Abstract
Heat shock proteins (Hsp) are highly conserved immunomodulatory molecules upregulated when cells are exposed to stressful stimuli, such as inflammation. Their involvement in various autoimmune diseases, including autoimmune bullous diseases and celiac disease, has been increasingly recognized. To further study the role of Hsp in autoimmune bullous diseases, we have investigated for the first time the humoral autoimmune response to Hsp40, Hsp60, Hsp70, and Hsp90 in patients with dermatitis herpetiformis (DH; n = 26), bullous pemphigoid (BP; n = 23), and pemphigus vulgaris (PV; n = 16), the first representing a cutaneous manifestation of celiac disease. While in patients with active BP and PV, serum levels of autoantibodies against these Hsp did not differ from the corresponding age- and gender-matched healthy controls (n = 9–14); circulating autoantibodies against Hsp60, Hsp70, and Hsp90 were found to be increased at the active disease stage of DH. Further analysis of this latter patient subgroup showed that these anti-Hsp autoantibodies decreased in parallel with serum autoantibodies against epidermal and tissue transglutaminase during remission of skin lesions following a gluten-free diet, revealing significantly positive correlations. Although further studies on larger groups of patients will be needed to confirm the present data, our results support the notion that autoantibodies against Hsp60, Hsp70, and Hsp90 deserve attention in the study of the mechanisms that promote the development and maintenance of DH and possibly also the underlying celiac disease as well as potential novel disease biomarkers.
Keywords: Autoantibody, Autoimmune bullous disease, Heat shock protein
Introduction
Heat shock proteins (Hsp) are cell stress- (e.g., inflammation) inducible molecules that are highly conserved among prokaryotes and eukaryotes. Hsp, for which an updated nomenclature has recently been proposed (Kampinga et al. 2009), are categorized into several families that are named on the basis of their molecular weight. As mainly intracellular proteins, they are essential for protein folding, transport within the cell, and structural maturation and conformational regulation of a number of signaling molecules as well as transcription factors, including those that are involved in synthesis of proinflammatory mediators (Li and Srivastava 2004). In addition, Hsp can act as potent activators of the immune system outside of cells, where they can also induce various proinflammatory cytokines, interact with (auto-) antigenic polypeptides and assist in (auto-) antigen presentation (Pockley et al. 2008). Therefore, Hsp, especially Hsp60 and Hsp90, have been implicated in the induction and propagation of inflammation and autoimmunity in several diseases, including atherosclerosis (Grundtman et al. 2011), rheumatic diseases (Huang et al. 2010; Shukla and Pitha 2012), and inflammatory bowel diseases (Rodolico et al. 2010; Tomasello et al. 2011b). On the other hand, some Hsp, especially Hsp40 and Hsp70, but also Hsp60 and endoplasmic reticulum-derived Hsp90, have the potential to induce a protective anti-inflammatory immune response (Pockley et al. 2008; Tukaj et al. 2010; Stocki and Dickinson 2012). The basis for the dichotomous properties of Hsp remains largely unknown, but is probably related to the context in which they are encountered by the complex cellular immune response network (Pockley et al. 2008). Regardless of whether Hsp exhibit a promotive or rather regulatory immune function, autoantibodies against these chaperones, including Hsp40, Hsp60, Hsp70, and Hsp90, have been found to be elevated in sera of patients with various inflammatory and autoimmune diseases compared to healthy controls (Stevens et al. 1992; Huang et al. 2010; Tukaj et al. 2010; Yokota and Fujii 2010; Grundtman et al. 2011; Shukla and Pitha 2012), implicating that they may play some pathophysiological role.
