Abstract
In the present study, the effects of 10- or 100-nm silica oxide (SiO2) NPs on human peripheral blood mononuclear cells (PBMC) were examined. Cytotoxic effects and oxidative stress effects, including glutathione (GSH) depletion, the formation of protein radical species, and pro-inflammatory cytokine responses, were measured. PBMC exposed to 10-nm NP concentrations from 50 to 4,000 ppm showed concentration-response increases in cell death; whereas, for 100-nm NPs, PBMC viability was not lost at <500 ppm. Interestingly, 10-nm NPs were more cytotoxic and induced more oxidative stress than 100-nm NPs. Immunoelectron micrographs show the cellular distribution of GSH and NPs. As expected based on the viability data, the 10-nm NPs disturbed cell morphology to a greater extent than did the 100-nm NPs. Antibody to the radical scavenger, 5,5-dimethyl-1-pyrroline N-oxide (DMPO), was used for Western blot analysis of proteins with radicals; more DMPO proteins were found after exposure to 10-nm NPs than 100-nm NPs. Examination of cytokines (TNF-α, IL-1ra, IL-6, IL-8, IL-1β, and IFN-γ) indicated that different ratios of cytokines were expressed and released after exposure to 10- and 100-nm NPs. IL-1β production was enhanced by 10- and 100-nm NPs;, the cytotoxicity of the NPs was associated with an increase in the IL-1β/IL-6 ratio and 100-nm NPs at concentrations that did not induce loss of cell viability enhanced IL-1β and IL-6 to an extent similar to phytohemagglutinin (PHA), a T cell mitogen. In conclusion, our results indicate that SiO2 NPs trigger a cytokine inflammatory response and induce oxidative stress in vitro, and NPs of the same chemistry, but of different sizes, demonstrate differences in their intracellular distribution and immunomodulatory properties, especially with regard to IL-1β and IL-6 expression.
Keywords: Silica (SiO2) nanoparticles; PBMCs; Oxidative stress; Glutathione (GSH); Radicals; 5,5-dimethyl-1-pyrroline N-oxide (DMPO); Inflammatory cytokines
Introduction
Today, a great deal of attention is focused on nanotechnology. The term “nanotechnology” is used to describe the manufacture of small materials at nanoscale (1 to 100 nm). According to the National Nanotechnology Initiative (www.nano.gov), nanoparticles (NPs) are those with one or more dimensions of ≤100 nm. Nanotechnology utilizes new fabrication technologies to manufacture NPs, and NPs are used to improve current materials or in the development of new materials and devices. A few examples of NPs include organic (C60 fullerenes, polymer) and inorganic (metal and metal oxide) NPs, carbon tubes, nanofibers, quantum dots, and nanoscale-engineered substrates (Medina et al. 2007). The unique physiochemical properties of NPs foster increasing production of manmade NPs and their applications in multiple areas, including medicine and electronics (www.malvern.com; Salata 2004; Mahmoudi et al. 2009; Lynch et al. 2006). For example, titanium dioxide (TiO2) NPs are used in sunscreens (Lanone et al. 2009), and silver (Ag) NPs, which possess antibacterial properties (Rai et al. 2009) and antiviral activity against HIV-1 (Lara et al. 2010), are used in medicinal applications; clusters of gold (Au) NPs have been used to target and damage leukemia cells by laser nano-thermolysis (Lapotko et al. 2006). Injection of fabricated NPs is being evaluated for numerous other medical purposes (Flynn and Bryant 2005).
Despite the fact that NPs are being used in a wide variety of applications, relatively little is known about their potential cytotoxic properties, environmental impact, and most importantly, the consequences of direct/indirect human exposures. Studies involving mice, and the classification of carbon-based NPs as a possible carcinogen by the International Agency for Research on Cancer (www.iarc.fr), raised concerns about the increased production of manmade NPs. Considering their size (<100 nm), NPs easily enter the human body by inhalation, ingestion, or absorption through the skin; thus, raising concerns of their potential adverse health effects, as they are highly reactive (high surface-to-volume ratio) (Oberdörster et al. 2005a, b). Given this reactivity, as well as their size, chemistry, and surface-charge characteristics, NPs readily interact with biological molecules and gain access to cell compartments by passing through cell membranes and/or biological barriers (blood–brain barrier), thereby producing oxidative stress (Stratmeyer et al. 2008; Balbus et al. 2007) and becoming deposited in organs (Kim et al. 2009).
Oxidative stress occurs when reactive oxygen species (ROS) create an imbalance between oxidants and antioxidants within a cell, via either an increase in oxidants (ROS) or a decrease in antioxidant defenses (Halliwell and Gutteridge 1999; Magder 2006). One indicator of oxidative stress is the generation of ROS such as superoxide (O2–), hydrogen peroxide (H2O2), hydroxyl radical (·HO), and other oxygen radicals; another is the depletion of antioxidant reserves, such as glutathione (GSH) (Shi et al. 1998; Thannickal and Fanburg 2000; Ault and Lawrence 2003; Halliwell and Gutteridge 1999). GSH is a low molecular weight antioxidant found in the cytoplasm, nuclei, and mitochondria of cells at millimolar concentrations; among its important biological functions, it serves as a substrate for GSH-S-transferase (GST) and as a scavenger for ROS such as singlet oxygen (1O2), .HO, O2– and to a lesser extent H2O2 (Ault and Lawrence 2003; Dickinson and Forman 2002). GSH also provides a reducing equivalent (H+ + e−) and is the cell’s last defense against oxidative stress (Ault and Lawrence 2003). ROS induced by transition metals, chemicals, or through interactions between NPs and cellular components can form free radicals which damage proteins, lipids, and DNA, increasing necrosis or apoptosis (Shi et al. 1998; Magder 2006; Valko et al. 2004). In addition, NPs modify cellular thiols and processes associated with a cell’s redox status, given that some transition metals have a high affinity for redox-reactive and protective thiol groups (Pelka et al. 2009; Pacurari et al. 2010).
Thus far, little is known about the consequences of prolonged direct or indirect (environmental) human exposure to NP or whether they increase susceptibility to autoimmune diseases or cancers of the immune and other organ systems, as well as to neurodegenerative and neuroinflammatory disorders (Pacurari et al. 2010; Sayre et al. 2008; Miranda et al. 2000), which may be related to oxidative stress. The lack of research on the immune system and effects of NPs, particles which have the potential to induce immunostimulatory or immunosuppressive reactions by interacting with immune cells, prompted the current study to examine the effects of NPs of the same chemistry but of different sizes on human PBMC in vitro. We hypothesized that smaller NPs would be more toxic to cells than larger NPs do to a greater induction of oxidative stress.
Methods and materials
Silica NPs
Commercially available silica NPs, 100-nm (5.68 wt%) and 10-nm (40 wt%), dispersed in water (H2O), were purchased from Polysciences, Inc. (Warrington, PA, USA). Silica oxide (SiO2) NPs (density = 2 g/mm3) were characterized by mass-volume (wt/v) percentage. In brief, a 1-ml aliquot of the NP aqueous dispersion was placed in a small container and weighed, and H2O was then removed by evaporation (Isotemp Oven Model 750G, Fisher Scientific). The container was re-weighed, and the mass difference was used to calculate the mass-volume (wt/v) percentage. An average of three determinations indicated 100-nm SiO2 NP to be 5.8 wt.%, whereas 10-nm SiO2 NPs were 32.6 wt.%. The mass-volume percentage concentration was used to make 500 to 4,000 ppm solutions of 100-nm NPs and 50 to 4,000 ppm solutions of 10-nm NPs.
Characterization of the NPs
The 100- and 10-nm SiO2 NPs were analyzed for possible endotoxin contamination with a Limulus Amebocyte Lysate QCL-1000 kit (Cambrex Bio Science Walkersville, Inc., Walkersville, MD) following the manufacturer’s instructions; this kit uses Escherichia coli 0111:B4 endotoxin (Cambrex Bio Science) as a standard. The NPs also were imaged by scanning electron microscopy (SEM; LEO 1550 Scanning Electron Microscope, Carl Zeiss International) for their shape and extent of aggregation when placed in medium. In addition the association of the NPs with serum proteins in the medium was assessed by analytical ultracentrifugation with a Optima XL-1 analytical centrifuge (Beckman Coulter). The sedimentation was scanned with absorbance optics at 310 nm for SiO2 NPs and at 280–289 for protein with the NPs.
Cell viability
Human PBMC isolated from EDTA-venous blood samples from three healthy donors using a standard Histopaque-1077 gradient (Sigma), were exposed in triplicate to 100- or 10-nm SiO2 NPs. In brief, PBMC were resuspended at a density of 1 × 106 cells/900 μl in complete RPMI-1640 (Sigma) containing 1.2 % sodium bicarbonate,1 % sodium pyruvate,1 % nonessential amino acids, 0.025 % gentamicin (at 40 mg/ml), and 5 % human AB serum. The PBMC were transferred to a 24-well plate (BD Falcon 352054, San Jose, CA, USA) and treated with 100 μl NPs in RPMI-1640 medium prepared immediately before use at the appropriate concentrations. Samples not exposed to NPs served as controls. PBMC were incubated for 24 or 48 h in an incubator at 37 °C in a 5 % CO2 and 8 % O2 humidified environment with gentle rocking. PBMC were then centrifuged at 550×g at 4 °C, isolated, washed with 3 ml PBS, resuspended in 500 μl PBS, followed by the addition of 5 μl propidium iodide (0.5 mg/ml solution; Sigma) to each sample. After a 5-min incubation period at RT, samples were analyzed with FACSCAN Flow Cytometer (BD Biosciences) using CellQuest software (BD Biosciences).
