Abstract
The aim of this study was to investigate the potential protective effect of the Hsp70 protein in the cardiac dysfunction induced by doxorubicin (DOX) and the mechanisms of its action. For this purpose, we used both wild-type mice (F1/F1) and Hsp70-transgenic mice (Tg/Tg) overexpressing human HSP70. Both types were subjected to chronic DOX administration (3 mg/kg intraperitoneally every week for 10 weeks, with an interval from weeks 4 to 6). Primary cell cultures isolated from embryos of these mice were also studied. During DOX administration, the mortality rate as well as weight reduction were lower in Tg/Tg compared to F1/F1 mice (P < 0.05). In vivo cardiac function assessment by transthoracic echocardiography showed that the reduction in left ventricular systolic function observed after DOX administration was lower in Tg/Tg mice (P < 0.05). The study in primary embryonic cell lines showed that the apoptosis after incubation with DOX was reduced in cells overexpressing Hsp70 (Tg/Tg), while the apoptotic pathway that was activated by DOX administration involved activated protein factors such as p53, Bax, caspase-9, caspase-3, and PARP-1. In myocardial protein extracts from identical mice with DOX-induced heart failure, the particular activated apoptotic pathway was confirmed, while the presence of Hsp70 appeared to inhibit the apoptotic pathway upstream of the p53 activation. Our results, in this DOX-induced heart failure model, indicate that Hsp70 overexpression in Tg/Tg transgenic mice provides protection from myocardial damage via an Hsp70-block in p53 activation, thus reducing the subsequent apoptotic mechanism.
Keywords: Heart failure, Hsp70, Doxorubicin, Apoptosis, p53-apoptosis
Introduction
Heat shock proteins (Hsps) constitute a large family that is divided into six subfamilies based on molecular mass: 100–110, 90, 70, 60, 40, and 18–30 kDa. Heat shock protein 70 or Hsp70, used herein to denote HSP70A1A (Kampinga et al. 2009), is the major member of the 70 kDa family, and its expression is induced after exposure to physical and chemical factors or stresses (Hightower 1991). Hsp70 acts in cooperation with co-chaperone proteins to form molecular protein machines (Minami et al. 1996; Höhfeld et al. 2001; Bozidis et al. 2002), and its function, performed by its monomeric form (Benaroudj et al. 1996; Angelidis et al. 1999), has been implicated in various cellular pathways. At the molecular level, Hsp70 participates in cell processes such as protein folding (Beckmann et al. 1990), protein degradation Saliba et al. 2002), protein translocation (Chirico et al. 1988), and DNA repair both in the nucleus and the nucleoli (Kotoglou et al. 2009). At the cellular level, Hsp70 has been related to heat resistance, cell viability (Angelidis et al. 1991; Angelidis et al. 1996), and also apoptosis (Jaattela et al. 1998a,b; Damalas et al. 2011). Finally, Hsp70 has been linked to disease and pathological states such as neurodegenerative diseases (Cummings et al. 2001; Adachi et al. 2003), cancer (Scott and Frydman 2003; Mosser and Morimoto 2004), cardiovascular conditions (Plumier et al. 1995; Lysitsas et al 2007), spinal cord ischemia (Kyrou et al. 2012), and PTZ kindling (Ammon-Treiber et al. 2007).
A protective effect of Hsp70 to the myocardium in the setting of ischemia–reperfusion (Okubo et al. 2001; Liu et al. 2007) and atrial fibrillation (Brundel et al. 2006) has been demonstrated, while the role of Hsp70 in heart failure has not been entirely overlooked (Knowlton et al 1998; Willis and Patterson 2010). Inhibition of apoptosis and pro-inflammatory cytokines, repair of ion channels, restoration of redox balance, and nitric-oxide-induced protection have been reported as mechanisms via which Hsp70 may protect heart cells (Delogu et al. 2002). The Hsp70-transgenic mice that overexpress Hsp70 (Angelidis et al. 1996) have been used as a major model for the role of Hsp70 in diseases and pathological states (Adachi et al. 2003; Plumier et al. 1995; Ammon-Treiber et al. 2007). Our research team, in collaboration with Canadian scientists, has previously shown that Hsp70 plays an important role in protecting the myocardium from apoptosis after ischemia/reperfusion (Plumier et al. 1995).
Doxorubicin (DOX), a cytotoxic antibiotic of the anthracycline group, has long been used as a potent chemotherapeutic agent for the treatment of solid and hematopoietic tumors in humans. DOX cytotoxicity has been attributed to a variety of mechanisms such as oxygen reactive species generation, topoisomerase-II inhibition, DNA crosslinks, double-strand breaks, and the mismatch repair pathway (Skladanowski and Konopa 1994; Larson and Drummond 2001). The main side effect of DOX is a dose-dependent cardiotoxicity that may result in the development of cardiomyopathy and heart failure (Singal and Iliskovic 1998; Sawyer et al 2010; Octavia et al. 2012), hence limiting its use. The exact mechanisms that mediate cardiotoxicity induced by the chronic administration of DOX still remain largely unknown. Increased oxidative stress and inflammation with DOX administration, leading cardiomyocytes to apoptosis, appears to be an important mechanism (Nozaki et al. 2004; Wang et al. 2004; Sawyer et al. 2010). Previous work has demonstrated a potential interference of Hsp70 with the cytotoxicity of DOX (Abe et al. 1996; Karlseder et al. 1996; Ciocca et al. 2003), while the potential protective role of Hsp70 in chronic DOX cardiotoxicity has also been little investigated, mainly in cellular models (Ito et al. 1999; Demidenko et al. 2006).
In this study, we utilized the Hsp70-transgenic mouse animal model and the Hsp70-transgenic mouse embryonic fibroblast to investigate whether Hsp70 overexpression in transgenic mice might have a protective role in the DOX-induced heart failure. Additionally, we sought to assess the action mechanism of Hsp70 protein.
Material and methods
Cell culture, heat shock, and cell and tissue extracts
Primary embryonic cells were isolated and cultured from 13-day mouse embryos (wild-type mice and Hsp70-transgenic mice overexpressing the human heat shock protein 70, strain CBA-C57BL/6J; more details are provided below). These cells, named F1/F1 (cells derived from embryos of wild type mice) or Tg/Tg cells (cells, derived from embryos of the Hsp70-transgenic mice, overexpress the Hsp70 protein), were spontaneously immortalized during their repeated passaging, which is likely due to the additional copy of certain chromosomes (Williams et al. 2008).
Sub-confluent primary cells growing as monolayers in DMEM supplemented with 10 % fetal calf serum (Angelidis et al. 1996) were subjected or not to heat shock at 42.5 °C for 60 min followed by 90-min recovery at 37 °C, in order to assess the expression of Hsp70.
Control or heat-treated cells were harvested, washed with NaCl/Pi, resuspended in RIPA buffer, and lysates prepared as described previously (Bozidis et al. 2002). The lysates were mixed with sodium dodecyl phosphate (SDS) sample buffer to 1× final concentration, boiled for 3 min, and stored to −30 °C.
Cardiac tissue fragments from control and transgenic animals were used for protein extract selection as described previously (Williams et al. 2008). Fresh mouse tissue samples fractionated using the Thermo Scientific Subcellular Protein Fractionation Kit for Tissues (cat. no. 87790). Normalized loads of each extract (20 μg) were analyzed by Western blotting.
Antibodies
The antibodies used in our experiments were the following: mouse anti-Hsp70 (StressGen Biotechnologies, Victoria, Canada: SPA 810, a mouse monoclonal antibody that binds to human HSP70 A1A specifically), rabbit anti-PARP-1 (Santa Cruz Biotechnology, sc-7150), anti-Bax (Santa Cruz, sc-7480), anti-phospho-p53-[ser15] (Cell Signalling, no. 9284), anti-active caspase 3 polyclonal antibody (BD Pharmingen, cat. no. 559565), anti-cleaved-caspase-9 (Cell Signalling, no. 9509), anti-α-tubulin (Sigma, T5168), and Annexin V-fluorescein isothiocynate (FITC) (Annexin V-FITC, 556420, BD Pharmigen TM). The B23 (H-106: sc-5564, Santacruz) and β-actin (N-21: sc-13065, Santacruz) antibodies were used as markers for the nuclei and the cytoplasm, respectively.
Western blotting analysis
Protein extracts (20 μg/sample) from cells or tissues were analyzed by SDS-PAGE and subjected to Western blotting analysis using specific antibodies and the enhanced chemiluminescence’s method (PIERCE, SuperSignal West Pico, Chemiluminescent Substrate CA 47079).
