Abstract
Endoplasmic reticulum (ER) stress has been implicated in various neurodegenerative diseases, including Alzheimer’s disease. We have previously observed amyloid production in the retina of the Tg2576 transgenic mouse model of Alzheimer’s disease. In this study, we used tunicamycin-induced ER stress in RGC-5 cells, a cell line identical to the photoreceptor cell line 661W, to investigate the effect of ER stress on production of amyloid-beta (Abeta) peptides. We found that the mRNA level of amyloid-beta precursor protein (APP) remained stable, while the protein level of amyloid-beta precursor protein (APP) was decreased, the amyloid-beta precursor protein cleaving enzymes beta-site APP-cleaving enzyme 1 and presenilin 1 were upregulated, Abeta1–40 and Abeta1–42 production were increased, and reactive oxygen species production and apoptosis markers were elevated following induction of ER stress. The protein level of Abeta degradation enzymes, neprilysin, endothelin-converting enzyme 1, and endothelin-converting enzyme 2 remained unchanged during the prolonged ER stress, showing that the generation of Abeta did not result from reduction of proteolysis by these enzymes. Inclusion of group II caspase inhibitor, Z-DEVD-FMK, increased the ER stress mediated Abeta production, suggesting that they are generated by a caspase-independent mechanism. Our findings provided evidence of a role of ER stress in Abeta peptide overproduction and apoptotic pathway activation in RGC-5 cells.
Keywords: Endoplasmic reticulum stress, Amyloid-beta peptide, RGC-5 cells, Amyloid-beta precursor protein, Enzymes beta-site APP-cleaving enzyme 1, Presenilin 1
Introduction
The endoplasmic reticulum (ER) is a cell organelle that forms an interconnected network of folded membranes (cisternae) where proteins are synthesized, posttranslationally modified, and properly folded. Various factors, such as excessive mutant proteins, energy or nutrient deprivation, and alteration in the redox status, can compromise the protein folding capacity of the ER, resulting in ER stress (Beer et al. 2013; Gorlach et al. 2006). Upon ER stress, accumulated unfolded proteins in the ER lumen compete for binding ER chaperone binding immunoglobulin protein (BiP), resulting in dissociation of BiP from the ER stress transducers (Pandol et al. 2011; Pincus et al. 2010), leading to their activation and triggering downstream protective cellular responses which attenuate the aggregation of unfolded or misfolded proteins and promote cell survival (Haynes et al. 2004; Paschen and Frandsen 2001; Yoshida 2007). However, prolonged or overwhelming ER stress will result in activation of an apoptotic cascade, leading to cell death (Jing et al. 2012; Katayama et al. 2004; Paschen and Frandsen 2001; Rao et al. 2004).
ER stress is implicated in a number of neurodegenerative diseases or aging-related disorders, such as Alzheimer’s disease (Ansari and Khodagholi 2013; Ghribi 2006; Hoozemans et al. 2012; Katayama et al. 2004), Parkinson’s disease (Brown and Naidoo 2012; Hoozemans et al. 2012; Lindholm et al. 2006), macular degeneration (Salminen et al. 2010), and diabetic retinopathy (Jing et al. 2012; Zhang et al. 2011). Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, memory loss, and neuronal loss in the brain. Increased production, oligomerization, and aggregation of toxic Abeta peptides are the crucial factors in the pathogenesis of AD (Selkoe 2001a; Selkoe 2001b; Tanzi and Bertram 2005). Abeta peptides are produced by sequential cleavage of the amyloid-beta precursor protein, a transmembrane glycoprotein which is folded and modified in the ER, by beta-site amyloid-beta precursor protein (APP)-cleaving enzyme 1 (BACE1, beta-secretase) and gamma-secretase complexes (Selkoe 2001a; Thinakaran and Koo 2008; Vetrivel et al. 2008; Zheng H, 2006). Inhibition of beta- or gamma-secretase could reduce amyloid-beta peptide production and subsequent aggregation in the brain, which could be a major therapeutic strategy (Basi et al. 2010; Ghosh et al. 2012; Panza et al. 2011).
We have previously found marked accumulation of Abeta peptides in the retina of Tg2576 AD transgenic mice and in hypoxia-treated RGC-5 cells (Li et al. 2011; Liu et al. 2009). The most senile plaque-like staining patterns were detected from the ganglion cell layer to the outer nuclear layer (photoreceptor) (Liu et al. 2009). ER stress has been shown to play a role in the accumulation of Abeta peptides in AD (Ghribi 2006), presumably through upregulating the APP-cleaving enzymes, BACE1 (O’Connor et al. 2008), and presenilin1 (PS1), a major component of gamma-secretase intramembrane protease complex (Ohta et al. 2011).
ER stress has also been reported to be associated with retinal cell death following chronic ocular hypertension in rat (Doh et al. 2010). Treatment with ER stress inducer tunicamycin caused apoptotic cell death in RGC-5 and also elevated production of ER stress-related proteins (Shimazawa et al. 2007). However, it remains unclear whether or not ER stress directly induces Abeta accumulation in the RGC-5 cells. In this study, we sought to investigate the effect of ER stress on production of Abeta peptides in RGC-5 cells, a cell line which was recently proved to be identical to 661W, a mouse SV-40 T antigen transformed photoreceptor cell line.