Immunoelectron microscopy
The distribution of NPs and GSH was investigated after human peripheral blood mononuclear cells (PBMC) were exposed to 100- or 10-nm SiO2 NPs, for 1 h, using a previously published procedure (Ault and Lawrence 2003). In brief, adherent cells (mainly macrophages) were cultured on cover slips and processed as follows. Experimental and control PBMC were fixed with cold (4 °C) 4 % paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4) for 20 min, treated with cold (4 °C) 10 mM N-ethylmaleimide (NEM; Aldrich Inc., St. Louis, MO, USA) in PBS (pH 7.4) for 20 min, post-fixed with cold 1 % glutaraldehyde in PBS (pH 7.4) for 1 h, and washed twice in PBS (pH 7.4). Samples were then dehydrated in an ascending ethanol series ending with 100 % ethanol, and embedded in LR White. Semi-thin (0.20–0.25 μm) sections were cut with a Diatome diamond knife on a Reichert Ultracut E ultramicrotome. The sections were blocked for 30 min in a blocking solution containing 50 μg/ml goat serum, 50 μg/ml rabbit IgG, 20 μg/ml bovine serum albumin (BSA) in Tris-buffered saline (TBS; containing 1 μg/ml BSA, 0.05 % Tween 20, 0.5 M NaCl, 20 mM NaN3, pH 7.4), exposed to 28 μg/ml 8.1-GSH antibody (StressGen, Victoria, BC, Canada) in TBS (pH 7.4) with 20 μg/ml rabbit IgG for 1 h, washed four times in TBS, labeled with a 1:100 dilution of nanogold particles conjugated to goat anti-mouse IgG antibodies (Ted Pella, Inc., Redding, CA) in TBS with 10 μg/ml goat serum for 1 h, washed five times in TBS, and stained with uranyl acetate for 20 min and lead for 1 min. The negative controls included this protocol except no NEM was used. The sections were viewed at 80 kV with a Zeiss 910 transmission electron microscope. The 8.1-GSH antibody (StressGen) is a mouse monoclonal IgG1 antibody against GSH adduct with N-ethylmaleimide (GS-NEM), and was previously described in depth (Ault and Lawrence 2003; Messina et al. 1987). Considering the greater density of the 10-nm gold NPs, they could be distinguished from the 10-nm SiO2 NPs, which were difficult to discern on an individual basis.
Cell culture and treatment with silica NPs
Human blood samples were provided by healthy volunteers, who had given informed consent, at the Wadsworth Center nursing station; the protocol was approved by the IRB of NYS Department of Health. Samples were obtained by venipuncture and collected into EDTA-containing tubes. Human PBMC were isolated using a standard Ficoll/Histopaque-1077 (Sigma-Aldrich, Inc. St. Louis, MO, USA) gradient and cultured in RPMI 1640 medium supplemented with 10 % AB human serum (Sigma), 1 mM sodium pyruvate, 1 mM non essential amino acids, 1 mM glutamine, and 20 μg/ml gentamicin. PBMC were added to a 24-well plate at a density of 1 × 106 cells ml−1 well−1. Triplicate cultures were exposed to 10- or 100-nm NPs at particular concentration. Cells not exposed to SiO2 NPs served as a negative control, whereas PBMC exposed to 10 μg/ml final concentration of PHA (a T cell mitogen) were used as positive controls. PBMC were placed in an incubator for 24–48 h at 37 °C in a humidified environment of 5 % CO2 in air.
After a 24-h incubation period, the 24-well plate was centrifuged at 180×g for 5 min at 4 °C; the cell culture medium collected and immediately frozen at −20 °C for cytokine analysis. PBMC were washed once with cold (4 °C) PBS (pH 7.4) and lysed with a freshly prepared solution of 1 % Triton X-100 and 1 mM EDTA in PBS for 5 min. The cell lysates were aliquoted and analyzed for oxidative stress by measurement of GSH levels and protein radical formation using the spin-trapping reagent 5,5-dimethyl-1-pyrroline N-oxide (DMPO; Sigma).
Protein concentration
The protein concentration was measured by a BCA™ Protein Assay Kit (Pierce Chemical Co., Rockford, IL), which uses BSA as a standard.
Cytokine analysis
To assess the extent of the cytokine response from NP exposure, a multiplex bead assay with specific antibodies was used to measure the cytokine levels of TNF-α, IL-1ra, IL-6, IL-8, IL-1β, and IFN-γ. The cytokine response was determined by taking a small volume of cell culture supernatant from human PBMC (106/ml) exposed to 100- or 10-nm SiO2 NPs for 24 h, as described above. A portion of the supernatant, 50 μl, was used to measure cytokine levels with a bead-based Fluorokine® MultiAnalyte Profiling Multiplex Base kit (R&D Systems, Inc., Minneapolis, MN) following the manufacturer’s instructions with a Luminex® 200™ (Luminex Co., Austin, TX) instrument.
DMPO Western immunoblotting analysis
Spin-trapping agent DMPO is radical scavenger with low cytotoxicity used to trap short-lived reactive radical species such as protein radicals (Ionita et al. 2008). DMPO covalently binds to the radical species, forming a stable adduct that may be detected with a commercially available DMPO antibody (Detweiler et al. 2002; Deterding et al. 2004). The technique involves the rapid addition of DMPO to the short-lived radical species; the radical is captured, resulting in the formation of a covalent bond between DMPO and the radical. Immediately after PBMC were lysed, a 200 μl aliquot of the cell lysate was treated with 100 μl of 100 mM DMPO and allowed to stand at room temperature (RT) in the dark for 1 h. The solution mixture was centrifuged at 11,750×g for 10 min at 4 °C, the supernatant was isolated, and the protein concentration measured using a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). A 40-μg sample of DMPO-protein nitrone adduct from the cell lysate was diluted in 10 μl of PBS, followed by the addition of 10 μl of 2× DTT reducing buffer (containing 4 % (wt/v) sodium dodecyl sulfate (SDS), 20 % (v/v) glycerol, 3 % (wt/v) dithiothreitol (DTT), 0.10 % (wt/v) bromophenol blue, and 0.5 M Tris–HCl buffer (pH 6.8)); the mixture was then heated in H2O at 90 °C for 5 min. This mixture was loaded onto a freshly prepared 0.75 mm 10-well 12 % SDS-polyacrylamide gel along with molecular weight standards (Precision Plus Protein unstained standards; Bio-Rad, Hercules, CA). SDS-PAGE protein separation was run under continuous conditions using a Mini PROTEAN 3 Cell system (Bio-Rad) at 140 V for 2 h. The separated proteins were blotted on a nitrocellulose membrane (0.45-μm pore size; Millipore, MA) following the manufacturer's instructions using a Trans-Blot SD Semi-dry transfer cell (Bio-Rad) for 25 min at 20 V. The membrane was blocked with 20 ml 5 % cold water fish gelatin in Tris-buffered saline solution (pH 7.4) containing 0.05 % Tween-20 (TBS-T) for 2 h at RT on a rocker platform (Bellco Biotechnologies, Vineland, NJ). The membrane was washed thrice with TBS-T, and 20 ml of rabbit anti-DMPO serum (1:4,000 in TBS-T containing 1 % cold water fish gelatin; Cayman Chemicals, Ann Arbor, MI, USA) was added and incubated overnight (16 h) at 4 °C. The membrane was washed thrice with TBS-T, and 20 ml goat anti-rabbit IgG (H + L) HRP conjugate (1:5,000 in washing buffer; Bio-Rad) was added and allowed to incubate for 1 h at RT in the dark. The membrane was washed with TBS-T, and the blot was developed by incubation with Super Signal chemiluminescent substrate (Pierce) for 5 min, and then assayed with a LAS-3000 plus (FujiFilm) instrument. It is important to note that the molecular weight standard lane was developed separately following the manufacturer’s instructions using Precision StrepTactin-HRP conjugate (Bio-Rad).
Immunoprecipitation of DMPO nitrone adducts
A 200-μg sample of PBMC lysate treated with DMPO was pre-cleared for 1 h at 4 °C in a 100-μl slurry (50 %) of agarose-conjugated goat IgG (Rockland, Inc., Gilbertsville, PA, USA). The slurry was centrifuged at 11,750×g for 10 min, and the supernatant was isolated, treated with 20 μl of anti-DMPO (diluted 1:500 in PBS), and incubated overnight at 4 °C with gentle rocking. A portion, 50 μl (100 μg), was added to 100 μl of 50 % goat anti-rabbit IgG-agarose in dilution buffer (1:1 (v/v) TBS, pH 8, containing 1 % NP-40; Sigma), incubated overnight at 4 ºC with gentle rocking, and centrifuged at 11,750×g for 10 min at 4 ºC. The agarose antigen-antibody pellet was isolated, treated with 40 μl of ×1 DTT-reducing buffer, heated at 90 ºC for 5 min in a water bath, and centrifuged at 11,750×g for 10 min at RT. The supernatant was isolated and immediately resolved on a freshly prepared 10 % Bis Tris gel using Kaleidoscope Prestained Standards (Bio-Rad) as a marker. After electrophoresis (90 min at 140 V), the gel was fixed with 100 ml of fixative solution (50 % methanol, 7 % acetic acid, 43 % water) with gentle agitation on an orbital shaker for 30 min, stained overnight with SYPRO Ruby protein staining (Molecular Probe S-12000), and developed with a LAS-3000 Plus (Fuji) instrument.
