Abstract
The Toll family of receptors is required for innate immune response to pathogen-associated molecules, but the mechanism of signaling is not entirely clear. In Drosophila the prototypic Toll regulates both embryonic development and adult immune response. We demonstrate here that the host protein Spätzle can function as a ligand for Toll because Spätzle forms a complex with Toll in transgenic fly extracts and stimulates the expression of a Toll-dependent immunity gene, drosomycin, in adult flies. We also show that constitutively active mutants of Toll form multimers that contain intermolecular disulfide linkages. These disulfide linkages are critical for the activity of one of these mutant receptors, indicating that multimerization is essential for the constitutive activity. Furthermore, systematic mutational analysis revealed that a conserved cysteine-containing motif, different from the cysteines used for the intermolecular disulfide linkages, serves as a self-inhibitory module of Toll. Deleting or mutating this cysteine-containing motif leads to constitutive activity. This motif is located just outside the transmembrane domain and may provide a structural hindrance for multimerization and activation of Toll. Together, our results suggest that multimerization may be a regulated, essential step for Toll-receptor activation.
Upon infection, insects mount a rapid but rather nonspecific response. This insect antimicrobial response is similar to the mammalian innate immune response (1–7). The recognition receptors of the innate immune system have limited diversity and are designed to recognize pathogen-associated molecules such as lipopolysaccharides and peptidoglycans, which are common molecules found in groups of microorganisms. Toll and Toll-like receptors (TLRs) are a conserved family of recognition receptors in insects and mammals (5, 6). The Toll family of receptors is essential for innate immunity, but it is unclear how the receptors interact with microbial substances and host ligands.
In Drosophila, the prototypic Toll receptor was first identified as an essential component for embryonic development (8). Subsequently, Toll was also shown to be essential for the induced expression of a subset of antimicrobial peptides during antifungal and anti-Gram-positive bacterial responses (9, 10). The regulation of the anti-Gram-negative bacterial response depends on a second pathway called the Immune deficiency (Imd) pathway, which controls the expression of another subset of antimicrobial peptides (2–4, 11). The induced expression of antimicrobial peptides through these two pathways is critical for Drosophila to survive microbial infections (12).
The mechanism by which Toll receptors transmit the signal of infection across the cell membrane is not clear. Many components of the Toll pathway in the Drosophila innate immune response have been identified. The extracellular components include a peptidoglycan recognition protein (PGRP-SA), a serine protease (Persephone), a serine protease inhibitor (Necrotic), and a putative ligand (Spätzle) (13–16). The intracellular components include two adaptor proteins (MyD88 and Tube), a kinase (Pelle), and a transcription factor (Dif) (9, 17–20).
We show here that the host protein Spätzle binds to Toll in transgenic fly extracts and activates a Toll-dependent immunity gene, drosomycin, suggesting that Spätzle is a ligand for Toll in vivo. Constitutively active mutants of Toll form multimers. The multimers contain disulfide linkages, which are critical for the constitutive activity. We also show that a separate cysteine-containing motif likely provides structural information to modulate the multimerization and activity of the receptor. Together, the results suggest that multimerization may be a regulated, essential step for Toll-receptor activation.
Materials and Methods
Fly Stocks and P Element Transformation. The Toll10b line was maintained with X-linked male lethal and TM3 balancer chromosomes, as described (21). Transgenic flies were generated by coinjecting 1 mg/ml of P element plasmid and 0.3 mg/ml of the turbo transposase plasmid into y w embryos. The chromosomal locations of the transgenes were determined by mating with double balancer flies and scoring for segregation. Meiotic recombination was performed by crossing two transgenic lines located on homologous chromosomes, collecting offspring to establish individual lines, and assaying for the presence of both transgenes based on protein expression of the constructs.
Molecular Cloning and Mutagenesis. The FLAG, MYC, and V5 tags were placed at the C termini of Spätzle and Toll. The FLAG sequence was contained in the 3′ PCR primer and was fused in frame with the last codon of Toll. The MYC and V5 clones were generated by fusing the last codons of Toll and spätzle in frame with the tag sequences through a BamHI site. The fusion clones were first generated in pBluescript and then subcloned into the pGem3Actin5C or pUAST vector. Site-directed mutagenesis of Toll at the cysteine-containing motif was performed on a 700-bp pBluescript clone encompassing the region. The Stratagene QuikChange mutagenesis kit was used on a double-stranded DNA template. The confirmed mutants were then subcloned back to the full-length Toll cDNA by using the StuI and PflmI sites. The ΔN2 to ΔN6 deletion mutants were constructed by PCR amplification of the appropriate regions of the Toll cDNA. The 5′ end points of the constructs correspond to aa 210, 472, 656, 743, and 802, respectively. The PCR products were cloned into a pBluescript vector containing a 5′ fragment of Toll, which encodes the first 34 aa and spans the extracellular signal sequence. At the junctions, the fusion constructs contain an XbaI site as the linker. The ΔC1 and ΔC2 constructs contain the tag sequence fused at aa 1,002 and 870, respectively. Mutagenesis of the ΔN6 constructs was performed similarly, by using pBluescript–ΔN6 as a template. The SpätzleΔN contained the N-terminal 21 aa fused to the C-terminal 106 aa. A HindIII site was introduced at the junction.
