Abstract
We have previously reported epithelial cellular hyperplasia in ventral prostates (VP) of mice lacking estrogen receptor β (ERβ). To investigate the causes of this phenomenon, we measured cellular proliferation and apoptosis in VP of ERβ-/- and WT mice. With BrdUrd labeling, the number of proliferating cells was 3.6-fold higher in ERβ-/- mice. There was also a decrease in apoptosis as measured by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling assay and an increase in expression of the anti-apoptotic bcl-2. The state of differentiation of the epithelial cells of the VP was studied by immunohistochemical staining, Western blotting, and fluorescence-activated cell sorting (FACS). In ERβ-/- mouse VP, the number of p63-positive cells (basal phenotype) was 2.6-fold higher, and expression level of cytokeratin (CK) 8, a luminal cell marker, was lower. FACS analysis with p63 showed that in WT mice the ratio of basal to intermediate/luminal cell populations expressing p63 was 1:2.5, whereas in ERβ-/- mice it was 1:9. The expression of basal/intermediate marker CK 19 in three FACS areas, g1, g2, and g3, gated according to cellular size and granularity, was 1:0.6:2 in WT and 1:4:6.7 in ERβ-/- mice, showing a shift of CK 19-positive cells toward a cell population of intermediate size and granularity. We conclude that, in ERβ-/- mouse VP, there is increased epithelial proliferation, decreased apoptosis, and accumulation of incompletely differentiated cells in an intermediate pool. The continued proliferation of intermediate cells leads to the prostatic epithelial hyperplasia observed in the absence of ERβ signaling.
Keywords: prostate cell isolation, fluorescence-activated cell sorter, prostate cancer, prostate intraepithelial neoplasia, phytoestrogens
Studies with mice lacking estrogen receptor β (ERβ) have revealed roles for ERβ in many tissues and organs, including the ovary, uterus, mammary gland, brain, immune system, and ventral prostate (VP) (1–3). In some tissues, both ERα and ERβ are expressed, and the specific function of each receptor is difficult to evaluate, particularly in cases where the two receptors oppose each other's actions. The influence of estradiol on the CNS–gonadal axis presents a further difficulty in distinguishing direct ERβ-mediated effects from indirect, systemic actions of estradiol. In the ovary and VP of ERβ -/- mice, there is overexpression of the androgen receptor (4, 5). The epithelium of the mouse VP expresses ERβ, not ERα, suggesting a possible direct influence of estrogens on prostatic epithelium mediated by ERβ. We have reported epithelial hypercellularity in the VP in ERβ-/- mice (4), but this description has not met with consensus in all laboratories (4, 6, 7). Many histologists caution against cutting artifacts, which can be mistaken for hyperplasia. The neoplastic foci seen in ERβ-/- mouse VP have the appearance of low-grade prostatic intraepithelial neoplasia (PIN). High-grade PIN, a precursor of prostate adenocarcinoma, is associated with alterations in the basement membrane and nuclei, whereas low-grade PIN has fewer signs of tissue and cellular atypia. Overexpression of the prolactin receptor and the absence of estradiol in aromatase-null mutant mice also lead to prostatic epithelial hyperplasia without development of malignancy (8–10).
Although there are clear anatomical differences between the VP of the mouse and the human prostate, respectively, there are similarities in cellular composition and hormonal control. Because, in both mouse and human prostates, ERβ is the only ER in the epithelium, if loss of ERβ results in abnormal growth of the prostatic epithelium with age in mice, the role of this receptor in controlling growth of the human prostate has to be considered. Several studies have investigated the expression of ERβ and its splice variants in prostate cancer. Some studies find no correlation between ERβ and prostate cancer (11), whereas others find a protective role for ERβ against prostate adenocarcinoma (12). It has also been reported that higher levels of ERβ are present in less differentiated tumors (13) and that differential expression of ERβ and its C-terminal splice variant, ERβcx, may be prognostic markers for prostate cancer (14). Furthermore, there are reports that malignant progression from a normal prostate epithelium to a cancerous one is associated with loss of ERβ expression, whereas those cancers that retain ERβ expression are associated with a higher rate of recurrence (15).
A better understanding of the role of ERβ in regulation of normal prostatic growth is required. In this study we compared the VP of WT and ERβ-/- mice for morphological differences and for changes in markers of proliferation, apoptosis, and differentiation of the epithelium. We report that loss of ERβ results in a higher rate of cellular proliferation, less apoptosis, and a shift toward an incompletely differentiated epithelium.