Glutathione competitive ELISA
PBMC were lysed as described earlier; a 200-μl aliquot was immediately treated with 200 μl of 10 mM NEM in PBS and incubated at RT for 1 hr in the dark. The mixture was centrifuged at 11,750×g for 10 min at 4 °C and the supernatant isolated for analysis. A 96-well ELISA plate (Costar, high binding, Corning, Inc., Corning, NY, USA) was coated with 100 μl per well of 1 % BSA in PBS, incubated at 4 °C overnight and washed with washing buffer (PBS containing 0.05 % Tween-20) using an ELISA plate washer. A 100-μl per well sample 1 mM BS3 (0.572 mg/ml; Pierce) in 0.05 M phosphate buffer (pH 8) was added and allowed to incubate for 15 min at RT, followed by the addition of 20 μl per well of 0.025 M GS-NEM (10.8 mg/ml) in PBS (pH 7.4) for 30 min. After incubation for 30 min at RT, the plate was washed and blocked with 100 μl per well 10 mM glycine (Sigma) in washing buffer for 30 min at RT. The plate was washed, and 50 μl per well of sample were incubated with mouse 8.1-GSH (1:50,000 in PBS containing 0.05 % Tween-20 and 0.1 % BSA; Stressgen; sample was incubated for 30 min with 8.1-GSH at RT prior to addition to the plate) was added and allowed to incubate at RT for 1 h. The plate was washed, and 50 μl per well of goat anti-mouse IgG (whole molecule)-peroxidase (1:5,000 in washing buffer; Sigma) was added. The plate was allowed to incubate in the dark for 1 h at RT; it was then washed and developed with 50 μl per well of 3,3′,5,5′-tetramethyl-benzidine liquid substrate (Sigma). The reaction was stopped with 25 μl per well of 1 M H2SO4, and read at 450 nm with an ELx808™ microplate reader (BIO-TEK® Instruments, Inc., Winooski, VT, USA). Standard curve stock GS-NEM solutions were prepared by slow addition of 3.9 mg (0.0127 mmol) of reduced GSH (Sigma) dissolved in 500 μl PBS to 1.29 mg (0.0127 mmol) NEM (Sigma) in 500 μl PBS at RT. The reaction was followed by UV–visible spectroscopy (λ 304–315 nm, NEM) until complete. Serial dilutions from the standard stock GS-NEM (5.5 mg/ml, 2.28 × 10−2 M) solution were prepared ranging from 2 × 10−4 to 6.25 × 10−6 M as well as a blank PBS solution without GS-NEM. An aliquot of each solution was then treated with an equal volume amount of 8.1-GSH (1:50,000 in PBS containing 0.05 % Tween-20 and 0.1 % BSA; solutions were incubated with 8.1-GSH for 30 min at RT prior to addition to the plate), added to the ELISA plate along with the samples, and developed as described earlier. The standard GSH curve was determined by plotting O.D. vs. log of the concentration, calculation of the amount of GSH, and determination of GSH concentration, in μg GSH/mg protein.
Statistical analysis
Unless otherwise stated, experimental results were analyzed as mean value ± standard deviation (SD) from three replicates (n = 3). One-way analysis of variance followed by appropriate post hoc tests were utilized. In all comparisons of numerical results, a p value < 0.05 indicates a significant difference.
Results
SiO2 NP endotoxin contamination analysis and evaluation of NP aggregation
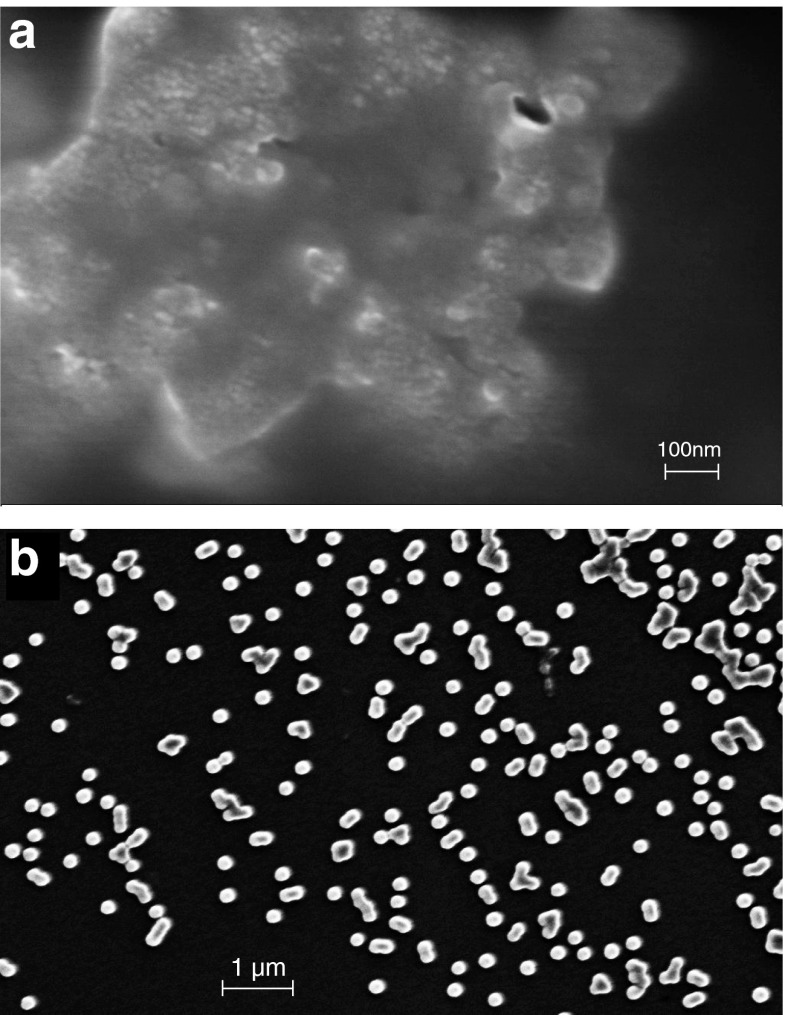
No endotoxin contamination was found for the suspension of either 10- or 100-nm NPs. We utilized SEM and analytical ultracentrifugation to assess the shape and aggregation of the NPs (Fig. 1). In the preparation of the 10-nm NPs for the SEM, the NPs aggregated too readily to allow analysis of their individual shapes (Fig. 1). The 100-nm NPs were both spherical and amorphous; they aggregated mainlyinto doublets and triplets, but the majority remained as singlets. As has been described in numerous previous reports, NPs in culture medium tend to aggregate. The 10-nm NPs aggregated into large masses, but they did disperse more readily when cultured in the PBMC cultures as shown in the latter figures with cells. There was no attempt to quantify the kinetics of aggregation; however, we did assess association of the 10- and 100-nm NP with serum proteins by analytical ultracentrifugation. The NPs readily associated with the proteins and the proteins sediment with the NPs. Analytical ultracentrifugation of the NPs was performed to investigate the interaction between the main serum proteins (IgG and albumin) and the NPs. Sedimentation of the 100- and 10-nm NPs and protein-NP complexes occurred at 3,000 rpm, whereas sedimentation of the free protein was best observed at speeds of 10,000–20,000 rpm. Essentially, all of the protein sedimented to the bottom of the cell with the NPs at low rotor speeds (2,500–3,500 rpm) in a relatively short amount of time (<30 scans) indicating that the protein strongly adsorbed to the silica NPs (data not shown). The sedimentation of free proteins observed at speeds of 10,000–20,000 rpm was scanned for Absorbance Optics at 280 vs. at 289 nm for protein-NPs; this is the same way we 'observed' the NPs at 3,000 rpm using Absorbance Optics at a wavelength suitable for viewing the proteins attached to them, the proteins in essence acting as 'reporter molecules' for the NPs.
Fig. 1.
SEM of NPs. The 10- (a) and 100-nm (b) SiO2 NPs were diluted into medium and immediately prepared for analysis. Scale bars are shown on each SEM image
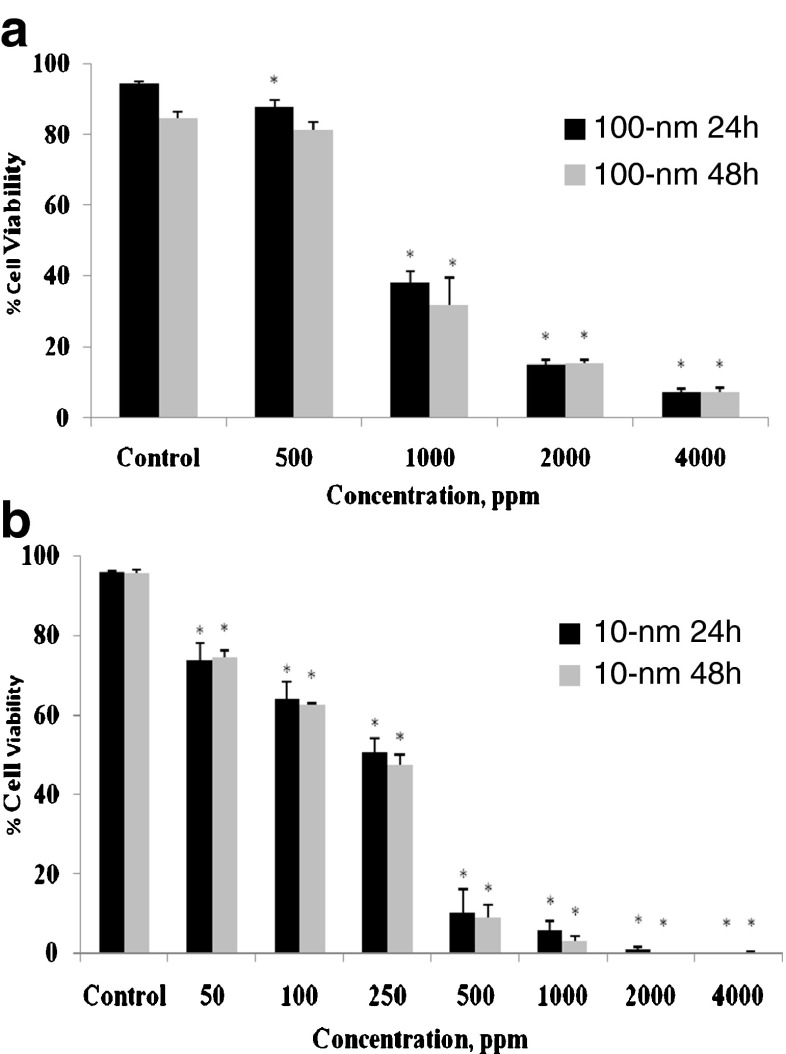
Effect of SiO2 NPs on cell viability
PBMC were exposed to 100-nm SiO2 NPs at concentrations of 500, 1,000, 2,000, or 4,000 ppm (Fig. 2a) or to 10-nm SiO2 NPs at concentrations of 50, 100, 250, 500, 1,000, 2,000, or 4,000 ppm (Fig. 2b) for 24 and 48 h. For both types of NPs, cell viability decreased as a function of both time and concentration (Fig. 2). The 10-nm SiO2 NPs (≥50 ppm) produced significant cytotoxicity; at 2,000 ppm, fewer than 1 % of the cells were viable for either exposure duration. Cell viability also gradually declined with 100-nmSiO2 NPs (≥500 ppm), indicating that the larger NPs are less cytotoxic, which is mainly due to significantly less surface area interaction with the cells when equivalent concentrations are used. The surface areas of the 10-nm and 100-nm NPs are similar when the 100-nm concentration is 10-fold greater, and at those concentrations, the different sized NPs produced near equivalent toxicity. Surprisingly, there were no significant viability differences between the 24- and 48-h exposures, for either NP size.