Recent results from our laboratory suggest that Hsp90 plays a pathogenetic role also in some autoimmune bullous diseases, which are generally characterized by cutaneous and/or mucosal blistering and autoantibodies against either desmosomal (pemphigus) or hemidesmosomal (pemphigoid and epidermolysis bullosa acquisita) components of the skin or against epidermal/tissue transglutaminase (eTG/tTG) (dermatitis herpetiformis (DH)) (Schmidt and Zillikens 2011). We recently showed that Hsp90 is overexpressed in both the skin of patients with bullous pemphigoid (BP) and BP serum-treated human keratinocyte (HaCaT) cells and that this stress protein is an effective treatment target in an experimental mouse model of epidermolysis bullosa acquisita (Kasperkiewicz et al. 2011; Tukaj et al. 2013). However, autoantibodies against this and other chaperones in serum of patients with autoimmune bullous diseases have so far not been explored. As Hsp have been implicated in intestinal pathology of celiac disease (CD) patients (Partanen et al. 1993; Iltanen et al. 1999; Ramos-Arroyo et al. 2001; Zanoni et al. 2006; Sziksz et al. 2010), the investigation of an anti-Hsp response in DH, which is induced by an underlying latent gluten-sensitive enteropathy in most of the patients (Kárpáti 2012), is of particular interest.
Therefore, the goal of our study was to screen circulating autoantibodies to Hsp40, Hsp60, Hsp70, and Hsp90 in a cohort of patients with DH, BP, and pemphigus vulgaris (PV), as well as in age- and gender-matched healthy controls.
Materials and methods
Patients
Sera from 26 patients with active DH (mean age 34.5 ± 15.8 years, 11 females, 15 males) and elevated circulating anti-eTG autoantibodies (mean 81.8 ± 87.2 U/ml, range 12.7 to 428.5 U/ml) and anti-tTG autoantibodies (mean 108.8 ± 103.5 U/ml, range 5.8 to 904.5 U/ml), 23 patients with active BP (mean age 79.4 ± 9.2 years, 14 females, 9 males) and elevated circulating anti-BP180 NC16A autoantibodies (mean 3,375.3 ± 4,226 U/ml, range 28 to 10,000 U/ml), 16 patients with active PV (mean age 55.8 ± 11.5 years, 8 females, 8 males) and elevated circulating anti-desmoglein 3 (Dsg3) autoantibodies (mean 565.4 ± 839.8 U/ml, range 44 to 2,297 U/ml) and anti-desmoglein 1 (Dsg1) autoantibodies (mean 1,090.2 ± 1,179.3 U/ml, range 188 to 3,224 U/ml), and the respective age- and gender-matched healthy control volunteers (n = 14, mean age 33.9 ± 9 years, 7 females, 7 males; n = 14, mean age 80.4 ± 7.8 years, 10 females, 4 males; n = 9, mean age 56.1 ± 10.4 years, 5 females, 4 males) were included into this study. In addition, another set of sera was analyzed from 11 of the 26 DH patients, from whom follow-up data was available, after a mean follow-up period of 7.8 ± 4.0 years, during which they received gluten-free diet leading to a decline in circulating anti-eTG and anti-tTG autoantibodies and complete remission of skin lesions. Three DH patients had a concomitant autoimmune disease (two with anti-thyroid peroxidase autoantibody-positive thyroid disease and one with type 1 diabetes mellitus). The investigations were conducted under approval from the ethics committees of the University of Lübeck, the Semmelweis University, and the University of Gdańsk, Poland and with written informed consent.
Autoantibody levels
Circulating IgG autoantibodies to BP180 NC16A, Dsg3, and Dsg1 as well as circulating IgA autoantibodies to eTG/tTG were detected by enzyme-linked immunosorbent assay (ELISA, Euroimmun) according to the manufacturer’s instructions. Serum IgG and, in case of DH sera, also IgA autoantibodies directed against human Hsp40 (HDJ2, Abcam), human Hsp60 (Stressgen), human Hsp70 (Stressgen), and human Hsp90 (Stressgen) were evaluated by homemade ELISAs as described previously with minor modification (Tukaj et al. 2010). Briefly, 96-well immunoplates (MaxiSorp) were coated with 50 μl Hsp at a concentration of 0.5 μg ml−1 in bicarbonate buffer (0.1 M) at 4 °C for 18 h. Wells were blocked with 100 μl 1 % bovine serum albumin (BSA) in phosphate-buffered saline (PBS) at room temperature for 2 h. After being washed three times with 300 μl PBS + 0.05 % Tween 20, 100 μl sera diluted 1:100 in PBS + 0.1 % BSA were incubated at room temperature for 2 h. After washing, plates were incubated with horseradish peroxidase(HRP)-conjugated anti-human IgG (Sigma) or anti-human IgA (Dako) specific secondary antibodies each diluted 1:5.000 in PBS containing 0.1 % BSA at room temperature for 1 h. TMB substrates were used to visualize HRP enzymatic reaction. The reaction was stopped by the addition of 0.5 M H2SO4. The optical density was measured at 450 nm using an ELISA plate reader.