Exposure of cells to DOX and flow cytometry to assess apoptosis
Mice F1/F1 and Tg/Tg cells were exposed to a range of DOX concentrations (0.1–25 μΜ) for 24 and 48 h. Previous studies have shown that DOX causes apoptosis of cardiomyocytes over a wide range of concentrations among 0.06–5 mM, while at concentrations higher than 10 mM, DOX induces necrosis rather than apoptosis (Kotamraju et al. 2000). The measurement of apoptosis was performed by flow cytometry and PI/Annexin V-FITC staining. Cells were washed twice with cold phosphate-buffered saline, collected and measured in a Neubauer hemocytometer. After measurement, cells were centrifuged and suspended in calcium buffer 1× at a rate 105 cells/100 μl. Then, cells were stained with 5 μl Annexin V-FITC (Annexin V-FITC, 556420, BD Pharmigen TM) and 5 μl of PI (propidium iodide solution, P4864, Sigma). The samples were incubated for 15 min at room temperature (25 °C) in the dark and then 1 ml of calcium buffer 1× was added in every sample. The cytometric analysis was performed in a Partec ML flow cytometer (CyFlow®ML, Partec, Munster, Germany), and the results were analyzed by Partec FloMax software. Apoptosis and necrosis were calculated over all viable cells and after subtracting the autofluorescence of DOX.
Experimental animals
Eighty-four male F1/F1 (WT: wild type) strain CBA-C57BL/6J and Tg/Tg (Hsp70-transgenic mice overexpressing the human Hsp70) (Angelidis et al. 1996) were studied at a mean age of 11 weeks, weighing 30–35 g at the beginning of the experiment. Genotypes were determined by PCR using genomic DNA isolated from tail biopsies as substrate. Ten nanograms DNA was used as template for a PCR reaction using two new primers that generate a 600-bp fragment: forward primer (5′- ttttatggtaataacgcgccggcccggcttcctttatccc-3′) and reverse primer (3′-tacgcctcggcgatctccttcatcttggtcagcaccatg-5′) (unpublished results). As internal marker of equal loading, two primers were used for the mouse 18S rRNA identification: the forward (5′- aggggagagagcgggtaagaga-3′) and the reverse (3′-ggacaggactaggcggaaca-5′) primer (F56 and R296 corresponding, Qiagen) that generate a fragment of 241 bp. The WT mice (F1/F1) and the homozygous Hsp70-transgenic mice (Tg/Tg) were selected and used in all experiments.
All animals were maintained in 21–22 °C and 50–60 % humidity, receiving commercial food and water in an inverted 12-h light/dark cycles. All experimental protocols were performed according to the rules of the European Community for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes. All conducted experiments were approved by the Veterinarian Services of the Prefecture of Ioannina in accordance with the Greek legislation for breeding and handling animals subject to the European Directives.
Chronic DOX administration
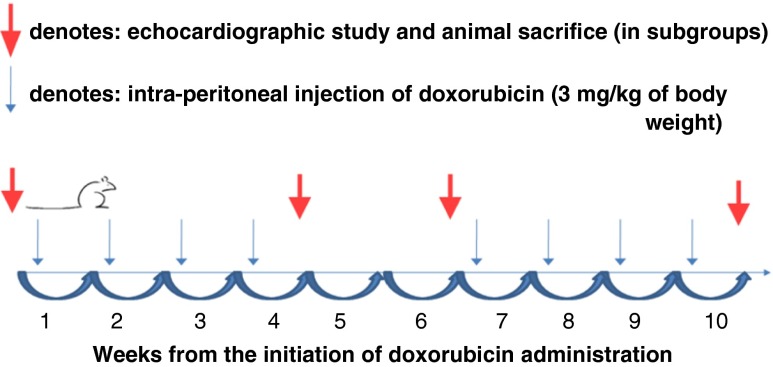
DOX was administered to both WT (n = 32) and transgenic (n = 29) mice in a chronic protocol as previously described (Nozaki et al. 2004). In brief, DOX was administered via an intraperitoneal injection at a dose of 3 mg/kg of body weight every week for a total of 10 weeks, with a 2-week interval after the first 4 weeks. The timeline of the study is shown in Fig. 1. At the beginning of the injection protocol, animals in each group were divided in prespecified subgroups so that they could be studied at three time-points, i.e., at 4, 6, and 10 weeks after DOX initiation: n = 8–13 in each subgroup of transgenic mice and n = 10–11 in each subgroup of WT mice. The mortality was examined in both WT and Tg/Tg groups during the 10 weeks of the experimental protocol of DOX administration. A control group of both WT (n = 12) and transgenic mice (n = 11) that did not have DOX injections was also included in the study to compare to animals that were subjected to chronic DOX administration. At the end of 4, 6, and 10 weeks (and 24 h after the last DOX injection), the animals at the corresponding subgroup were studied echocardiographically and then killed (Fig. 1). Animals in the control groups were also studied echocardiographically and then killed. Subsequently, their hearts were excised following in situ perfusion with ice-cold phosphate-buffered saline. Heart tissues were split in half, frozen in optimal cutting temperature (OCT) compound, freezing medium for cryosections and immunofluorescence, and immunobloting with apoptotic markers.
Fig. 1.
Diagram showing the timeline of the study
Echocardiography
Mice were anesthetized with ketamine (100–125 mg/kg intraperitoneally) and maintained on a heated platform in a left lateral decubitus position. Their chest was shaved, and prewarmed coupling gel was applied. Transthoracic echocardiography was performed using a GE Vividi ultrasonograph with an 11.5-MHz transducer (GE Healthcare, USA) for acquisition of two-dimensionally guided M-mode images of the left ventricle in the short axis at the level of the papillary muscles. All images were saved for offline analysis.
Anterior and posterior wall thickness at end-diastole and left ventricular (LV) diameters diameter at end-diastole and end-systole (LVDd and LVDs respectively) were measured from the M-mode images using leading edge-to-edge conventions as recommended by the American Society of Echocardiography. All parameters were measured over at least three consecutive cardiac cycles and averaged. LV fractional shortening (FS) was calculated as [(LVDd − LVDs)/LVDd] × 100] as an index of LV systolic function. Heart rate was determined from at least three consecutive intervals from the continuous wave Doppler tracings of the aortic valve. The same operator (KKN) obtained all images and measures and was blinded to the animal genotype as well as the duration of DOX administration.
Immunofluorescence staining of frozen heart tissue sections
Five-micrometer-thick F1/F1 and Tg/Tg heart tissue cryosections stored in OCT freezing medium were analyzed for the activation of p53 after injection with saline solution or doxorubicin by indirect immunofluorescence, following the directions of the manufacturer (Cell Signalling Technology). Briefly, tissues were washed in TBS, fixed in 4 % paraformaldehyde for 15 min, and permeabilized for 5 min in ice-cold absolute methanol. Then, the tissues were washed with TBS, blocked in 3 % bovine serum albumin to prevent nonspecific staining and incubated overnight with phospho-p53 (Ser15) polyclonal antibody (Cell Signaling no. 9284S) at a dilution of 1:400 in TBS. Then, tissues were washed and incubated with the appropriate secondary antibody (Alexa Fluor 568—Invitrogen no. A11036) at a dilution of 1: 100 followed by nuclear SYBR-Green I staining (Sigma, S-9340), mounted in coverslips with Vectashield, and observed in a confocal Leica microscope.
Statistical analysis
Data are expressed as mean ± SD unless otherwise stated. Comparisons among different time-points within the same group (WT or Tg/Tg) were performed using the one-way analysis of variance model (ANOVA) and Tukey’s or Dunn’s tests for post hoc comparisons. Comparisons between the two groups (F1/F1 vs. Tg/Tg) for any given time-point were made using unpaired t test. The chi-square test was used to compare mortality rates at each time-point between transgenic and control animals treated with DOX. A calculated P < 0.05 was considered to be statistically significant.
Results
Hsp70 confers antiapoptotic activity in embryonic primary cells of Hsp70-transgenic mice
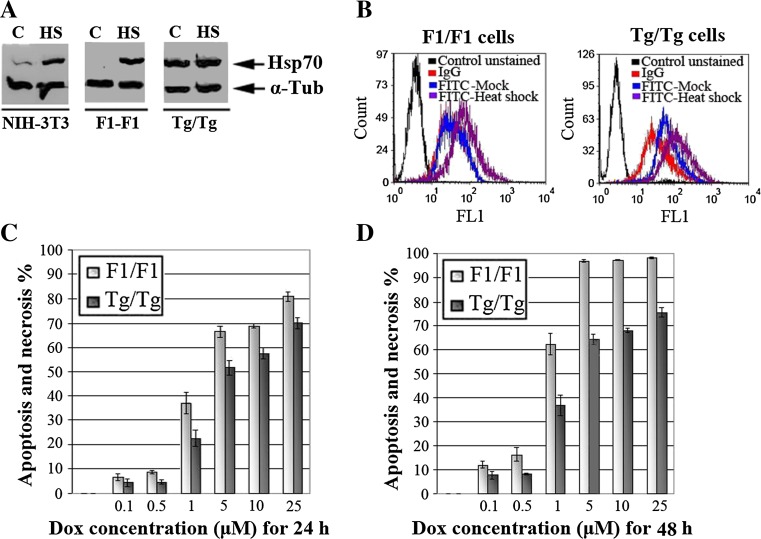
In primary embryonic cell lines (80 passages) isolated from mice embryos of Tg/Tg (Hsp70-transgenic animals) and F1/F1 (wild-type animals) mice, the Hsp70 overexpression was examined using Western blotting before and after heat shock at 43 °C for 90 min and recovery at 37 °C for 0 or 3 h. As shown in Fig. 2a, no detectable levels of Hsp70 were observed in control F1/F1 cells, while in the Tg/Tg cells, the Hsp70 expression was similar to that expressed in F1/F1 cells when exposed to heat shock (Fig. 2a). Similar results were obtained under the same conditions, using anti-Hsp70 antibody (StressGen SPA 810) and flow cytometry analysis (Fig. 2b). Therefore, the acquired Tg/Tg cells expressed Hsp70 continuously without exposure of cells to heat shock.
