Abstract
LyP-1 is a peptide selected from a phage-displayed peptide library that specifically binds to tumor and endothelial cells of tumor lymphatics in certain tumors. Fluorescein-conjugated LyP-1 and a related peptide, LyP-1b, strongly accumulated in primary MDA-MB-435 breast cancer xenografts and their metastases from i.v. peptide injections, allowing visualization of orthotopic tumors in intact mice. The LyP peptide accumulation coincided with hypoxic areas in tumors. LyP-1 induced cell death in cultured human breast carcinoma cells that bind and internalize the peptide. Melanoma cells that do not bind LyP-1 were unaffected. Systemic LyP-1 peptide treatment of mice with xenografted tumors induced with the breast cancer cells inhibited tumor growth. The treated tumors contained foci of apoptotic cells and were essentially devoid of lymphatics. These results reveal an unexpected antitumor effect by the LyP-1 peptide that seems to be dependent on a proapoptotic/cytotoxic activity of the peptide. As LyP-1 affects the poorly vascularized tumor compartment, it may complement treatments directed at tumor blood vessels.
Keywords: phage display, tumor targeting, live imaging, therapy
Tumor blood vessels express molecular markers that distinguish them from normal blood vessels. Many of these tumor vessel markers are related to angiogenesis, but some are selective for certain tumors (1). Markers that distinguish the vasculature of tumors at the premalignant stage from the vasculature of fully malignant tumors in the same tumor system have also been described (2, 3). Recent data from our laboratory indicate that lymphatic vessels in tumors are also specialized, because a cyclic 9-amino acid peptide, LyP-1, binds to the lymphatic vessels in certain tumors, but not to the lymphatics of normal tissues (4).
The lymphatic system is an important route of tumor metastasis. Many cancers preferentially spread through the lymphatics. Recent discoveries of growth factors and molecular markers for lymphatic endothelial cells have made possible detailed studies of the relationship of tumor cells and the lymphatic vasculature of tumors (5–9). The use of marker proteins such as LYVE-1 (6), podoplanin (5), and Prox-1 (10) has shown that lymphatic vessels are abundant in the periphery of tumors and that many tumors also contain lymphatics within the tumor mass (4, 11). However, the intratumoral lymphatic vessels are generally not functional in transporting tissue fluid (12) and are often filled with tumor cells (4, 13). Recent experimental and clinical data strongly suggest that the number of lymphatics in a tumor, perhaps their size as well, and the expression of lymphangiogenic growth factors are important determinants in the ability of a tumor to metastasize (14–18).
Thus, it may become possible to reduce metastasis by specifically targeting tumor lymphatics (and the tumor tissue adjacent to these vessels) for destruction. The LyP-1 peptide, which specifically binds to tumor lymphatics (4), provides one potential avenue for developing reagents that can specifically destroy tumor lymphatics. This peptide also binds to the tumor cells in tumors that contain LyP-1-positive lymphatics, further expanding the potential of this peptide.
We show here that i.v. injected LyP-1 strongly and specifically accumulates in breast cancer xenografts over time, localizing preferentially in hypoxic areas. We also report that LyP-1 has a proapoptotic/cytotoxic effect on tumor cells and that systemic administration of the LyP-1 peptide inhibits breast cancer xenograft growth in mice. The treated tumors contain foci of apoptotic cells and reduced numbers of lymphatic vessels. These findings suggest that LyP-1 may provide a starting point for the development of new antitumor agents.
Materials and Methods
Cell Lines and Tumors. MDA-MB-435 human breast carcinoma cells and C8161 human melanoma cells were maintained in DMEM supplemented with 10% FCS. Nude BALB/c nu/nu mice were injected s.c. or into the mammary fat pad with 1 × 106 tumor cells to induce tumors. A vascular endothelial growth factor (VEGF)-C-transfected MDA-MB-435 cell line was prepared as previously reported for MCF7 cells (13).
Antibodies and Immunohistology. Blood vessels were visualized by staining tissue sections with monoclonal antibodies against CD-31, CD-34, or MECA-32 (all rat anti-mouse antibodies from Pharmingen). A polyclonal rabbit anti-mouse LYVE-1 antibody (4) and a rat monoclonal anti-mouse podoplanin antibody (provided by Kari Alitalo, University of Helsinki) were used to visualize lymphatic vessels. The primary antibodies were detected with goat anti-rabbit or anti-rat Alexa 594 (Molecular Probes).
Biodistribution of fluorescein-conjugated peptides was examined after i.v. injection (100 μl of 1 mM peptide solution in 200 μl of PBS) into the tail vein of a mouse. The peptide was allowed to circulate for various periods of time, and the mouse was perfused through the heart with 4% paraformaldehyde. Tissues were removed, soaked in 30% sucrose in PBS overnight, and frozen in OCT embedding medium (Tissue-Tek). Alternatively, tumor-bearing mice were i.v. injected with 500 μl of 1 mM fluorescein-conjugated peptide in PBS, and the peptide was allowed to circulate for 16–20 h.
