Abstract
Bullous pemphigoid (BP) is the most common subepidermal autoimmune blistering skin disease characterized by autoantibodies against the hemidesmosomal proteins BP180 and BP230. The cell stress chaperone heat shock protein 90 (Hsp90) has been implicated in inflammatory responses, and recent evidence suggests that it represents a novel treatment target in autoimmune bullous diseases. The aim of the study was to investigate the contribution of Hsp90 to the proinflammatory cytokine production in keratinocytes induced by autoantibodies to BP180 from BP patient serum. HaCaT cells were treated with purified human BP or normal IgG in the absence or presence of the Hsp90 blocker 17-DMAG and effects on viability, interleukin 6 (IL-6) and IL-8 (cytokines critical for BP pathology), NFκB (their major transcription factor), and Hsp70 (marker of effective Hsp90 inhibition and potent negative regulator of inflammatory responses) were investigated. We found that BP IgG stimulated IL-6 and IL-8 release from HaCaT cells and that non-toxic doses of 17-DMAG inhibited this IL-8, but not IL-6 secretion in a dose- and time-dependent fashion. Inhibition of this IL-8 production was also observed at the transcriptional level. In addition, 17-DMAG treatment blunted BP IgG-mediated upregulation of NFκB activity and was associated with Hsp70 induction. This study provides important insights that Hsp90 is involved as crucial regulator in anti-BP180 IgG-induced production of keratinocyte-derived IL-8. By adding to the knowledge of the multimodal anti-inflammatory effects of Hsp90 blockade, our data further support the introduction of Hsp90 inhibitors into the clinical setting for treatment of autoimmune diseases, especially for BP.
Keywords: Autoantibody, Autoimmune bullous disease, Bullous pemphigoid, Heat shock protein 90
Introduction
Bullous pemphigoid (BP) is the most common subepidermal autoimmune blistering skin disease characterized by an autoimmune response to two hemidesmosomal proteins within the dermal-epidermal junction, designated BP180 and BP230. The extracellular linker domain of BP180 termed noncollagenous 16A (NC16A) domain is considered to harbor major pathogenically relevant epitopes and is recognized by autoantibodies, which correlate with disease activity in the majority of BP patients (Schmidt and Zillikens 2013). Insights into the functionally relevant pathogenic mechanisms in BP, which include complement activation, mast cell degranulation, recruitment and activation of neutrophils and eosinophils, and release of basal membrane zone-degradading proteinases from these effector cells, have been provided by in vitro studies using cultured human keratinocytes and ex vivo studies using cryosections of human skin as well as various mouse models, although the role of mast cells in models of antibody-mediated autoimmunity has recently been questioned (Kasperkiewicz and Zillikens 2007; Feyerabend et al. 2011; Schmidt and Zillikens 2013). Although the precise sequence of these events is largely unknown, it has been proposed that one of the first steps leading to blister formation in BP comprise the binding of autoantibodies to BP180, thereby initiating Fc receptor-independent events leading to the release of interleukin 6 (IL-6) and IL-8 from basal keratinocytes (Schmidt et al. 2000, 2001; Messingham et al. 2011). IL-8 is a potent chemoattractant for pathophysiologically relevant leukocyte effector cells and together with IL-6 known to be elevated in sera and blister fluids of BP patients and critical for blister formation in mouse models (Schmidt et al. 1996a, b; Liu et al. 1997; Inaoki and Takehara 1998; Chen et al. 2001; Nelson et al. 2006; Kobayashi 2008).
Heat shock protein 90 (Hsp90) belongs to the group of ubiquitous molecular chaperones essential for protein folding and transport within the cell. It is strongly involved in participation of structural maturation and conformational regulation of a number of signaling molecules as well as transcription factors, which have been linked to synthesis of proinflammatory mediators and cytokines such as TNFα, IL-1, IL-6, and IL-8 (Salminen et al. 2008; Taipale et al. 2010; Henderson and Kaiser 2013).
Pharmacological inhibition of Hsp90 function has therefore emerged as a promising method to ameliorate inflammatory cascades, as it has been demonstrated in various experimental mouse models of autoimmune diseases, including autoimmune encephalomyelitis (Dello Russo et al. 2006), rheumatoid arthritis (Rice et al. 2008; Yun et al. 2011), and systemic lupus erythematosus (Han et al. 2010; Shimp et al. 2012a). Our own recent research work showed that, by downregulating T cell responses, this kind of treatment is also effective in mice with the experimentally induced subepidermal autoimmune blistering skin disease epidermolysis bullosa acquisita (Kasperkiewicz et al. 2011). Moreover, we recently found first evidence suggesting that Hsp90 may play a pathophysiological role and represent a novel potential treatment target in patients with BP (Tukaj et al. 2013).
In the present study, we show that pharmacological blockade of Hsp90 by the geldanamycin derivative 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) leads to suppression of IL-8 production from anti-BP180 IgG-stimulated HaCaT cells in a dose- and time-dependent manner.
Materials and methods
Purification of IgG
Total serum IgG from a healthy blood donor and patient diagnosed with BP (based on typical clinical findings as well as on detection of linear deposits of IgG and C3 at the dermal-epidermal junction and circulating IgG autoantibodies against BP180 NC16A by direct immunofluorescence microscopy and enzyme-linked immunosorbent assay [ELISA; Euroimmun, Lübeck, Germany], respectively) was isolated using Protein G Sepharose Fast Flow affinity column chromatography (GE Healthcare, Munich, Germany) as described previously (Tukaj et al. 2013). Antibodies were eluted with 0.1 M glycin buffer (pH 2.8), neutralized with Tris-HCl (pH 9.0), and concentrated using Amicon Ultra-15 filters (Millipore, Bradford, MA, USA). Immunoreactivity of the affinity-purified BP IgG preparations was confirmed by indirect immunofluorescence microscopy on salt-split normal human skin (staining at the epidermal side of the artificial split) and by anti-BP180-NC16A-4X ELISA (Euroimmun, Lübeck, Germany). No circulating anti-BP230 IgG autoantibodies were found in the patient’s serum by ELISA (Euroimmun, Lübeck, Germany), and IL-6 and IL-8 concentrations in the IgG preparations were below the ELISA detection limit.