Materials and Methods
Animals. Animals were used under the Guidelines for Care and Use of Experimental Animals issued by Stockholm's Södra Djurförsöksetiska Nämnd. Mice were maintained under standard conditions, with free access to food and water. WT and ERβ-/- mice were bred from heterozygous mice. Genotyping by PCR was performed on DNA isolated from tails of 2-week-old mice (16). Mice were killed by CO2 asphyxiation. The prostates were removed, and VPs were dissected. For immunohistochemical studies, VPs were fixed in 4% paraformaldehyde and embedded in paraffin; for Western blotting, VPs were frozen in liquid nitrogen; and for fluorescence-activated cell sorter (FACS) analysis, tissues were processed immediately upon dissection.
Chemicals and Antibodies. BrdUrd was purchased from Roche (Mannheim, Germany). Rabbit antibodies were anti-BrdUrd (3D4) and anti-Bcl-2. Monoclonals were cytokeratin (CK) 5/8 (RCK 102), p63 (4A4), and CK 19 (Ab-1), all purchased from BD Biosciences Pharmingen. Biotinylated goat anti-rabbit IgG and avidin-biotin complex kits were from Vector Laboratories.
Treatment with BrdUrd. For measurement of proliferation, eight mice were treated i.p. with BrdUrd at 100 mg/kg every 12 h for 2 days (total of four injections).
Immunohistochemistry. For immunohistochemical studies, 15 WT and 15 ERβ-/- mice, 10–24 months old, were used. Paraffin sections (4 μm) were dewaxed in xylene and rehydrated through graded ethanol to water. Antigens were retrieved by boiling in 10 mM citrate buffer (pH 6.0) for 30 min. The cooled sections were incubated in 1% H2O2 for 30 min to quench endogenous peroxidase and then in 0.3% Triton X-100 in PBS for 10 min. To block the nonspecific binding, sections were incubated in normal serum from the host of secondary antibodies for 1 h at 4°C. Sections were then incubated with the following antisera: anti-BrdUrd (1:500), anti-bcl-2 (1:200), and anti-p63 (1:1,000), in 3% BSA overnight at 4°C. Negative controls were incubated with only 3% BSA without primary antibody. The avidin-biotin complex method was used to visualize the signal, according to the manufacturer's manual (Vector Laboratories). The sections were incubated in appropriate biotinylated Ig solution (1:200) for 2 h, followed by washing with PBS and incubation in avidin-biotin-horseradish peroxidase for 1 h. After washing in PBS, sections were developed with 3,3′-diaminobenzidine tetrahydrochloride substrate (DAKO), lightly counterstained with Mayer′s hematoxylin, dehydrated through ethanol series and xylene, and mounted.
Terminal Deoxynucleotidyltransferase-Mediated dUTP Nick End Labeling (TUNEL). An in situ cell death detection kit was purchased from Roche. After dewaxing and antigen retrieval, the cooled sections were incubated with a TUNEL labeling mixture for 60 min at 37°C with subsequent mounting and inspection under a microscope with the excitation wavelength in the range of 450–500 nm and detection in the range of 515–565 nm. Selected sections were incubated with labeling solution only for negative control.
Western Blotting. Nuclear, membrane, or cytosolic fractions were separated on 4–20% Tris-glycine SDS gels (Invitrogen) and transferred to a poly(vinylidene difluoride) membrane. The membrane was blocked with blocking buffer (5% nonfat milk in PBS containing 0.1% Nonidet P-40), followed by incubation with the primary antibody, 1 μg/ml at 4°C, overnight. The membrane was washed for 10 min with blocking buffer and then washed in PBS. Signals were detected by using a peroxidase-conjugated secondary antibody and the Amersham Biosciences enhanced chemiluminescence reagent ECL Plus.
Prostatic Epithelial Cell Isolation. VP from six WT and six ERβ-/- age-matched mice were isolated under the dissection microscope in DMEM containing 4% FCS on ice. Samples were then chopped into pieces and transferred to a 170 units/ml solution of collagenase type 3 (Worthington) in DMEM containing 4% FCS and incubated on a shaker for 1 h at 37°C. The supernatant was removed and centrifuged at 515 × g for 5 min. Pelleted cells were resuspended in PBS. After washing in PBS, the total number of cells was calculated by using a hematometer.
FACS Analysis. Isolated prostate cells (1 × 106) from WT and ERβ-/- mouse tissues were treated with IntraPrep permeabilization reagent (Beckman Coulter). Cells were then preincubated for 30 min on ice in FACS buffer (PBS/1% BSA/0.1% sodium azide) with 0.5 μg of mouse serum as an isotype-matched antibody. Mouse IgG, anti-mouse p63, and CK 19 antibodies were added (2 μg/ml) and incubated on ice for 30 min. FITC-conjugated affinity purified F(ab′)2 fragment goat anti-mouse IgG (H+L) (Beckman Coulter) was added to each sample. Control mouse and goat sera as isotype-matched antibodies were used throughout the experiment. The cells were washed with PBS resuspended in 1% formaldehyde and analyzed with a FACSCalibur flow cytometer (Becton Dickinson).