Immunoprecipitation and Western Blots. Tissue-culture cells or adult flies were homogenized in the lysis buffer [50 mM Hepes, pH 7.5/150 mM NaCl/1.5 mM MgCl2/1 mM EGTA/10% glycerol/1% Triton X-100/0.5 mM PMSF/protease inhibitor mixture P2714 (Sigma)]. The homogenates were incubated on ice for 10 min and subsequently spun at 15,000 × g for 10 min at 4°C. The supernatants were then taken as extracts. For Western blots, the extracts were separated by SDS/PAGE and transferred to Millipore Immobilon-P membrane. The membrane was treated with 5% nonfat dry milk in Tris-buffered saline at room temperature for 1 h and then incubated overnight with primary antibody at 4°C in Tris-buffered saline plus 0.25% Tween 20 (TBST) and 5% nonfat dry milk. The membrane was washed four times with TBST and incubated with secondary antibody in TBST plus 5% milk for 1.5 h at room temperature. After washing the membrane four times with TBST (10 min each time), the signal was developed by using chemiluminescence detection (NEN, NEN104). M2 antibody (F-3165, 1:5,000 dilution, Sigma), c-Myc antibody (SC-40, 1:200 dilution, Santa Cruz Biotechnology), and V5 antibody (R960-25, 1:5,000 dilution, Invitrogen) were used as primary antibodies. The secondary antibody was horseradish peroxidase-conjugate, anti-mouse antibody (W4021, Promega).
Immunoprecipitation was performed by using 200 μl of extract, which is equivalent to approximately four flies. The extracts were precleared for 30 min with 40 μl of 50:50 protein G beads (no. 17-0618-01, Amersham Biosciences) in lysis buffer with a final volume of 500 μl. The supernatants obtained were incubated with antibodies at 4°C for 1 h. Forty microliters of protein G beads in lysis buffer was added and incubated for another hour. The beads that contained the immunocomplex were collected by centrifugation, washed three more times with lysis buffer, and used for subsequent SDS/PAGE analysis. Transfection, Northern blot, and luciferase activity assays were conducted as described (22).
Results
A Truncated Spätzle Multimerizes and Interacts with Toll. Activity assays in transgenic flies show that a truncated Spätzle can activate a Toll target gene (Fig. 1A). Spätzle is an upstream component of Toll. The full-length Spätzle is cleaved during activation, and the mature C-terminal polypeptide is the active moiety in injection assays (14, 16, 23, 24). We generated a truncated version called SpätzleΔN that resembles the mature polypeptide. V5- or Myc-epitope-tagged, full-length Spätzle and SpätzleΔN were expressed in transgenic flies by the Gal4-UAS system. The yolk protein 1 (YP1)-Gal4 was used to direct the expression in adult female fat bodies (18). Expression of SpätzleΔN in transgenic flies led to increased expression of drosomycin, an antifungal gene that is activated by the Toll pathway (Fig. 1A). This result shows that in vivo expression of a truncated Spätzle in adult flies can mimic microbial induction of the Toll pathway. The full-length Spätzle, however, did not have the same effect of inducing drosomycin expression.
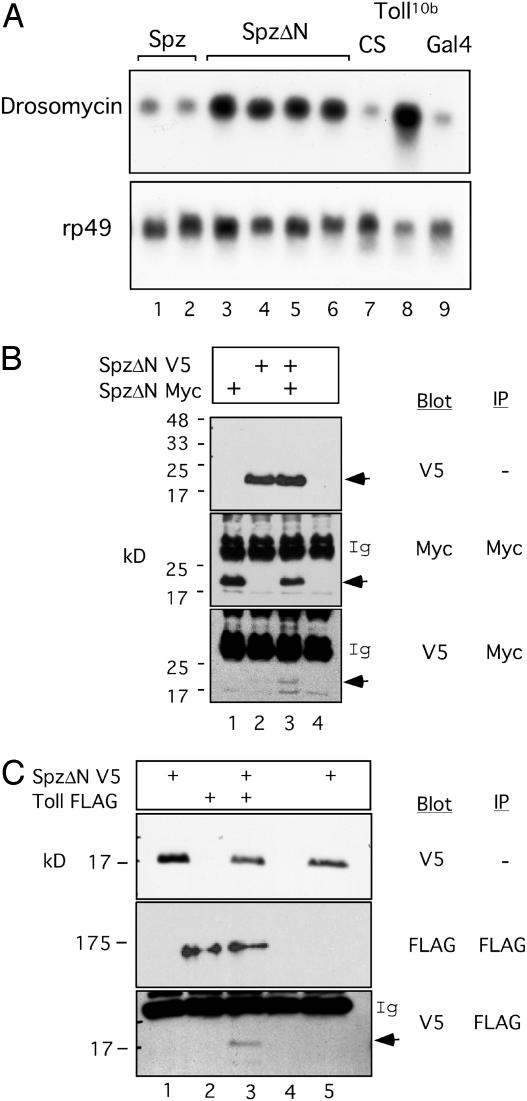
Fig. 1.
SpätzleΔN multimerization and interaction with Toll. (A) Northern blots of female transgenic lines containing YP1-Gal4 and UAS-SpätzleV5 (Spz) or UAS-SpätzleΔNV5 (SpzΔN). drosomycin or ribosomal protein 49 (rp49) probes were used for hybridization. Two independent Spz (lanes 1 and 2) and four independent SpzΔN transgenic lines (lanes 3–6) were assayed. The Can-tonS (CS) wild-type flies, Toll10b mutant flies, and YP1-Gal4 (Gal4) flies were included for comparison. It can be seen that SpzΔN can activate drosomycin expression efficiently. (B) Co-IP assays of fly lines containing SpätzleΔNV5, SpätzleΔNMyc, or both. The control line used was Gal4 (lane 4 in each panel). Antibodies used for Western blot or IP are as indicated. The proteins were separated on an SDS/15% PAGE gel. Ig indicates the immunoglobulin bands, and the arrows indicate SpätzleΔN, which runs as an ≈20-kDa protein. The blot shows that the two tagged proteins form a complex in the extract (bottom panel). (C) Co-IP assay of transgenic lines containing the indicated constructs crossed with YP1-Gal4. Lanes 1 and 5 were from two independent crosses of SpätzleΔNV5 with YP1-Gal4. Lane 4 was from Gal4 control flies. Antibodies used for Western blot or IP are as indicated. Toll-FLAG runs as a 175-kDa protein. Co-IP was detected only when both proteins were present in the same fly extract (bottom panel, lane 3).