Statistical analysis
Data was analyzed using Mann–Whitney U test for unpaired samples, Wilcoxon signed-rank test for related samples, and Spearman’s rank or Pearson’s correlation test. P values less than 0.05 were considered significant.
Results
Serum levels of anti-Hsp60, anti-Hsp70, and anti-Hsp90 autoantibodies are increased in patients with active DH
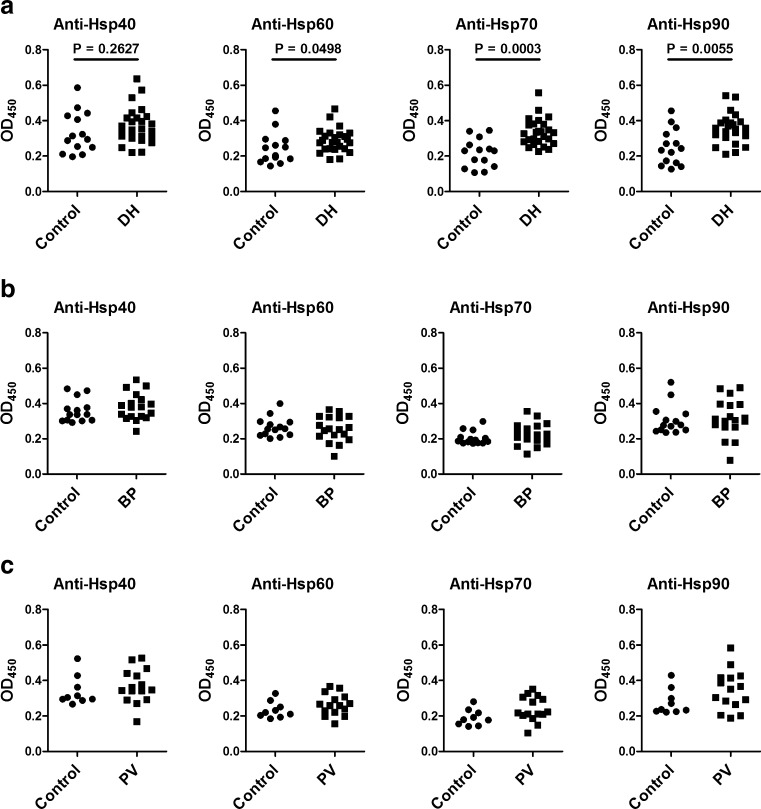
In comparison with sera from age- and gender-matched healthy individuals, sera from patients with active DH contained significantly higher levels of circulating IgG autoantibodies (but not IgA autoantibodies; data not shown) against Hsp60, Hsp70, and Hsp90 (Fig. 1a), whereas levels of circulating IgG autoantibodies directed against Hsp40, Hsp60, Hsp70, and Hsp90 were similar in patients with active BP (Fig. 1b) and PV (Fig. 1c) as measured by ELISA.
Fig. 1.