Materials and methods
Materials
The RGC-5 cells were kindly provided by Dr. Zhiqun Tan at the University of California Irvine School of Medicine in the Department of Neurology (Irvine, CA, USA). Low glucose Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco Life Technologies (Carlsbad, CA, USA). Tunicamycin, cell lysis buffer, and protease inhibitor cocktail were purchased from Sigma-Aldrich (St Louis, MO, USA). Mouse anti-Abeta1–40 was purchased from Abcam (Cambridge, UK). Primary antibodies [rabbit anti-APP, rabbit anti-BiP, rabbit anti-BACE1, rabbit anti-p-eIF2α, rabbit anti-total eIF2α, mouse anti-CHOP, and rabbit anti-cleaved caspase 3 (Asp175)], rabbit anti-full length caspase 3, were purchased from Cell Signaling Technology (Boston, MA, USA), and corresponding secondary antibodies conjugated to horseradish peroxidase (HRP) or fluorescein isothiocyanate (FITC) were purchased from Multisciences Biotech (Hangzhou, Zhejiang, China). Rabbit anti-CD10 (neprilysin) was purchased from Epitomics (CA, USA). Rabbit anti-endothelin-converting enzyme 1 (ECE1) was purchased from Boster (Wuhan, Hubei, China). Goat anti-endothelin-converting enzyme 2 (ECE2) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Polyvinylidene difluoride (PVDF) membranes were purchased from Bio-Rad (Hercules, CA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for Abeta1–40 and Abeta1–42 were purchased from Wako (Osaka, Japan). The 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) fluorescent probe for flow cytometric detection of cellular reactive oxygen species (ROS) was purchased from Invitrogen Molecular Probes. The group II caspase inhibitor, Z-D(OMe)E(Ome)VD(OMe)-FMK (Z-DEVD-FMK) was purchased from EMD Millipore (MA, USA).
Cell treatment and sample preparation
The RGC-5 cells were cultured in low glucose DMEM supplemented with 10 % FBS in 25 mm cell culture flasks, or six-well cell culture plates with cover slips for 24 h and then treated with 2 μg/ml tunicamycin or vehicle DMSO in replenished fresh culture medium. Cell lysates were collected at 2, 4, 8, 12, 24, and 48 h after tunicamycin treatment for western blot analysis and Elisa analysis of Abeta1–40 and Abeta1–42. For experiments involving caspase inhibition, cells were pretreated with 10 μM Z-DEVD-FMK or 20 μM Z-DEVD-FMK in normal culture media for 2 hours prior to induction of ER stress. Cells were then exposed to 2 μg/ml tunicamycin in the presence of the inhibitor. Cell lysates were collected at 4, 24, and 48 h after tunicamycin treatment for Elisa analysis of Abeta1–40 and Abeta1–42. Cells from separate culture flasks were trypsinized and resuspended for flow cytometric analysis of reactive oxygen species (ROS), and cells cultured on cover slips in 24-well plates were fixed in 4 % paraformaldehyde for immunocytochemical staining of APP, and Abeta1–40.
RT-PCR analysis
RGC-5 cells treated with either tunicamycin or vehicle were rinsed with cold phosphate-buffered saline (PBS). Total RNA was then isolated from the cells using Trizol Reagent (Invitrogen) according to manufacturer’s instructions. cDNA was synthesized with a SuperScript™ One-Step RT-PCR System (Invitrogen) in a GeneAmp PCR System (PerkinElmer, Shelton, CT). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. Endpoint PCR amplifications for APP, and GAPDH were carried out in 30 cycles using primers with the following sequences: mouse APP forward CAGAATGGAAAGTGGGAGTCA and reverse TAGGCAACGGTAAGGAATCAC and GAPDH forward ACCACAGTCCATGCCATCAC and reverse TCCACCACCCTGTTGCTGTA. The amplified products were visualized on 1 % agarose gels stained with ethidium bromide and photographed under UV illumination for analysis of the bands.
Western blot analysis
Cells treated with either tunicamycin or vehicle were rinsed with phosphate-buffered saline (PBS) and lysed with cell lysis buffer containing protease inhibitors. Cell lysates with equal amount of protein were loaded and resolved by SDS–PAGE, and transferred onto a polyvinylidene difluoride (PVDF) membrane. The PVDF membrane was blocked with 5 % bovine serum albumin (BSA) for 1 h and incubated with the primary antibody rabbit anti-APP (1:500 dilution), rabbit anti-BiP (1:1000 dilution), rabbit anti-BACE1 (1:1000 dilution), rabbit anti-p-eIF2α (1:500 dilution), rabbit anti-total eIF2α (1:1000 dilution), mouse anti-CHOP (1:500 dilution), rabbit anti-cleaved caspase3 (Asp175) (1:500 dilution), rabbit anti-full length caspase 3 (1:500 dilution), rabbit anti-neprilysin (1:1000 dilution), rabbit anti-ECE1 (1:250 dilution), or goat anti-ECE2 (1:500) in 5 % BSA at 4 °C overnight, followed by incubation with corresponding HRP-conjugated secondary antibody before visualized by chemiluminescence. The intensity of target bands on the blot was analyzed with Image J software and normalized to that of housekeeping control beta-actin or GAPDH. The intensity data from blots of three independent experiments were presented as mean ± SEM and further analyzed by one-way ANOVA (SPSS) (p < 0.05 was conceived as statistically significant).