We then examined whether Spätzle could form homomultimers, which is the case for many ligands, such as epidermal growth factor and transforming growth factor β (25, 26). For this purpose, we generated recombinant fly lines that contained both UAS-Spätzle-Myc and UAS-Spätzle-V5. We also generated similar fly lines containing the two tagged SpätzleΔN. After mating these Spätzle lines with the YP1-Gal4 line, transgenic flies expressing the fusion proteins were used for extract preparation and coimmunoprecipitation (co-IP) experiments. Our results demonstrate that both tagged forms of SpätzleΔN are present in the precipitated complex (Fig. 1B), suggesting the formation of SpätzleΔN homomultimers in vivo. However, our data on the multimerization of full-length Spätzle was inconclusive (data not shown).
Co-IP assays showed that SpätzleΔN interacts with Toll in transgenic fly extracts (Fig. 1C). We generated FLAG-tagged, wild-type Toll transgenic flies and crossed them with the SpätzleΔN-V5 transgenic flies to establish stable lines. After mating the individual or combined Spätzle and Toll lines with the YP1-Gal4 line, flies containing all of the transgenes were used for extract preparation and co-IP assays. Our results showed that co-IP of the complex could be detected only when Toll-FLAG and SpätzleΔN-V5 were present in the same flies (Fig. 1C). Thus, we conclude that the truncated form of Spätzle interacts, directly or indirectly, with the Toll receptor. Because SpätzleΔN activates a Toll target gene, forms a homomultimer, and interacts with Toll, we postulate that the proteolytically processed Spätzle likely functions as a ligand for Toll in vivo during immune responses.
Constitutively Active Toll Proteins Form Multimers. Receptor multimerization is a widely used mechanism to transmit signals of extracellular stimulations to the cytoplasm (25). To examine whether multimerization can occur for Toll, we took advantage of the Toll10b gain-of-function mutation. Toll10b contains a point mutation and is a ligand-independent, constitutively active receptor in the embryo and the adult (9, 27, 28). We generated transgenic fly lines that expressed different combinations of FLAG- or Myc-tagged wild-type Toll and mutant Toll10b. The extracts obtained from these Gal4-UAS flies were then used for co-IP experiments.
The wild-type Toll and Toll10b proteins have different mobility on SDS gels due to differential glycosylation (27, 29), and they can be distinguished conveniently (Fig. 2A, bracket). We found that both tagged versions of the Toll10b proteins were present in the immunoprecipitated complex (Fig. 2A, arrow), suggesting the formation of homomultimers. However, we did not detect any wild-type Toll multimerization. The Toll–Toll10b combination also showed no multimerization. These results suggest that multimerization can be detected by co-IP only when all subunits in the receptor complex are in an active conformation.
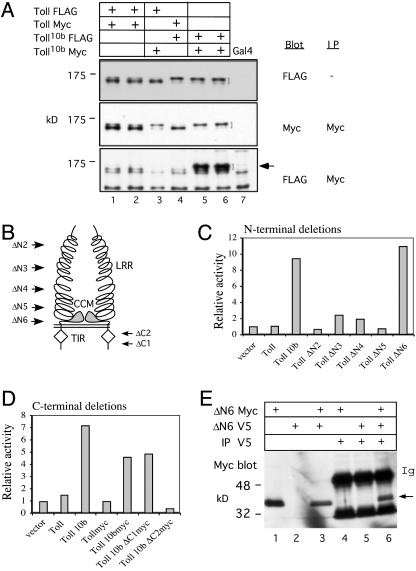
Fig. 2.
Multimerization of constitutively active Toll mutants. (A) Co-IP assays of recombinant fly lines containing various combinations of epitope-tagged Toll and Toll10b constructs. Two independent lines of Toll-FLAG/TollMyc (lanes 1 and 2), one line each of Toll-FLAG/Toll10bMyc and TollMyc/Toll10bFLAG (lanes 3 and 4), and two independent lines of Toll10bFLAG/Toll10bMyc (lanes 5 and 6) were used for the assay. Toll10b is more highly glycosylated than wild-type Toll and runs as a bigger protein (indicated by the brackets) (27, 29). The bottom panel shows that when both copies of tagged Toll10b are present, significant co-IP can be observed (indicated by the arrow). We did not observe any substantial complex formation for wild-type Toll, even after repeated trials. (B) Schematic representation of a Toll dimer. The extracellular LRR, the cysteine-containing motif (CCM), and the intracellular TIR domain are as labeled. The N-terminal deletion constructs all contain the first 34 aa fused with different lengths of C termini by a XbaI linker. The arrows indicate the approximate fusion points of the deletions (see Materials and Methods). ΔN1 is the same as the full-length protein except it contains a linker after aa 34, and, therefore, it is not included here. (C) Gene regulation by the N-terminal deletion constructs. N-terminal deletion constructs were expressed under the control of the Actin5C promoter, and their gene regulatory activities were assayed by cotransfection in S2 cells. The drosomycin-luciferase reporter was cotransfected, and the relative luciferase activity based on the Actin5C vector control is shown. All transfection results represent the average of three independent experiments. (D) Cotransfection assay of the C-terminal deletion constructs. These two deletion constructs also contain the Toll10b point mutation. The constructs were generated in the pUAST vector, and Actin5C-Gal4 and drosomycin-luciferase plasmids were included in the cotransfection. These two constructs contain the Myc tag, and, therefore, the Myc-tagged Toll and Toll10b constructs were included for comparison. (E) Co-IP assay of V5- and Myc-tagged ΔN6 in transfected S2 cell extracts. The cell extracts (lanes 1–3) and immunoprecipitates of V5 antibodies (lane 4–6) were analyzed by SDS/PAGE. Anti-Myc antibody was used for the Western blot. The blot shows that the region containing the transmembrane and cytoplasmic domains is sufficient for multimerization.