Levels of anti-Hsp40, anti-Hsp60, anti-Hsp70, and anti-Hsp90 IgG autoantibodies in sera of patients with active a dermatitis herpetiformis (DH) (n = 26), b bullous pemphigoid (BP) (n = 23), and c pemphigus vulgaris (PV) (n = 16) as well as of age- and gender-matched healthy controls (a–c) (n = 14, n = 14, and n = 9, respectively), measured by enzyme-linked immunosorbent assay
Circulating anti-Hsp60, anti-Hsp70, and anti-Hsp90 autoantibodies are decreased after therapy and positively correlated with the disease status and serum anti-eTG/tTG autoantibodies in DH patients
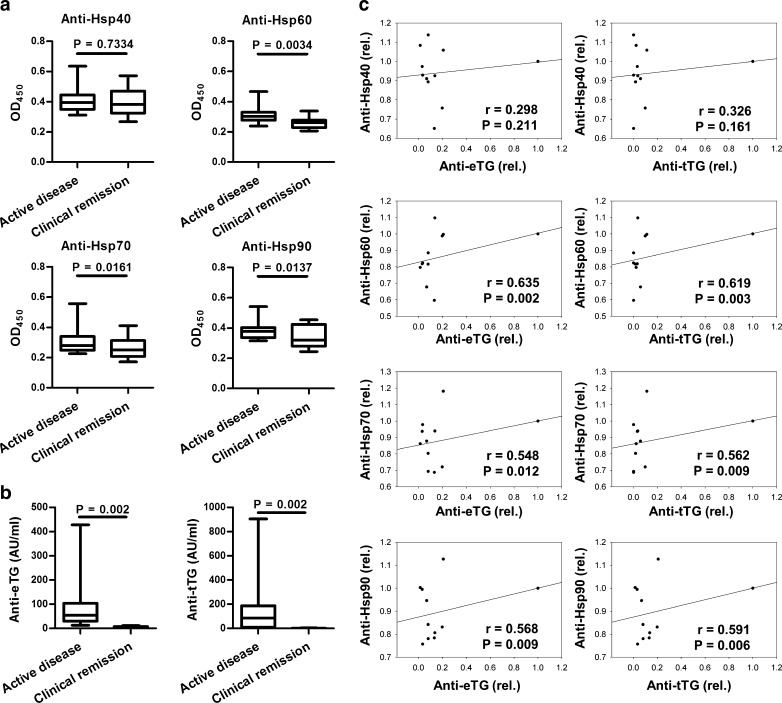
Considering the above mentioned results, further analysis focused on the DH patient group and revealed that serum levels of anti-Hsp60, anti-Hsp70, and anti-Hsp90 IgG autoantibodies (but not IgA autoantibodies; data not shown) decreased significantly in parallel with healing of skin lesions after a mean follow-up period of 7.8 years, during which the patients received a gluten-free diet (Fig. 2a). In addition, a concomitant significant fall in circulating anti-eTG/tTG IgA autoantibodies was observed in these DH patients (Fig. 2b). A statistically significant positive relationship was found between the serum levels of anti-Hsp60, anti-Hsp70, and anti-Hsp90 IgG autoantibodies and the status of skin disease in the patients (r = 642, P = 0.004; r = 746, P < 0.001; r = 739, P < 0.001, respectively) as well as their levels of circulating anti-eTG/tTG IgA autoantibodies (Fig. 2c).
Fig. 2.
Box plots of a serum levels of anti-Hsp40, anti-Hsp60, anti-Hsp70, and anti-Hsp90 IgG autoantibodies and b anti-eTG and anti-tTG IgA autoantibodies at time of cutaneous symptoms and during remission of skin lesions in dermatitis herpetiformis patients (n = 11) after a mean period of 7.8 years of therapy with gluten-free diet, measured by enzyme-linked immunosorbent assay. Box plots show the median (center horizontal line), interquartile range (the 25th to the 75th percentile (box)), and the 5th and 95th percentiles (whiskers). c Analysis of a relationship between levels of circulating anti-Hsp40, anti-Hsp60, anti-Hsp70, and anti-Hsp90 IgG autoantibodies and serum levels of anti-eTG and anti-tTG IgA autoantibodies during follow-up of these dermatitis herpetiformis patients
Discussion
In this study, we investigated for the first time the humoral autoimmune response directed to Hsp in sera of patients with autoimmune bullous diseases using recombinant human Hsp40, Hsp60, Hsp70, and Hsp90.