Results
ERβ-/- VP Phenotype. Morphological studies of H–E-stained sections of VP from mice 12 months of age or older revealed hyperplastic foci in ERβ-/- mice (Fig. 1). The VP of WT mice were of normal appearance, with ducts lined by a single layer of epithelium overlying a discontinuous layer of flattened cells on the basal membrane (Fig. 1 A). The hyperplastic foci in ERβ-/- mice looked like piled-up, normal epithelial cells without signs of nuclear atypia or visible alterations of the basement membrane (Fig. 1 B–D). This hyperplasia is, therefore, not homologous to high-grade PIN and seems to have a different etiology and developmental course. Epithelial hyperplasia starts to develop in ERβ-/- animals by 6 months of age. At this age the mouse VP is mature, and the incidence of epithelial hyperplasia increases with age. After 18 months, almost every ERβ-/- mouse VP has foci of epithelial hyperplasia.
Fig. 1.
Epithelial cellular hyperplasia in ERβ-/- VP. H–E-stained sections of VP from 12-month-old WT and ERβ-/- mice show that the VP of WT mice appear normal (A), with ducts lined by a single layer of columnar epithelium overlying a discontinuous layer of flattened cells on the basal membrane. (B–D) In ERβ-/- mouse VP, there are foci composed of piled-up, normal-looking epithelial cells. Blue arrows show normal branching with visible basement membrane. Red arrows show foci of epithelial hyperplasia.
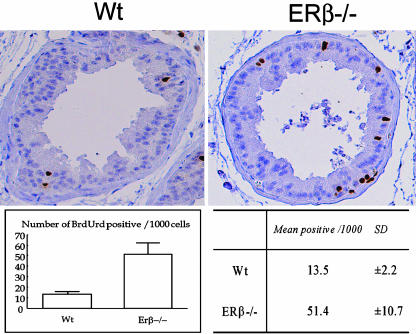
Measurement of Epithelial Cellular Proliferation. To understand the mechanism behind the hyperplasia, indices of proliferation and the rate of apoptosis in ERβ-/- and WT VPs were compared. To quantify and compare proliferation in VPs of ERβ-/- and WT mice, BrdUrd incorporation into DNA was examined. Four 10-month-old ERβ-/- mice and four WT littermates were dosed with BrdUrd every 12 h for 48 h. With this protocol, one to two BrdUrd-labeled cells were detected in some of the VP ducts of WT mice (Fig. 2). There was no labeling of the cells next to the basement membrane. In ERβ-/- mice, there were labeled cells in most ducts. These cells were in both the basal and the luminal layers, and there were usually pairs of labeled cells, suggesting that cell division had occurred. BrdUrd-positive and -negative epithelial cells were counted, and proliferation indices were calculated by normalization to 1,000 cells. The percentage of proliferating cells in the VP epithelium in the absence of ERβ was 3.6-fold higher than it was in WT littermates.
Fig. 2.
Incorporation of BrdUrd into DNA of VP in 10-month-old WT and ERβ-/- mice. Labeled cells are present in some of the VP ducts of WT mice. There is no labeling of the cells next to the basement membrane. In ERβ-/- mice, there are labeled cells in both basal and luminal layers in most ducts. In most cases there are pairs of labeled cells, suggesting that cell division has occurred.
Apoptotic Activity in ERβ-/- and WT Mice. Apoptosis was detected by enzymatic in situ labeling of apoptosis-induced DNA strand breaks. In VP of WT mice, apoptotic signals were detected in the epithelial cell layer lining the ducts, whereas in ERβ-/- mice there were very few positive signals in the epithelium (Fig. 3 A and B). In both cases strong immunofluorescent signals were emitted by the cellular debris in the lumen of ducts, suggesting detection of necrotic cells shed into the lumen. To investigate the reason for the apparent suppression of apoptosis in ERβ-/- VP, expression of the anti-apoptotic protein bcl-2 was examined by immunohistochemistry and Western blotting. Expression of bcl-2 in WT mice was limited to the basal cell layer of VP epithelium; in ERβ-/- mice all layers of ductal epithelium expressed bcl-2, suggesting that apoptosis is inhibited (Fig. 3 C and D). Immunohistochemical staining was supported by Western blotting of total VP protein extracts for bcl-2. In the ERβ-/- VP, the expression of the protein was markedly increased (Fig. 3E).