Immunocytochemical staining
Cells cultured on coverslips in 24-well plates were fixed with iced cold 4 % paraformaldehyde for 30 min at 0, 2, 4, 8, 12, and 24 h after tunicamycin treatment, permeabilized with 0.2 % Triton X-100 in PBS for 5 min, blocked with 5 % BSA, and incubated overnight at 4° C with rabbit anti-APP (1:200 dilution) and mouse anti-Abeta1–40 (1:250 dilution) in 1 % BSA in PBS, followed by two washes with PBS and incubation with FITC-conjugated goat anti-mouse IgG or goat anti-rabbit IgG secondary antibody (1:50 dilution) in 1 % BSA. The cells were then counterstained with DAPI and mounted with anti-fade fluorescence mounting medium. Images were obtained with a Zeiss LSM 510 confocal microscope.
ELISA analysis of Abeta1–40 and Abeta1–42
The levels of Abeta1–40 and Abeta1–42 were examined by ELISA according to the manufacturer’s protocols (Wako, Japan). One hundred microliters of cell lysate (1:10 dilution) from each sample was assayed in the study, with equal volume of standard solution as internal control. The data were presented as Abeta peptides (pmol) per gram (g) of total cell lysate protein (pmol/g), and the tunicamycin treated groups were compared with control by one-way ANOVA (p < 0.05 was conceived as statistically significant).
Measurement of intracellular ROS by flow cytometry
Intracellular ROS was detected by using oxidation-sensitive fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA), as described previously (Chen et al. 2011). Cells were harvested at 12, 24, and 48 h after tunicamycin or vehicle treatment, incubated with 1 μM H2DCFDA at 37 ºC for 30 min in the dark, rinsed twice with PBS, and analyzed immediately by Becton Dickinson FACSAria™ flow cytometer (BD Biosciences, San Jose, CA, USA) using excitation and emission wavelengths of 488 and 530 nm, respectively. The fluorescent probe H2DCFDA is deacetylated by nonspecific esterase and further oxidized by ROS in the cell to highly fluorescent 2′,7′-dichlorofluorescein (DCF), which then serve as an indicator for intracellular ROS. Ten thousand cells were analyzed for each sample, and data from three independent experiments were collected.
Results
ER stress is induced by tunicamycin in RGC-5 cells
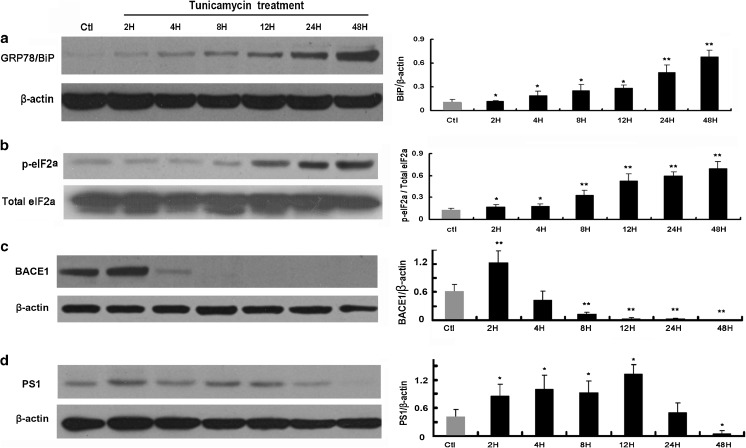
Inhibition of N-linked glycosylation will lead to protein misfolding and cause ER stress (Elbein 1987). In this study, we sought to create a cellular model to study the effect of ER stress on Abeta peptide accumulation by using tunicamycin, an inhibitor of N-linked glycosylation (Elbein 1987), to induce ER stress in RGC-5 cells. Upon stimulation of unfolded or misfolded proteins, ER stress transducer IRE1 is activated to splice the mRNA of the transcription factor XBP1 and produce the active form XBP1s, which then upregulates ER chaperones to enhance the protein folding capacity of the ER (Beer et al. 2013). On the other hand, activation of the ER stress transducer PERK increases phosphorylation of eIF2α, leading to a global attenuation of protein synthesis and hence decreasing the protein load to the ER. Here, we examined the protein expression levels of the ER chaperone BiP/GRP78 and phosphorylated eIF2α (p-eIF2α) by western blot to assess ER stress induced by tunicamycin treatment of the cells and found that significant ER stress was induced by treating RGC-5 cells with 2 μg/ml tunicamycin. Western blot analysis showed that the ER stress markers BiP/GRP78 and p-eIF2α were upregulated in a time-dependent manner following tunicamycin treatment. The expression level of BiP/GRP78 and p-eIF2α was significantly increased 2 h after tunicamycin treatment (Fig. 1a, b). These data showed that ER stress has been successfully induced in the RGC-5 cells, which we then used as a cellular model to study the effect of ER stress on APP-cleaving enzymes, Abeta production, and other downstream targets.
Fig. 1.