The collection of blood samples was approved by the Ethics Committee of the University of Lübeck, and informed consent was obtained according to the Declaration of Helsinki.
Cell culture
HaCaT cells were cultured in keratinocyte growth medium (Promo Cell, Heidelberg, Germany) supplemented with 10 % FCS at 37 ° C in 5 % CO2 atmosphere. Cells were seeded on 24- or 12-well plates (Greiner Bio-One, Solingen, Germany) and grown to 80 % confluence. Cells were incubated with medium alone or with purified total IgG from a healthy blood donor and BP patient and cultured in absence or presence of 17-DMAG (InvivoGen, San Diego, CA, USA). After 24 h, cells were washed twice with PBS, trypsinized, harvested, washed with PBS again, lysed, and stored for further analysis at −20 ° C. For cytokine evaluation, culture supernatants were collected after 6, 12, and 24 h of incubation, and stored for further analysis at −20 ° C.
Lactate dehydrogenase cytotoxicity assay
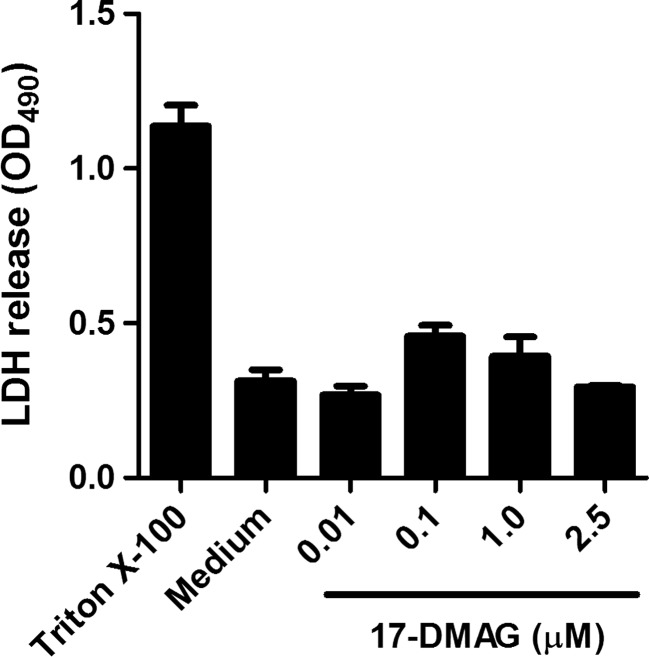
Cytotoxicity of 17-DMAG was measured by a lactate dehydrogenase (LDH)-releasing assay using a Cytotoxicity Detection Kit (Roche, Mannheim, Germany) according to the manufacturer’s protocol and quantified using an ELISA plate reader. Briefly, HaCaT cells were incubated with 17-DMAG at different concentrations (0.01, 0.1, 1, and 2.5 μM) for up to 24 h. Cell lysis was determined by measuring the amount of LDH released into culture medium.
Cytokine measurement
BP IgG-treated HaCaT cells were cultured without or with different concentrations (0.01, 0.1, 1, and 2.5 μM) of 17-DMAG for 6, 12, and 24 h. IL-6 (BD, Heidelberg, Germany) and IL-8 (BD, Heidelberg, Germany) levels were analyzed in cell culture supernatants by ELISA according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction
BP IgG-treated HaCaT cells were cultured without or with 1 μM of 17-DMAG for 12 h. Total RNA was isolated from cultured HaCaT cells using TRIzol reagent (Life Technologies) according to the manufacturer’s instruction. Complementary DNA (cDNA) was reverse-transcribed from isolated RNA by incubating 1 μg of RNA with the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific) following the manufacturer’s instructions. Quantitative real-time polymerase chain reaction (RT-PCR) was performed in a Mastercycler (Eppendorf) using SYBR® Select Master Mix (Life Technologies). PCR mixtures contained 0.5 μM of each forward and reverse primers. Each reaction mixture was subjected to 2 min at 50 °C and 2 min at 95 °C followed by 40 cycles with 15 s at 95 °C and 1 min at 60 °C. Data was normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers used in RT-PCR were as follows: IL-8 messenger RNA (mRNA), forward, 5′-ACC GGA AGG AAC CAT CTC AC -3′, and reverse, 5′-AAA CTG CAC CTT CAC ACA GAG -3′ and GAPDH, forward, 5′-ACA ACT TTG GTA TCG TGG AAG G -3′, and reverse, 5′-GCC ATC ACG CCA CAG TTT -3′.
Quantification of NFκB activation
BP IgG-treated HaCaT cells were cultured without or with 1 μM of 17-DMAG for 24 h. NFκB activity was analyzed in cell culture lysates by an NFκB p65 ELISA (Enzo, Lorrach, Germany) following the manufacturer’s instruction.
Quantification of Hsp70 expression
BP IgG-treated HaCaT cells were cultured without or with 1 μM of 17-DMAG for 24 h. Hsp70 protein expression was analyzed in cell culture lysates by ELISA (Enzo, Lorrach, Germany) following the manufacturer’s instruction.
Statistical analysis
Data was analyzed using Student’s t test or one-way analysis of variance (ANOVA). A P value <0.05 was considered to indicate a statistically significant difference.