Fig. 2.
The Hsp70 overexpression is followed by decreased apoptotic activity in primary embryonic cells. a NIH-3T3, F1/F1 and Tg/Tg cells were exposed or not to heat shock (90 min at 43 °C and 90-min recovery at 37 °C) as indicated. Protein lysates (10 μg per sample) were analyzed to SDS-PAGE and subjected to Western blot using a MAb specific for the inducible Hsp70 (C92). bThe same primary embryonic cells were used for flow cytometric analysis of intracellular Hsp70, using the same anti-Hsp70 antibody as in a. Control unstained sample, IgG incubation only with secondary anti-mouse-FITC, FITC-Mock incubation with anti-Hsp70 and anti-mouse-FITC, FITC-heat shock incubation with anti-Hsp70 and anti-mouse-FITC, after 90-min heat shock at 42.5 °C and 3-h recovery to 37 °C. c, d The same cells were exposed to a range of doxorubicin (DOX) concentrations for 24 h (c) or 48 h (d) and analyzed for PI/Annexin V FITC by flow cytometry. In c and d, each point represents the mean ± SD of three measurements in three separate experiments. In all points, except for treatment with 0.1 μM of DOX (P = NS), P values were <0.01 and <0.005 for positive pI/Annexin V cells %: apoptosis and necrosis %, respectively
When the apoptosis, after incubation with DOX in concentrations (0.1, 0.5, 1, 5, and 25 μΜ) for 24 and 48 h, was studied using flow cytometry and PI/Annexin V FITC staining, it was found that the Tg/Tg cells conferred an increased antiapoptotic activity compared to that of F1/F1 cells (Fig. 2c, d). Our measurements with pI/Annexin V showed that the percentage of necrotic cells was too low and with no significant differences among doses and the time of doxorubicin treatment (range, 0–5 %). Thus, we concluded that apoptotic activity was responsible for the significant changes between F1/F1 and Tg/Tg cells after specific doses and time of incubation with DOX. The histograms of the percentage of apoptotic cells showed that control F1/F1 cells with no detectable amounts of Hsp70 presented an increased apoptotic activity compared to the Tg/Tg cells during 24 h (P < 0.01 at all points) or 48 h (P < 0.005 at all points) of DOX treatment, suggesting that the Hsp70 presence in Tg/Tg cells reduced apoptosis (Fig. 2c, d). These cells were initially used to investigate the apoptotic mechanism triggered by DOX, and then to determine whether the same mechanism is activated after chronic administration of DOX to mice.
The apoptotic pathway activated by DOX in mouse primary embryonic cells and the role of Hsp70
Given that DOX enters into the nucleus and exerts its effect creating DNA damages (Lee et al. 2004), the stimulation of mitochondrial apoptotic pathway that is triggered after DNA damage was examined. More specifically, the activation of all p53, Bax, caspase-9, caspase-3, and PARP-1 that are implicated on this pathway (Erster et al. 2004; Rich et al. 2000) and simultaneously the regulatory role of Hsp70 was investigated.
For this purpose, F1/F1 and Tg/Tg cells were exposed to DOX, and protein cell extracts were analyzed with SDS-PAGE/Western blotting/ECL system, using specific antibodies against Hsp70, PARP-1. phospho-p53-[ser15], Bax, cleaved-caspase-9, and active caspase-3.
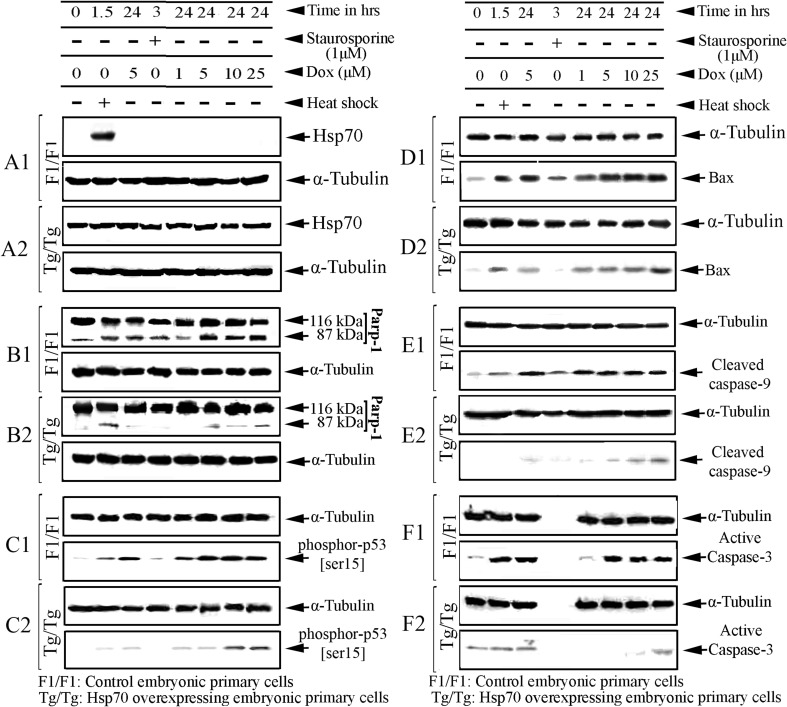
As may be seen in Fig. 3 (A1–A2), the DOX incubation of assay cells did not appear to significantly affect the expression of Hsp70 in any cell type. After exposure of assay cells to DOX (1–25 μM), Hsp70 was not detected in F1/F1 cells, whereas its expression remained almost constant in Tg/Tg cells (Fig. 3, A2).
Fig. 3.
Hsp70 blocks the doxorubicin-induced apoptotic cascade upstream of p53 phosphorylation. F1/F1 and Tg/Tg cells were exposed to 1, 5, 10, or 25 μM DOX for 24 h. Simultaneously, the same cells were exposed to heat shock (60 min at 42.5 °C and 90-min recovery at 37 °C) or staurosporine (3 h at 1 μM) that were used as markers of Hsp70 expression or as markers of apoptosis activation. Ten micrograms of each cellular fraction was analyzed by Western blot with anti-Hsp70 (A1, A2), anti-PARP-1 (B1, B2) anti-phospho-p53-[ser15] (C1, C2), anti-Bax (D1, D2), anti-cleaved caspase-9 (E1, E2), anti-active caspase-3 (F1, F2), and anti-tubulin antibodies as indicated
Comparing PARP-1 under the same conditions in assay cells, we observed that PARP-1 inactivation was disabled when the cells expressed Hsp70 (Fig. 3, B2) in agreement with previously published results (Kotoglou et al. 2009). These findings showed that Hsp70 appears to be involved in the regulation of DNA damage produced by DOX and detected by PARP-1.
Then, the p53 accumulation, as the first activated molecule during DOX-induced apoptotic pathway, was studied. Given that p53 is involved in the path under its phosphorylated form (Shieh et al. 1997), an antibody that recognizes the phosphorylated p53 form at ser15 was used. Thus, the increased accumulation of the p53-phosphorylated form indirectly indicates the promotion of the mitochondrial apoptotic pathway. As shown in Fig. 2, the levels of p53(ser15) was found to be reduced in cells that overexpress Hsp70. (Fig. 3, C2).
To characterize this apoptotic pathway in greater detail, the levels of Bax accumulation, caspase-9 and caspase-3 activation were studied under the same conditions of DOX exposure. As expected, the same phenomenon was observed when the accumulation of Bax (Fig. 3, D1–D2) and activation of caspase-9 (Fig. 3, E1–E2) and caspase-3 (Fig. 3F1-F2) were comparatively examined in assay cells. These results show that the presence of Hsp70 partially inhibits the activation and further promotion of p53-apoptotic pathway (Fig. 3).
Combined, these results indicate that DOX, by causing DNA damage to cells, activates the mitochondrial apoptotic pathway that passes via the p-53, Bax, caspase-9, caspase-3, and ends in the inactivation of the PARP-1. Hsp70 appears to inhibit the activation of this apoptotic pathway, and its action is located upstream of p53 activation.