ER stress upregulates BACE1 and PS1. Western blot analysis of ER stress markers GRP78/BiP (a) and p-eIF2α (b) and APP cleavage enzymes BACE1 (c) and PS1 (d). Right panel shows densitometric analysis of corresponding bands. The band of p-eIF2α of individual time points was normalized to corresponding band of total eIF2α. Others were normalized to beta-actin. The data were presented as Mean ± SEM (one-way ANOVA, *p < 0.05, **p < 0.01)
ER stress decreases protein level of APP and promotes Abeta1–40 and Abeta1–42 production in RGC-5 cells
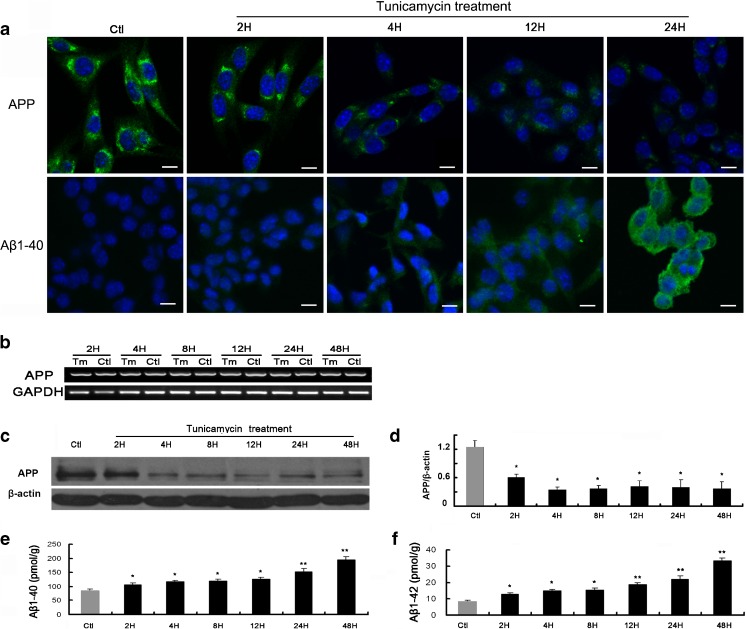
Our results revealed that BACE1 was significantly upregulated 2 h after tunicamycin treatment, then dramatically decreased at 4 h, and decreased to undetectable level after 8 h (Fig. 1c). PS1 was upregulated from 2 h after tunicamycin treatment and gradually decreased after 24 h (Fig. 1d). We then asked whether the upregulation of APP-cleaving enzymes increases the cleavage of APP. We checked the protein level of full-length APP by immunocytochemical staining following tunicamycin treatment and found that APP level was gradually decreased after tunicamycin treatment (Fig. 2a upper panel). RT-PCR showed that the mRNA level of APP remained stable during the 48 h tunicamycin treatment, suggesting that the protein synthesis of APP was not inhibited by the prolonged ER stress (Fig. 2b). Further western blot analysis confirmed the observation from immunostaining analysis and revealed APP was significantly decreased 2 h after tunicamycin treatment, reached its lowest level at 4 h, and remained low until the longest observation time of 48 h (Fig. 2c–d). Immunocytochemical staining showed that ER stress increased production of Abeta1–40 following tunicamycin treatment, which was in accord with the decreased level, or increased cleavage of APP (Fig. 2a, lower panel). ELISA analysis showed that the production of both Abeta1–40 (Fig. 2e) and Abeta1–42 (Fig. 2f) was significantly increased. Abeta1–40 was found to be at a much higher level compared with Abeta1–42.
Fig. 2.
ER stress decreases full-length APP and increases Abeta1–40 and Abeta1–42 production. a. Immunofluorescence staining of APP (upper panel) and Abeta1–40 (lower panel). The antibody was FITC-labeled (green fluorescence). Nuclei were counterstained with DAPI (blue fluorescence). Scale bar = 10 μm. b. RT-PCR analysis of APP. c. Western blot analysis of APP. d. Densitometric analysis of APP normalized to corresponding beta-actin. The data were presented as Mean ± SEM, and analyzed by one-way ANOVA (*p < 0.05). e. ELISA analysis of Abeta1–40. f. ELISA analysis of Abeta1–42. The Abeta data were presented as Mean ± SEM (pmol/g) (one-way ANOVA, *p < 0.05, **p < 0.01)
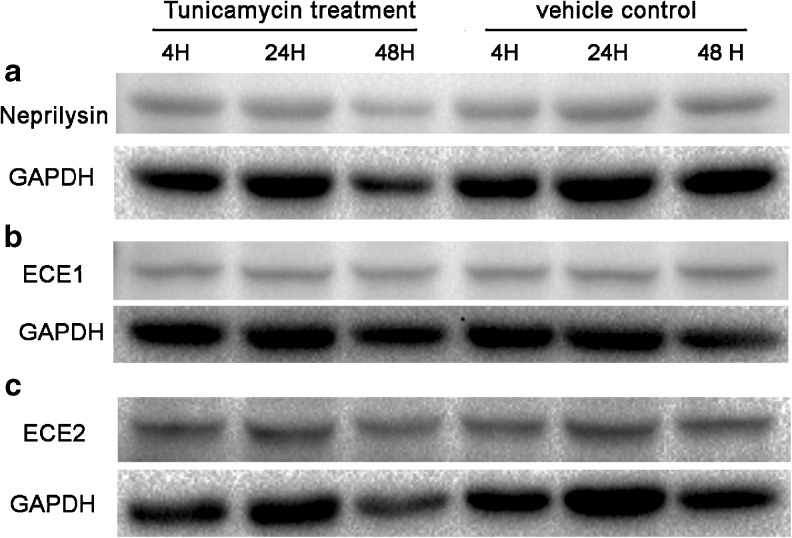
ER stress did not change the protein levels of Abeta degradation enzymes
Given that sustained ER stress may eventually lead to inhibition of protein synthesis and cell death, we asked whether the Abeta degradation enzymes were inhibited by the prolonged ER stress. We found that the protein levels of Abeta degradation enzymes, neprilysin, ECE1, and ECE2 all remained stable during the time points observed (Fig. 3). The results suggested that the elevation of Abeta level was not achieved by reducing the neprilysin, ECE1, or ECE2 related proteolysis.