Results
17-DMAG dampens IL-8, but not IL-6 release from HaCaT cells, mediated by BP IgG
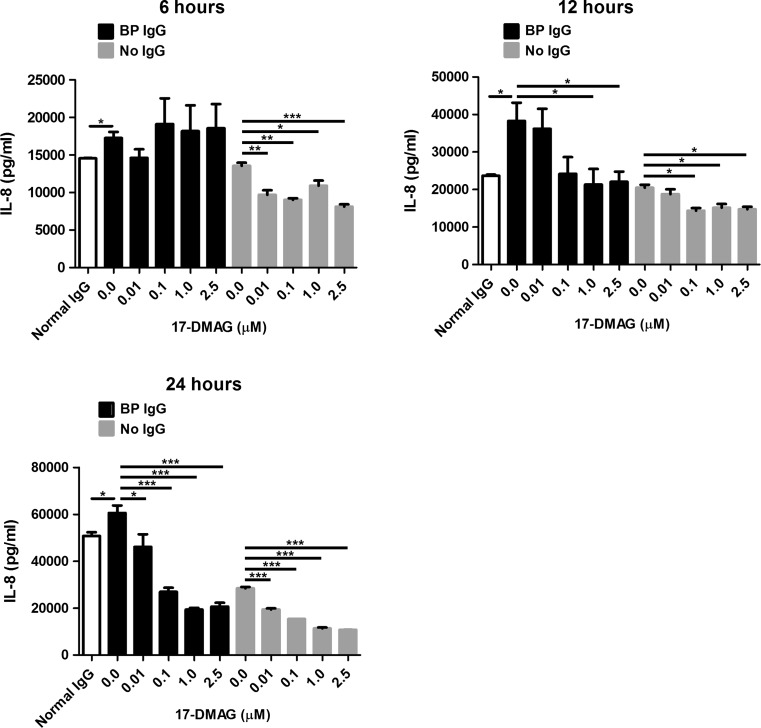
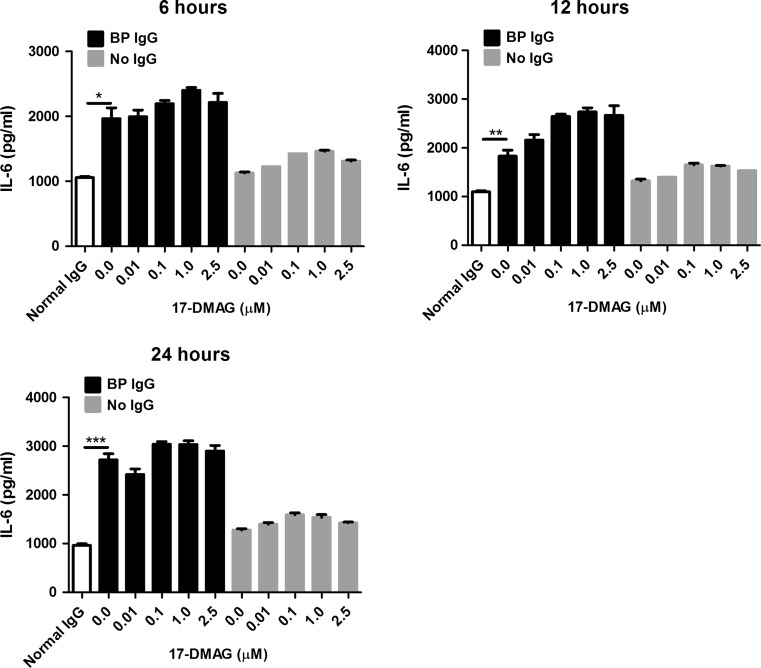
Using ELISA, we measured the effect of 17-DMAG, which was used in non-toxic doses throughout our experiments (Fig. 1), on secretion of proinflammatory IL-6 and IL-8 cytokines by HaCaT cells stimulated with BP IgG. In the absence of 17-DMAG, BP IgG led to a significant release of both cytokines compared to normal IgG-treated cells (Figs. 2 and 3). The addition of 17-DMAG significantly inhibited the secretion of IL-8 in a dose- and time-dependent manner in both BP IgG-stimulated cells and cells cultured without IgG (Fig. 2). In contrast, we found no significant inhibitory influence of 17-DMAG on IL-6 secretion (Fig. 3).
Fig. 1.
Lactate dehydrogenase (LDH)-based cytotoxicity measurement in cell culture medium after 6-h incubation of HaCaT cells with different concentrations of 17-DMAG. LDH release from cells lysed with 1 % Triton X-100 was regarded as positive control (maximum LDH secretion). Non-toxicity was also seen with longer 17-DMAG exposure times (up to 24 h; data not shown). Results are mean ± SEM of two independent experiments
Fig. 2.
Evaluation of the effects of pharmacological Hsp90 inhibition on IL-8 secretion into culture medium by HaCaT cells treated with medium alone, 2 mg/ml IgG from a healthy volunteer (normal IgG), and 2 mg/ml IgG from a bullous pemphigoid patient (BP IgG) for 6, 12, and 24 h. These BP IgG-stimulated and IgG-non-treated HaCaT cells were cultured in absence or presence of different non-toxic concentrations of 17-DMAG. IL-8 levels in cell culture supernatants were analyzed by ELISA. Results are mean ± SEM of two separate experiments, each performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001
Fig. 3.