Hsp70 protects mice from doxorubicin-induced heart failure
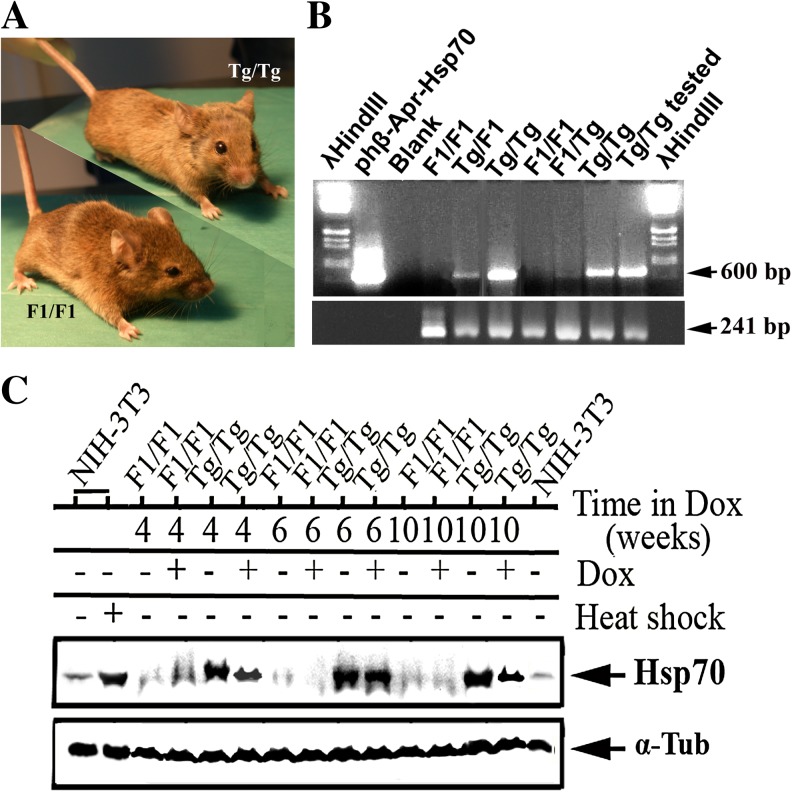
As aforementioned, 84 male F1/F1 and Tg/Tg mice were selected by PCR analysis (Fig. 4b) and were exposed or not to DOX. The expression of Hsp70 was monitored in heart tissue using Western blot and antibodies against Hsp70 and α-tubulin. As shown in Fig. 3c, Hsp70 was expressed highly only in Tg/Tg mice. A small increase in the amount of Hsp70 was observed in F1/F1 mice at 4 weeks of DOX treatment. The latter was either not evaluable or its accumulation was regulated by a mechanism at low levels (see “Discussion”). Paradoxically, a downregulation of Hsp70 was observed in Tg/Tg mice at 4, 6, and 10 weeks after DOX treatment (Fig. 4c).
Fig. 4.
The Hsp70 expression in F1/F1 and Tg/Tg mice during their exposure to DOX treatment. a Real picture of wild-type control (left) and Hsp70-transgenic mice (right). b Determination of Hsp70-transgenic mice genotype: PCR analysis of DNAs isolated from control (F1/F1 mice), heterozygous (F1/Tg mice), and homozygous (Tg/Tg mice) Hsp70-transgenic mice. The amplification of the 18S mouse rRNA was used as internal marker of equal loading (241 bp). c Cardiac tissue protein extracts (20 μg/sample) were analyzed in SDS-PAGE and subjected to Western blotting using specific antibodies. The α-tubulin was used as marker for equal sample loading
In the group of Tg/Tg mice that received DOX, the percentage of mice that died by week 4, 6, and 10 after the initiation of DOX administration (0, 0, and 15 %, respectively) was significantly lower compared to the group of F1/F1 mice (18, 30, and 45 %, respectively, P < 0.05 at all time-points) (Table 1, upper panel). The percentage of weight reduction in the transgenic mice that received DOX at week 4, 6, and 10 after the initiation of DOX administration compared to their weight before the initiation of DOX (reduction by 2.0, 5.0, and 6.0 %, respectively) was significantly lower compared to the F1/F1 group that received DOX (reduction of 5.5, 13.9, and 19.3 %, respectively, P < 0.02 at all time points between the two groups (Table 1, lower panel).
Table 1.
Mortality and weight reduction (percent changes compared to baseline body weight), following chronic DOX administration between F1/F1 and Tg/Tg mice
| 4 weeks | 6 weeks | 10 weeks | |
|---|---|---|---|
| Mortality | |||
| Mice | After DOX initiation | ||
| F1/F1 | (2/11) 18 % | (3/10) 30 % | (5/11) 45 % |
| Tg/Tg | (0/8) 0 % | (0/8) 0 % | (2/13) 15 % |
| P value | <0.001 | <0.005 | <0.05 |
| Weight reduction | |||
| Mice | After DOX initiation | ||
| F1/F1 | 5.5 % | 13.9 % | 19.3 % |
| Tg/Tg | 2.0 % | 5.0 % | 6.0 % |
| P value | <0.02 | <0.002 | <0.0002 |
The numbers in the parentheses indicate the number of animals that died (numerator) and the total number of animals that were used (denominator) in each experimental condition
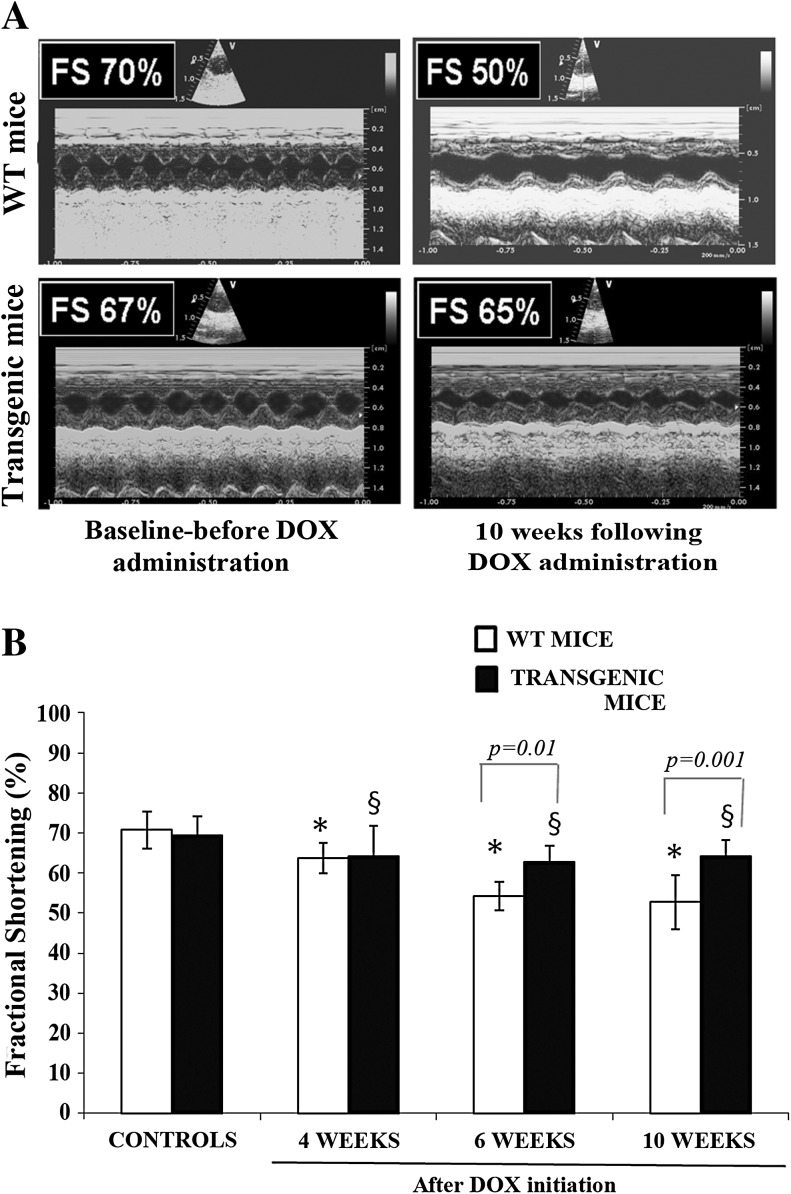
The echocardiographic measurements are shown in representative M-mode measurements (Fig. 5a). According to Fig. 5b, LV systolic function as assessed by the FS index did not differ between the two controls groups. FS was significantly reduced in both groups of animals that received DOX compared to their corresponding controls (P < 0.05 at 4, 6, and 10 weeks for both groups), but the reduction in LV systolic function was greater in the F1/F1 mice compared to the Tg/Tg mice (P < 0.05) (Fig. 5b). The difference in FS between the Tg/Tg and the F1/F1 mice reached statistical significance at 6 and 10 weeks after DOX initiation; FS at 10 weeks was reduced in WT animals from 70.8 ± 4.6 % to 54.2 ± 3.6 % and to 52.7 ± 6.8 % at 6 and 10 weeks, respectively (P < 0.0005 for both) and in Tg/Tg from 69.5 ± 4.7 % to 62.7 ± 4.8 % and to 64.2 ± 4.1 % (P < 0.05 for both) (P < 0.05 between the two groups) (Fig. 5b).
Fig. 5.