It has previously been reported by several independent groups that patients with inflammatory and autoimmune diseases, such as atherosclerosis, rheumatoid arthritis, systemic lupus erythematosus, Crohn’s disease, and ulcerative colitis, exhibit a pronounced humoral autoimmune reactivity against these immunomodulatory chaperones when compared with healthy controls (Stevens et al. 1992; Huang et al. 2010; Tukaj et al. 2010; Yokota and Fujii 2010; Grundtman et al. 2011; Shukla and Pitha 2012). Similarly, significantly higher levels of circulating autoantibodies against Hsp60, Hsp70, and Hsp90 but not Hsp40 were found in our patients with active DH compared with healthy subjects. A more detailed analysis of this patient group further revealed that these autoantibodies decreased significantly (except anti-Hsp40 autoantibodies) by the time the patients, who were treated by a gluten-free diet, showed a complete remission of skin symptoms. In addition to the observed significant positive correlation between autoantibodies against Hsp60, Hsp70, and Hsp90 and the cutaneous disease activity of DH patients, a significant positive relationship was found between the humoral autoimmune response towards these Hsp and levels of circulating autoantibodies against eTG and tTG, which also decreased significantly during follow-up of our patients. Autoantibodies against eTG are believed to play a central role in the pathogenesis and maintenance of the cutaneous disease in patients with DH (Sárdy et al. 2002; Rose et al., 2009), while autoantibodies to tTG are known to reflect the extent of histopathologic changes of the small bowel and to decrease under a gluten-free diet (Caproni et al. 2001; Tursi et al. 2003).
In contrast, our study showed no elevation of serum levels of autoantibodies against all investigated Hsp in patients with either active BP or PV compared with healthy individuals. In this context, it is worth noting that in both of these patient cohorts, the expression of Hsp90 has been recently investigated by our research group. In this previous study, a high intracellular expression of Hsp90 was found in keratinocytes and peripheral blood mononuclear cells from patients with active BP. However, only a low or similar expression of secreted (circulating) Hsp90 was observed in these and PV patients compared with healthy controls, respectively (Tukaj et al. 2013). At least for autoantibodies with specificity for Hsp90, this latter finding might therefore at least in part explain the lack of an increased humoral autoimmune response to this extracellular chaperone in these subgroups of autoimmune bullous disease patients, although the limited sample size does not yet allow definitive conclusions.
Another reason for why the immune response towards these Hsp differs between patients with DH and the other investigated autoimmune bullous diseases with respect to healthy controls may be that DH is regularly associated with latent CD (Kárpáti 2012), which itself may lead to potent Hsp induction due to inflammatory processes in the gut. In fact, similar to mucosal expression of a wide range of proinflammatory Hsp which correlated with disease activity in patients with inflammatory bowel disease (Tomasello et al. 2011a; Tomasello et al. 2011b), increased levels of immune response promoting jejunal Hsp65 and duodenal Hsp72 as well as polymorphisms in the Hsp70 gene have been previously reported in CD patients (Partanen et al. 1993; Iltanen et al. 1999; Ramos-Arroyo et al. 2001; Zanoni et al. 2006; Sziksz et al. 2010). Increased anti-Hsp serum autoantibody levels in our cohort of DH patients could likely occur as the result of Hsp release from injured gut epithelium as it has been previously described in Crohn’s disease and ulcerative colitis (Stevens et al. 1992). Yet it is unclear, however, whether this exaggerated autoimmune response towards Hsp is primarily related to skin inflammation or underlying CD in DH patients. Further studies are needed to clarify the origin of the observed autoimmunity towards Hsp.