Evaluation of the effects of pharmacological Hsp90 inhibition on IL-6 secretion into culture medium by HaCaT cells treated with medium alone, 2 mg/ml IgG from a healthy volunteer (normal IgG), and 2 mg/ml IgG from a bullous pemphigoid patient (BP IgG) for 6, 12, and 24 h. These BP IgG-stimulated and IgG-non-treated HaCaT cells were cultured in absence or presence of different non-toxic concentrations of 17-DMAG. IL-6 levels in cell culture supernatants were analyzed by ELISA. Results are mean ± SEM of two separate experiments, each performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001
17-DMAG blocks IL-8 mRNA expression in HaCaT cells, mediated by BP IgG
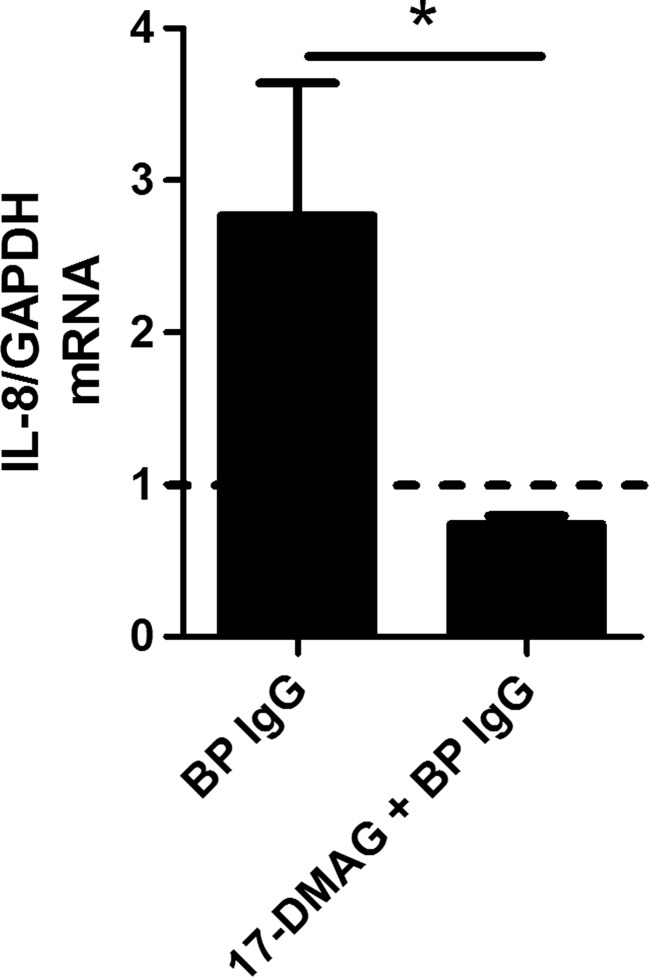
To investigate whether IL-8 inhibition by 17-DMAG takes place at the transcriptional level, IL-8 expression was assayed in BP IgG-stimulated HaCaT cells by RT-PCR. In fact, the addition of 17-DMAG significantly inhibited mRNA expression of this cytokine (Fig. 4).
Fig. 4.
RT-PCR-based investigation of the impact of pharmacological Hsp90 inhibition on IL-8 mRNA expression in HaCaT cells treated with medium alone (dotted line) and 2 mg/ml IgG from a bullous pemphigoid patient (BP IgG) for 12 h. These BP IgG-stimulated HaCaT cells were cultured in absence or presence of 1 μM 17-DMAG. Data was normalized to the housekeeping gene GAPDH. Results are mean ± SEM of two separate experiments, each performed in duplicate. *P < 0.05
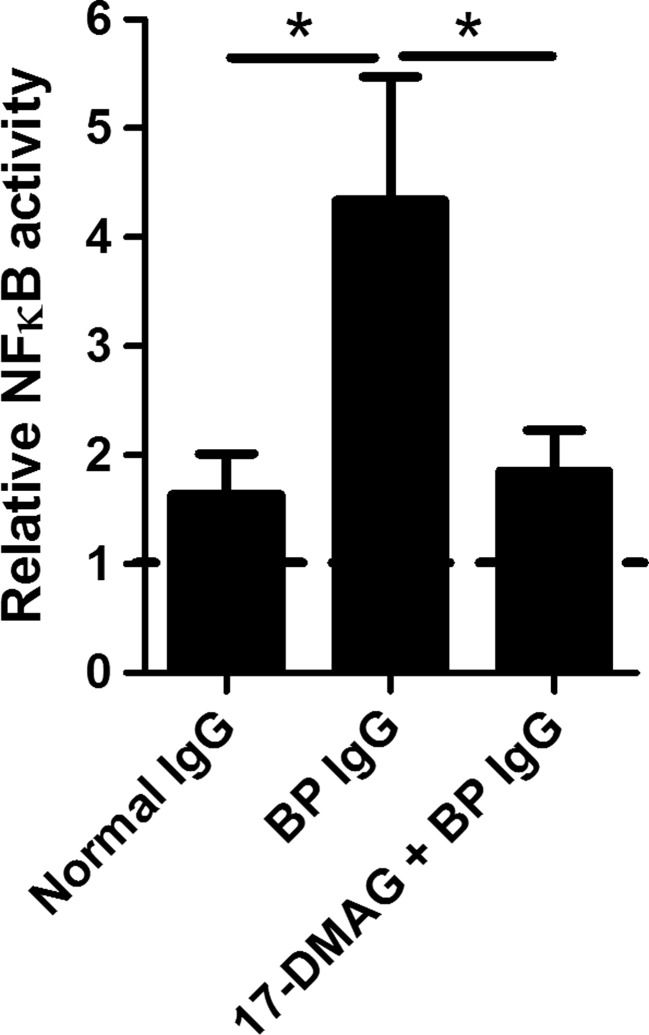
17-DMAG suppresses NFκB activity in HaCaT cells that were stimulated with BP IgG
To test whether Hsp90 inhibition had an impact on the activation status of NFκB, a major transcription factor regulating IL-8 synthesis (Hoffmann et al. 2002), NFκB p65 subunit activity was measured in BP IgG-stimulated HaCaT cell lysates by ELISA. In the absence of 17-DMAG, BP IgG significantly stimulated NFκB p65 activity compared to normal IgG-treated cells. The presence of 17-DMAG led to a significant suppression of the activity of NFκB p65 (Fig. 5).