To search for other constitutively active forms of Toll and study their multimerization, we generated and analyzed a series of deletion mutants. The activities of these deletion mutants were examined by a drosomycin-luciferase reporter assay in transiently transfected S2 cells (22, 30). A ΔN1 construct was designed as the full-length protein plus two additional amino acids after aa 34 by means of an added XbaI linker; therefore, the construct is not a deletion and is not included here. We found that the ΔN2–ΔN5 constructs that retained the various lengths of the extracellular leucine-rich repeats (LRR) of Toll did not display a significant change in the activity of regulating drosomycin (Fig. 2 B and C). In contrast, the deletion mutant ΔN6, which, in addition to the deletion of LRR also has a deleted cysteine-containing motif, displayed a high constitutive activity. The ΔN6 construct has the same C-terminal sequence as the previously described ΔLRR construct (27, 30) but a shorter N terminus (34 aa versus 100 aa) that still includes the extracellular signal sequence. Although both ΔLRR and ΔN6 exhibit constitutive gene regulatory activities, our serial deletion analysis suggests that it is not the deletion of LRR but the removal of the cysteine-containing motif that leads to the increase in receptor activity.
Analysis of C-terminal Toll deletions shows that the Toll/IL-1 resistance (TIR) domain is essential for the activity of regulating target genes. The two constructs ΔC1 and ΔC2 also contained the Toll10b point mutation, because we expected a loss of activity. Although Toll10b-ΔC1 had the nonconserved C-terminal tail deleted, we found this construct to be active, whereas Toll10b- ΔC2, which had the tail and most of the TIR domain deleted, showed a complete loss of activity (Fig. 2D). These results indicate that the TIR domain is essential for receptor function, consistent with previous reports (31, 32). Although the C-terminal tail was not essential in our assay, a previous report showed that there might be a weak inhibitory function associated with this region (31). This discrepancy in findings may be explained by the differences in the reporters used and in our use of Toll10b-mutation-containing constructs.
Overall, our deletion analyses demonstrated that ΔN6 is the only mutant that showed constitutive activity in activating drosomycin. Therefore, we performed a co-IP assay by using two tagged versions of ΔN6 in transiently transfected S2 cells to test for multimerization. We found that the two differentially tagged ΔN6 proteins formed complexes in the extract (Fig. 2E). Thus, analysis of Toll10b and TollΔN6 together suggests a strong correlation between receptor multimerization and increased gene regulatory activity.
The Constitutively Active Toll Multimers Contain Disulfide Linkages. To gain support for the idea that multimerization is critical for constitutive activity, we first analyzed how the multimers were formed. The tagged proteins in transgenic fly extracts were examined by native PAGE (Fig. 3A). In the Toll10b extract there was a distinctively bigger complex of around 750 kDa (Fig. 3A, lane 4, indicated by arrow). This Toll10b complex formation depended on disulfide linkage because β-mercaptoethanol (β-ME) treatment of the extract before gel analysis abolished the band (Fig. 3A, lane 5). Wild-type Toll and β-ME-treated Toll10b both ran approximately as 500-kDa proteins. Under nondenaturing conditions, the biophysical properties and the association with other proteins contribute to the apparent size of a protein. We do not yet know the composition of this complex. Nonetheless, the presence of a bigger complex in the extracts of Toll10b but not of Toll is consistent with our co-IP results.
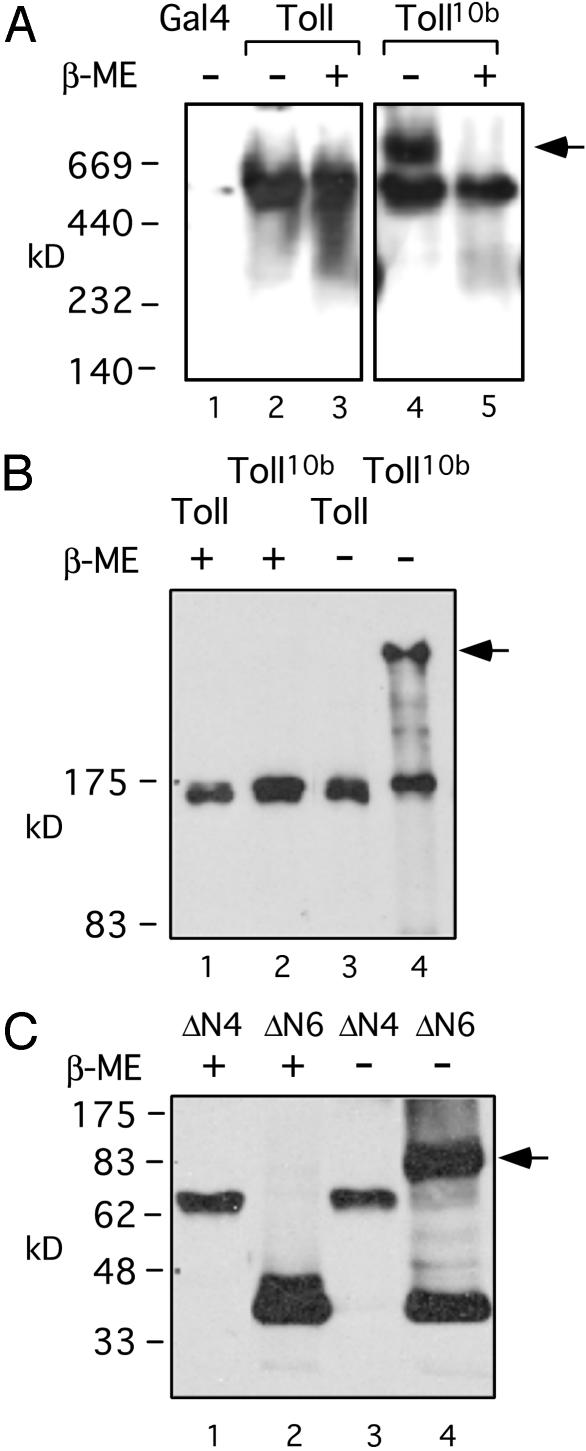
Fig. 3.