Fig. 3.
Apoptosis in the VP of WT and ERβ-/- mice. A TUNEL assay was performed. In WT mice (A), apoptotic signals are seen in the epithelial cell layer lining the ducts. In ERβ-/- mice (B), there are few positive signals in the epithelial layer. In both WT and ERβ-/- mice, there is strong fluorescence emitted by the dead cells in the lumen of ducts. Expression of bcl-2 in WT mice (C) was limited to the basal cell layer of VP, whereas in ERβ-/- mice (D), most of the epithelial cells express bcl-2. (E) Western blotting of VP extracts for bcl-2 shows increased expression in ERβ-/- VP.
Cellular Composition of the Prostatic Epithelium in ERβ-/- and WT Mice. The pattern of differentiation of the VP epithelium in the absence of ERβ signaling was studied by immunohistochemical staining, Western blotting, and FACS of p63-, CK 5/8-, and CK 19-containing cells. p63 (a basal cell marker) was confined to the most basal cells in the epithelial cellular compartment in the VP of WT mice, whereas in ERβ-/- mice, p63 was expressed in both basal and luminal cells. Overall, there was a 2.6-fold increase in the number of p63-positive cells in ERβ-/- mouse VP (Fig. 4). CK 8, specific for luminal epitheliocytes, was decreased in the ERβ-/- VP, whereas there was no difference in expression of CK 5, a marker for basal cells. CK 19, a marker for both basal and intermediate cells, was more abundant in the ERβ-/- mouse prostates (Fig. 5). These changes in the ratio of CK markers indicate that, in ERβ -/- mice, the differentiation of prostatic epithelium is altered (Table 1).
Fig. 4.
Expression of p63 in the VP of WT and ERβ -/- mice. The basal cell marker p63 is confined to basal cells in the VP of WT mice. In ERβ-/- mice, p63 is expressed in both basal and luminal cells. Overall, there is a 2.6-fold increase in the number of p63-positive cells in ERβ-/- mouse VP.
Fig. 5.
Western blot for CKs 5, 8, and 19. In WT mice there is equal expression of CK 8 (luminal) and CK 5 (basal). In ERβ-/- mice, CK 5 is as abundant as it is in WT, but CK 8 is poorly expressed. CK 19 (basal/intermediate) is more abundant in the ERβ-/- VP.
Table 1. Expression of differentiation markers in WT and ERβ-/- VP.
| Markers | WT | ERβ-/- |
|---|---|---|
| Basal | ||
| p63 | + | +++ |
| CK 5 | + | + |
| CK 19 | + | ++ |
| Luminal | ||
| CK 8 | ++ | + |
Levels of expression: +, low; ++, moderate; +++, high.
The differentiation in the absence of ERβ signaling was further characterized by using FACS. For flow cytometry analysis, VPs from WT and ERβ-/- mice were digested with collagenase to isolate epithelial cells and analyze the distribution of p63 and CK 19. The extent of tissue digestion was checked under the microscope and stopped when there was a single-cell suspension of digested cells (Fig. 6A). Two clear cell populations could be distinguished on the basis of size and granularity: the smaller cells probably representing the basal cells, and the larger cells representing the luminal cells. Consistently, side scatter (SSC)/forward scatter (FSC) plots showed two cell populations separated by size (FSC) and granularity (SSC).
Fig. 6.
FACS analysis of p63 expression. (A) Microscopic view of single-cell suspension after collagenase treatment of VP. Arrows indicate cells of different sizes. (B) FITC–p63 histogram of the distribution of p63-positive cells. R indicates a gated area of all p63-positive cells (blue, WT; red, ERβ-/-). (C and D) Distribution of p63-expressing cells on SSC/FCS plots of WT (C) and ERβ-/- (D) VP cell isolates. The horizontal axis shows the FSC, and the vertical axis shows the SSC.
For analysis of expression of p63, two cell populations were gated on the SSC/FSC plot: the g1 population represented cells of small size and granularity, and the g2, cells of larger size and high granularity. Analysis of p63 showed a strong shift in expression of p63 toward the g2 population in ERβ-/- mice. This expression of p63 in larger, more granular cells in ERβ-/- VP explains the observed immunohistochemical distribution of p63-positive cells in the luminal compartment of the duct (Fig. 6). The g1/g2 ratio in WT VP was 1:2.5, whereas in ERβ-/- mouse VP it was 1:9. Hence, there are more cells in the luminal compartment of ERβ-/- VP ducts expressing p63, a marker of undifferentiated basal cells.
