not require a cSMAC during initial APC contact.
The lack of PKCθ focusing in naive CD8+ T cells should not detract from the overall importance of PKCθ in T cell activation. PKCθ still localized to the interface in our studies and became activated as indicated by the accumulation of phospho-PKCθ, even if it did not focus to a defined cSMAC. PKCθ has been reported to be the predominant PKC isoform that translocates to the T cell/APC interface (13) and to integrate TCR/CD28 costimulatory signals (21–23). Moreover, PKCθ-/- T cells exhibit a deficiency in IL-2 production and proliferation in response to TCR/CD28 engagement (24). With regard to immunological synapse formation, CD28 has been suggested to play a critical role in the localization of PKCθ to the cSMAC in naive CD4+ T cells (25). This finding contrasts with the data of Bromley et al. (26), in which B7-1 engagement was not required for cSMAC generation. Our data support a role for CD28 in the partitioning PKCθ to the cSMAC in effector cells, but, nonetheless, the addition of CD28 coengagement did not enable PKCθ focusing in naive CD8+ T cells.
CD28 costimulation has been suggested to promote recruitment of lipid rafts to the immunological synapse (27), and PKCθ localizes to lipid rafts after TCR ligation (28). Moreover, recent data have suggested that human CD8+ T cells do not reorient lipid rafts to the T cell/APC interface during activation (29). Taken with our present results, it is conceivable that the membrane lipid composition of naive CD8+ T cells does not favor cSMAC/pSMAC segregation and that the plasma membrane composition becomes altered during CD8+ T cell differentiation into effector cells. This hypothesis is worth exploring in future studies.
Using a different model system, Potter et al. (30) described the immunological synapse in CD8+ TCR transgenic T cells. cSMAC formation was observed in their system, but several differences are noteworthy. They used OT1 mice that were not on a RAG-deficient background. Thus, the T cell population was not guaranteed to be homogeneous. They also used lymph node as a source of T cells, whereas we used splenic T cells. Recent observations have suggested that T cells in the lymph node environment may exhibit precapped TCRs and indicate partial activation (31), making it possible that a property of the lymph node environment may alter the architecture of the T cell/APC interface. The Potter report clearly indicated a role for the CD8 coreceptor in immunologic synapse formation and illustrated that a cSMAC could be generated in CD8+ T cells. At the same time, our results clearly demonstrate that signal strengths sufficient to induce IL-2 production by naive CD8+ T cells failed to generate a detectable cSMAC.
If cSMAC formation is not required for CD8+ T cell activation, then what is the role of the cSMAC in CD8+ T cells? It is plausible that the cSMAC architecture contributes to polarized secretion in effector T cells. Recently, it has been shown that lytic granules are targeted to the cSMAC when effector CD8+ T cells are triggered to lyse targets (7). MTOC polarization also has been shown to correlate with lytic activity (32). Although we observed MTOC polarization to the T cell/APC interface in both naive and effector conjugates, arguing that a cSMAC is not necessary for MTOC reorientation, it is conceivable that the combination of MTOC polarization and formation of a cSMAC regulate the directional release of cytolytic effector function. Therefore, it will be of interest to correlate cSMAC formation in CD8+ effector cells with increased specificity and decreased bystander killing during the process of cytolysis.
Acknowledgments
We thank Jim Miller, Fabiola Rivas, and Candace Cham for helpful advice and Janel Washington for assistance with mouse breeding. This work was supported by National Institutes of Health Grant R01 AI47919.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: APC, antigen-presenting cell; CMAC, 7-amino-4-chloromethylcoumarin; cSMAC, central supramolecular activation cluster; CTL, cytolytic T lymphocytes; MTOC, microtubule organizing center; pSMAC, peripheral supramolecular activation cluster; QL9, superagonist peptide QLSPFPFDL; RAG, recombination-activating gene; TCR, T cell receptor.