DOX-induced cardiac dysfunction is attenuated in Tg/Tg transgenic mice overexpressing Hsp70. a Echocardiographic M-mode images of murine hearts. Upper row wild-type animals, control that served as baseline (left) and 10 weeks after DOX initiation (right). Lower row transgenic mice overexpressing Hsp70, control that served as baseline (left), and 10 weeks after DOX initiation (right). FS represents fractional shortening, an index of left ventricular systolic function. b Fractional shortening, an index of systolic function in wild-type (WT) and transgenic mice overexpressing Hsp70. *P < 0.05 compared to control wild-type (WT) mice group (without DOX administration), § P < 0.05 compared to control transgenic mice group (without DOX administration)
Hsp70 attenuates the DOX-induced cardiac dysfunction in transgenic mice overexpressing Hsp70 via inhibition of apoptosis, acting upstream of p53 activation
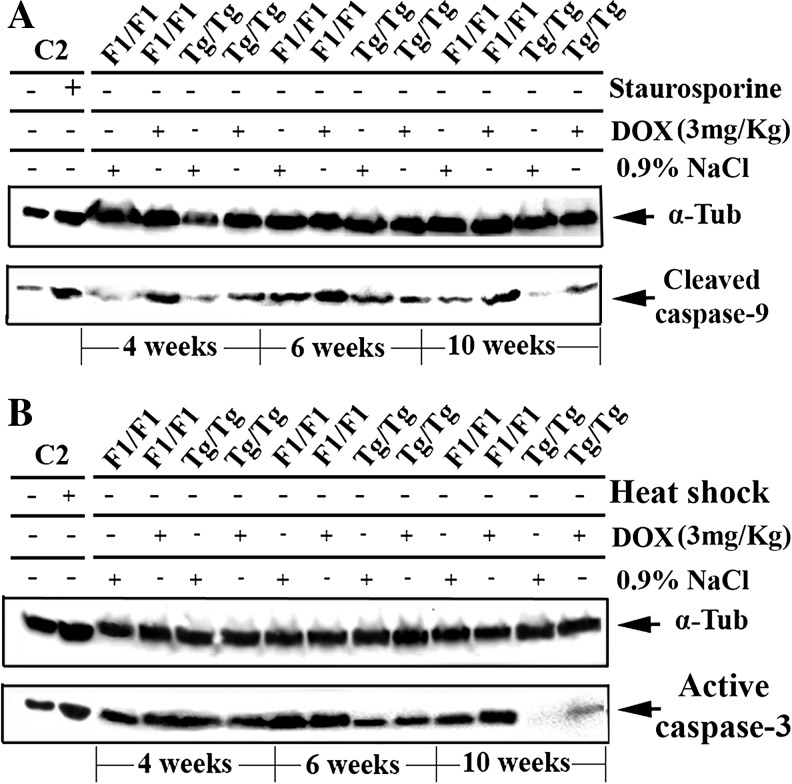
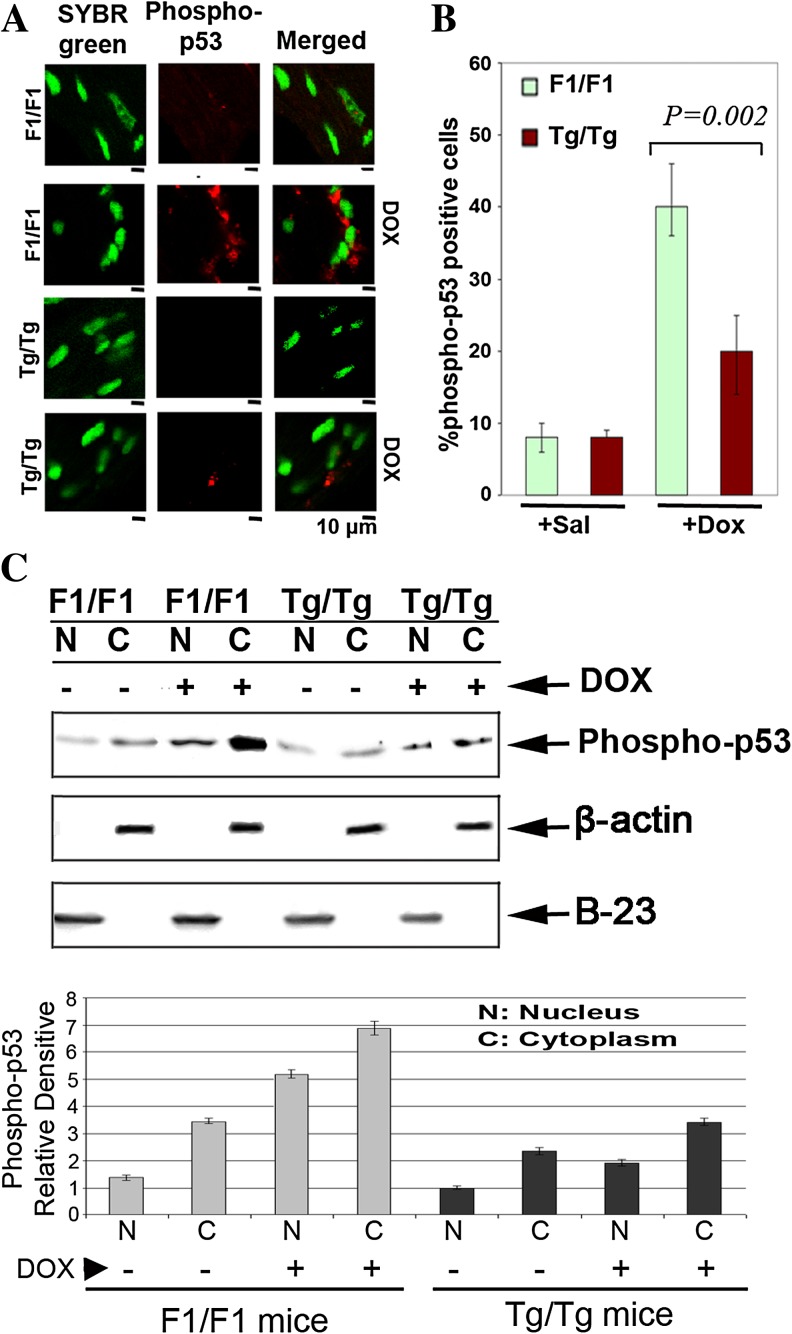
Having studied the apoptotic pathway that is activated by DOX in primary embryonic cells, the DOX-induced apoptotic pathway was examined also in cardiac tissues of experimental animals that were exposed to DOX (3 mg/kg) for 4, 6, or 10 weeks as indicated in Fig. 6. Myocardial protein extracts of the experimental animals analyzed by Western blotting using specific antibodies against caspase-9 (Fig. 6a) and caspase-3 (Fig. 6b). As observed, less procaspase-9 and procaspase-3 processing was observed following DOX treatment in the Tg/Tg cells compared to those of F1/F1 mice, indicating that the same apoptotic pathway, involving caspase-9/caspase-3, was activated after DOX exposure in both groups of mice. Thus, the point of Hsp70 action appears to be upstream of caspase-9 activation and possibly, as demonstrated in primary embryonic cells, upstream of p53 activation. In order to detect and compare the activation of p53, heart tissue sections of F1/F1 and Tg/Tg mice exposed to DOX were collected and immediately subjected to indirect immunofluorescence using antibodies specific for phosphorylated-p53. Confocal microscopy analysis showed that the phospho-p53 positive cells (red stained cells) (Fig. 7a) were significantly (P = 0.002) reduced in tissue slides derived from sections of Tg/Tg mice compared to F1/F1 mice after DOX administration (Fig. 7b). To further characterize the phospho-p53 cytoplasmic accumulation, cytoplasmic (C) and nuclear (N) protein extract from fresh myocardial tissues were analyzed in SDS-PAGE and subjected to Western blotting using specific antibodies against phosphor-p53-[ser15]. In agreement with the previous confocal studies, the phospho-p53 translocates to the cytoplasm at the period of 10 weeks of DOX administration. Quantification of phosphorylated-p53(ser15) was done using image J software (Fig. 7c, lower panel). This phenomenon was inhibited in myocardial protein extracts obtained from Tg/Tg mice (Fig. 7c).
Fig. 6.
Inhibition of caspace-9 and caspace-3 in cardiac tissues of Hsp70-transgenic mice. F1/F1 and Tg/Tg mice were exposed to DOX (3 mg/kg) for 4, 6, or 10 weeks. Cardiac tissue protein extract (20 μg/sample) obtained from F1/F1 and Tg/Tg were analyzed in SDS-PAGE and subjected to Western blotting using specific antibodies against cleaved caspase-9 (a) and active caspase-3 (b). The α-tubulin was used as marker for equal loading of samples. C2 cells were exposed to heat shock (60 min at 42.5 °C and 90 min recovery at 37°C) or staurosporine (3 h at 1 μM) to be used as markers of Hsp70 expression or as markers of apoptosis activation
Fig. 7.
Activation via phosphorylation of p53 in mouse myocardial tissue after doxorubicin administration. a Confocal microscopy analysis of phospho-p53 positive cells (red stained cells) derived from F1/F1 and Tg/Tg myocardial sections 10 weeks after DOX (3 mg/kg) initiation. Tissues were fixed for immunofluorescence staining of phosphorylated-p53, followed by co-staining with Alexa-Fluor 568 and SYBR Green I. b Percentages of p53 activation were determined by cell counting. Data are expressed as mean ± SD (n = 5), P = 0.002. c The same amounts of nuclear and cytoplasmic proteins derived from cells of fresh myocardial tissues of F1/F1 and Tg/Tg mice were analyzed by SDS-PAGE and in turn subjected to Western blotting using specific antibodies against the phospho-p53
These results indicate that the myocardiocytes undergoing apoptosis, after DOX exposure of F1/F1 mice, as indicated by the translocation of the p53-phosphorylated form to the cytoplasm (Fig. 7a), were unable to downregulate the promotion of p53-apoptotic pathway. In contrast, the presence of Hsp70 effectively inhibited the p53-apoptosis in Tg/Tg cardiomyocytes increasing thus their survival.