To analyze the expression of basal/intermediate marker CK 19, we gated two cell populations in the SSC/FSC plots. The areas r1 and r2 were estimated to represent cells within the differentiation continuum of increasing size and granularity. (Fig. 7 A and B). Subsequently, two areas of CK 19-positive cells were gated on FITC-CK 19 histograms (Fig. 7 C and D). All cells positive for CK 19 were plotted on SSC/FSC plots, and it became evident that all cells were redistributed into three populations. In ERβ-/- VP, expression of CK 19-positive cells was shifted toward the g2 cell population, most probably representing an intermediate cell phenotype (Fig. 7 E and F). The ratio g1/g2/g3 in WT VP was 1:0.6:2; in ERβ-/- VP, the ratio changed to 1:4:6.7.
Fig. 7.
FACS analysis of CK 19 expression. (A and B) SSC/FSC plots of all cells in WT (A) and ERβ-/- (B) samples, with areas r1 and r2 gated according to the size and granularity. (C and D) Gated areas on FITC–CK 19 histograms corresponding to A and B (blue, WT; red, ERβ-/-). WT (E) and ERβ-/- (F) SSC/FSC plots of CK 19-positive cell populations from areas gated r1 and r2 in B and C; g1–g3 areas representing possible localization of basal, intermediate, and luminal cells, respectively.
Hence, in the absence of ERβ signaling, more prostatic epithelial cells of intermediate and luminal size and granularity expressed CK 19, indicating that these cells were not terminally differentiated.
Discussion
The role of ERβ in epithelial cellular hyperplasia in the VP has become a contentious point between different laboratories studying ERβ-/- mice (4, 6, 7). Part of the disagreement is likely related to the lack of universal methods of morphological processing of rodent prostates, i.e., embedding and cutting. Thus, almost any change of tissue architecture can be described as a “cutting artifact.” We have reported and continue to observe areas of cellular accumulation in H–E-stained VP of ERβ-/- mice, whereas none is seen in WT sections processed in the same way (Fig. 1). This observation early on prompted us to postulate a role for ERβ in cellular homeostasis in mouse VP (4).
The major aim of the present study was not to obtain proof of the epithelial hyperplasia in ERβ-/- mice, but to characterize the phenomenon. BrdUrd incorporation into DNA in the mature mouse VP showed a marked increase in the proliferative activity in the absence of ERβ. The proliferation indices of ERβ-/- VP were 3.6-fold higher than those of WT littermates. In addition to the high proliferation in ERβ-/- mice, apoptosis seemed to be suppressed in these animals.
Although the mature rodent VP is not an actively proliferating tissue, there is some cellular turnover. Epithelial renewal is achieved by cellular shredding into the lumen of the duct, and new cells are formed by ongoing proliferation. In ERβ-/- mice, acinar lumini of the prostate sections contain an increased amount of cellular debris and necrotic cells, and TUNEL reaction gave a strong background signal, indicative of dead cells in the lumen. The high sensitivity and moderate specificity of TUNEL is associated with certain difficulties in calculation of apoptotic indices; despite this, the difference between WT and ERβ-/- VPs is clear (Fig. 3 A and B). Additional evidence for a reduction in apoptosis in ERβ-/- VP comes from the high level of expression of the anti-apoptotic protein bcl-2. Down-regulation of bcl-2 in the mouse VP is the first and most important step in the sequence of events involved in the apoptotic death signaling (17). Moreover, bcl-2 overexpression inhibits cell death and promotes morphogenesis, but not tumorigenesis, in human mammary epithelial cells (18). These changes bear certain resemblance to the situation in ERβ-/- VP epithelium. Immunohistochemical detection revealed a significant increase in the number of cells expressing bcl-2 in the absence of ERβ signaling, a result supported by Western blotting with the same antibody (Fig. 3 C–E). Bcl-2 is an estrogen-regulated gene, suggesting that ERβ mediates the regulation of bcl-2 in the VP. In WT mice, bcl-2 expression was confined to the basal cell layer of the prostate, whereas in ERβ-/- mice, all epithelial cells were bcl-2-positive (Fig. 3 C and D). On the basis of the high proliferative activity, low apoptotic rate, cellular accumulation, and altered expression of bcl-2 in ERβ-/- VP, we hypothesize that, in the absence of ERβ signaling, the prostatic epithelium fails to differentiate completely.