Fig. 2.
In vitro effects of 100- or 10-nm SiO2 NPs on human PBMC viability. PBMCs (1 × 106/ml) were cultured with 100- (a; 500, 1,000, 2,000, or 4,000 ppm) or 10-nm NPs (b; 50, 100, 250, 500, 1,000, 2,000, or 4,000 ppm) for 24 and 48 h. Control PBMCs were cultured in absence of NPs. Cell viability was determined by propidium iodide permeability assessed by flow cytometry. Values shown are the mean ± SD from three separate experiments. Significant differences from control values are indicated by *ρ < 0.05
NP uptake and cellular GSH distribution
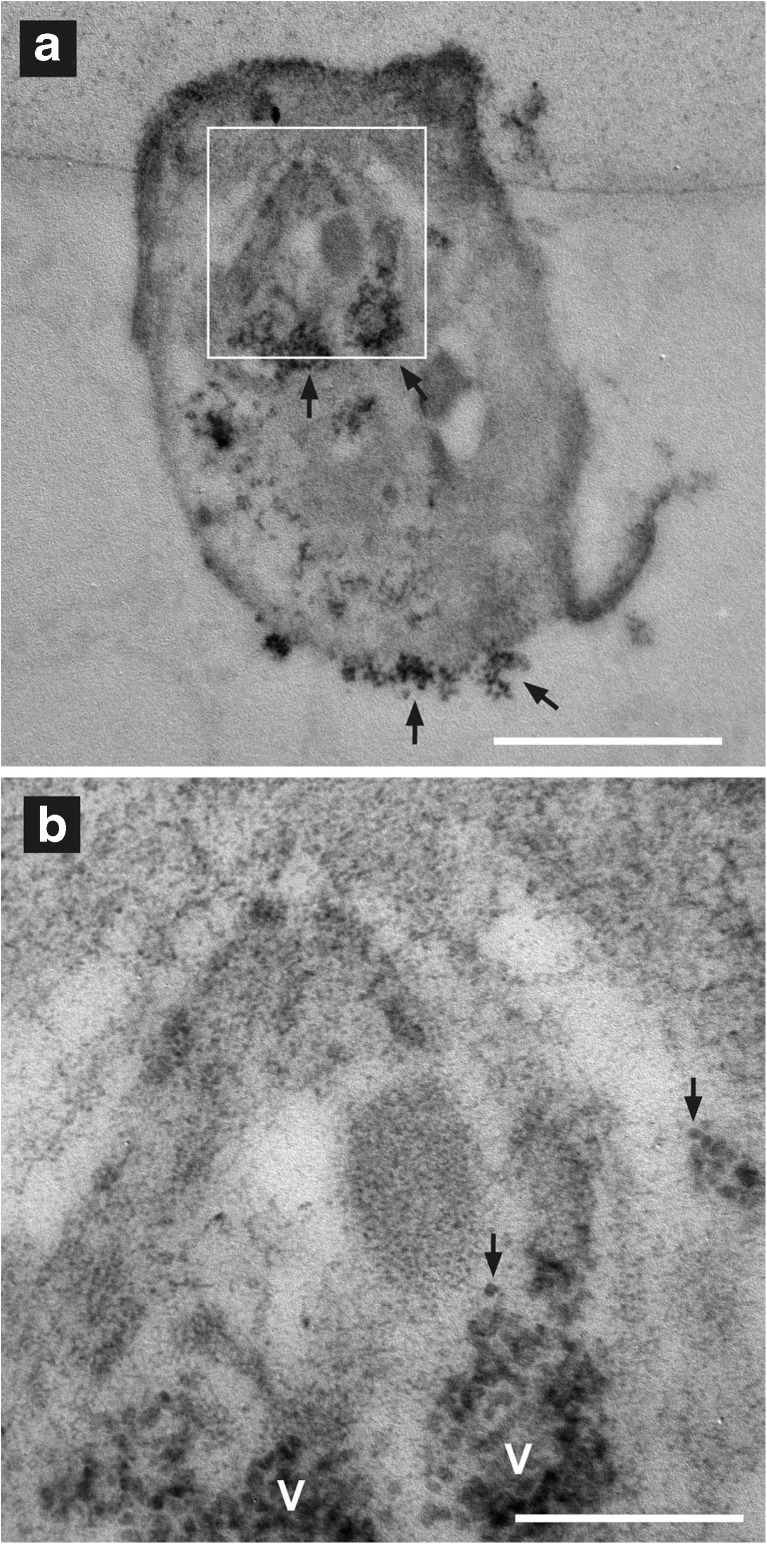
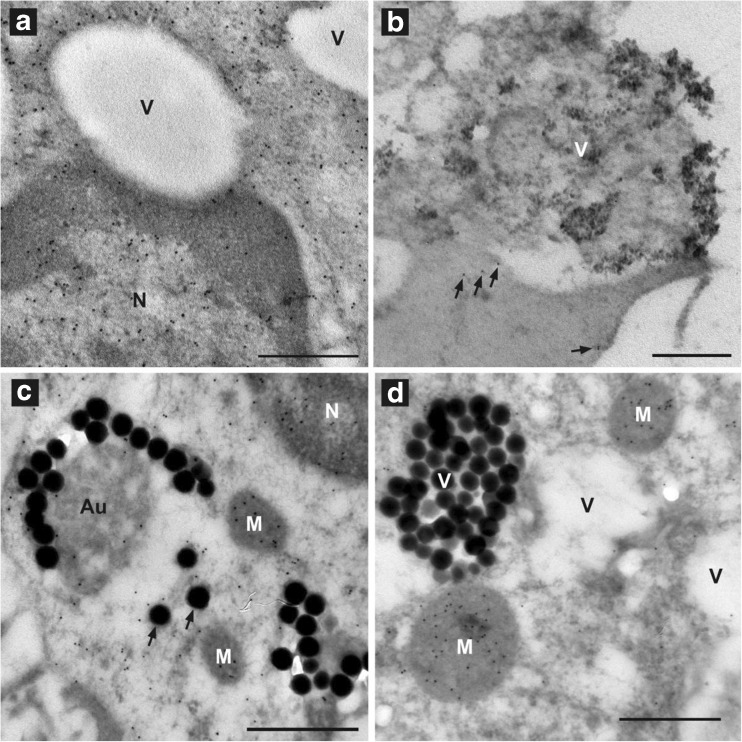
Immunoelectron microscopy was used to study earlier (1 h) effects of the NPs on GSH distribution in PBMC while tracking the uptake and behavior of the SiO2 NPs. The 100-nm NPs were easier to observe within a cell than were the 10-nm NPs. The larger diameter (and thus volume) made the 100-nm NPs more electron dense, and an individual NP could easily be identified (Fig. 3). The less electron-dense 10-nm silica NPs were hard to identify individually within a similar cellular matrix (Fig. 4). Aggregates of several 10-nm NPs could be seen on the cell surface (Fig. 4a) and within the cell (Fig. 4a, b). Aggregates of 3 or 4 10-nm NPs were at the limits of detection for identification of 10-nm SiO2 NPs within the cellular matrix (Fig. 4b). The 100-nm SiO2 NPs, and presumably also the 10-nm NPs, entered the cell by phagocytosis. NPs initially adsorbed to the cell surface (Figs. 3a and 4a). During phagocytosis, the cell surface invaginated (Fig. 3b), and groups of NPs were transported into the cell in vacuoles called phagosomes. The majority of the 100-nm SiO2 NPs remained in vacuoles, either phagosomes or phagolysosomes, with only a few individual NP escaping into the cytoplasm (Fig. 4c, d). Given the difficulty of identifying individual 10-nm SiO2 NPs in the cellular matrix, it was not possible to determine the proportion of 10-nm NPs that escaped to the cytoplasm. Eventually, some NPs were expelled from the cell by exocytosis (Fig. 5b).