Fig. 5.
Analysis of the impact of pharmacological Hsp90 inhibition on the activation status of NFκB in HaCaT cells treated with medium alone, 2 mg/ml IgG from a healthy volunteer (normal IgG), and 2 mg/ml IgG from a bullous pemphigoid patient (BP IgG) for 24 h. These BP IgG-stimulated and IgG-non-treated HaCaT cells were cultured in absence or presence of 1 μM 17-DMAG. NFκB p65 subunit activity in cell culture lysates was analyzed by ELISA and expressed relative to control cells incubated with medium alone. The dotted line corresponds to both cells treated with medium in absence and presence of 17-DMAG alone. Results are mean ± SEM from triplicate determinants. *P < 0.05
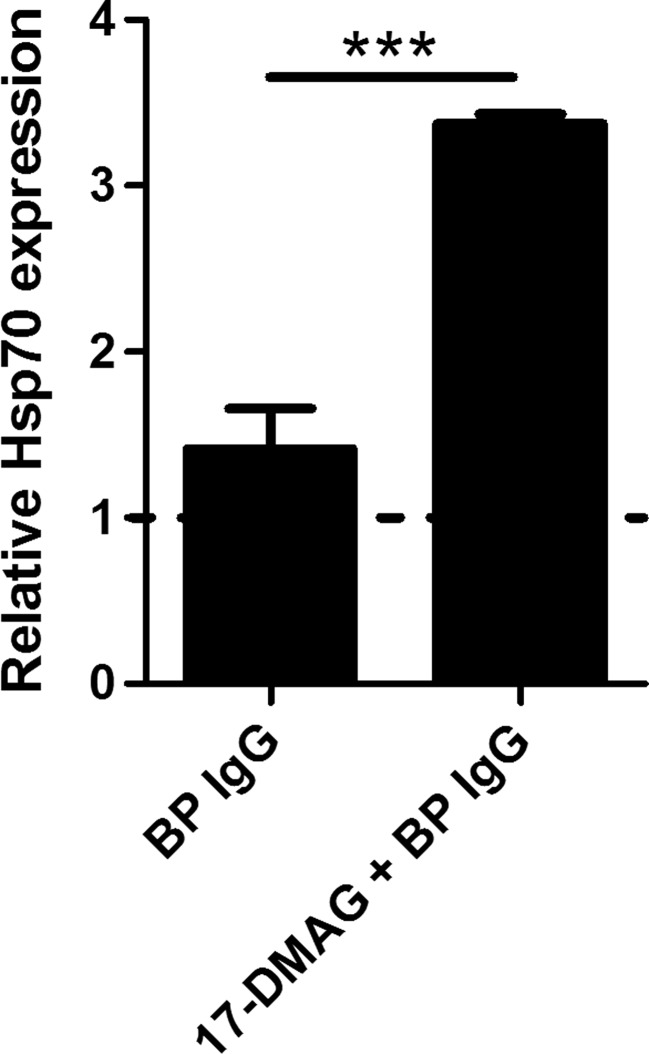
17-DMAG upregulates Hsp70 expression in HaCaT cells that were stimulated with BP IgG
To confirm that the above-mentioned results were induced by Hsp90 inhibition, we investigated the expression of Hsp70, a common marker of effective Hsp90 inhibition (Dakappagari et al. 2010), by ELISA using cell lysates of BP IgG-stimulated HaCaT cells. As expected, the addition of 17-DMAG resulted in significant induction of Hsp70 protein expression (Fig. 6).
Fig. 6.
Analysis of the impact of pharmacological Hsp90 inhibition on Hsp70 protein expression in HaCaT cells treated with medium alone and 2 mg/ml IgG from a bullous pemphigoid patient (BP IgG) for 24 h. These BP IgG-stimulated and IgG-non-treated HaCaT cells were cultured in absence or presence of 1 μM 17-DMAG. Hsp70 protein levels in cell culture lysates were analyzed by ELISA and expressed relative to control cells incubated with medium alone. The dotted line corresponds to both cells treated with medium in absence and presence of 17-DMAG alone. Results are mean ± SEM from triplicate determinants. ***P < 0.001
Discussion
The rationale of our study to probe the effects of 17-DMAG on BP IgG-induced IL-6 and IL-8 production by HaCaT cells was mainly based on three documented findings: (i) both inflammatory cytokines play a key role in the pathophysiology of BP (Schmidt et al. 1996a, b; Liu et al. 1997; Inaoki and Takehara 1998; Schmidt et al. 2000; Chen et al. 2001; Schmidt et al. 2001; Nelson et al. 2006; Messingham et al. 2011), (ii) the extensive list of Hsp90 client proteins includes many proinflammatory molecules (Salminen et al. 2008; Taipale et al. 2010; Henderson and Kaiser 2013), and (iii) recent evidence suggests that Hsp90 represents a novel treatment target in autoimmune bullous diseases (Kasperkiewicz et al. 2011; Tukaj et al. 2013).
The biological importance of proinflammatory cytokines in BP, especially of the strong leukocyte chemoattractant IL-8 (Kobayashi 2008), has been implicated by the fact that (i) the concentration of this cytokine in serum and blister fluid of BP patients is correlated with disease activity and that (ii) blistering in complement component C5- or C4-deficient mice as well as mast cell-deficient mice, which are otherwise resistant to the pathogenic effect of antimurine BP180 IgG in the passive-transfer BP mouse model, is restored by intradermal injections of neutrophil-recruiting IL-8 (Schmidt et al. 1996a, b; Liu et al. 1997; Chen et al. 2001; Nelson et al. 2006; Kasperkiewicz and Zillikens 2007).