Fig. 3.
ER stress did not change the protein levels of Abeta degradation enzymes. a. Western blot analysis of neprilysin. b. Western blot analysis of ECE1. c. Western blot analysis of ECE2. Densitometric analysis of neprilysin, ECE1, and ECE2 normalized to corresponding GAPDH by one-way ANOVA showed that there were no significant changes between the group means (p > 0.05)
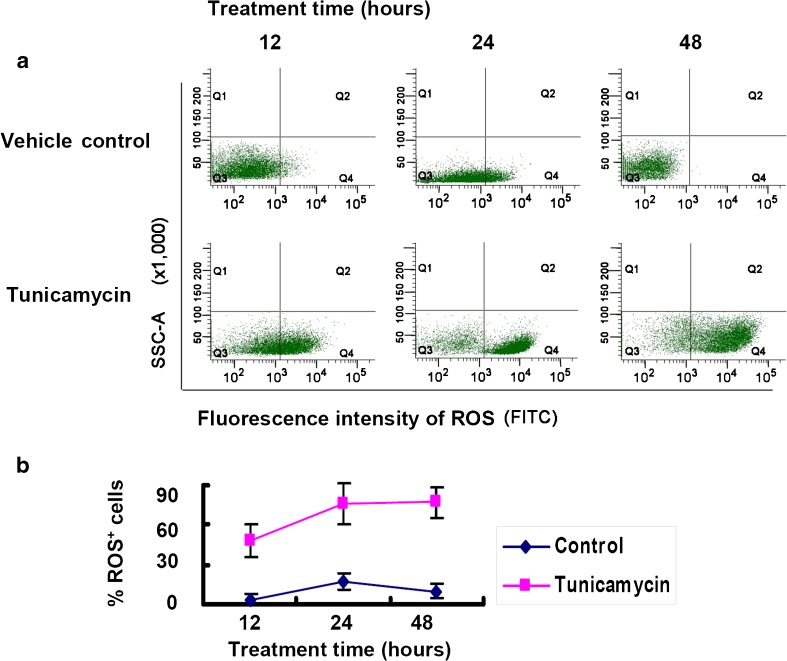
ER stress increases production of ROS in RGC-5 cells
We detected the intracellular ROS by flow cytometry using oxidation-sensitive fluorescent probe H2DCFDA, which is oxidized by ROS to give out fluorescence. The result showed that the percentage of ROS-positive fluorescent cells were significantly increased at 12 h after tunicamycin treatment, and reached the peak at 24 h, compared to the vehicle control (Fig. 4).
Fig. 4.
ER stress increases the number of ROS-positive cells by flow cytometry. a. Scatter plot of flow cytometry in RGC-5 cells treated with 2 μg/ml tunicamycin or vehicle. Quadrant 4 (Q4) represents ROS-positive cells. Quadrant 3 (Q3) represents ROS-negative cells. b. Quantitative analysis of flow cytometric data. The ratio of ROS-positive cells to total number of cells was calculated for each time point and plotted as Mean ± SEM
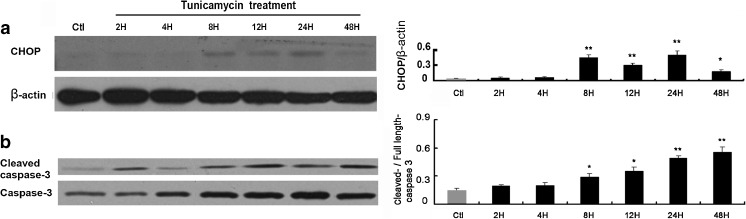
ER stress increases expression of CHOP and cleaved caspase-3 in RGC-5 cells
Here, we aimed to examine whether apoptotic signaling pathways are activated upon ER stress in RGC-5 cells. Western blot analysis revealed that CHOP expression was increased 8 h after tunicamycin treatment (Fig. 5a). Caspases, such as caspase-3, are well-known proapoptotic components in the ER stress-induced cell death signaling pathways and are a family of the cysteine-aspartic acid proteases (caspases) that are synthesized as inactive precursors that have to be cleaved at key aspartate residues to be activated. Following induction of apoptosis, proteolytic cleavage of procaspase-3 occurs to generate an active 17 kDa caspase-3 fragment, which targets key modulators of the apoptotic pathway, including poly-ADP-ribose polymerase and other caspases, for cleavage, leading to apoptotic cell death. Cleaved caspase-3 is therefore a useful marker for apoptosis. Western blot analysis showed that cleaved caspase-3 was increased 8 h following tunicamycin treatment (Fig. 5b), which was in accord with the increase of CHOP level.
Fig. 5.