References
- 1.Grakoui, A., Bromley, S. K., Sumen, C., Davis, M. M., Shaw, A. S., Allen, P. M. & Dustin, M. L. (1999) Science 285, 221-227. [DOI] [PubMed] [Google Scholar]
- 2.Dustin, M. L. & Cooper, J. A. (2000) Nat. Immunol. 1, 23-29. [DOI] [PubMed] [Google Scholar]
- 3.Monks, C. R., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. (1998) Nature 395, 82-86. [DOI] [PubMed] [Google Scholar]
- 4.Wulfing, C. & Davis, M. M. (1998) Science 282, 2266-2269. [DOI] [PubMed] [Google Scholar]
- 5.Lee, K. H., Holdorf, A. D., Dustin, M. L., Chan, A. C., Allen, P. M. & Shaw, A. S. (2002) Science 295, 1539-1542. [DOI] [PubMed] [Google Scholar]
- 6.Reichert, P., Reinhardt, R. L., Ingulli, E. & Jenkins, M. K. (2001) J. Immunol. 166, 4278-4281. [DOI] [PubMed] [Google Scholar]
- 7.Stinchcombe, J. C., Bossi, G., Booth, S. & Griffiths, G. M. (2001) Immunity 15, 751-761. [DOI] [PubMed] [Google Scholar]
- 8.Cham, C. M., Xu, H., O'Keefe, J. P., Rivas, F. V., Zagouras, P. & Gajewski, T. F. (2003) J. Biol. Chem. 278, 17044-17052. [DOI] [PubMed] [Google Scholar]
- 9.Fields, P. E., Finch, R. J., Gray, G. S., Zollner, R., Thomas, J. L., Sturmhoefel, K., Lee, K., Wolf, S., Gajewski, T. F. & Fitch, F. W. (1998) J. Immunol. 161, 5268-5275. [PubMed] [Google Scholar]
- 10.Morgan, M. M., Labno, C. M., Van Seventer, G. A., Denny, M. F., Straus, D. B. & Burkhardt, J. K. (2001) J. Immunol. 167, 5708-5718. [DOI] [PubMed] [Google Scholar]
- 11.Sedwick, C. E., Morgan, M. M., Jusino, L., Cannon, J. L., Miller, J. & Burkhardt, J. K. (1999) J. Immunol. 162, 1367-1375. [PubMed] [Google Scholar]
- 12.Guehler, S. R., Finch, R. J., Bluestone, J. A. & Barrett, T. A. (1998) J. Immunol. 160, 5341-5346. [PubMed] [Google Scholar]
- 13.Monks, C. R., Kupfer, H., Tamir, I., Barlow, A. & Kupfer, A. (1997) Nature 385, 83-86. [DOI] [PubMed] [Google Scholar]
- 14.Sykulev, Y., Brunmark, A., Tsomides, T. J., Kageyama, S., Jackson, M., Peterson, P. A. & Eisen, H. N. (1994) Proc. Natl. Acad. Sci. USA 91, 11487-11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loss, G. E., Jr., Elias, C. G., Fields, P. E., Ribaudo, R. K., McKisic, M. & Sant, A. J. (1993) J. Exp. Med. 178, 73-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kane, L. P., Mollenauer, M. N., Xu, Z., Turck, C. W. & Weiss, A. (2002) Mol. Cell. Biol. 22, 5962-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan, A. C., Irving, B. A., Fraser, J. D. & Weiss, A. (1991) Proc. Natl. Acad. Sci. USA 88, 9166-9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan, A. C., Iwashima, M., Turck, C. W. & Weiss, A. (1992) Cell 71, 649-662. [DOI] [PubMed] [Google Scholar]
- 19.van der Merwe, P. A. (2002) Curr. Opin. Immunol. 14, 293-298. [DOI] [PubMed] [Google Scholar]
- 20.Rivas, F. V., O'Keefe, J. P., Alegre, M. L. & Gajewski, T. F. (2004) Mol. Cell. Biol. 24, 1628-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coudronniere, N., Villalba, M., Englund, N. & Altman, A. (2000) Proc. Natl. Acad. Sci. USA 97, 3394-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, X., O'Mahony, A., Mu, Y., Geleziunas, R. & Greene, W. C. (2000) Mol. Cell. Biol. 20, 2933-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herndon, T. M., Shan, X. C., Tsokos, G. C. & Wange, R. L. (2001) J. Immunol. 166, 5654-5664. [DOI] [PubMed] [Google Scholar]
- 24.Sun, Z., Arendt, C. W., Ellmeier, W., Schaeffer, E. M., Sunshine, M. J., Gandhi, L., Annes, J., Petrzilka, D., Kupfer, A., Schwartzberg, P. L. & Littman, D. R. (2000) Nature 404, 402-407. [DOI] [PubMed] [Google Scholar]
- 25.Huang, J., Lo, P. F., Zal, T., Gascoigne, N. R., Smith, B. A., Levin, S. D. & Grey, H. M. (2002) Proc. Natl. Acad. Sci. USA 99, 9369-9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bromley, S. K., Iaboni, A., Davis, S. J., Whitty, A., Green, J. M., Shaw, A. S., Weiss, A. & Dustin, M. L. (2001) Nat. Immunol. 2, 1159-1166. [DOI] [PubMed] [Google Scholar]
- 27.Viola, A., Schroeder, S., Sakakibara, Y. & Lanzavecchia, A. (1999) Science 283, 680-682. [DOI] [PubMed] [Google Scholar]
- 28.Bi, K., Tanaka, Y., Coudronniere, N., Sugie, K., Hong, S., van Stipdonk, M. J. & Altman, A. (2001) Nat. Immunol. 2, 556-563. [DOI] [PubMed] [Google Scholar]
- 29.Kovacs, B., Maus, M. V., Riley, J. L., Derimanov, G. S., Koretzky, G. A., June, C. H. & Finkel, T. H. (2002) Proc. Natl. Acad. Sci. USA 99, 15006-15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potter, T. A., Grebe, K., Freiberg, B. & Kupfer, A. (2001) Proc. Natl. Acad. Sci. USA 98, 12624-12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefanova, I., Dorfman, J. R. & Germain, R. N. (2002) Nature 420, 429-434. [DOI] [PubMed] [Google Scholar]
- 32.Radoja, S., Saio, M., Schaer, D., Koneru, M., Vukmanovic, S. & Frey, A. B. (2001) J. Immunol. 167, 5042-5051. [DOI] [PubMed] [Google Scholar]