Similar to previous experiments, which showed that IgG or IgE autoantibodies from BP patients stimulated Fc receptor-independent production of IL-6 and IL-8 in primary cultures of normal human epithelial keratinocytes (NHEK) (Schmidt et al. 2000, 2001; Messingham et al. 2011), we also observed a significant BP IgG-induced secretion of both cytokines compared to normal IgG using the HaCaT cell line. Cytokine background levels that were seen in medium containing normal IgG and which have also been reported by previous investigators may reflect the culture conditions on a plastic surface (and not human dermis) or may result from unspecific stimuli produced by keratinocytes (Schmidt et al. 2000, 2001). Like in the previous studies, the purified BP IgG reacted with the NC16A domain of BP180 that is known to contain the major antigenic sites for most BP sera (Schmidt and Zillikens 2013). Together with these previous observations and the recent finding that BP180-deficient epidermal keratinocytes display an abnormally high IL-8 response under various inflammatory stimuli (Van den Bergh et al. 2012), our current results support the assumption that targeting BP180 directly affects the epidermal keratinocyte proinflammatory cytokine response (Schmidt et al. 2000, 2001; Messingham et al. 2011; Van den Bergh et al. 2012).
Through Hsp90 blockade, we observed a dose- and time-dependent suppression of this BP IgG-mediated IL-8 release from HaCaT cells, which was at least in part mediated by inhibition of mRNA expression of this cytokine. In contrast, the IL-6 secretion was not disturbed in the presence of 17-DMAG under these conditions. Inhibition of IL-8 secretion by 17-DMAG was also found in cells not treated with the autoantibody, which may reflect suppression of constitutively expressed IL-8 in HaCaT cells rather than cytotoxic effects of this Hsp90 inhibitor as no such cytokine release was observed for IL-6 and cytotoxicity of 17-DMAG in the concentrations used was ruled out using the LDH assay. Similar results were previously obtained by Schmidt and colleagues using another immunomodulatory agent, the sulfone derivative dapsone (Schmidt et al. 2001). This drug is applied successfully in the treatment of different autoimmune bullous diseases associated with neutrophil or eosinophil skin infiltration, including BP, although hemolysis and concomitant anemia secondary to hemolysis are expected in most patients receiving this therapy (Gürcan and Ahmed 2009). In their experiments, dapsone analogously inhibited the anti-BP IgG-induced IL-8 release from NHEK without affecting IL-6 secretion (Schmidt et al. 2001).
The capacity of Hsp90 inhibitors to blunt IL-8 production was recently reported in the context of bacterial- and viral-induced inflammation, including Helicobacter pylori-infected gastric epithelial cells (Yeo et al. 2004), Legionella pneumophila-infected lung epithelial cells (Teruya et al. 2007), Bacteroides fragilis enterotoxin-treated intestinal epithelial cells (Kim et al. 2009), and oncogenic herpes virus-infected endothelial cells and fibroblasts (Defee et al. 2011). In these experimental studies, Hsp90 inhibitors acted via deactivation of NFκB, a client of Hsp90 and one of the major transcription factors for IL-8 (Hoffmann et al. 2002; Salminen et al. 2008). Similarly, we could demonstrate that the activity of this transcription factor was upregulated in BP IgG-stimulated HaCaT cells and that this effect was abrogated in the presence of 17-DMAG. In this regard, it is worth noting that blockade of NFκB by its specific inhibitor Bay-11-7082 has recently been shown to result in normalization of the above-mentioned abnormally high IL-8 response in activated BP180-deficient epidermal keratinocytes (Van den Bergh et al. 2012). Taken together, this suggests that NFκB plays an important role in mediating anti-BP180 effects on the keratinocyte IL-8 response and that this inflammatory cell signaling event can be efficiently interrupted by Hsp90 blockade.
It remains unclear, why anti-BP IgG-induced IL-6 expression was not hampered in response to Hsp90 inhibition in our study, although NFκB is also known to be an important transcription factor for this cytokine and impairment of the inflammatory IL-6 pathway by Hsp90 blockade was previously demonstrated in various cell types (except keratinocytes) (Sato et al. 2003; Wax et al. 2003; Mitsiades et al. 2006; Lang et al. 2007; Ambade et al. 2012; Petersen et al. 2012; Shimp et al. 2012a, b). Similar to our study, however, inhibition of NFκB by Bay-11-7082 suppressed the upregulation of different proinflammatory cytokines, but also did not compromise the IL-6 response in diaphragm muscle cells exposed to oxidative stress (Sigala et al. 2011). One explanation for this and our finding could be that cytokine regulation may be cell type and tissue specific. It can be speculated that in case of keratinocyte-derived IL-6, for which the molecular events that regulate its induction are not yet fully clarified, production of this cytokine may be compensated by other transcription factors than NFκB, which may be less or not prone to Hsp90 inhibition.
Finally, we found upregulated Hsp70 protein levels in BP-IgG-stimulated HaCaT cells after treatment with 17-DMAG. Hsp70 is generally accepted as a good marker for effective Hsp90 inhibition as its increased expression is known to result from Hsp90 blockade-induced activation and nuclear translocation of the transcription factor heat shock factor-1, which then drives the synthesis of this chaperone (Dakappagari et al. 2010; Collins et al. 2013). Hsp70, in turn, is also regarded as a potent negative regulator of inflammatory responses through, but not limited to, its negative feedback effect on NFκB signaling activity (de Jong et al. 2009; Stocki and Dickinson 2012). This suggests that, in addition to Hsp90 inhibition per se, Hsp70 itself may account for the anti-inflammatory effects seen in our study and warrants further investigation.