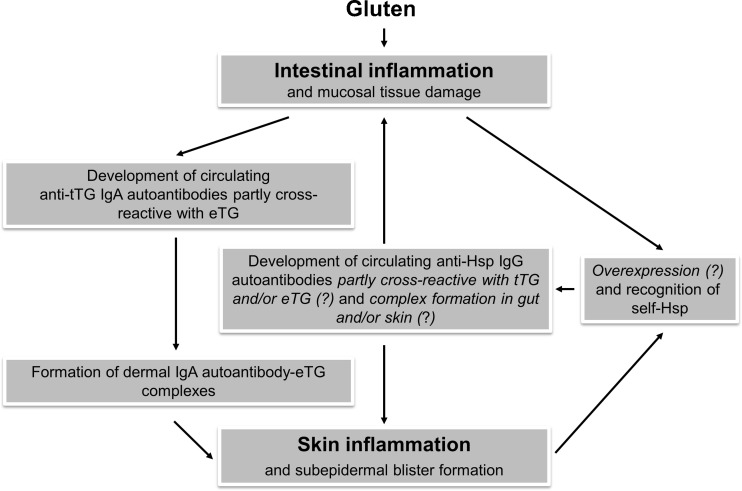
Another question that remains open is whether these anti-Hsp autoantibodies play a true pathophysiological role or just represent an epiphenomenon in DH or associated CD. However, several pieces of evidence suggest that, by cross-linking of Hsp and probably their receptors, these autoantibodies may enhance intracellular Hsp signaling leading to an intensified proinflammatory cytokine production and facilitated autoantigen presentation (Yokota et al. 2006; Yokota and Fujii 2010). Moreover, it was reported that in patients with active CD, a subset of autoantibodies against tTG also recognizes self-Hsp60. These Hsp60 cross-reactive autoantibodies, which disappeared following gluten-free diet, proved to be pathogenetically relevant for their ability to alter the intestinal barrier integrity and to activate monocytes, thus amplifying the damage of the intestinal mucosa with increased intestinal permeability (Zanoni et al. 2006). In addition, it should be noted that several other autoimmune diseases exist in which high structural similarity between human Hsp, especially Hsp60, and other human molecules (e.g., thyroglobulin/thyroid peroxidase and glutamic acid decarboxylase in Hashimoto’s thyroiditis and type 1 diabetes mellitus, respectively) has been described (Cappello et al. 2014). As some of our DH patients had a concurrent autoimmune thyroid disease or diabetes mellitus and association of DH and CD with these and other autoimmune disorders is a well-documented finding (Kárpáti 2012), it may be speculated that this type of cross-reactivity was also involved in generating autoimmunity to those concomitant diseases. In contrast, however, the observed decline in anti-Hsp autoimmune response after a strict gluten-free diet, representing the mainstay of treatment in DH and CD (Kárpáti 2012), favors a rather skin- or gut-specific role of these Hsp antibodies in DH patients. It is also worth mentioning in this context that tTG ELISA positivity has been reported in patients with autoimmune diseases independent of gluten-sensitive disease, which was explained by possible impurities in recombinant human tTG used such as copurification of chaperones (Sárdy et al. 2007). Although the true contribution of Hsp autoantibodies to the pathophysiology of DH is currently unknown, their possible involvement in this disease is proposed in Fig. 3.
Fig. 3.
Putative pathophysiologic role of IgG autoantibodies to Hsp 60, 70, and 90 in patients with dermatitis herpetiformis. Anti-Hsp autoantibodies partly cross-reactive with tTG and/or eTG may originate from inflamed intestinal tissue in the context of underlying celiac disease, the pathologic skin condition itself or both. Resulting immune complexes formed in the skin and/or gut may amplify and perpetuate inflammatory processes (e.g., by augmented proinflammatory cytokine production and costimulatory MHC-II expression via enhanced Hsp (receptor) cross-linking-mediated intracellular signaling events and/or by mimicking pathogenic activity of anti-tTG/eTG autoantibodies in terms of cross-reactivity) known to occur in dermatitis herpetiformis patients
In summary, while the humoral response towards self-Hsp was comparable between healthy subjects and patients with active BP or PV, levels of circulating autoantibodies against Hsp60, Hsp70, and Hsp90 were elevated and positively correlated with both cutaneous disease activity and serum anti-eTG/tTG autoantibody levels in patients with DH. Although further studies on larger groups of patients will be needed to confirm the present data, our results support the notion that these anti-Hsp autoantibodies deserve attention in the study of the mechanisms that promote the development and maintenance of DH and possibly also the underlying CD as well as potential novel disease biomarkers.
Acknowledgments
We would like to thank Prof. P. Trzonkowski (Medical University of Gdańsk) for providing us with sera from healthy control individuals. This work was supported by Deutsche Forschungsgemeinschaft (DFG) Excellence Cluster “Inflammation at Interfaces” (EXC 306/2), DFG KA 3438/1-1, Medical Faculty of the University of Lübeck (E22-2013), and Focus Program “Autoimmunity” at the University of Lübeck.
Footnotes
Michael Kasperkiewicz and Stefan Tukaj contributed equally in this work.