The whole-body imaging was done under a blue light, by using the imaging system of a fluorescence stereo microscope (model LZ12; Leica, Deerfield, IL) equipped with a mercury 50-W lamp (19).
Determination of Vessel Density in Tissues. Frozen tumor sections were stained with antibodies against CD-34 and podoplanin (5) to visualize the tumor-associated blood and lymphatic vessels. Using ×200 magnification, each microscopic field in the horizontal and the vertical directions was counted for the presence of the two types of vessels.
Hypoxia. Hypoxic areas in the tumor were visualized by i.v. injection of a hypoxia marker 2-nitroimidazole (EF5) (20) into tumor-bearing mice (10 μl of 10 mM EF5 per g), followed by Cy3-conjugated mouse anti-EF5 (provided by Randall S. Johnson, University of California at San Diego). Cultured MDA-MB-435 cells were grown on coverslips and incubated overnight at 37°C to allow for attachment and spreading of the cells. Half of the cells were transferred to a hypoxia chamber (0.1% oxygen/5% CO2) and incubated overnight under hypoxic conditions. Fluorescein-conjugated peptides (10 μM) were added to the cells in 1% BSA in DMEM and incubated for 3 h, followed by fixation with 4% paraformaldehyde in PBS. The coverslips were mounted on glass slides by using Vecta-Shield mounting media with 4′,6-diamidino-2-phenylindole (Vector Laboratories).
Cytotoxicity Assay. Cytotoxic efficacy of the different peptides was judged by measuring the release of a cytoplasmic enzyme, lactate dehydrogenase, from damaged cells into the supernatant by using a colorimetric assay Cytotoxicity Detection Kit (LDH assay; Roche Diagnostics). MDA-MB-435 cells were plated on 96-well plates (6,000 cells per well) and incubated overnight at 37°C to allow for attachment and spreading of the cells. Cells were washed once with PBS, and 50 μl of 2% BSA in DMEM was added to the cells. Peptides were added in 50 μl of H2O and incubated for 24–72 h at 37°C. After the incubation, the cells were spun down (1,000 rpm, 10 min), and the supernatant was transferred to a new plate. The color reaction was added to the cells and incubated for 25 min before the absorbance was read at 492 nm. Cells incubated with 50 μl of H2O and 50 μl of 2% BSA in DMEM served as a background control, and cells incubated with 1% Nonidet P-40 showed the maximal cytotoxic value. The cytotoxicity was determined as a percentage of the maximal value after the subtraction of the background.
Tumor Treatment Studies. Tumor-bearing mice were treated with i.v. injections of peptides beginning 4 weeks after tumor cell inoculation. The injections were administered twice a week for 4–5 weeks. Tumor volumes were measured once a week and were calculated according to the formula V = width × height × depth/2, derived from the formula for the volume of an ellipsoid (21). Student's t test was used for statistical analysis of the results. The animal experiments reported here were approved by The Burnham Institute Animal Research Committee.
Synthesis of Fluorescein-Conjugated Peptides. Peptides were synthesized by using Fmoc-protected amino acids (Nova Biochem) and HATU (PE Biosystems, Foster City, CA) as a coupling reagent in dimethylformamide activated with diisiopropylethylamine. All peptides were amide-capped at the C terminus by the use of Fmoc-PAL-PEG-PS resin (PE Biosystems). The peptides were conjugated with fluorescein at the N terminus by reacting with fluorescein isothiocyanate isomer (FITC, Aldrich) in dimethylformamide for 20 h in the presence of diisiopropylethylamine.
Results
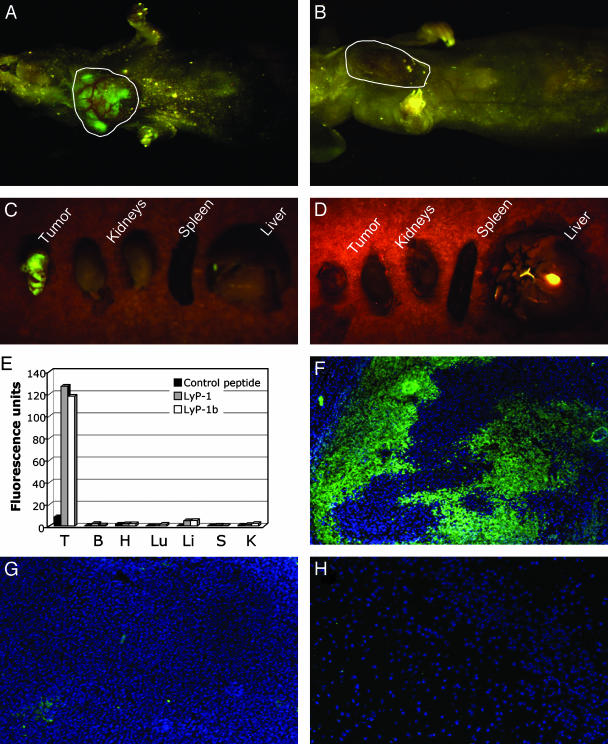
Fluorescein-Conjugated Homing Peptides Accumulate in Tumor Tissue. Intravenously injected LyP-1 peptide was observed to home to tumor-associated lymphatic vessels and tumor cells in MDA-MB-435 xenografts and some other tumors (4). In this earlier work, the peptide was allowed to circulate for <20 min. To optimize the accumulation of LyP-1 in these tumors, we studied the distribution of the peptide for longer periods of time. We found striking accumulation of fluorescein-conjugated LyP-1 in tumors several hours after the injection. At 16–20 h, the tumors of the LyP-1-injected mice were brightly fluorescent in whole-body fluorescent imaging (19) of intact mice (Fig. 1A). Tumors from the mice injected with a control peptide showed no fluorescence (Fig. 1B). Imaging of dissected tumors and organs from the same animals revealed strong fluorescence in tumors from mice injected with LyP-1, whereas no fluorescence was detectable in the tumors from the control peptide-injected mice. Other tissues showed no specific fluorescence with either peptide (Fig. 1 C and D).