Discussion
The results of the current study in transgenic mice overexpressing Hsp70 indicate that Hsp70 may provide protection from the DOX-induced myocardial damage as assessed by the lower mortality, the preservation of body weight, and left ventricular systolic function in the transgenic mice compared to WT mice. Chronic DOX administration activates the mitochondrial apoptotic pathway that passes via the p-53/Bax-1/caspase-9/caspase-3. Hsp70 appears to inhibit the activation of this apoptotic pathway, and its action is located upstream of p53 activation.
Previous studies at the cellular level have demonstrated that Hsp70 may block several steps in the apoptotic cascade. Hsp70 has been shown to block apoptosis upstream of mitochondria by preventing Bax translocation (Stankiewicz et al. 2005), release of cytochrome C and apoptosis-inducing factor (AIF), nuclear import of AIF, activation of procaspase-9 and procaspase-3, and even downstream of active caspase-3 (Mosser et al. 1997; Jaattela et al. 1998a,b; Beere et al. 2000; Ravagnan et al. 2001; Guo et al. 2005). Several prior studies supported the protective effect of Hsp70 on cells treated by DOX. A large study that assessed the mechanisms mediating cytoprotection from DOX studied 71 genes and Saccharomyces cerevisiae deletion strains that displayed varying degrees of hypersensitivity to DOX (Xia et al. 2007). Genes involved with multiple pathways including DNA repair, RNA metabolism, chromatin remodeling, amino acid metabolism, and heat shock response have been found to mediate cytoprotection from DOX; among those, the Hsp70 gene appears to play an important role. Thermal preconditioning protected effectively cardiomyocytes against DOX-induced apoptosis, and this protection was attenuated by knockdown Hsp70 in cardiomyocytes by antisense messenger RNA (Ito et al. 1999). Hsp70 overexpression has been previously shown to provide protection against DOX cytotoxicity Karlseder et al. 1996), and Hsp70 accumulation following DOX administration has been associated with a shortening of the DOX-mediated cell cycle arrest in the G2 phase and a restart of cell proliferation (Abe et al. 1996). Other Hsps have also been involved in cardiac protection from DOX-induced cardiotoxicity, among them, Hsp60 (Tanonaka et al. 2001), Hsp20 (Fan et al. 2008), and Hsp27 Venkatakrishnan et al. 2006). Important addition to this field is the work on the role of the HSF1—a transcription factor, which has the ability to activate simultaneously many heat shock genes with heat shock element-in DOX-induced heart failure (Vedam et al. 2010). Therefore, we still do not know which of the induced genes are involved in the installation of heart failure. Furthermore, the mechanism of action of the protective effect of Hsp70 in DOX-induced cardiomyopathy has not been previously studied in an animal model.
In order to examine the apoptotic pathway that was activated following exposure to DOX, we used physical immortalized primary embryonic cells (over passages 80) isolated from pregnant paternal (F1/F1 cells) or homozygous transgenic mice (Tg/Tg cells). We assumed that these cell lines could serve as a convenient tool in order to determine, on the one hand, the apoptotic mechanism induced by DOX and, on the other hand, the point of hsp70 action on path.
The expression of Hsp70 in Tg/Tg cells was similar to that of F1/F1 cells exposed to heat shock (Fig. 2a). Western blotting analysis revealed that treatment of cells with DOX activates the p53 that then promotes mitochondrial apoptotic signals and the caspases’ cascade. Specifically, p53 activates the Bax, caspase-9 and caspase-3, while the activation of PARP-1 reveals the DOX-induced DNA damage. This apoptotic pathway appeared to be inhibited (Fig. 3, C2, D2, E2, and F2) by the high expression of human Hsp70 in the primary embryonic Tg/Tg cells (Fig. 3, A2), and this inhibitory effect was initiated from p53 activation (Fig. 3, C2). In conclusion, Hsp70 may prevent the promotion of apoptosis induced by DOX, acting at a site upstream of p53 activation (Fig. 3) as illustrated in Fig. 8.
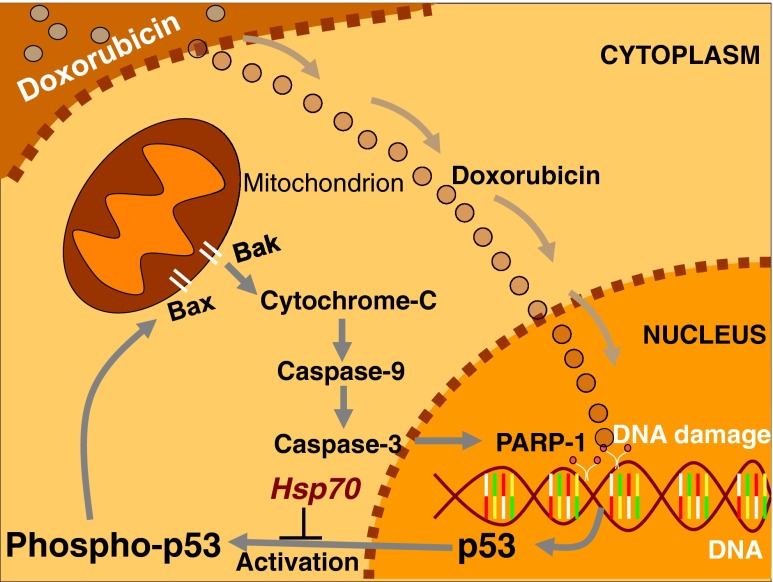
Fig. 8.
Hypothetical model of the Hsp70 function on the response to doxorubicin treatment, in the cytoplasm and nucleus. Doxorubicin translocates to the nucleus and produces DNA damages that promote the activated p53-apoptotic pathway via p53, Bax, caspase-9, and caspase-3
Having previously analyzed the apoptotic pathway that is activated by DOX administration, the mechanism of cardiac protection at the animal level was then investigated. Eighty-four mice (F1/F1 and Hsp70 homozygous Tg/Tg) were selected by PCR (Fig. 4a, b) and used in our experiments. The unexpected lower expression of Hsp70 was observed in Tg/Tg mice at 4, 6, and 10 weeks after DOX treatment (Fig. 3c). The same phenomenon was observed in Tg/Tg cells when exposed for 48 h at 5, 10, or 25 μM DOX (data not shown). These results were in agreement with previous studies showed that Hsp70 downregulation after DOX treatment in Wistar rats may be attributed to the degradation of Hsp70 by DOX (Simoncikova et al. 2008). A relatively recent work showed that a therapeutic dose of doxorubicin activates ubiquitin-proteasome system-mediated proteolysis by acting on both the ubiquitination apparatus and proteasome (Liu et al. 2008). Furthermore, it was shown that CHIP mediates during the stress recovery process by sequential ubiquitination of substrates and Hsp70 (Qian et al. 2006), although further research is needed in order to clarify this aspect of the particular protein. The Hsp70 protective effect of the primary cells was confirmed in F1/F1 mice and Tg/Tg mice that overexpress the human Hsp70 in all cells including cardiac cells (Angelidis et al. 1996). Our current results in mice following chronic administration of DOX that induces cardiac dysfunction indicated that Hsp70 may provide protection from the DOX-induced myocardial damage as assessed by the lower mortality, the preservation of body weight (Table 1), and left ventricular systolic function in the transgenic mice compared to F1/F1 mice. As assessed by transthoracic echocardiography, the reduction in LV systolic function after treatment with DOX was attenuated in Tg/Tg compared to F1/F1 mice (Fig. 5), indicating that overexpression of Hsp70 may have offered cardiac protection against DOX in mice.
Subsequently, the inhibition of p53-apoptosis in tissues of both F1/F1 and Tg/Tg mice was studied. Western blotting analysis of myocardial cell protein extracts showed an increased presence of Hsp70 in samples from Hsp70-transgenic animals (Fig. 4c), consistent with an increased protection from p53-apoptosis (Fig. 6a, b). Specifically, both cleaved caspase-9 and active caspase-3 were downregulated in tissue cells overexpressing Hsp70. Immunofluorescence study in tissue sections and confocal microscope using specific antibodies against the activated form of p53 or phosphorylated p53(ser15) added strength to this observation. It should be considered that these p53-phosphorylated molecules were implicated to the mitochondrial apoptotic pathway via activation of Bax-1 (Seong and Ha 2012). Subsequently, we investigated the activation of p53, as it is translocated out of the nucleus in the path to the mitochondria. As shown in Fig. 7a and b, in F1/F1 sections, the phosphorylated-p53 form is mainly detected in the cytoplasm of cells and accumulated at higher levels compared to that of Tg/Tg cells. The p53 accumulation in the cytoplasm in F1/F1 mice sections (Fig. 7) is in agreement with previous studies that showed the p53 phosphorylated form translocation to the cytoplasm or mitochondria (Helton and Chen 2007).