It is thought that there are four different cell types in prostatic epithelia of mammals (19). These cell types represent a continuum of a self-renewal process of the prostatic epithelium (20, 21). Each type possesses a unique protein expression profile that can be used to distinguish one cell type from another one. The four cell types are: basal, luminal, neuroendocrine, and transiently proliferating/amplifying. Basal cells are relatively undifferentiated, multipotent cells, some of which possess stem-cell characteristics. This population consists of slowly cycling cells that are independent of androgen for growth but are androgen sensitive. They are located along the basement membrane. This cellular pool gives rise to all prostatic epitheliocytes. Basal cells express bcl-2 under normal conditions and are thought to be resistant to apoptosis (22, 23). They uniquely express p63 and CKs 5 and 15. Luminal cells represent terminally differentiated, specialized cell populations and produce components of the prostatic secretion. This cell type does not express bcl-2 and is subjected to apoptosis and subsequent shredding into the lumen. Luminal cells express CK 8. They are both androgen sensitive and dependent, and require androgen stimulation for functioning and survival. Neuroendocrine cells are a specialized population of cells possibly playing a role in the regulation of functional and proliferative activity. This cell type is positive for chromogranin A. Transiently proliferating/amplifying cell populations serve as intermediate between basal and luminal phenotypes. These intermediate cells express both luminal and basal cell markers. They are androgen dependent and highly androgen sensitive, and they are capable of rapid proliferation under androgen stimulation and rapid death upon androgen withdrawal.
Basal and luminal cells represent two extremities of the differentiation continuum, possessing the unique expression pattern of markers: undifferentiated basal cells express CK 5, CK 14, CK 19, and p63, and terminally differentiated luminal cells express CK 8, androgen receptor, and prostate-specific antigen. Intermediate cells within the continuum do not have a universal immunohistochemical signature because they are on their way from undifferentiated to terminally differentiated phenotypes. This intermediate pool consists of cells of different size, granularity, and antigen marker profile. Some authors propose distinguishing “basal intermediate” and “luminal intermediate” cells based upon the localization of the cell in the continuum (24–26). Thus, although cells may have the same antigen profile, differences in size and granularity help to distinguish intermediate cells at various stages on the way from basal to luminal phenotype.
In ERβ-/- VP, there was a 2.7-fold increase in the basal cell marker p63 (Fig. 4) and a decrease in expression of the luminal cell marker CK 8. The basal cell marker CK 5 remained unchanged, but the basal/intermediate marker, CK 19, was higher in VP of ERβ-/- mice (Fig. 5).
Under normal conditions, p63 is expressed only in basal, i.e., undifferentiated, cells. This marker is distributed between g1 and g2 populations at a ratio of 1:2.5. In the VP of ERβ-/- mice, the distribution of p63-positive cells in g1 and g2 populations showed a clear shift toward g2, indicating that more cells of luminal size and granularity are positive for p63, a marker for undifferentiated cells (Fig. 6).
The expression pattern of the basal/intermediate marker CK 19 was also different. All cells in the SSC/FSC plot were initially divided into two gated populations. However, when only CK 19-positive cells were plotted on SSC/FSC, they were redistributed in three distinct populations. The population of cells of middle size and granularity was more prominent in ERβ-/- mice (Fig. 7 E and F). This population most likely represents cells of intermediate phenotype. The ratio g1/g2/g3 was 1:0.6:2 in WT VP; in ERβ-/- it was 1:4:6.7. Cells expressing CK 19 in the absence of ERβ distributed in favor of g2 and g3 populations, with the strongest change in g2. This fraction of CK 19-positive cells in g2 and g3 areas possibly represents an accumulated pool of intermediate epitheliocytes (Fig. 7).
The population of cells of larger size and higher granularity includes both true luminal and intermediate cells, and the shift of expression of basal and basal/intermediate markers toward these cells represents accumulation of incompletely differentiated cells in the absence of ERβ signaling. Consequently, ERβ is likely to have a prodifferentiative role in the mouse VP.
Evidence for the involvement of ERβ in the pathogenesis of prostate cancer is accumulating. Studies of methylation of the ERβ promoter in prostate cancer show that methylation increases with the stage of cancer as well as with the progression of the disease (27). Thus, there is a silencing of ERβ with the advancement of prostate cancer. Interestingly, ERβ expression is lost in primary cultures of malignant prostate cancer cells (28). One of the most important clinical characteristics of prostatic adenocarcinoma is degree of differentiation. This aspect of the tumor biology has a great prognostic value for clinicians and may currently be evaluated and quantified by using the Gleason score (29). We suggest a possible role of ERβ in promotion of differentiation of the prostate epithelium.
According to current concepts, PIN is a histological lesion that is strongly associated with prostate cancer and believed to represent a premalignant stage of adenocarcinoma. High-grade PIN is directly linked to carcinoma in situ and in most cases is associated with elevated serum prostate-specific antigen.