ER stress increases apoptosis markers CHOP and cleaved caspase-3. Western blot analysis of CHOP (a), cleaved caspase-3, and full-length caspase 3 (b). Right panel shows densitometric analysis of corresponding bands. The band of CHOP of individual time points was normalized to corresponding band of beta-actin. The cleaved caspase-3 was normalized to corresponding full-length caspase 3. The data were presented as Mean ± SEM (one-way ANOVA, *p < 0.05, **p < 0.01)
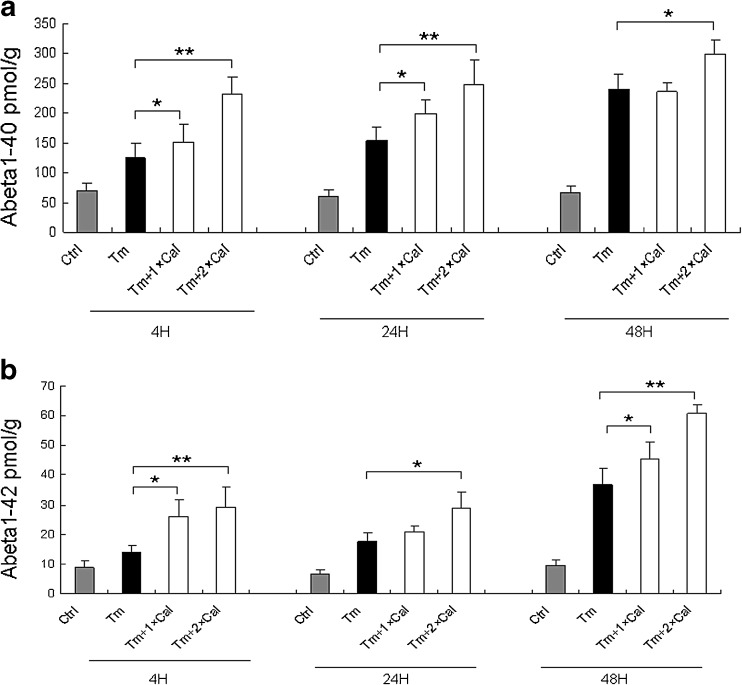
Z-DEVD-FMK promotes the ER stress-mediated production of Abeta1–40 and Abeta1–42 in RGC-5 cells
Activation of caspase 3 was reported to be associated with APP cleavage and Abeta production. We then asked whether the activation of caspase-3 is responsible for the generation of Abeta in the tunicamycin treated RGC-5 cells. Elisa analysis showed that the group II caspase inhibitor, Z-DEVD-FMK, a potent, cell-permeable, and irreversible inhibitor of caspase-3 as well as caspase-6, caspase-7, caspase-8, and caspase-10, significantly promoted the ER stress-induced generation of Abeta1–40 and Abeta1–42 in a dose-dependent manner (Fig. 6), indicating a caspase-independent mechanism in the production of Abeta in our cellular model.
Fig. 6.
Z-DEVD-FMK promotes the ER stress mediated production of Abeta1–40 and Abeta1–42. a. ELISA analysis of Abeta1–40. b. ELISA analysis of Abeta1–42. Tm tunicamycin; 1 × CaI, 10uM Z-DEVD-FMK; 2 × CaI, 20uM Z-DEVD-FMK. The data were presented as Mean ± SEM (pmol/g) (one-way ANOVA, *p < 0.05, **p < 0.01)
Discussion
In this study, we found that the ER stress markers BiP/GRP78 and p-eIF2α were significantly increased in a time-dependent manner following tunicamycin treatment, indicating that ER stress has been successfully induced in the RGC-5 cells. Interestingly, we found that upon induction of ER stress, both BACE1 and PS1 were increased immediately and then decreased following tunicamycin treatment. We then examined whether the upregulation of APP-cleaving enzymes decreases the APP level and found that the mRNA level remained unchanged, while the protein level of APP was gradually decreased after tunicamycin treatment and remained low until the longest observation time 48 h. Further analysis revealed increased production of both Abeta1–40 and Abeta1–42 peptides, which was in accord with the increased cleavage of APP by enzymes BACE1 and PS1. After the initial upregulation, the decrease of BACE1 was very fast, while the decline of PS1 was more gradual. On the other hand, the protein level of Abeta degradation enzymes, neprilysin, ECT1, and ECE2 remained stable in the time points examined, suggesting that the degeneration of Abeta peptides was not inhibited by the prolonged tunicamycin treatment. These results may indicate that upregulation of BACE1 and PS1 may contribute to increased Abeta peptide production and APP cleavage, while PS1 may also play a role in sustaining these effects. We speculate that there might be a positive feedback loop to regulate the levels of APP and BACE1/PS1 (Li et al. 2013), in which the decreased APP level or the cleaved APP fragments may account for the subsequent decrease of BACE1 and PS1 levels following induction of ER stress in the RGC-5 cells. This may also indicate a protective mechanism to break the vicious circle and mitigate the detrimental effect of sustained ER stress.
Recently, the origin of the RGC-5 line has been recharacterized (Van Bergen et al. 2009). The RGC-5 line, designated as a transformed rat retinal ganglion cell line, is actually of mouse origin. Using cryopreserved RGC-5 cells obtained from the laboratory where the original cell line was established, Krishnamoorthy and colleagues report that the RGC-5 cells are virtually identical to the photoreceptor cell line 661W (Krishnamoorthy et al. 2013). Thus, our results might represent in vitro cellular response of photoreceptor cells to ER stress.