Overall, the above results show that transgenic mice overexpressing Hsp70 demonstrate an increased resistance to the p53-apoptotic pathway, which, in turn, may cause delays in DOX-induced heart failure. The exact mechanism by which this is achieved is not known. Binding experiments of Hsp70 with populations of p53 suggest the involvement of Hsp70 in the activation of p53 (data not shown).
A proposed model of Hsp70-action in heart failure regulation is presented in Fig. 8. According to this model, the DΟΧ enters in the nucleus of cells and causes DNA damages and p53 activation. Subsequently, the p53-phosphorylated form is translocated to the cytoplasm promoting the p53-apoptotic mitochondrial pathway. The inhibitory effect of Hsp70 is located upstream of p53 activation, while PARP-1 reveals the DNA damage caused to the cell by the DOX. The proposed model could also be completed after studies on the phosphorylation of p53 and upstream thereof. Previous studies have shown that the p53 protein is activated and phosphorylated on serine-15 in response to various DNA damaging agents. At high levels of DNA damage, p53 pro-apoptotic function is enabled, leading to programmed cell death (Helton and Chen 2007). We assume that, in the model studied here (Hsp70-transgenic mice), Hsp70 overexpression could cause mitigation of the p53-apoptotic mechanism by influencing the phosphorylation of p53 via ATM and / or DNA-PK (Tomita 2010; Achanta et al. 2001). Since previous studies show binding of Hsp70 with p53 (data not shown), such an approach should not be overlooked.
In conclusion, in this DOX-induced heart failure model in Hsp70-transgenic mice Hsp70 appears to provide protection from myocardial damage caused by DOX via a reduction in the p53 activation-dependent apoptosis. Whether Hsp70 serves as a potential therapeutic target against DOX-induced cardiotoxicity-especially as a therapeutic strategy to decrease side effects of chemotherapy without affecting its therapeutic efficacy warrants further research. Taking into consideration the anti-apoptotic properties of Hsp70, the role of Hsp70 in other types of heart failure needs to be investigated. However, further research is also needed to investigate other mechanisms that may be involved in this protective effect of Hsp70 in DOX-induced cardiac dysfunction. A direct effect of Hsp70 on the inflammatory response and/or oxidative stress as well as other pathways via which Hsp70 may inhibit apoptosis should not be ruled out.
Acknowledgments
The study was partly funded by a grant from the Hellenic Cardiological Society.
Abbreviations
- DOX
Doxorubicin
- F1/F1 mice
Wild type mice
- Tg/Tg mice
Hsp70-transgenic mice
- F1/F1 cells
Control embryonic primary cells
- Tg/Tg cells
Hsp70 overexpressing embryonic primary cells
Contributor Information
Katerina Naka K, Email: anaka@cc.uoi.gr.
Patra Vezyraki, Email: pvezirak@cc.uoi.gr.
Alexandros Kalaitzakis, Email: akalaitz@cc.uoi.gr.
Stelios Zerikiotis, Email: szerik@cc.uoi.gr.
Lampros Michalis, Email: lmihalis@cc.uoi.gr.
Charalampos Angelidis, FAX: +30-2651-007863, Email: chaggeli@cc.uoi.gr.
References
- Abe T, Fukamachi Y, Kanazava Y, Purukawa H, Shimizu K, Hirano T, Kasai H, Kashimura M, Higashi K. Inhibition of nucleolar function and morphological change by adriamycin associated with heat shock protein 70 accumulation. Jpn J Cancer Res. 1996;87:945–951. doi: 10.1111/j.1349-7006.1996.tb02124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achanta G, Pelicano H, Feng L, Plunkett W, Huang P. Interaction of p53 and DNA-PK in response to nucleoside analogues: potential role as a sensor complex for DNA damage. Cancer Res. 2001;61:8723–8729. [PubMed] [Google Scholar]
- Adachi H, Katsuno M, Minamiyama M, Sang C, Pagoulatos G, Angelidis C, Kusakabe M, Yoshiki A, Kobayashi Y, Doyu M, Sobue G. Heat shock protein 70 chaperone overexpression ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model by reducing nuclear-localized mutant androgen receptor protein. J Neurosci. 2003;23:2203–2211. doi: 10.1523/JNEUROSCI.23-06-02203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammon-Treiber S, Grecksch G, Angelidis C, Vezyraki P, Höllt V, Becker A. Pentylenetetrazol-kindling in mice overexpressing heat shock protein 70. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:115–121. doi: 10.1007/s00210-007-0143-0. [DOI] [PubMed] [Google Scholar]
- Angelidis C, Lazaridis I, Pagoulatos G. Constitutive expression of heat-shock protein 70 in mammalian cells confers thermoresistance. Eur J Biochem. 1991;199:35–39. doi: 10.1111/j.1432-1033.1991.tb16088.x. [DOI] [PubMed] [Google Scholar]
- Angelidis CE, Nova C, Lazaridis I, Kontoyannis D, Kollias G, Pagoulatos G. Overexpression of HSP70 in transgenic mice results in increased cell thermotolerance. Transgenics. 1996;2:111–117. [Google Scholar]
- Angelidis CE, Lazaridis I, Pagoulatos GN. Aggregation of HSP70 and HSC70 in vivo is distinct and temperature dependent and their chaperon function directly related to non aggregated forms. Eur J Biochem. 1999;259:505–512. doi: 10.1046/j.1432-1327.1999.00078.x. [DOI] [PubMed] [Google Scholar]
- Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Benaroudj N, Triniolles F, Ladjimi MM. Effect of nucleotides, peptides, and unfolded proteins on the self-association of the molecular chaperone HSC70. J Biol Chem. 1996;271:18471–18476. doi: 10.1074/jbc.271.31.18471. [DOI] [PubMed] [Google Scholar]
- Bozidis P, Lazaridis I, Pagoulatos GN, Angelidis CE. Mydj2 as a potent partner of hsc70 in mammalian cells. Eur J Biochem. 2002;269:1553–1560. doi: 10.1046/j.1432-1033.2002.02807.x. [DOI] [PubMed] [Google Scholar]
- Brundel BJ, Shiroshita-Takeshita A, Qi X, Yeh YH, Chartier D, van Gelder IC, Henning RH, Kampinga HH, Nattel S. Induction of heat shock response protects the heart against atrial fibrillation. Circ Res. 2006;99:1394–1402. doi: 10.1161/01.RES.0000252323.83137.fe. [DOI] [PubMed] [Google Scholar]
- Chirico WJ, Waters MG, Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988;332:805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Rozados VR, Cuello Carrión FD, Gervasoni SI, Matar P, Scharovsky OG. Hsp25 and Hsp70 in rodent tumors treated with doxorubicin and lovastatin. Cell Stress Chaperones. 2003;8:26–36. doi: 10.1379/1466-1268(2003)8<26:HAHIRT>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CJ, Sun Y, Opal P, Antalffy B, Mestril R, Orr HT, Dillmann WH, Zoghbi HY. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet. 2001;10:1511–1518. doi: 10.1093/hmg/10.14.1511. [DOI] [PubMed] [Google Scholar]
- Damalas A, Velimezi G, Kalaitzakis A, Liontos M, Papavassiliou AG, Gorgoulis V, Angelidis C. Loss of p14(ARF) confers resistance to heat shock- and oxidative stress-mediated cell death by upregulating β-catenin. Int J Cancer. 2011;128:1989–1995. doi: 10.1002/ijc.25510. [DOI] [PubMed] [Google Scholar]
- Delogu G, Signore M, Mechelli M, Famularo G. Heat shock proteins and their role in heart injury. Curr Opin Crit Care. 2002;8:411–416. doi: 10.1097/00075198-200210000-00007. [DOI] [PubMed] [Google Scholar]
- Demidenko ZN, Vivo C, Halicka HD, Li CJ, Bhalla K, Broude EV, Blagosklonny MV. Pharmacological induction of Hsp70 protects apoptosis-prone cells from doxorubicin: comparison with caspase-inhibitor- and cycle arrest-mediated cytoprotection. Cell Death Differ. 2006;13:1434–1441. doi: 10.1038/sj.cdd.4401812. [DOI] [PubMed] [Google Scholar]
- Erster S, Mihara M, Kim RH, Petrenko O, Moll UM. In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol Cell Biol. 2004;24:6728–6741. doi: 10.1128/MCB.24.15.6728-6741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G-C, Zhou X, Wang X, Song G, Qian J, Nicolaou P, Chen G, Ren X, Kranias EG. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res. 2008;103:1270–1279. doi: 10.1161/CIRCRESAHA.108.182832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Sigua C, Bali P, George P, Fiskus W, Scuto A, Annavarapu S, Mouttaki A, Sondarva G, Wei S, Wu J, Djeu J, Bhalla K. Mechanistic role of heat shock protein 70 in Bcr-Abl-mediated resistance to apoptosis in human acute leukemia cells. Blood. 2005;105:1246–1255. doi: 10.1182/blood-2004-05-2041. [DOI] [PubMed] [Google Scholar]