In summary, this study provides important insights that Hsp90 is involved as crucial regulator in anti-BP180 IgG-induced production of keratinocyte-derived IL-8, one of the significant chemokines related to BP pathogenesis. By adding to the knowledge of the anti-inflammatory effects of Hsp90 blockade, our data further support the introduction of Hsp90 inhibitors into the clinical setting for treatment of autoimmune diseases, especially for BP.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft (DFG) Excellence Cluster “Inflammation at Interfaces” (EXC 306/2), DFG KA 3438/1-1, Medical Faculty of the University of Lübeck (E22-2013), and Focus Program “Autoimmunity” at the University of Lübeck.
References
- Ambade A, Catalano D, Lim A, Mandrekar P. Inhibition of heat shock protein (molecular weight 90 kDa) attenuates proinflammatory cytokines and prevents lipopolysaccharide-induced liver injury in mice. Hepatology. 2012;55:1585–1595. doi: 10.1002/hep.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Ning G, Zhao ML, et al. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J Clin Invest. 2001;108:1151–1158. doi: 10.1172/JCI11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CB, Aherne CM, Yeckes A, et al. Inhibition of N-terminal ATPase on HSP90 attenuates colitis through enhanced Treg function. Mucosal Immunol. 2013;6:960–971. doi: 10.1038/mi.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakappagari N, Neely L, Tangri S, et al. An investigation into the potential use of serum Hsp70 as a novel tumour biomarker for Hsp90 inhibitors. Biomarkers. 2010;5:31–38. doi: 10.3109/13547500903261347. [DOI] [PubMed] [Google Scholar]
- de Jong PR, Schadenberg AW, Jansen NJ, Prakken BJ. Hsp70 and cardiac surgery: molecular chaperone and inflammatory regulator with compartmentalized effects. Cell Stress Chaperones. 2009;14:117–131. doi: 10.1007/s12192-008-0066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defee MR, Qin Z, Dai L, Toole BP, Isaacs JS, Parsons CH. Extracellular Hsp90 serves as a co-factor for NF-κB activation and cellular pathogenesis induced by an oncogenic herpes virus. Am J Cancer Res. 2011;1:687–700. [PMC free article] [PubMed] [Google Scholar]
- Dello Russo C, Polak PE, Mercado PR, et al. The heat-shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin suppresses glial inflammatory responses and ameliorates experimental autoimmune encephalomyelitis. J Neurochem. 2006;99:1351–1362. doi: 10.1111/j.1471-4159.2006.04221.x. [DOI] [PubMed] [Google Scholar]
- Feyerabend TB, Weiser A, Tietz A, et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832–844. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Gürcan HM, Ahmed AR. Efficacy of dapsone in the treatment of pemphigus and pemphigoid: analysis of current data. Am J Clin Dermatol. 2009;10:383–396. doi: 10.2165/11310740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Han JM, Kwon NH, Lee JY, et al. Identification of gp96 as a novel target for treatment of autoimmune disease in mice. PLoS One. 2010;5:e9792. doi: 10.1371/journal.pone.0009792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B, Kaiser F. Do reciprocal interactions between cell stress proteins and cytokines create a new intra-/extra-cellular signalling nexus? Cell Stress Chaperones. 2013;18:685–701. doi: 10.1007/s12192-013-0444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Inaoki M, Takehara K. Increased serum levels of interleukin (IL)-5, IL-6 and IL-8 in bullous pemphigoid. J Dermatol Sci. 1998;16:152–157. doi: 10.1016/S0923-1811(97)00044-3. [DOI] [PubMed] [Google Scholar]
- Kasperkiewicz M, Zillikens D. The pathophysiology of bullous pemphigoid. Clin Rev Allergy Immunol. 2007;33:67–77. doi: 10.1007/s12016-007-0030-y. [DOI] [PubMed] [Google Scholar]
- Kasperkiewicz M, Müller R, Manz R, et al. Heat-shock protein 90 inhibition in autoimmunity to type VII collagen: evidence that nonmalignant plasma cells are not therapeutic targets. Blood. 2011;117:6135–6142. doi: 10.1182/blood-2010-10-314609. [DOI] [PubMed] [Google Scholar]
- Kim JM, Lee DH, Kim JS, et al. 5,7-Dihydroxy-3,4,6-trimethoxyflavone inhibits the inflammatory effects induced by Bacteroides fragilis enterotoxin via dissociating the complex of heat shock protein 90 and I kappaB alpha and I kappaB kinase-gamma in intestinal epithelial cell culture. Clin Exp Immunol. 2009;155:541–551. doi: 10.1111/j.1365-2249.2008.03849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- Lang SA, Moser C, Gaumann A, et al. Targeting heat shock protein 90 in pancreatic cancer impairs insulin-like growth factor-I receptor signaling, disrupts an interleukin-6/signal-transducer and activator of transcription 3/hypoxia-inducible factor-1alpha autocrine loop, and reduces orthotopic tumor growth. Clin Cancer Res. 2007;13:6459–6468. doi: 10.1158/1078-0432.CCR-07-1104. [DOI] [PubMed] [Google Scholar]