Fig. 3.
Binding to and uptake of 100-nm SiO2 NPs by human adherent PBMCs, which are mainly monocytes. NPs (arrow) are initially adsorbed to the cell surface (a). Bar indicates 1 μm. b Some NPs, on the outside of the cell, lay within an invagination (arrow) of the cell surface; such invagination is an early step in phagocytosis. The cell is a negative control (no NEM; thus no gold particles; see “Methods and materials”). M mitochondrium. Bar indicates 1 μm. c Two 100-nm NPs are within a vacuole (V), presumably a phagolysosome; GSH distribution is indicated by the 10-nm gold particles. d An apoptotic cell (Ap) bound (start of efferocytosis) to a monocyte with NPs is shown. The 100-nm NPs are in V, either phagosomes or phagolysosomes. One of these V contains material other than silica particles, indicating that it is an autophagolysosome (Au); N nucleus. The apoptotic cell contains no silica NPs and may have started the process of apoptosis before being exposed to the silica NPs. Bar indicates 1 μm
Fig. 4.
The 10-nm SiO2 NPs appear dispersed within the PBMCs. a A PBMC has clumps of 10-nm NPs (arrows) at the cell surface and dispersed within the cell. The area within the white box is enlarged in (b). Bar indicates 1 μm. b Clumps of 10-nm NPs are seen in vacuoles (V). The lesser-electron-dense 10-nm silica NPs are hard to identify individually within similarly dense cellular matrix. Groups of two or three 10-nm NPs (arrows) can be resolved within the clumps; bar indicates 0.25 μm
Fig. 5.
The distribution of GSH in PBMCs exposed to 10-nm or 100-nm SiO2 NPs is similar to that seen in PBMCs during oxidative stress (Ault and Lawrence 2003). a An unexposed control PBMC showing GSH (indicated by 10-nm gold-anti-mouse IgG) distributed throughout the nucleus (N) and cytoplasm, but not within vacuoles (V); bar indicates 0.5 μm. b A PBMC exposed to 10-nm NPs is shown with clumps of NPs in a vacuole (V) and NPs being taken up or exocytosed by the cell. GSH levels (indicated by the number of gold particles) are low compared with those in unexposed control PBMC in (a). Only five gold particles (arrows) are observed in the cytoplasm; bar indicates 0.5 μm. c A PBMC exposed to 100-nm NPs showing depletion of GSH (indicated by a few gold particles in the N and cytoplasm than in those of the unexposed PBMC in (a). GSH remains concentrated in the mitochondria (M). The 100-nm NPs are found in V and individually in the cytoplasm (arrows). The nonsilica material of an autophagolysosome (Au) has some labeling (gold particles) indicating the presence of GSH. This material may be that of autophagocytized mitochondria; bar indicates 0.5 μm. d A PBMC exposed to 100-nm NPs is shown with the cytoplasm being almost depleted of GSH (absence of gold particles). GSH still remains in the mitochondria (M) as previously observed with PMBC enzymatically depleted of GSH (Ault and Lawrence 2003); 100-nm silica particles are contained in a V; bar indicates 0.5 μm
The effects of SiO2 NPs on cellular GSH distribution were similar to the cellular indicators of oxidative stress (Ault and Lawrence 2003). In untreated PBMC, GSH was distributed relatively evenly throughout the nucleus, mitochondria, and cytoplasm (Fig. 5a). From gold particle counts that indicate GSH localization, it appeared that SiO2 NPs may stimulate GSH production, possibly during macrophage activation (Table 1). However, even in the short incubation time of 1 h, GSH was depleted when the cells showed signs of stress, such as presence of many vacuoles and the appearance of washed-out cytoplasm. GSH was lost from the cytoplasm and nucleus (Fig. 5c); the last reservoirs of GSH were found in mitochondria (Fig. 5c, d); it had been previously reported that when cells are undergoing oxidative stress due to blockage of GSH synthesis the last amounts of GSH are observed in the mitochondria (Ault and Lawrence 2003). Eventually, most of the GSH in the cytoplasm and nucleus was depleted (Fig. 5b, d).
Table 1.
Silica NPs alter GSH concentration of PBMCs
| Cellular treatment and condition | Mean ± SEM (N)* |
|---|---|
| Untreated control cells showing no stress | 24 ± 0.9 (351) |
| Cells exposed to 100-nm particles and showing no stress | 60 ± 2.8 (130) |
| Cells exposed to 100-nm particles and showing stress | 5 ± 1.2 (48) |
| Cells exposed to 10-nm particles and showing no stress | 76 ± 3.8 (264) |
| Cells exposed to 10-nm particles and showing stress | 13 ± 2.0 (28) |
*GSH concentration is presented as the mean number of gold particles, indicating GSH, per square micron ± S.E.M. N is the total number of 0.5 micron squared areas where gold particles were counted in each group. Each group was compared with each other using the Student’s t test and was found to be significantly different from each other using the P-level of 0.005.
Effects of SiO2 NPs on intracellular GSH levels
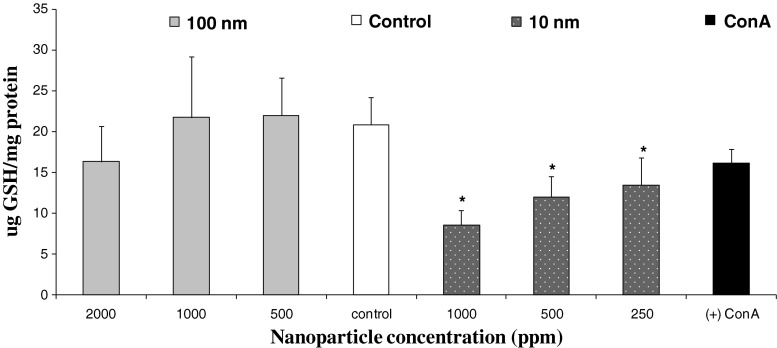
A competitive ELISA assay was used to measure intracellular GSH levels in the cultures of the PBMC after exposed to SiO2 NPs for 24 h. GSH levels were measured from cell lysates treated with NEM. The addition of NEM forms a GS-NEM adduct as previously described (Ault and Lawrence 2003); this adduct is recognized by the 8.1-GSH antibody, which is specific only to GS-NEM; GS-SG and protein-S-SG is not measured. There was a significant decrease of GSH in PBMC exposed to 10-nm SiO2 NP (Fig. 6). As the NP concentration declined, the level of GSH increased. A slight, but not significant, decrease in GSH levels relative to GSH levels in controls was measured for PBMC exposed to 100-nm NPs at a concentration of 2,000 ppm; however, no significant differences were measured at 1,000 or 500 ppm of the 100-nm NPs. As expected, depletion of GSH levels was associated with loss of cell viability; however it is noteworthy that 1,000 ppm of 100-nm NPs, which produced slightly more cytotoxicity than 250 ppm of 10-nm NPs, did not significantly lower GSH levels, suggesting that the 10-nm NPs induce more oxidative stress regardless of cell viability.
Fig. 6.
Effects of 100- or 10-nm SiO2 NPs on intracellular GSH (micrograms GSH per milligrams protein) levels of human PBMCs (n = 4). Cells were treated with 100-nm SiO2 NPs (2,000, 1,000, or 500 ppm) or 10-nm SiO2 NPs (1,000, 500, or 250 ppm) for 24 h. The negative control was cells cultured in the absence of NPs; positive control was cells cultured with concanavalin A (stimulated control) and absence of NEM (no GS-NEM adduct for 8.1GSH antibody). Values reported are the mean ± SD from four separate PBMC donors and experiments. *ρ < 0.05, significant differences from the control values
Western blotting and immunoprecipitation analysis of DMPO nitrone adducts
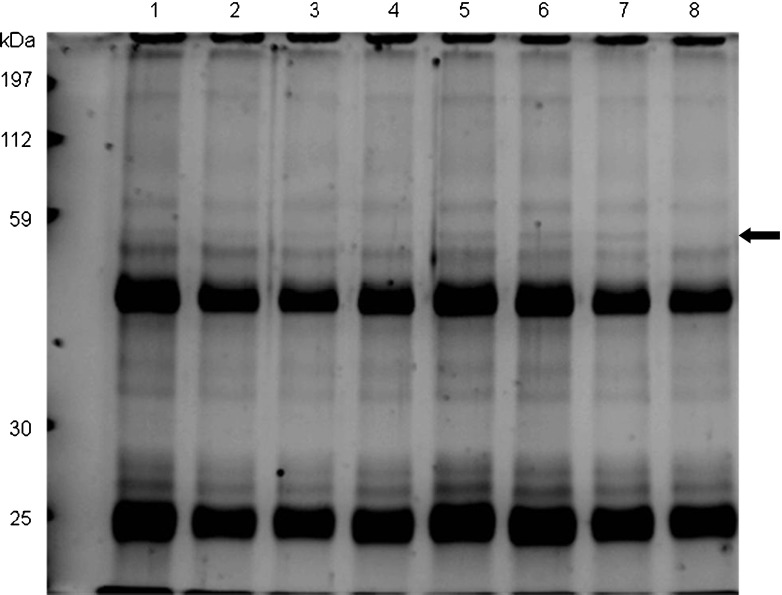
Formation of protein radicals from NP exposure was assessed by determination of the amount of protein-DMPO nitrone adduct with an anti-DMPO polyclonal antiserum. Increased amounts of DMPO adducts are expected with increased oxidative stress. Western blot analysis (Fig. 7) indicated no significant differences between samples exposed to 100-nm NPs, lanes 1 (2,000 ppm), 2 (1,000 ppm), and 3 (500 ppm) in comparison to control and lane 4 (not exposed to NPs). The lysates from cells exposed to 10-nm NPs, under the same conditions as 100-nm NPs, showed significantly increased amounts of protein-DMPO adduct, as shown in lanes 5 (1,000 ppm), 6 (500 ppm), and 7 (250 ppm) (Fig. 7). The amount of DMPO adducts with 10-nm NPs declined with the NP dose similar to the decline in cytotoxicity. For all groups, the most dense anti-DMPO bands from PBMC were between the 70- and 80-kDa markers. While exposure to 10-nm NPs produced DMPO protein adducts in a concentration-dependent manner, no such concentration response was apparent with the 100-nm NPs.