Dietary factors, particularly phytoestrogens, are believed to play a role as oncoprotective agents and supposedly lower the risk of prostate cancer. The protective effect of soybeans was known before the discovery of the functional effects of phytoestrogens (30). Genistein, one of the most studied phytoestrogens, has been shown to act as a prodifferentiative agent for the treatment of prostate cancer (31). Ligand-binding studies have shown that ERβ exhibits a 40-fold higher affinity for genistein than does ERα, suggesting that this compound may act through ERβ-signaling pathways (32). In the rat prostate, isoflavones up-regulate heat shock protein HSP-27 and inhibit progression of PIN to adenocarcinoma (33). Because ERβ is the only ER in the prostate, and heat shock protein HSP-27 is known to be induced by estrogen (34), it is tempting to speculate that isoflavones might act through ERβ.
Our study shows that there is epithelial hyperplasia and insufficient differentiation of the epithelium in ERβ-/- mouse VP. ERβ signaling seems to be essential for maintenance of cellular homeostasis and to drive the cellular differentiation. The results suggest the possible use of ERβ as a target for the development of new modalities of prevention and medical treatment of prostate cancer.
Acknowledgments
We thank Dr. Sari Makela and Teija Teivonen (Institute of Biomedicine, University of Turku, Turun Yliopisto, Finland) for animals and Annemarie Witte and Patricia Humire for excellent technical support. This work was supported by grants from the Swedish Cancer Fund and from KaroBio AB.
Abbreviations: CK, cytokeratin; ERα/β, estrogen receptor α or β; VP, ventral prostate; PIN, prostatic intraepithelial neoplasia; FACS, fluorescence-activated cell sorter; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling; FSC, forward scatter; SSC, side scatter.
References
- 1.Kuiper, G. G., Enmark, E., Pelto-Huikko, M., Nilsson, S. & Gustafsson, J.-Å. (1996) Proc. Natl. Acad. Sci. USA 93, 5925-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shim, G. J., Wang, L., Andersson, S., Nagy, N., Kis, L. L., Zhang, Q., Makela, S., Warner, M. & Gustafsson, J.-Å. (2003) Proc. Natl. Acad. Sci. USA 100, 6694-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilsson, S., Makela, S., Treuter, E., Tujague, M., Thomsen, J., Andersson, G., Enmark, E., Pettersson, K., Warner, M. & Gustafsson, J.-Å. (2001) Physiol. Rev. 81, 1535-1565. [DOI] [PubMed] [Google Scholar]
- 4.Weihua, Z., Makela, S., Andersson, L. C., Salmi, S., Saji, S., Webster, J. I., Jensen, E. V., Nilsson, S., Warner, M. & Gustafsson, J.-Å. (2001) Proc. Natl. Acad. Sci. USA 98, 6330-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, G., Weihua, Z., Makinen, S., Makela, S., Saji, S., Warner, M., Gustafsson, J.-Å. & Hovatta, O. (2002) Biol. Reprod. 66, 77-84. [DOI] [PubMed] [Google Scholar]
- 6.Dupont, S., Krust, A., Gansmuller, A., Dierich, A., Chambon, P. & Mark, M. (2000) Development (Cambridge, U.K.) 127, 4277-4291. [DOI] [PubMed] [Google Scholar]
- 7.Krege, J. H., Hodgin, J. B., Couse, J. F., Enmark, E., Warner, M., Mahler, J. F., Sar, M., Korach, K. S., Gustafsson, J.-Å. & Smithies, O. (1998) Proc. Natl. Acad. Sci. USA 95, 15677-15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Coppenolle, F., Slomianny, C., Carpentier, F., Le Bourhis, X., Ahidouch, A., Croix, D., Legrand, G., Dewailly, E., Fournier, S., Cousse, H., et al. (2001) Am. J. Physiol. 280, E120-E129. [DOI] [PubMed] [Google Scholar]
- 9.Li, X., Nokkala, E., Yan, W., Streng, T., Saarinen, N., Warri, A., Huhtaniemi, I., Santti, R., Makela, S. & Poutanen, M. (2001) Endocrinology 142, 2435-2442. [DOI] [PubMed] [Google Scholar]