Disulfide linkages in constitutively active Toll complexes. (A) Western blot of Myc-tagged protein extracts from various fly strains in native gel. The Toll or Toll10b protein extracts from adult female flies, as well as Gal4 fly extracts (lane 1), were separated on a 4–15% gradient native acrylamide gel (Bio-Rad). Some samples were treated with β-ME as indicated before loading onto the gel. The arrow indicates a complex of ≈750 kDa in the Toll10b extract (lane 4). After β-ME treatment, both Toll and Toll10b behaved as ≈500-kDa proteins. We cannot determine whether these proteins represent monomers, dimers, or bigger complexes, because the apparent size of a protein on native gel depends on many factors, such as charge, shape, and other associated proteins. (B) Western blot of transgenic fly extracts in denaturing gel. The transgenic fly extracts, with or without β-ME treatment as indicated, were analyzed. Only Toll10b forms a complex under nonreducing condition, and the size indicates a probable homodimer (lane 4, arrow). There is no commercially available high-molecular-weight marker at this range for denaturing gels, and we were not able to calculate the size of the complex more accurately. (C) Western blot of transfected S2 cells extracts. The extracts were analyzed by using denaturing SDS gels and Western blotting, and by using anti-V5 antibodies. Only the ΔN6, not the inactive ΔN4, forms a disulfide-linked complex. The size indicates that it is probably a homodimer (lane 4, arrow).
Denaturing gel analysis showed that the Toll10b complex contains at least a homodimer linked by intermolecular disulfide bonds (Fig. 3B). We performed experiments similar to those described above but used denaturing conditions by boiling the transgenic extracts in SDS loading buffer, either in the presence or the absence of β-ME. The extracts were then analyzed by SDS/PAGE. Under such conditions, all noncovalently bound proteins should be dissociated. We observed that only Toll10b formed a detectable complex (Fig. 3B, arrow). This complex contained disulfide linkages. However, because it is much bigger than the denaturing gel markers, we could not determine its exact size. Because we suspected it to be a homodimer of Toll10b, we performed the same denaturing gel experiment for ΔN6, which is much smaller. We detected a ΔN6 complex under nonreducing conditions in S2 cell extracts after transient transfection (Fig. 3C, lane 4, indicated by arrow). The size of this complex, ≈80 kDa, appears to be that of a homodimer because the monomer is ≈40 kDa. The ΔN4-truncated control protein, which is not active, did not form such a complex (Fig. 3C, lanes 1 and 3). Because we used denaturing conditions, we could not determine whether the native Toll proteins form dimers or tetramers. Therefore, we conclude that constitutively active forms of Toll exist in vivo as homomultimeric complexes that contain disulfide linkages.
Disulfide Linkages in the Multimers Are Essential for the Constitutive Activity. To determine whether multimerization is critical for the constitutive activity of Toll, we used site-directed mutagenesis and single amino acid-deletion strategies aimed at disrupting the disulfide linkages. The full-length Toll protein has 18 cysteine residues in its extracellular domain and 1 cysteine in its intracellular domain. The ΔN6 protein has only 1 extracellular cysteine, in addition to the cysteine in the intracellular domain (Fig. 4A). We established various mutants of ΔN6 by deleting or mutating its two cysteines. The activities of various mutants were determined by transient transfection in S2 cells by using the drosomycin-luciferase reporter assay (Fig. 4B). Our results showed that the intracellular cysteine (C892) by itself is not essential for the activity of ΔN6. Mutating or deleting the extracellular cysteines, as in the mutants ΔN6C34A and ΔN6SS, caused partial reduction of the activity. Removing the two cysteines simultaneously caused a total loss of activity, demonstrating that these two cysteines are critical but act redundantly for ΔN6.
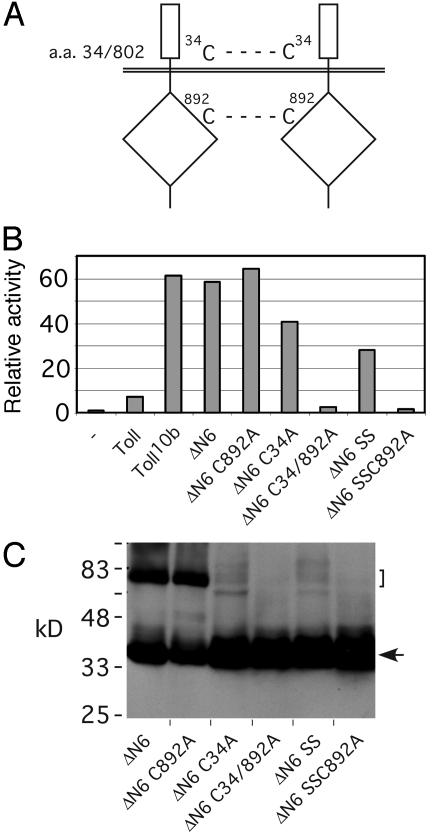
Fig. 4.