References
- Cappello F, Marino Gammazza A, Palumbo Piccionello A, Campanella C, Pace A, Conway de Macario E, Macario AJ. Hsp60 chaperonopathies and chaperonotherapy: targets and agents. Expert Opin Ther Targets. 2014;18:185–208. doi: 10.1517/14728222.2014.856417. [DOI] [PubMed] [Google Scholar]
- Caproni M, Cardinali C, Renzi D, Calabrò A, Fabbri P. Tissue transglutaminase antibody assessment in dermatitis herpetiformis. Br J Dermatol. 2001;144:196–197. doi: 10.1111/j.1365-2133.2001.03981.x. [DOI] [PubMed] [Google Scholar]
- Grundtman C, Kreutmayer SB, Almanzar G, Wick MC, Wick G. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:960–968. doi: 10.1161/ATVBAHA.110.217877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MN, Yu H, Moudgil KD. The involvement of heat-shock proteins in the pathogenesis of autoimmune arthritis: a critical appraisal. Semin Arthritis Rheum. 2010;40:164–175. doi: 10.1016/j.semarthrit.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iltanen S, Rantala I, Laippala P, Holm K, Partanen J, Maki M. Expression of HSP-65 in jejunal epithelial cells in patients clinically suspected of coeliac disease. Autoimmunity. 1999;31:125–132. doi: 10.3109/08916939908994056. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kárpáti S. Dermatitis herpetiformis. Clin Dermatol. 2012;30:56–59. doi: 10.1016/j.clindermatol.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Kasperkiewicz M, Müller R, Manz R, Magens M, Hammers CM, Somlai C, Westermann J, Schmidt E, Zillikens D, Ludwig RJ, Orosz A. Heat-shock protein 90 inhibition in autoimmunity to type VII collagen: evidence that nonmalignant plasma cells are not therapeutic targets. Blood. 2011;117:6135–6142. doi: 10.1182/blood-2010-10-314609. [DOI] [PubMed] [Google Scholar]
- Li Z, Srivastava P (2004) Heat-shock proteins. Curr Protoc Immunol Appendix 1:Appendix 1 T. doi:10.1002/0471142735.ima01ts58 [DOI] [PubMed]
- Partanen J, Milner C, Campbell RD, Mäki M, Lipsanen V, Koskimies S. HLA-linked heat-shock protein 70 (HSP70-2) gene polymorphism and celiac disease. Tissue Antigens. 1993;41:15–19. doi: 10.1111/j.1399-0039.1993.tb01971.x. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008;33:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Ramos-Arroyo MA, Feijoó E, Sánchez-Valverde F, Aranburu E, Irisarri N, Olivera JE, Valiente A. Heat-shock protein 70–1 and HLA class II gene polymorphisms associated with celiac disease susceptibility in Navarra (Spain) Hum Immunol. 2001;62:821–825. doi: 10.1016/S0198-8859(01)00277-4. [DOI] [PubMed] [Google Scholar]
- Rodolico V, Tomasello G, Zerilli M, Martorana A, Pitruzzella A, Gammazza AM, David S, Zummo G, Damiani P, Accomando S, Conway de Macario E, Macario AJ, Cappello F. Hsp60 and Hsp10 increase in colon mucosa of Crohn’s disease and ulcerative colitis. Cell Stress Chaperones. 2010;15:877–884. doi: 10.1007/s12192-010-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose C, Armbruster FP, Ruppert J, Igl BW, Zillikens D, Shimanovich I. Autoantibodies against epidermal transglutaminase are a sensitive diagnostic marker in patients with dermatitis herpetiformis on a normal or gluten-free diet. J Am Acad Dermatol. 2009;61:39–43. doi: 10.1016/j.jaad.2008.12.037. [DOI] [PubMed] [Google Scholar]