Fig. 7.
Western blot of protein-DMPO nitrone adducts (40 μg/lane, 12 % acrylamide-SDS gel) from PBMCs exposed to 100- or 10-nm silica NPs for 24 h; lane 1, 2,000 ppm (100 nm); 2, 1,000 ppm (100 nm); 3, 500 ppm (100 nm); 4, control PBMCs not exposed to NPs; 5, 1,000 ppm (10 nm); 6, 500 ppm (10 nm); 7, 250 nm (10 nm); and 8, PHA (stimulated control). The arrow indicates the nitrone antibody specifically bound to the DMPO-protein adduct via a radical derived protein oxidation following exposure to 100- or 10-nm silica NPs
Immunoprecipitation of cell lysates with anti-DMPO polyclonal antiserum (Fig. 8) showed a pattern similar to that noted in the Western blot analysis. An immunoprecipitation experiment performed with rabbit anti-DMPO yielded a protein between 55 and 59 kDa that reacted with the anti-DMPO antiserum. This protein was immunoreactive in PBMC lysates from cells exposed to 10-nm NPs (lanes 5, 6, and 7 in Fig. 8). The protein also was present in samples exposed to 100-nm NPs (lanes 1, 2, and 3); however, the protein demonstrated only weak immunoreactivity.
Fig. 8.
Immunoprecipitation of protein-DMPO nitrone adducts (50 μg/lane, 12 % acrylamide-SDS gel) from PBMCs exposed to 100- or 10-nm silica NPs for 24 h; lanes 1, 2,000 ppm (100 nm); 2, 1,000 ppm (100 nm); 3, 500 ppm (100 nm); 4, control PBMCs not exposed to NPs; 5, 1,000 ppm (10 nm); 6, 500 ppm (10 nm); 7, 250 nm (10 nm); and 8, PHA (stimulated control). The arrow indicates a protein that immunoprecipitated with anti-DMPO. This protein showed strong immunoreactivity with anti-DMPO for samples exposed to 10-nm NPs
Cytokine analysis following exposure to SiO2 NPs
The elicited cytokine response pattern was different for 100 and 10-nm NPs (Fig. 9). At 24 h, IFN-γ expression was below the detectable level (<4 pg/ml) for all of the culture supernatants of PBMC exposed to NPs regardless of size and concentration, including the control PBMC (not exposed to NPs); however, IFN-γ expression was significantly elevated for PBMC exposed to PHA (positive control, 33 pg/ml). There was no significant difference in IL-8 or TNFα expression by PBMC exposed to 100-nm NPs, although TNFα expression did increase with decreasing concentrations of 100-nm NPs, a trend that also was observed for IL-1ra and IL-6. For IL-6, 100-nm NPs (500 ppm) increased expression above that of the control cells and to a level equivalent with PHA. Interestingly, the lower concentrations of 10-nm NPs (250 and 500 ppm) and all concentrations of 100-nm NPs enhanced IL-1β expression. Overall, cytokine expression was inhibited to a greater extent by 10-nm NPs than that observed for the 100-nm NPs. Cytokine expression was dependent on the NP size and concentration. Especially interesting to note is that as the toxicity of the 10-nm NPs rose with increasing concentration, the ratio of IL-1β to IL-6 significantly elevated. This shift in the IL-1β/IL-6 ratio was observed only at 2,000 ppm, a toxic dose, of the 100-nm NPs.
Fig. 9.
Effects of 100- or 10-nm SiO2 NPs on cytokine production by human PBMCs. PBMC from three separate donors were cultured with different doses of NPs, and after 24 h supernatants are harvested to quantify cytokine expression by Luminex analysis. Negative control was cell cultured without stimulant or NPs; positive control was cells cultured with PHA, but no NPs. Values are the mean ± SD from three separate experiments. *ρ < 0.05, significant differences of each cytokine value from that of the nonstimulated (control) cells
Discussion
In this study, the differential cytotoxic and immunomodulatory activities of SiO2 NPs were shown to be dependent on size/diameter and concentration. The cytotoxic properties of the SiO2 NPs were investigated with regard to their dissemination within the cells and their induction of oxidative stress and cytokines associated with inflammation. Although the 10-nm NPs appear to be more cytotoxic than the 100-nm NPs, the equivalent concentration is not based on ppm but on surface area. Thus, the 10- and 100-nm NP exert similar cytotoxicity, in that the surface areas are near equal when the 100-nm NP concentration is ten times greater. Most likely, the smaller NP size at 50 ppm induced greater increases in cell death than 500 ppm of 100-nm NPs due to more rapid entry and dissemination with the cells. Previous studies showed that as NP size decreases, the relative surface area rises, and a proportionately greater number of atoms are exposed at the surface (Oberdörster et al. 2005; Nel et al. 2006). Thus, smaller NPs, which cross the cell membrane more readily than do larger NP, probably provoke greater cytotoxicity because of greater interactions with cell surface components per square nanometer, producing more aggregation of adsorbed proteins. Structural disorganization or aggregation of intracellular proteins may lead to endoplasmic reticulum (ER) stress producing an elevation of oxidative stress (Dimcheff et al. 2004; Lindholm et al. 2006). Our study showed that the 10-nm NPs, which do appear to aggregate intracellularly more readily than the 100-nm NPs, provoked significant loss of GSH and formation of protein radicals. Other studies reported that NP exposure increased the production of ROS, which affects GSH levels (Chang et al. 2007), pro-inflammatory responses (Park and Park 2009), cellular signaling (Unfried et al. 2008), and apoptosis (Park et al. 2008). Furthermore, the interaction of proteins with NPs may directly influence their folding, aggregation, and functioning (Fei and Perrett 2009). NP-adsorbed proteins were reported to undergo conformation changes (Wu and Narsimhan 2008) due to the nonspecific, noncovalent interactions of proteins with the synthetic surfaces (Senarath-Yapa et al. 2007).
Scanning electron microscopic analysis indicated that NPs aggregate in cell culture medium, which is probably mainly due to the presence of proteins (Wang et al. 2008). Aggregation or agglomeration of NPs is known to elicit the generation of ROS (Bhattacharya et al. 2009; Fahmy and Cormier 2009; Eom and Choi 2009; Wang et al. 2009) and thereby induce oxidative stress. Exposure of PBMC to NP initially occurs at the cell surface, where some NPs are seen to form aggregates (Fig. 3a and 4a). The NPs were suggested to be transported into cells by phagocytosis or endocytosis (Eom and Choi 2009). The 100-nm NPs mainly remained in vesicles of the monocytes/macrophages, which likely were trying to digest the NPs (Fig. 3c, d); however, some individual NPs appeared to have escaped into the cytoplasm (Fig. 5b). A larger proportion of the 10-nm than the 100-nm NPs seem to have escaped into the cytoplasm (Fig. 4b), rather than eventually being expelled from the cell via exocytosis (Fig. 5d), which is probably why the smaller NPs induced more oxidative stress. Exocytosed NPs or NPs released from lysed cells are potentially more cytotoxic, due to release of either harmful lysosomal constituents or released cytoplasmic molecules, such as enzymes, that may be harmful in an extracellular location. Furthermore, the NPs may provoke a disequilibrium of cation fluxes affecting cell survival; exaggerated increases in intracellular Ca2+ are known to be associated with oxidative and ER stress (Zhang 2010; Liu et al. 1998).
As noted earlier, exposure to NPs increases cell production of ROS. Under normal conditions, cellular GSH is in a reduced state (>99 %); however, an increase in the production of ROS induces GSH to be converted to oxidized glutathione (GS-SG or other mixed disulfides), as the cell attempts to neutralize the ROS. If the enzymes that synthesize new GSH or convert GS-SG back to GSH are unable to cope with the amount of ROS, the GSH/GSSG ratio decreases, and oxidative stress rises (Patsoukis and Georgiou 2004). Once GSH is depleted, the cell has no defense against ROS and is susceptible to apoptosis; apoptotic progression includes protein oxidation, ER stress, mitochondrial damage, and DNA digestion (Wang et al. 2009). PBMC exposed to the 10-nm NPs exhibited a concentration-dependent response in which the antioxidant defense was overwhelmed, as apparent from the depletion of GSH in the lysates of PBMC. In contrast, the 100-nm NPs showed no significant changes in intracellular GSH in the lysates of exposed PBMC; however, the immunoelectron microscopy analysis of adherent cells (mainly macrophages) indicated that cellular GSH was lower after both the exposure to100- and 10-nm NPs. Interestingly, it appears that GSH synthesis was first (1 h) upregulated by both 100- and 10-nm NPs in those cells not morphological displaying signs of cytotoxicity (Table 1). The difference in intracellular GSH levels in lysates of PBMC exposed to 100- and 10-nm NPs may be the result of a greater number of the lymphocytes in the PBMC preparations being affected by the 10-nm NPs since in an equal ppm solution there are more 10-nm NP than 100-nm NP; thus, the smaller NPs will be able to be disbursed among more cells, and lymphocytes represent the majority of the PBMC population.