Disulfide linkages in ΔN6 are critical for the constitutive activity. (A) Schematic representation of the ΔN6 construct. It contains the first 34 aa of Toll fused with its own C terminus at residue 802. The whole fusion protein contains only two cysteines, labeled as C34 and C892. (B) drosomycinluciferase activities of various ΔN6 constructs. Activities were assayed by transient transfection by using the drosomycin-luciferase reporter. Cysteine- to-alanine mutations in the constructs at the specified sites are as labeled. The SS constructs contain the first 33 aa, and C34 has been deleted. (C) Western blot of S2 cell extracts. S2 cell extracts containing the various constructs with V5 tags were analyzed by SDS/PAGE and Western blotting. All samples were previously boiled in SDS buffer without β-ME. The arrow indicates the monomers, and the bracket indicates the disulfide-linked complexes. Our results indicate that the disulfide linkages are critical for constitutive activity.
Gel analysis shows that the formation of disulfide-linked complexes correlates with the activity of gene regulation (Fig. 4C). Extracts containing the various ΔN6 proteins were obtained from transiently transfected cells and analyzed by SDS/PAGE in the absence of β-ME treatment. All of the proteins also contained V5 tags and were detected by Western blots. Mutating or deleting the extracellular cysteine, as in the mutants ΔN6C34A and ΔN6SS, caused significant reduction of complex formation. However, some residual disulfide-linked complexes were still detectable, correlating with the observed reduced but still substantial activities of these constructs (Fig. 4B). The intracellular cysteine mutation (C892A) did not cause a detectable change in complex formation or activity. Removing both cysteines, as in mutants ΔN6C34/892A and ΔN6SSC892A, led to a complete loss of complex formation, consistent with a complete loss of activity observed for these constructs. Although we cannot completely rule out that the point mutations disrupted the overall protein structure of ΔN6, it appears unlikely to us, because all of the constructs had similar expression levels and single mutations did not disrupt the activity. These results suggest that constitutively active Toll proteins form multimers through disulfide linkages critical for regulating drosomycinluciferase activity.
The Conserved Cysteine-Containing Motif Provides Structural Information for Self-Inhibition. Deleting or mutating the highly conserved cysteine-containing motif, as in ΔN6 or Toll10b, leads to constitutive gene regulatory activities. This observation suggests that the extracellular domain, particularly the cysteine-containing motif, serves as a self-inhibitory module for the Toll receptor activity. To examine this theory, we analyzed this motif by systematic mutagenesis.
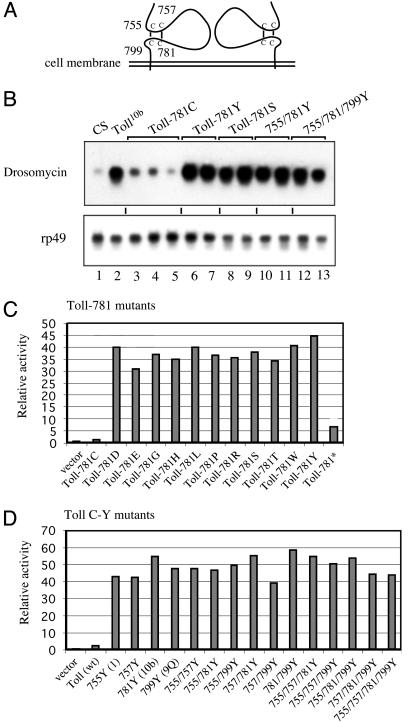
We changed the cysteine-781 to serine, a conservative amino acid substitution, and changed more than one of the cysteines to tyrosines simultaneously (Fig. 5A). Transgenic flies harboring the wild type (781C) and the different mutants were expressed in vivo under the Gal4-UAS system and analyzed by examining the RNA levels of endogenous drosomycin. We found that the 781Y protein caused a high level expression of drosomycin. The 781Y mutation is the same mutation as the ethyl methanesulfonate-induced Toll10b, and its drosomycin-activating ability is comparable to that of the original Toll10b mutant flies (Fig. 5B). The 781S, as well as the double and triple tyrosine mutants, also stimulated the expression of the target gene. The expression level of the Toll constructs driven by the Gal4-UAS system is not the only cause that activates the pathway; the drosomycin expression levels in the 781C wild-type construct lines were similar to those in wild-type flies.
Fig. 5.
The cysteine-containing motif as an autoinhibitory module. (A) Cysteine-containing motifs of a Toll dimer. The numbering of the cysteine residues is indicated. The two disulfide bonds are speculative, but possible, according to the structure of a related protein, glycoprotein 1bα (28, 38, 39). (B) Northern blot of mutants of the cysteine-containing motif. The Toll-781C is wild type, and other amino acid substitutions are as indicated. The proteins were expressed in transgenic flies by using the YP1-Gal4-UAS system. The expression of drosomycin and rp49 was analyzed by Northern blotting. Three independent 781C lines (lanes 3–5) and two independent lines of each of the other mutants were assayed (lanes 6–13). The CS and Toll10b flies were included for comparison (lanes 1 and 2, respectively). (C) Transfection assays of cysteine-781 mutants. The cysteine-781 was changed to other amino acids as indicated, and the activities of the mutants were assayed by cotransfection with a drosomycin-luciferase reporter in S2 cells. The Toll-781* contains a stop codon and, therefore, should produce a truncated protein. (D) Transfection assays of single or multiple cysteine-to-tyrosine mutants. Single or multiple cysteine-to-tyrosine changes were introduced into the wild-type Toll, and the activities were assayed by transfection as described for C. The mutations of the four cysteines in any combination, including quadruple mutation, have the same result of constitutive receptor activation. Overall, these data show that the cysteine-containing motif is an autoinhibitory module.