ER has an oxidizing environment that is necessary for disulfide bond formation for protein folding, and ER stress will result in overproduction of ROS, which will in turn exacerbate protein misfolding and amplify ER stress response (Malhotra and Kaufman 2007). Here, we found that the percentage of ROS-positive fluorescent cells were significantly increased in the RGC-5 cells 12 h after tunicamycin treatment, indicating that oxidative stress is also involved in our cellular model. ER directly communicates with mitochondria through close contacts that promote calcium transfer from ER to mitochondria thus maintaining mitochondrial metabolism and cell survival (Giorgi et al. 2009; Rizzuto et al. 1998). ER stress and increased production of ROS can lead to mitochondrial dysfunction, which is intimately linked to further production of ROS (Costa et al. 2012; Richter et al. 1995). On the other hand, it may be indicated that oxidative stress could also contribute to increased APP cleavage and Abeta peptide generation, based on previous observations that oxidative stress stimulates amyloid peptide production by enhancing BACE1 and gamma-secretase activities (Tamagno et al. 2008; Xiong et al. 2007). Taken together, ER stress and correlated oxidative stress increase Abeta peptide production by upregulating BACE1/PS1, and overproduction of Abeta peptides will exacerbate ER stress by overloading the ER, which will then further boost ROS production. Therefore, there seems to be a vicious circle encompassing ER stress, Abeta production, and oxidative stress (Ferreiro et al. 2012), which may ultimately culminate into apoptotic cell death.
In response to prolonged ER stress, the PERK-ATF4-mediated and the ATF6-mediated signaling pathways will be activated to upregulate the proapoptotic transcription factor CHOP and initiate signal transduction resulting in apoptotic cell death (Ferreiro et al. 2012; Malhotra and Kaufman 2007). In this study, we found that both CHOP and cleaved caspase-3, a well-known apoptosis marker downstream of CHOP, were both upregulated in the RGC-5 cells following induction of ER stress. The relationship between caspase-3 activation and Abeta production remains unclear. Abeta-mediated toxicity initiates caspase-3 related APP cleavage at the C-terminus and generation of a 31 amino acid fragment, C31 (Lu et al. 2003). Recently, apoptosis in H4 neuroglioma cells was shown to be associated with a decrease in the generation of Abeta, as well as an increase in the formation of alternatively processed APP fragments (25–35 kDa) (Fiorelli et al. 2013). Co-localization of cleaved caspase-3 and amyloid plaques with caspase-cleaved APP has been observed in the brain of AD (Gervais et al. 1999); however, this correlation does not necessarily prove that caspase-initiated cleavage of APP increases Abeta production, given that the internalization of APP could be reduced by caspase mediated truncation which may in fact decrease Abeta generation as endocytosis is required for APP cleavage via the amyloidogenic pathway (Soriano et al. 2001). Our data support this hypothesis and showed that treatment with the group II caspase inhibitor, Z-DEVD-FMK, was associated with an increase of Abeta40 and Abeta42 generation, indicating a caspase independent mechanism in the ER stress mediated Abeta production. The association between caspase activation and APP cleavage and Abeta generation warrants further investigation in our model.
In this study, we investigated the effect of ER stress on Abeta accumulation in RGC-5 cells by using an induced ER stress cellular model and found increased BACE1 and PS1 levels, increased APP cleavage, increased Abeta peptides generation, increased ROS production, and increased apoptotic markers, indicating that ER stress plays an important role in Abeta peptides overproduction and apoptotic pathway activation in RGC-5 cells.
Acknowledgments
We thank Mr. Bingshen Li for technical assistance in Western blot, RT-PCR, and cytometry assays. This study was supported by the National Natural Science Foundation of China (81270992 and 81000376) and Key Project of Natural Science Foundation of Guangdong Province (10251008901000028).
Footnotes
Bingqian Liu, Yingting Zhu, and Jiayi Zhou contributed equally to this work.
References
- Ansari N, Khodagholi F. Molecular mechanism aspect of ER stress in Alzheimer’s disease: current approaches and future strategies. Curr Drug Targets. 2013;14:114–122. doi: 10.2174/138945013804806532. [DOI] [PubMed] [Google Scholar]
- Basi GS, et al. Amyloid precursor protein selective gamma-secretase inhibitors for treatment of Alzheimer’s disease. Alzheimers Res Ther. 2010;2:36. doi: 10.1186/alzrt60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer S et al (2013) Comprehensive functional analysis of chymotrypsin C (CTRC) variants reveals distinct loss-offunction mechanisms associated with pancreatitis risk. Gut 62:1616–1624 [DOI] [PMC free article] [PubMed]
- Brown MK, Naidoo N. The endoplasmic reticulum stress response in aging and age-related diseases. Front Physiol. 2012;3:263. doi: 10.3389/fphys.2012.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, et al. Mitochondria-targeted peptide MTP-131 alleviates mitochondrial dysfunction and oxidative damage in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:7027–7037. doi: 10.1167/iovs.11-7524. [DOI] [PubMed] [Google Scholar]
- Costa RO, et al. Amyloid beta-induced ER stress is enhanced under mitochondrial dysfunction conditions. Neurobiol Aging. 2012;33(824):e5–e16. doi: 10.1016/j.neurobiolaging.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Doh SH, KJ, Lee KM et al (2010) Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Res 13: 158–66 [DOI] [PubMed]