The generation of cellular ROS directly or indirectly produces depletion of GSH levels and promotes the formation of free radical species, which are capable of directly damaging cell components including proteins and DNA (Eom and Choi 2009). The generation of free radicals naturally occurs at low levels in cells due to routine biological processes (Schreck and Baeurle 1991); however, because of their reactivity, the life of these free radicals is relatively short (Harman 1956). Spin-trapping agents, such as DMPO, are commonly used for the detection of short-lived radical species that include protein radicals, hydroxyl radicals and superoxide radicals (Deterding et al. 2004). Protein radical products were detected in our study by using commercially available DMPO-protein conjugate and anti-DMPO polyclonal antiserum, after PBMC was exposed to SiO2 NPs for 24 h. Western blotting and immunoprecipitation analyses indicated that proteins formed greater quantities of DMPO nitrone adducts as a result of exposure to 10-nm SiO2 NPs. Not surprisingly, since as discussed, the 10-nm NPs accumulated more readily in the interior of cells than the 100-nm NPs. The increased production of cellular ROS by the 10-nm NPs may be due either to their action as a catalyst (an electron donor, in which Si–O bonds are cleaved, generating radicals on the surface of Si) or to the modifying Si surface disrupting normal electron transport (Hamilton et al. 2008). The by-products of such reactions are modifying proteins with susceptible amino acids that undergo oxidation upon exposure to ROS (Stadtman and Berlett 1998). Proteins with sulfur-containing amino acids (methionine and cysteine), amino acids containing aromatic functional groups (tyrosine, tryptophan, and phenylalanine), and histidine, all of which have relatively fast reaction rates with radicals, are particularly sensitive to oxidative modification (Stadtman and Berlett 1998). The increased presence of more DMPO-protein conjugates after exposure to 10-nm NPs than to 100-nm NPs correlated with their differential effects on the depletion of GSH. Thus, less GSH seems to lead to more formation of protein radicals, both outcomes of which likely stem from generation of ROS due to the more widespread dispersal of smaller NPs within cells, which increases their cellular interactions generating more proteins with radicals.
PBMC exposed to SiO2 NPs also generated a pro-inflammatory response, which indicates that at least for some initial period cell responsiveness must in intact or a percentage of cells are more resistant to cytotoxicity and are activated by the NPs. However, the cytokines that were enhanced in production were pro-inflammatory cytokines and are known to be induced by the redox-sensitive transcription factor nuclear factor kappa B (NF-κB; De la Fuente and Miquel 2009; Li et al. 2008). As for the redox associated aspects of the NPs, the cytokine expression pattern also was unique to NP size and concentration for expression of IFN-γ, TNF-α, IL-1ra, IL-6, IL-8, and IL-1β. Previous studies showed that NP exposure influences the generation of inflammatory cytokines. For example, human PBMC exposed to silver NP at concentrationsof >15 ppm lost viability, and their cytokine responses were concentration dependent (Shin et al. 2007). Additionally, human PBMC exposed to cobalt NPs demonstrated increased expression of TNFα and IFN-γ, but reduced expression of IL-10 and IL-2 (Petrarca et al. 2006). A study involving human lung epithelial cells exposed to TiO2 NPs showed induction of IL-8, which was related to the NP surface area (Singh et al. 2007). A few studies (Park and Park 2009; Lin et al. 2006; Sayes et al. 2007; Kim et al. 2009; Singh et al. 2009) involving SiO2 NPs reported that these NPs induce oxidative stress both in vivo and in vitro as well as an inflammatory response that arises via activation of NF-κB. Activation of redox-sensitive transcription factors (NF-κB and AP-1) and cytokine production are regulated by cellular production of ROS, which play important roles in an array of several physiological responses, including cell-signaling pathways (Eom and Choi 2009). As NPs contribute to the increased or sustained formation of ROS via reactive radical species, and also to the induction of oxidative stress and/or oxidative damage to proteins, it is plausible that activation of these transcription factors may lead to an inflammatory response. Whether NPs of different sizes differentially induce certain transcription factors remains to be determined.
One important aspect of the inflammatory cytokine response is the IL-1β/IL-6 ratio; both of these cytokines are usually considered pro-inflammatory cytokines. PBMC exposed to 100- or 10-nm SiO2 NPs expressed IL-1β. It is likely that the activation of the inflammasome (Martinon 2010) due to enhanced stress or perceived threat of a toxicant, promoted an inflammatory response in which IL-1β was produced, and the discrepancy observed at the highest concentration of 10-nm NP (1,000 ppm) likely was due to entrapment of the cytokine due to the high concentration. Secretion of IL-6, on the other hand, increased with decreasing NP concentration for both 100- and 10-nm NPs. In a previous study (Fujii et al. 2002), secretion of IL-1β and TNF-α amplified the expression of IL-6; however, IL-6 was expressed at a high concentration in control cells whereas IL-1β was down regulated and TNF-α was low. Although IL-1β and TNF-α are known to enhance IL-6 production, it is surprising that with increasing concentrations of 10-nm and 100-nm NPs IL-6 expression declined and IL-1β remained elevated. As IL-6 expression is induced downstream of that of IL-1β, it is likely that the oxidative stress induced by NPs was interfering with intermediate signaling mechanisms.
In conclusion, the effects of oxidative stress from the SiO2 NP-cell interaction in vitro resulted in (1) increased formation of cellular ROS, (2) depletion of GSH levels, (3) production of protein radicals, which were a measure of generation of ROS, and (4) induction of pro-inflammatory cytokine responses. NP size and concentration are both important factors since it is the total surface area of NP that affects cellular outcome. However, the smaller NPs (10 nm) even when assessed based on surface area induced more damage than larger NPs, which seems to be due to their more diverse distribution within the cell causing more oxidative modifications. Whether the inflammatory response and cytotoxicity are directly related to differential stochiometric effects of NPs of different sizes due to cellular interactions per square nanometer, uptake mechanisms, aggregate effects, and/or ROS generated during the NP-cell interaction remain to be delineated in future investigations.
Acknowledgments
The authors would like to thank The Nanobiotechnology Center at Cornell University (2007) and Texas Southern University Research Enhancement office (2007) for their funding support of J.A.T-H. (summer of 2007). Furthermore, we would like to thank The Quality Education Minorities Networks for research funding (2008) for support of J.A. T-H. AM was supported by a supplement to U01 ES016014 to DAL. The authors also want to recognize The Biochemistry and Immunology Cores of Wadsworth Center for their collaboration and assistance. The immunoelectron microscopy was done in collaboration between the Wadsworth Center Immunology and Electron Microscopy Core Facilities. The authors also want to thank Dr. Jane Kasten-Jolly for her assistance in obtaining volunteer donors and Adrianna Verschoor for her editorial assistance.
Footnotes
The first three authors are equal contributors to the study.