- Helton ES, Chen X. p53 modulation of the DNA damage response. J Cell Biochem. 2007;100:883–896. doi: 10.1002/jcb.21091. [DOI] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Höhfeld J, Cyr DM, Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2001;2:885–890. doi: 10.1093/embo-reports/kve206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Shimojo T, Fujisaki H, Tamamori M, Ishiyama S, Adachi S, Abe S, Marumo F, Hiroe M. Thermal preconditioning protects rat cardiac muscle cells from doxorubicin-induced apoptosis. Life Sci. 1999;64:755–761. doi: 10.1016/S0024-3205(98)00617-1. [DOI] [PubMed] [Google Scholar]
- Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 1998;17:6124–6134. doi: 10.1093/emboj/17.21.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 1998;17:6124–6134. doi: 10.1093/emboj/17.21.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14:105–111 doi:10.1007/s12192-008-0068-7 [DOI] [PMC free article] [PubMed]
- Karlseder J, Wissing D, Holzer G, Orel L, Sliutz G, Auer H, Jäättelä M, Simon MM. HSP70 overexpression mediates the escape of a doxorubicin-induced G2 cell cycle arrest. BBRC. 1996;220:153–159. doi: 10.1006/bbrc.1996.0373. [DOI] [PubMed] [Google Scholar]
- Knowlton AA, Kapadia S, Torre-Amione G, Durand J-B, Bies R, Young J, Mann DL. Differential expression of heat shock proteins in normal and failing human hearts. J Mol Cell Cardiol. 1998;30:811–818. doi: 10.1006/jmcc.1998.0646. [DOI] [PubMed] [Google Scholar]
- Kotamraju S, Konorev EA, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen, role of reactive role of oxygen and nitrogen species. J Biol Chem. 2000;275:33585–33592. doi: 10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- Kotoglou P, Kalaitzakis A, Vezyraki P, Tzavaras T, Michalis LK, Dantzer F, Jung JU, Angelidis C. Hsp70 translocates to the nuclei and nucleoli, binds to XRCC1 and PARP-1, and protects HeLa cells from single-strand DNA breaks. Cell Stress Chaperone. 2009;14:391–406. doi: 10.1007/s12192-008-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou IE, Papakostas JC, Ioachim E, Koulouras V, Arnaoutoglou E, Angelidise C, Matsagkas MI. Early ischaemic preconditioning of spinal cord enhanced the binding profile of heat shock protein 70 with neurofilaments and promoted its nuclear translocation after thoraco-abdominal aortic occlusion in pigs. Eur J Vasc Endovasc Surg. 2012;43:408–414. doi: 10.1016/j.ejvs.2011.12.028. [DOI] [PubMed] [Google Scholar]
- Larson ED, Drummond JT. Human mismatch repair and G*T mismatch binding by MutSalpha in vitro is inhibited by adriamycin, actinomycin D, and nogalamycin. J Biol Chem. 2001;276:9775–9783. doi: 10.1074/jbc.M006390200. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Kang JS, Kim MS, Lee KP, Lee MS (2004) The study of doxorubicin and its complex with DNA by SERS and UV-resonance Raman spectroscopy. Bull Korean Chem Soc 25: No. 8
- Liu JC, Wan L, He M, Cheng XS. Protection of myocardiocytes against anoxia-reoxygeneration injury by heat shock protein 70 gene transfection: experiment with rats. Zhonghua Yi Xue Za Zhi. 2007;87:3436–3439. [PubMed] [Google Scholar]
- Liu J, Zheng H, Tang M, Ryu Y-C, Wang X. A therapeutic dose of doxorubicin activates ubiquitin-proteasome system-mediated proteolysis by acting on both the ubiquitination apparatus and proteasome. Am J Physiol Heart Circ Physiol. 2008;295:H2541–H2550. doi: 10.1152/ajpheart.01052.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysitsas DN, Katsouras CS, Papakostas JC, Toumpoulis IK, Angelidis C, Bozidis P, Thomas CG, Seferiadis K, Psychoyios N, Frillingos S, Pavlidis N, Marinos E, Khaldi L, Sideris DA, Michalis LK. Antirestenotic effects of a novel polymer-coated d-24851 eluting stent. Experimental data in a rabbit iliac artery model. Cardiovasc Intervent Radiol. 2007;30:1192–1200. doi: 10.1007/s00270-007-9027-4. [DOI] [PubMed] [Google Scholar]
- Minami Y, Höhfeld J, Ohtsuka K, Hartl F-U. Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. J Biol Chem. 1996;271:19617–19624. doi: 10.1074/jbc.271.32.19617. [DOI] [PubMed] [Google Scholar]
- Mosser D, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23:2907–2918. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki N, Shishido T, Takeishi Y, Kubota I. Modulation of doxorubicin-induced cardiac dysfunction in toll-like receptor-2-knockout mice. Circulation. 2004;110:2869–2874. doi: 10.1161/01.CIR.0000146889.46519.27. [DOI] [PubMed] [Google Scholar]
- Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijnsa HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Okubo S, Wildner O, Shah M, Chelliah JC, Hess ML, Kukreja RC. Gene transfer of heat shock protein 70 reduces infarct size in vivo after ischemia/reperfusion in the rabbit heart. Circulation. 2001;103:877–881. doi: 10.1161/01.CIR.103.6.877. [DOI] [PubMed] [Google Scholar]
- Plumier JC, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest. 1995;95:1854–1860. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S-B, McDonough H, Boellmann F, Cyr DM, Patterson C. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature. 2006;440:551–555. doi: 10.1038/nature04600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- Rich T, Allen R, Wyllie A. Defying death after DNA damage. Nature. 2000;407:777–783. doi: 10.1038/35037717. [DOI] [PubMed] [Google Scholar]
- Saliba RS, Munro PM, Luthert PJ, Cheetham ME. The cellular fate of mutant rhodopsin: quality control, degradation and aggresome formation. J Cell Sci. 2002;115:2907–2918. doi: 10.1242/jcs.115.14.2907. [DOI] [PubMed] [Google Scholar]
- Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010;53:105–113. doi: 10.1016/j.pcad.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MD, Frydman J. Aberrant protein folding as the molecular basis of cancer. Methods Mol Biol. 2003;232:67–76. doi: 10.1385/1-59259-394-1:67. [DOI] [PubMed] [Google Scholar]
- Seong HA, Ha H. Murine protein serine-threonine kinase 38 activates p53 function through Ser15 phosphorylation. Biol Chem. 2012;287:20797–20810. doi: 10.1074/jbc.M112.347757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/S0092-8674(00)80416-X. [DOI] [PubMed] [Google Scholar]
- Simoncikova P, Ravingerova T, Barancik M (2008) The effect of chronic doxorubicin treatment on mitogen-activated protein kinases and heat stress proteins in rat hearts. Physiol Res 57 (Suppl. 2), S97–S102. [DOI] [PubMed]
- Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- Skladanowski A, Konopa J. Interstrand DNA crosslinking induced by anthracyclines in tumour cells. Biochem Pharmacol. 1994;47:2269–2278. doi: 10.1016/0006-2952(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- Tanonaka K, Yoshida H, Toga W, Furuhama K, Takeo S. Myocardial heat shock proteins during the development of heart failure. BBRC. 2001;283:520–525. doi: 10.1006/bbrc.2001.4801. [DOI] [PubMed] [Google Scholar]
- Tomita M. Involvement of DNA-PK and ATM in radiation- and heat-induced DNA damage recognition and apoptotic cell death. J Radiat Res. 2010;51:493–501. doi: 10.1269/jrr.10039. [DOI] [PubMed] [Google Scholar]
- Vedam K, Nishijima Y, Druhan LJ, Khan M, Moldovan NI, Zweier JL, Ilangovan G. Role of heat shock factor-1 activation in the doxorubicin-induced heart failure in mice. Am J Physiol Heart Circ Physiol. 2010;298:H1832–H1841. doi: 10.1152/ajpheart.01047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatakrishnan CD, Tewari AK, Moldovan L, Cardounel AJ, Zweier JL, Kuppusamy P, Ilangovan G. Heat shock protects cardiac cells from doxorubicin-induced toxicity by activating p38 MAPK and phosphorylation of small heat shock protein 27. Am J Physiol Heart Circ Physiol. 2006;291:H2680–H2691. doi: 10.1152/ajpheart.00395.2006. [DOI] [PubMed] [Google Scholar]
- Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B. Intermediacy of H2O2 and p53 depentent pathways. J Biol Chem. 2004;279:25535–25543. doi: 10.1074/jbc.M400944200. [DOI] [PubMed] [Google Scholar]
- Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker C, Housman DE, Amon A. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322:703–709. doi: 10.1126/science.1160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis MS, Patterson C. Hold me tight. Role of the heat shock protein family of chaperones in cardiac disease. Circulation. 2010;122:1740–1751. doi: 10.1161/CIRCULATIONAHA.110.942250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Jaafar L, Cashikar A, Flores-Rozas H. Identification of genes required for protection from doxorubicin by a genome-wide screen in Saccharomyces cerevisiae. Cancer Res. 2007;67:11411–11418. doi: 10.1158/0008-5472.CAN-07-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]