- 10.McPherson, S. J., Wang, H., Jones, M. E., Pedersen, J., Iismaa, T. P., Wreford, N., Simpson, E. R. & Risbridger, G. P. (2001) Endocrinology 142, 2458-2467. [DOI] [PubMed] [Google Scholar]
- 11.Linja, M. J., Savinainen, K. J., Tammela, T. L., Isola, J. J. & Visakorpi, T. (2003) Prostate 55, 180-186. [DOI] [PubMed] [Google Scholar]
- 12.Fixemer, T., Remberger, K. & Bonkhoff, H. (2003) Prostate 54, 79-87. [DOI] [PubMed] [Google Scholar]
- 13.Torlakovic, E., Lilleby, W., Torlakovic, G., Fossa, S. D. & Chibbar, R. (2002) Hum. Pathol. 33, 646-651. [DOI] [PubMed] [Google Scholar]
- 14.Fujimura, T., Takahashi, S., Urano, T., Ogawa, S., Ouchi, Y., Kitamura, T., Muramatsu, M. & Inoue, S. (2001) Biochem. Biophys. Res. Commun. 289, 692-699. [DOI] [PubMed] [Google Scholar]
- 15.Horvath, L. G., Henshall, S. M., Lee, C. S., Head, D. R., Quinn, D. I., Makela, S., Delprado, W., Golovsky, D., Brenner, P. C., O'Neill, G., et al. (2001) Cancer Res. 61, 5331-5335. [PubMed] [Google Scholar]
- 16.Windahl, S. H., Vidal, O., Andersson, G., Gustafsson, J.-Å. & Ohlsson, C. (1999) J. Clin. Invest. 104, 895-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki, A., Matsuzawa, A. & Iguchi, T. (1996) Oncogene 13, 31-37. [PubMed] [Google Scholar]
- 18.Lu, P. J., Lu, Q. L., Rughetti, A. & Taylor-Papadimitriou, J. (1995) J. Cell Biol. 129, 1363-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, Y., Hayward, S., Cao, M., Thayer, K. & Cunha, G. (2001) Differentiation (Berlin) 68, 270-279. [DOI] [PubMed] [Google Scholar]
- 20.Isaacs, J. T. & Coffey, D. S. (1989) Prostate Suppl. 2, 33-50. [DOI] [PubMed] [Google Scholar]
- 21.Bonkhoff, H., Stein, U. & Remberger, K. (1994) Hum. Pathol. 25, 42-46. [DOI] [PubMed] [Google Scholar]
- 22.Hayward, S. W., Brody, J. R. & Cunha, G. R. (1996) Differentiation (Berlin) 60, 219-227. [DOI] [PubMed] [Google Scholar]
- 23.English, H. F., Drago, J. R. & Santen, R. J. (1985) Prostate 7, 41-51. [DOI] [PubMed] [Google Scholar]
- 24.Verhagen, A. P., Ramaekers, F. C., Aalders, T. W., Schaafsma, H. E., Debruyne, F. M. & Schalken, J. A. (1992) Cancer Res. 52, 6182-6187. [PubMed] [Google Scholar]
- 25.Robinson, E. J., Neal, D. E. & Collins, A. T. (1998) Prostate 37, 149-160. [DOI] [PubMed] [Google Scholar]
- 26.Van Leenders, G., Dijkman, H., Hulsbergen-van de Kaa, C., Ruiter, D. & Schalken, J. (2000) Lab. Invest. 80, 1251-1258. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki, M., Tanaka, Y., Perinchery, G., Dharia, A., Kotcherguina, I., Fujimoto, S. & Dahiya, R. (2002) J. Natl. Cancer Inst. 94, 384-390. [DOI] [PubMed] [Google Scholar]
- 28.Pasquali, D., Rossi, V., Esposito, D., Abbondanza, C., Puca, G. A., Bellastella, A. & Sinisi, A. A. (2001) J. Clin. Endocrinol. Metab. 86, 2051-2055. [DOI] [PubMed] [Google Scholar]
- 29.Gleason, D. F. (1966) Cancer Chemother. Rep. 50, 125-128. [PubMed] [Google Scholar]
- 30.Messina, M. J., Persky, V., Setchell, K. D. & Barnes, S. (1994) Nutr. Cancer 21, 113-131. [DOI] [PubMed] [Google Scholar]
- 31.Mentor-Marcel, R., Lamartiniere, C. A., Eltoum, I. E., Greenberg, N. M. & Elgavish, A. (2001) Cancer Res. 61, 6777-6782. [PubMed] [Google Scholar]
- 32.Kuiper, G. G., Lemmen, J. G., Carlsson, B., Corton, J. C., Safe, S. H., van der Saag, P. T., van der Burg, B. & Gustafsson, J.-Å. (1998) Endocrinology 139, 4252-4263. [DOI] [PubMed] [Google Scholar]
- 33.Hikosaka, A., Asamoto, M., Hokaiwado, N., Kato, K., Kuzutani, K., Kohri, K. & Shirai, T. (2004) Carcinogenesis 25, 381-387. [DOI] [PubMed] [Google Scholar]
- 34.Porter, W., Wang, F., Wang, W., Duan, R. & Safe, S. (1996) Mol. Endocrinol. 10, 1371-1378. [DOI] [PubMed] [Google Scholar]