- Liu Z, Giudice GJ, Zhou X, et al. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest. 1997;100:1256–1263. doi: 10.1172/JCI119639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messingham KN, Srikantha R, DeGueme AM, Fairley JA. FcR-independent effects of IgE and IgG autoantibodies in bullous pemphigoid. J Immunol. 2011;187:553–560. doi: 10.4049/jimmunol.1001753. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades NS, McMullan CJ, et al. Antimyeloma activity of heat shock protein-90 inhibition. Blood. 2006;107:1092–1100. doi: 10.1182/blood-2005-03-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KC, Zhao M, Schroeder PR, et al. Role of different pathways of the complement cascade in experimental bullous pemphigoid. J Clin Invest. 2006;116:2892–2900. doi: 10.1172/JCI17891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AL, Guedes CE, Versoza CL, et al. 17-AAG kills intracellular Leishmania amazonensis while reducing inflammatory responses in infected macrophages. PLoS One. 2012;7:e49496. doi: 10.1371/journal.pone.0049496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JW, Veal JM, Fadden RP, et al. Small molecule inhibitors of Hsp90 potently affect inflammatory disease pathways and exhibit activity in models of rheumatoid arthritis. Arthritis Rheum. 2008;58:3765–3775. doi: 10.1002/art.24047. [DOI] [PubMed] [Google Scholar]
- Salminen A, Paimela T, Suuronen T, Kaarniranta K. Innate immunity meets with cellular stress at the IKK complex: regulation of the IKK complex by HSP70 and HSP90. Immunol Lett. 2008;117:9–15. doi: 10.1016/j.imlet.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Sato N, Yamamoto T, Sekine Y, et al. Involvement of heat-shock protein 90 in the interleukin-6-mediated signaling pathway through STAT3. Biochem Biophys Res Commun. 2003;300:847–852. doi: 10.1016/S0006-291X(02)02941-8. [DOI] [PubMed] [Google Scholar]
- Schmidt E, Zillikens D (2013) Pemphigoid diseases. Lancet 381:320–332 [DOI] [PubMed]
- Schmidt E, Ambach A, Bastian B, Bröcker EB, Zillikens D. Elevated levels of interleukin-8 in blister fluid of bullous pemphigoid compared with suction blisters of healthy control subjects. J Am Acad Dermatol. 1996;34:310–312. doi: 10.1016/S0190-9622(96)80146-0. [DOI] [PubMed] [Google Scholar]
- Schmidt E, Bastian B, Dummer R, Tony HP, Bröcker EB, Zillikens D. Detection of elevated levels of IL-4, IL-6, and IL-10 in blister fluid of bullous pemphigoid. Arch Dermatol Res. 1996;288:353–357. doi: 10.1007/BF02507102. [DOI] [PubMed] [Google Scholar]
- Schmidt E, Reimer S, Kruse N, et al. Autoantibodies to BP180 associated with bullous pemphigoid release interleukin-6 and interleukin-8 from cultured human keratinocytes. J Investig Dermatol. 2000;115:842–848. doi: 10.1046/j.1523-1747.2000.00141.x. [DOI] [PubMed] [Google Scholar]
- Schmidt E, Reimer S, Kruse N, Bröcker EB, Zillikens D. The IL-8 release from cultured human keratinocytes, mediated by antibodies to bullous pemphigoid autoantigen 180, is inhibited by dapsone. Clin Exp Immunol. 2001;124:157–162. doi: 10.1046/j.1365-2249.2001.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp SK, 3rd, Chafin CB, Regna NL, et al. Heat shock protein 90 inhibition by 17-DMAG lessens disease in the MRL/lpr mouse model of systemic lupus erythematosus. Cell Mol Immunol. 2012;9:255–266. doi: 10.1038/cmi.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimp SK, 3rd, Parson CD, Regna NL, et al. HSP90 inhibition by 17-DMAG reduces inflammation in J774 macrophages through suppression of Akt and nuclear factor-κB pathways. Inflamm Res. 2012;61:521–533. doi: 10.1007/s00011-012-0442-x. [DOI] [PubMed] [Google Scholar]
- Sigala I, Zacharatos P, Toumpanakis D, et al. MAPKs and NF-κB differentially regulate cytokine expression in the diaphragm in response to resistive breathing: the role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1152–R1162. doi: 10.1152/ajpregu.00376.2010. [DOI] [PubMed] [Google Scholar]
- Stocki P, Dickinson AM. The immunosuppressive activity of heat shock protein 70. Autoimmune Dis. 2012;2012:617213. doi: 10.1155/2012/617213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- Teruya H, Higa F, Akamine M, et al. Mechanisms of Legionella pneumophila-induced interleukin-8 expression in human lung epithelial cells. BMC Microbiol. 2007;7:102. doi: 10.1186/1471-2180-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukaj S, Kleszczyński K, Vafia K, et al. Aberrant expression and secretion of heat shock protein 90 in patients with bullous pemphigoid. PLoS One. 2013;8:e70496. doi: 10.1371/journal.pone.0070496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh F, Eliason SL, Burmeister BT, Giudice GJ. Collagen XVII (BP180) modulates keratinocyte expression of the proinflammatory chemokine, IL-8. Exp Dermatol. 2012;21:605–611. doi: 10.1111/j.1600-0625.2012.01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax S, Piecyk M, Maritim B, Anderson P. Geldanamycin inhibits the production of inflammatory cytokines in activated macrophages by reducing the stability and translation of cytokine transcripts. Arthritis Rheum. 2003;48:541–550. doi: 10.1002/art.10780. [DOI] [PubMed] [Google Scholar]
- Yeo M, Park HK, Lee KM, Lee KJ, Kim JH, Cho SW. Blockage of HSP 90 modulates Helicobacter pylori-induced IL-8 productions through the inactivation of transcriptional factors of AP-1 and NF-kappaB. Biochem Biophys Res Commun. 2004;320:816–824. doi: 10.1016/j.bbrc.2004.05.214. [DOI] [PubMed] [Google Scholar]
- Yun TJ, Harning EK, Giza K, et al. EC144, a synthetic inhibitor of heat shock protein 90, blocks innate and adaptive immune responses in models of inflammation and autoimmunity. J Immunol. 2011;186:563–575. doi: 10.4049/jimmunol.1000222. [DOI] [PubMed] [Google Scholar]