To show that the high activities were not rare coincidences of the specific mutations tested so far, we generated 10 more single amino acid substitutions at the 781 position and all combinations of cysteine-to-tyrosine changes in the four positions, including a quadruple mutation. These mutants were tested in transfection assays in S2 cells (Fig. 5 C and D). The drosomycin-luciferase reporter assays demonstrated that all these mutants were equally active. Therefore, the four cysteines have equal functions, and changing any one in any way leads to constitutive activation. It seems unlikely to us that the single-point mutants such as Toll10b and Toll9Q (27) use the unpaired cysteines to interact with other proteins. Instead, we postulate that these four cysteines are essential to maintain a structure that counteracts the Toll-receptor activity, possibly through inhibiting multimerization (Figs. 2B and 5A).
Discussion
Our results obtained from analyzing Spätzle and Toll in transgenic fly extracts show that the host protein Spätzle can function as a ligand for Toll because Spätzle forms a complex with Toll in transgenic fly extracts and stimulates the expression of a Toll-dependent immunity gene in adult flies. These findings are consistent with a recent report demonstrating that a truncated form of Spätzle purified from a viral expression system can bind to the ectodomain of Toll in vitro (16). The report also showed that a dimer of cleaved Spätzle interacts with two Toll ectodomains and that injecting the cleaved Spätzle can stimulate drosomycin expression (16).
The formation of multimeric complexes of Spätzle and Toll in this study suggests that induced receptor multimerization may be a mechanism for Toll activation. By using an extracellular epidermal growth factor receptor and an intracellular Toll-receptor fusion construct, it has been shown that induced multimerization of this fusion protein by the epidermal growth factor ligand can activate the Toll pathway (33). Evidence from the studies of mammalian TLRs also suggests that multimeric complex formation is possible (32, 34–37). For example, complex formation can be detected by expressing TLR4 in transfected cells (36). However, induced multimerization of TLRs has not been shown convincingly, suggesting that Toll proteins may have a low affinity toward each other.
In the cases of constitutively active Toll mutants, our results demonstrate that disulfide linkages may stabilize the multimers and cause increased expression of a Toll-dependent immunity gene. There is concern that expression of constitutively active mutant receptors to a higher level may cause aggregation and that the aggregation can occur at other steps not related to activation. By using site-directed mutagenesis we showed that in ΔN6 the disulfide linkage-dependent multimerization is essential for the activity, dissipating some of these concerns. Moreover, the ethyl methanesulfonate-induced Toll10b and other gain-of-function fly strains demonstrate that at physiological levels these mutants also cause constitutive activity.
The highly conserved cysteine-containing motif, which is located just outside the transmembrane domain and is different from the cysteines involved in the intermolecular linkage, may form a self-inhibitory module to regulate Toll activity. Systematic mutagenesis suggests that the constitutive activity does not result from a freed cysteine that forms a disulfide bond with a neighboring protein. Instead, the cysteine-containing motif may form two disulfide bonds to maintain a rigid structure (28, 38, 39). We postulate that this structure may provide steric hindrance, preventing the wild-type Toll from forming stable multimers and becoming active. Thus, disassembling the structure by deletion or mutation may permit the subunits to come close together. Under the right subcellular environment, the disulfide bond formation may stabilize the multimer, leading to stimulation of the pathway. Interestingly, the wild-type Toll-9 protein lacks the full complement of cysteines within this motif and exhibits similarly high drosomycin-stimulating activity (22, 40), indicating that a wild-type Toll member can use such a mechanism to regulate the signaling pathway.
It is possible that multimerization and formation of disulfide linkages are part of the activation mechanism of the wild-type Toll receptor. We tested for this activation mechanism of the tagged wild-type Toll constructs in adult flies after stimulating the pathway by septic injury, by combining with SpätzleΔN, or by combining with a Necrotic mutation. After assaying for complex formation by using co-IP and nonreducing SDS gel, we did not observe an increase in multimerization. One possible explanation is that the amount of induced multimerization in vivo is lower than the detection limits of the assays. Nonetheless, further analysis based on the results presented in this report should help to elucidate the mechanism of Toll-receptor activation.
Acknowledgments
We thank Dominique Ferrandon for providing the Yolk-Gal4 driver line and Li Zeng for comments on the manuscript. This work was supported by National Institutes of Health Grant GM53269.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: β-ME, β-mercaptoethanol; co-IP, coimmunoprecipitation; LRR, leucine-rich repeats; TIR, Toll/IL-1 resistance; TLR, Toll-like receptor; YP1, yolk protein 1.