- Sárdy M, Kárpáti S, Merkl B, Paulsson M, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med. 2002;195:747–757. doi: 10.1084/jem.20011299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sárdy M, Csikós M, Geisen C, Preisz K, Kornseé Z, Tomsits E, Töx U, Hunzelmann N, Wieslander J, Kárpáti S, Paulsson M, Smyth N. Tissue transglutaminase ELISA positivity in autoimmune disease independent of gluten-sensitive disease. Clin Chim Acta. 2007;376:126–135. doi: 10.1016/j.cca.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Schmidt E, Zillikens D. The diagnosis and treatment of autoimmune blistering skin diseases. Dtsch Arztebl Int. 2011;108:399–405. doi: 10.3238/arztebl.2011.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla HD, Pitha PM. Role of hsp90 in systemic lupus erythematosus and its clinical relevance. Autoimmune Dis. 2012;2012:728605. doi: 10.1155/2012/728605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens TR, Winrow VR, Blake DR, Rampton DS. Circulating antibodies to heat-shock protein 60 in Crohn’s disease and ulcerative colitis. Clin Exp Immunol. 1992;90:271–274. doi: 10.1111/j.1365-2249.1992.tb07941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocki P, Dickinson AM. The immunosuppressive activity of heat shock protein 70. Autoimmune Dis. 2012;2012:617213. doi: 10.1155/2012/617213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sziksz E, Veres G, Vannay A, Prókai A, Gál K, Onody A, Korponay-Szabó IR, Reusz G, Szabó A, Tulassay T, Arató A, Szebeni B. Increased heat shock protein 72 expression in celiac disease. J Pediatr Gastroenterol Nutr. 2010;51:573–578. doi: 10.1097/MPG.0b013e3181ea0092. [DOI] [PubMed] [Google Scholar]
- Tomasello G, Rodolico V, Zerilli M, Martorana A, Bucchieri F, Pitruzzella A, Marino Gammazza A, David S, Rappa F, Zummo G, Damiani P, Accomando S, Rizzo M, de Macario EC, Macario AJ, Cappello F. Changes in immunohistochemical levels and subcellular localization after therapy and correlation and colocalization with CD68 suggest a pathogenetic role of Hsp60 in ulcerative colitis. Appl Immunohistochem Mol Morphol. 2011;19:552–561. doi: 10.1097/PAI.0b013e3182118e5f. [DOI] [PubMed] [Google Scholar]
- Tomasello G, Sciumé C, Rappa F, Rodolico V, Zerilli M, Martorana A, Cicero G, De Luca R, Damiani P, Accardo FM, Romeo M, Farina F, Bonaventura G, Modica G, Zummo G, Conway de Macario E, Macario AJ, Cappello F. Hsp10, Hsp70, and Hsp90 immunohistochemical levels change in ulcerative colitis after therapy. Eur J Histochem. 2011;55:e38. doi: 10.4081/ejh.2011.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukaj S, Kotlarz A, Jozwik A, Smolenska Z, Bryl E, Witkowski JM, Lipinska B. Hsp40 proteins modulate humoral and cellular immune response in rheumatoid arthritis patients. Cell Stress Chaperones. 2010;15:555–566. doi: 10.1007/s12192-010-0168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukaj S, Kleszczyński K, Vafia K, Groth S, Meyersburg D, Trzonkowski P, Ludwig RJ, Zillikens D, Schmidt E, Fischer TW, Kasperkiewicz M. Aberrant expression and secretion of heat shock protein 90 in patients with bullous pemphigoid. PLoS One. 2013;8:e70496. doi: 10.1371/journal.pone.0070496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursi A, Brandimarte G, Giorgetti GM. Prevalence of antitissue transglutaminase antibodies in different degrees of intestinal damage in celiac disease. J Clin Gastroenterol. 2003;36:219–221. doi: 10.1097/00004836-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Yokota S, Fujii N. Immunomodulatory activity of extracellular heat shock proteins and their autoantibodies. Microbiol Immunol. 2010;54:299–307. doi: 10.1111/j.1348-0421.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- Yokota S, Minota S, Fujii N. Anti-HSP auto-antibodies enhance HSP-induced pro-inflammatory cytokine production in human monocytic cells via Toll-like receptors. Int Immunol. 2006;18:573–580. doi: 10.1093/intimm/dxh399. [DOI] [PubMed] [Google Scholar]
- Zanoni G, Navone R, Lunardi C, Tridente G, Bason C, Sivori S, Beri R, Dolcino M, Valletta E, Corrocher R, Puccetti A. In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. PLoS Med. 2006;3:e358. doi: 10.1371/journal.pmed.0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]