Jose A. Torres-Hernandez NBTC Research Experience for Undergraduates participant
References
- Ault JG, Lawrence DA. Glutathione distribution in normal and oxidatively stressed cells. Exp Cell Res. 2003;285:9–14. doi: 10.1016/S0014-4827(03)00012-0. [DOI] [PubMed] [Google Scholar]
- Balbus JM, Maynard AD, Colvin VL, Castranova V, Daston GP, Denison RA, Dreher KL, Goering PL, Goldberg AM, Kulinowski KM, Monteiro-Riviere NA, Oberdörster G, Omenn GS, Pinkerton KE, Ramos KS, Rest KM, Sass JB, Silbergeld EK, Wong BA. Meeting report: hazard assessment for nanoparticles report from an interdisciplinary workshop. Environ. Health Persp. 2007;115:1654–1659. doi: 10.1289/ehp.10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya K, Davoren M, Boertz J, Schins RPF, Hoffmann E, Dopp E. Titanium dioxide nanoparticles induce oxidative stress and DNA-adduct formation but not DNA-breakage in human lung cells. Particle Fibre Toxicol. 2009;6:17. doi: 10.1186/1743-8977-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J-S, Chang K, Hwang D-F, Kong Z-L. In vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environ Sci Technol. 2007;41:2064–2068. doi: 10.1021/es062347t. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des. 2009;15:3003–3026. doi: 10.2174/138161209789058110. [DOI] [PubMed] [Google Scholar]
- Detweiler CD, Deterding LJ, Tomer KB, Chignell CF, Germolec D, Mason RP. Immunological identification of the heart myoglobin radical formed by hydrogen peroxide. Free Radic Biol Med. 2002;33:364–369. doi: 10.1016/S0891-5849(02)00895-X. [DOI] [PubMed] [Google Scholar]
- Deterding LJ, Ramirez DC, Dubin JR, Mason RP, Tomer KB. Identification of free radicals on hemoglobin from its self-peroxidation using mass spectrometry and immuno-spin trapping: observation of a histidinyl radical. J Biol Chem. 2004;279:11600–11607. doi: 10.1074/jbc.M310704200. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Forman HJ. Cellular glutathione and thiols metabolism. Biochem Pharmacol. 2002;64:1019–1026. doi: 10.1016/S0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- Dimcheff DE, Faasse MA, McAtee FJ, Portis JL. Endoplasmic reticulum (ER) stress induced by a neurovirulent mouse retrovirus is associated with prolonged BiP binding and retention of a viral protein in the ER. J Biol Chem. 2004;279:33782–33790. doi: 10.1074/jbc.M403304200. [DOI] [PubMed] [Google Scholar]
- Eom H-J, Choi J. Oxidative stress of silica nanoparticles in human bronchial epithelial cell, Beas-2B. Toxicol in Vitro. 2009;23:1326–1332. doi: 10.1016/j.tiv.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Fahmy B, Cormier SA. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol in Vitro. 2009;23:1365–1371. doi: 10.1016/j.tiv.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei L, Perrett S. Effect of nanoparticles on protein folding and fibrillogenesis. Int J Mol Sci. 2009;10:646–655. doi: 10.3390/ijms10020646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn ER, Bryant HC. A biomagnetic system for in vivo cancer imaging. Phys Med Biol. 2005;50:1273–1293. doi: 10.1088/0031-9155/50/6/016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Hayashi S, Hogg JC, Mukae H, Suwa T, Goto Y, Vincent R, Van Eeden SF. Interaction of alveolar macrophages and airway epithelial cells following exposure to particulate matter produces mediators that stimulate the bone marrow. Am J. Respir. Cell Molec. Biol. 2002;27:34–41. doi: 10.1165/ajrcmb.27.1.4787. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. New York: Oxford University Press; 1999. [Google Scholar]
- Hamilton RF, Jr, Thakur SA, Holian A. Silica binding and toxicity in alveolar macrophages. Free Radic Biol Med. 2008;44:1246–1258. doi: 10.1016/j.freeradbiomed.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Ionita P, Spafiu E, Ghica C. Dual behavior of gold nanoparticles, as generators and scavengers for free radicals. J Mater Sci. 2008;43:6571–6574. doi: 10.1007/s10853-008-2987-1. [DOI] [Google Scholar]
- Kim HW, Ahn E-K, Jee BK, Yoon H-K, Lee KH, Lim Y. Nanoparticulate-induced toxicity and related mechanism in vitro and in vivo. J. Nanopart. Res. 2009;11:55–65. doi: 10.1007/s11051-008-9447-3. [DOI] [Google Scholar]
- Lanone S, Rogerieux F, Geys J, Dupont A, Maillot-Marechal E, Boczkowski J, Lacroix G, Hoet P. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Particle Fibre Toxicol. 2009;6:14. doi: 10.1186/1743-8977-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapotko DO, Lukianova E, Oraevsky AA. Selective laser nano-thermolysis of human leukemia cells with microbubbles generated around clusters of gold nanoparticles. Lasers SurgMed. 2006;38:631–642. doi: 10.1002/lsm.20359. [DOI] [PubMed] [Google Scholar]
- Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. JNanobiotechnol. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WS, Huang YW, Zhou XD, Ma YF. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol. 2006;217:252–259. doi: 10.1016/j.taap.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- Liu H, Miller E, Van de Water B, Stevens JL. Endoplasmic reticulum stress proteins block oxidant-induced Ca2+ increases and cell death. J Biol Chem. 1998;273:12858–12862. doi: 10.1074/jbc.273.21.12858. [DOI] [PubMed] [Google Scholar]
- Lynch I, Dawson KA, Linse S. Detecting cryptic epitopes created by nanoparticles. Science. 2006;327:pe14. doi: 10.1126/stke.3272006pe14. [DOI] [PubMed] [Google Scholar]
- Magder S. Reactive oxygen species: toxic molecules or spark of life? Crit Care. 2006;10:208. doi: 10.1186/cc3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi M, Simchi A, Imani M. Cytotoxicity of uncoated and polyvinyl alcohol coated superparamagnetic iron oxide nanoparticles. J Phys Chem C. 2009;113:9573–9580. doi: 10.1021/jp9001516. [DOI] [Google Scholar]
- Martinon F. Activation mechanisms: signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40:595–653. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- Medina C, Santos-Martinez MJ, Radomski A, Corrigan OI, Radomski MW. Nanoparticles: pahrmacological and toxicological significance. Br J Pharmacol. 2007;150:552–558. doi: 10.1038/sj.bjp.0707130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina JP, Mazurkiewicz J, Lawrence DA. Production and characterization of monoclonal antibodies to thiol-modified glutathione. In: Cerutti PA, Nygaard MG, Simic MG, editors. Anticarcinogenesis and radiation protection. NY: Plenum New York; 1987. pp. 407–412. [Google Scholar]
- Miranda S, Opazo C, Larrondo LF, Muñoz FJ, Ruiz R, Leighton F, Inestrosa NC. The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer's disease. Prog Neurobiol. 2000;62:633–648. doi: 10.1016/S0301-0082(00)00015-0. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H (2005a) Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Particle Fibre Toxicol 2:1–35 [DOI] [PMC free article] [PubMed]
- Oberdörster G, Oberdörster E, Oberdörster J (2005b) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Persp 113:823–839 [DOI] [PMC free article] [PubMed]
- Pacurari M, Castranova V, Vallyathan V. Single- and multi-wall carbon nanotubes versus asbestos: are the carbon nanotubes a new health risk to humans? J Toxicol Environ Health A. 2010;73:378–95. doi: 10.1080/15287390903486527. [DOI] [PubMed] [Google Scholar]
- Park E-J, Park K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicology Lett. 2009;184:18–25. doi: 10.1016/j.toxlet.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Park E-J, Yi J, Chung K-H, Ryu D-Y, Choi J, Park K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicology Lett. 2008;180:222–229. doi: 10.1016/j.toxlet.2008.06.869. [DOI] [PubMed] [Google Scholar]
- Patsoukis N, Georgiou CD. Determination of the thiol redox state of organisms: new oxidative stress indicators. Anal Bioanal Chem. 2004;378:1783–1792. doi: 10.1007/s00216-004-2525-1. [DOI] [PubMed] [Google Scholar]
- Pelka J, Gehrke H, Esselen M, Türk M, Crone M, Bräse, Muller ST, Blank H, Send W, Zibat V, Brenner P, Schneider R, Gerthsen D, Marko D. Cellular uptake of platinum nanoparticles in human colon carcinoma cells and their impact on cellular redox systems and DNA integrity. Chem Res Toxicol. 2009;22:649–659. doi: 10.1021/tx800354g. [DOI] [PubMed] [Google Scholar]
- Petrarca C, Perrone A, Verna N, Verginelli F, Ponti J, Sabbioni E, Di Giampaolo L, Dadorante V, Schiavone C, Boscolo P, Mariani-Costantini R, Di Gioacchino M. Cobalt nanoparticles modulate cytokine in vitro release by human mononuclear cells mimicking autoimmune disease. Int J Immunopathol Pharmacol. 2006;19:11–14. [PubMed] [Google Scholar]
- Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Salata OV. Applications of nanoparticles in biology and medicine. Journal of Nanobiotechnol. 2004;2:3–9. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayes CM, Reed KL, Warheit DB. Assessing toxicity of fine and nanoparticles: comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol Sci. 2007;97:163–180. doi: 10.1093/toxsci/kfm018. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem Res Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- Schreck R, Baeurle PA. A role of oxygen radicals as second messengers. Trends Cell Biol. 1991;1:39–42. doi: 10.1016/0962-8924(91)90072-H. [DOI] [PubMed] [Google Scholar]
- Senarath-Yapa MD, Phimphivong S, Coym JW, Wirth MJ, Aspinwall CA, Saavedra SS. Preparation and characterization of poly(lipid)-coated, fluorophore-doped silica nanoparticles for biolabeling and cellular imaging. Langmuir. 2007;23:12624–12633. doi: 10.1021/la701917w. [DOI] [PubMed] [Google Scholar]
- Shi X, Castranova V, Halliwell B, Vallyathan V (1998) Reactive oxygen species and silica-induced carcinogenesis. J Toxicol Environ Health B 1:181–197 [DOI] [PubMed]
- Shin SH, Ye MK, Kim HS, Kang HS. The effects of nanosilver on the proliferation and cytokine expression by peripheral blood mononuclear cells. Int Immunopharmacol. 2007;7:1813–1818. doi: 10.1016/j.intimp.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Singh N, Manshian B, Jenkins GJS, Griffiths SM, Williams PM, Maffeis TGG, Wright CJ, Doak SH. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;30:3891–3914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Singh S, Shi T, Duffin R, Albrecht C, Van Berlo D, Höhr D, Fubini B, Martra G, Fenoglio I, Borm PJA, Schins RPF. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: role of the specific surface area and of surface methylation of the particles. Toxicol Appl Pharmacol. 2007;222:141–151. doi: 10.1016/j.taap.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab Rev. 1998;30:225–243. doi: 10.3109/03602539808996310. [DOI] [PubMed] [Google Scholar]
- Stratmeyer, M.E., Goering, P.L., Hitchins, V.M., and Umbreit, T.H. (2008) Biomedical microdevices. Springer Science+Business Media, LLC 2008
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Unfried K, Sydlik U, Bierhals K, Weissenberg A, Abel J. Carbon nanoparticle-induced lung epithelial cell proliferation is mediated by receptor-dependent Akt activation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L358–L367. doi: 10.1152/ajplung.00323.2007. [DOI] [PubMed] [Google Scholar]
- Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/B:MCBI.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- Wang F, Gao F, Lan M, Yuan H, Huang Y, Liu J. Oxidative stress contributes to silica nanoparticle-induced cytotoxicity in human embryonic kidney cells. Toxicol in Vitro. 2009;23:808–815. doi: 10.1016/j.tiv.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Wang S, Lu W, Tovmachenko O, Shanker Rai U, Yu H, Chandra Ray P. Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem Phys Lett. 2008;463:145–149. doi: 10.1016/j.cplett.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Narsimhan G. Effect of surface concentration on secondary and tertiary conformational changes of lysozyme adsorbed on silica nanoparticles. Biochim Biophys Acta. 2008;1784:1694–1701. doi: 10.1016/j.bbapap.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Zhang K. Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med. 2010;3:33–40. [PMC free article] [PubMed] [Google Scholar]