References
- 1.Boman, H. G., Nilsson, I. & Rasmuson, B. (1972) Nature 237, 232-235. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann, J. A. (2003) Nature 426, 33-38. [DOI] [PubMed] [Google Scholar]
- 3.Hultmark, D. (2003) Curr. Opin. Immunol. 15, 12-19. [DOI] [PubMed] [Google Scholar]
- 4.Khush, R. S., Leulier, F. & Lemaitre, B. (2002) Science 296, 273-275. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov, R. & Biron, C. A. (2003) Curr. Opin. Immunol. 15, 2-4. [DOI] [PubMed] [Google Scholar]
- 6.Takeda, K. & Akira, S. (2003) Cell Microbiol. 5, 143-153. [DOI] [PubMed] [Google Scholar]
- 7.Ulevitch, R. J. (2000) Immunol. Res. 21, 49-54. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto, C., Hudson, K. L. & Anderson, K. V. (1989) Cell 52, 269-279. [DOI] [PubMed] [Google Scholar]
- 9.Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J.-M. & Hoffmann, J. A. (1996) Cell 86, 973-983. [DOI] [PubMed] [Google Scholar]
- 10.Rutschmann, S., Kilinc, A. & Ferrandon, D. (2002) J. Immunol. 168, 1542-1546. [DOI] [PubMed] [Google Scholar]
- 11.Anderson, K. V. (2000) Curr. Opin. Immunol. 12, 13-19. [DOI] [PubMed] [Google Scholar]
- 12.Tzou, P., Reichhart, J.-M. & Lemaitre, B. (2002) Proc. Natl. Acad. Sci. USA 99, 2152-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michel, T., Reichhart, J.-M., Hoffmann, J. A. & Royet, J. (2001) Nature 414, 756-759. [DOI] [PubMed] [Google Scholar]
- 14.Levashina, E. A., Langley, E., Green, C., Gubb, D., Ashburner, M., Hoffmann, J. A. & Reichhart, J.-M. (1999) Science 285, 1917-1919. [DOI] [PubMed] [Google Scholar]
- 15.Ligoxygakis, P., Pelte, N., Hoffmann, J. A. & Reichhart, J.-M. (2002) Science 297, 114-116. [DOI] [PubMed] [Google Scholar]
- 16.Weber, A. N., Tauszig-Delamasure, S., Hoffmann, J. A., Leliévre, E., Gascan, H., Ray, K. P., Morse, M. A., Imler, J. L. & Gay, N. J. (2003) Nat. Immunol. 4, 794-800. [DOI] [PubMed] [Google Scholar]
- 17.Charatsi, I., Luschnig, S., Bartoszewski, S., Nusslein-Volhard, C. & Moussian, B. (2003) Mech. Dev. 120, 219-226. [DOI] [PubMed] [Google Scholar]
- 18.Tauszig-Delamasure, S., Bilak, H., Capovilla, M., Hoffmann, J. A. & Imler, J.-L. (2002) Nat. Immunol. 3, 91-97. [DOI] [PubMed] [Google Scholar]
- 19.Rutschmann, S., Jung, A. C., Hetru, C., Reichhart, J.-M., Hoffmann, J. A. & Ferrandon, D. (2000) Immunity 12, 569-580. [DOI] [PubMed] [Google Scholar]
- 20.Meng, X., Khanuja, B. S. & Ip, Y. T. (1999) Genes Dev. 13, 792-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdelyi, M. & Szabad, J. (1989) Genetics 122, 111-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ooi, J. Y., Yagi, Y., Hu, X. & Ip, Y. T. (2002) EMBO Rep. 3, 82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morisato, D. & Anderson, K. V. (1994) Cell 76, 677-688. [DOI] [PubMed] [Google Scholar]
- 24.Schneider, D. S., Jin, Y., Morisato, D. & Anderson, K. V. (1994) Development (Cambridge, U.K.) 120, 1243-1250. [DOI] [PubMed] [Google Scholar]
- 25.Schlessinger, J. (2002) Cell 110, 669-672. [DOI] [PubMed] [Google Scholar]
- 26.Shi, Y. & Massague, J. (2003) Cell 113, 685-700. [DOI] [PubMed] [Google Scholar]
- 27.Winans, K. A. & Hashimoto, C. (1995) Mol. Biol. Cell 6, 587-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider, D. S., Hudson, K. L., Lin, T.-Y. & Anderson, K. V. (1991) Genes Dev. 5, 797-807. [DOI] [PubMed] [Google Scholar]
- 29.Kubota, K., Keith, F. J. & Gay, N. J. (1995) FEBS Lett. 365, 83-86. [DOI] [PubMed] [Google Scholar]
- 30.Tauszig, S., Jouanguy, E., Hoffmann, J. A. & Imler, J.-L. (2000) Proc. Natl. Acad. Sci. USA 97, 10520-10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norris, J. L. & Manley, J. L. (1995) Genes Dev. 9, 358-369. [DOI] [PubMed] [Google Scholar]
- 32.Xu, Y., Tao, X., Shen, B., Horng, T., Medzhitov, R., Manley, J. L. & Tong, L. (2000) Nature 408, 111-115. [DOI] [PubMed] [Google Scholar]
- 33.Sun, H., Towb, P., Chiem, D. N., Foster, B. A. & Wasserman, S. A. (2004) EMBO J. 23, 100-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozinsky, A., Underhill, D. M., Fontenot, J. D., Hajjar, A. M., Smith, K. D., Wilson, C. B., Schroeder, L. & Aderem, A. (2000) Proc. Natl. Acad. Sci. USA 97, 13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandor, F., Latz, E., Re, F., Mandell, L., Repik, G., Golenbock, D. T., Espevik, T., Kurt-Jones, E. A. & Finberg, R. W. (2003) J. Cell Biol. 162, 1099-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, H. K., Dunzendorfer, S. & Tobias, P. S. (2004) J. Biol. Chem. 279, 10564-10574. [DOI] [PubMed] [Google Scholar]
- 37.Dunne, A., Ejdeback, M., Ludidi, P. L., O'Neill, L. A. & Gay, N. J. (2003) J. Biol. Chem. 278, 41443-41451. [DOI] [PubMed] [Google Scholar]
- 38.Dumas, J. J., Kumar, R., Seehra, J., Somers, W. S. & Mosyak, L. (2003) Science 301, 222-226. [DOI] [PubMed] [Google Scholar]
- 39.Uff, S., Clemetson, J.-M., Harrison, T., Clemetson, K. J. & Emsley, J. (2002) J. Biol. Chem. 277, 35657-35663. [DOI] [PubMed] [Google Scholar]
- 40.Luna, C., Wang, X., Huang, Y., Zhang, J. & Zheng, L. (2002) Insect Biochem. Mol. Biol. 32, 1171-1179. [DOI] [PubMed] [Google Scholar]