- Elbein AD. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Ferreiro E, et al. Mitochondrial- and endoplasmic reticulum-associated oxidative stress in Alzheimer’s disease: from pathogenesis to biomarkers. Int J Cell Biol. 2012;2012:735206. doi: 10.1155/2012/735206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorelli T, Kirouac L, Padmanabhan J. Altered processing of amyloid precursor protein in cells undergoing apoptosis. PLoS One. 2013;8:e57979. doi: 10.1371/journal.pone.0057979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais FG, et al. Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell. 1999;97:395–406. doi: 10.1016/S0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Brindisi M, Tang J. Developing beta-secretase inhibitors for treatment of Alzheimer’s disease. J Neurochem. 2012;120(Suppl 1):71–83. doi: 10.1111/j.1471-4159.2011.07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghribi O. The role of the endoplasmic reticulum in the accumulation of beta-amyloid peptide in Alzheimer’s disease. Curr Mol Med. 2006;6:119–133. doi: 10.2174/156652406775574514. [DOI] [PubMed] [Google Scholar]
- Giorgi C, et al. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- Haynes CM, Titus EA, Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell. 2004;15:767–776. doi: 10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, et al. Activation of the unfolded protein response is an early event in Alzheimer’s and Parkinson’s disease. Neurodegener Dis. 2012;10:212–215. doi: 10.1159/000334536. [DOI] [PubMed] [Google Scholar]
- Jing G, Wang JJ, Zhang SX. ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res. 2012;2012:589589. doi: 10.1155/2012/589589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T, et al. Induction of neuronal death by ER stress in Alzheimer’s disease. J Chem Neuroanat. 2004;28:67–78. doi: 10.1016/j.jchemneu.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy RR, et al. A forensic path to RGC-5 cell line identification: lessons learned. Invest Ophthalmol Vis Sci. 2013;54:5712–5719. doi: 10.1167/iovs.13-12085. [DOI] [PubMed] [Google Scholar]
- Li X, U.K., Hashimoto T (2013) Neuronal activity and secreted amyloid β lead to altered amyloid β precursor protein and presenilin 1 interactions. . Neurobiol Dis. 50: 127–34 [DOI] [PMC free article] [PubMed]
- Li J, et al. Hypoxia induces beta-amyloid in association with death of RGC-5 cells in culture. Biochem Biophys Res Commun. 2011;410:40–44. doi: 10.1016/j.bbrc.2011.05.101. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- Liu B, et al. Amyloid-peptide vaccinations reduce {beta}-amyloid plaques but exacerbate vascular deposition and inflammation in the retina of Alzheimer’s transgenic mice. Am J Pathol. 2009;175:2099–2110. doi: 10.2353/ajpath.2009.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DC, et al. Caspase cleavage of the amyloid precursor protein modulates amyloid beta-protein toxicity. J Neurochem. 2003;87:733–741. doi: 10.1046/j.1471-4159.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- O’Connor T, et al. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, et al. Endoplasmic reticulum stress enhances gamma-secretase activity. Biochem Biophys Res Commun. 2011;416:362–366. doi: 10.1016/j.bbrc.2011.11.042. [DOI] [PubMed] [Google Scholar]
- Pandol SJ, Gorelick FS, Lugea A. Environmental and genetic stressors and the unfolded protein response in exocrine pancreatic function - a hypothesis. Front Physiol. 2011;2:8. doi: 10.3389/fphys.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, et al. Interacting with gamma-secretase for treating Alzheimer’s disease: from inhibition to modulation. Curr Med Chem. 2011;18:5430–5447. doi: 10.2174/092986711798194351. [DOI] [PubMed] [Google Scholar]
- Paschen W, Frandsen A. Endoplasmic reticulum dysfunction—a common denominator for cell injury in acute and degenerative diseases of the brain? J Neurochem. 2001;79:719–725. doi: 10.1046/j.1471-4159.2001.00623.x. [DOI] [PubMed] [Google Scholar]
- Pincus D, et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8:e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- Richter C, et al. Oxidants in mitochondria: from physiology to diseases. Biochim Biophys Acta. 1995;1271:67–74. doi: 10.1016/0925-4439(95)00012-S. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Salminen A, et al. Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010;16:535–542. doi: 10.2119/molmed.2010.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- Shimazawa M, et al. Involvement of ER stress in retinal cell death. Mol Vis. 2007;13:578–587. [PMC free article] [PubMed] [Google Scholar]
- Soriano S, et al. The amyloidogenic pathway of amyloid precursor protein (APP) is independent of its cleavage by caspases. J Biol Chem. 2001;276:29045–29050. doi: 10.1074/jbc.M102456200. [DOI] [PubMed] [Google Scholar]
- Tamagno E, et al. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem. 2008;104:683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bergen NJ, et al. Recharacterization of the RGC-5 retinal ganglion cell line. Invest Ophthalmol Vis Sci. 2009;50:4267–4272. doi: 10.1167/iovs.09-3484. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, et al. Evidence that CD147 modulation of beta-amyloid (Abeta) levels is mediated by extracellular degradation of secreted Abeta. J Biol Chem. 2008;283:19489–19498. doi: 10.1074/jbc.M801037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, et al. Mitochondrial respiratory inhibition and oxidative stress elevate beta-secretase (BACE1) proteins and activity in vivo in the rat retina. Exp Brain Res. 2007;181:435–446. doi: 10.1007/s00221-007-0943-y. [DOI] [PubMed] [Google Scholar]
- Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Sanders E, Wang JJ. Endoplasmic reticulum stress and inflammation: mechanisms and implications in diabetic retinopathy. J Ocul Biol Dis Infor. 2011;4:51–61. doi: 10.1007/s12177-011-9075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng HKE. The amyloid precursor protein: beyond amyloid. Mol Neurodegener. 2006;1:5. doi: 10.1186/1750-1326-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]