Fig. 1.
Specific accumulation of lymphatic homing peptides in tumors. Mice bearing orthotopic MDA-MB-435 xenograft tumors were i.v. injected with fluorescein-conjugated LyP-1 (A)ora fluorescein-conjugated control peptide (ARALPSQRSR) (B). The mice were anesthetized 16–20 h later and examined for fluorescence under blue light. Tumor fluorescence of a LyP-1-injected tumor mouse is shown in A. No fluorescence was detected in tumors of mice injected with the control peptide (B). After the external examination, the mice were killed, and tumor, kidneys, spleen, and liver were excised and examined for fluorescence. LyP-1 produced intense fluorescence in the tumor, whereas no fluorescence was detectable in other organs (C). Even when imaged directly, no fluorescence was observed in the control peptide-injected tumor (D). The gallbladder is autofluorescent and appears as a green spot in C and D. E shows quantification of the imaging results for LyP-1 and for LyP-1b, which was analyzed in similar experiments. Mice that did not receive any fluorescent compound were used to determine the level of autofluorescence in tissues, and this background was subtracted from the experimental values. The graph shows a representative experiment of three. (F–H) Mice injected with peptides as in A and B were perfused through the heart, and their tumors were examined microscopically. Strong LyP-1 fluorescence is seen in the nuclei (visualized by 4′,6-diamidino-2-phenylindole staining) of tumor cells (F). No appreciable fluorescence from the control peptide is seen in tumor tissue (G), and all normal tissues tested were negative for all peptides (the result for LyP-1 in the brain is shown in H). T, tumor; B, brain; H, heart; Lu, lungs; Li, liver; S, spleen; K, kidneys. Magnification: F and G, ×100; H, ×200.
We then confirmed the tumor-specificity of LyP-1 by quantifying the fluorescence in the dissected tissues. Tumor fluorescence from the control peptide injection was too low to be accurately distinguished from the background, but LyP-1 concentration was at least 15- to 40-fold higher in the tumors than that of the control peptide, whereas fluorescence in other tissues was not significantly different from the background (Fig. 1E). A peptide closely related to LyP-1 (CNKRTRGGC; H.B., J. A. Hoffman, P.L., and E.R., unpublished data) also strongly accumulated in the tumors (LyP-1b in Fig. 1E). These results show that the LyP-1 peptides accumulate in the MDA-MB-435 tumors with extraordinary efficiency and that the accumulation is specific. The LyP-1 fluorescence was mainly present in tumor cell nuclei (Fig. 1F), whereas the control peptide was essentially negative in tumor tissue (Fig. 1G). No fluorescence was detected in other tissues with any of the peptides (shown for LyP-1 in brain tissue in Fig. 1H).
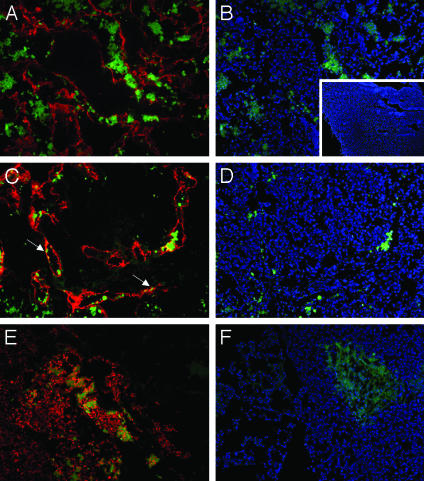
LyP-1 Recognizes Metastatic Lesions. MDA-MB-435 tumor cells transfected with the lymphangiogenic growth factor VEGF-C produce tumors with increased number of lymphatic vessels and enhanced propensity to metastasize into regional lymph nodes and the lungs (13, 16, 22). In agreement with the ability of LyP-1 to recognize tumor lymphatics, LyP-1 accumulation in the VEGF-C-expressing tumors seemed to be stronger than in the parental-line tumors (data not shown). LyP-1 peptide also homed to the metastatic lymph nodes of the MDA-MB-435/VEGF-C tumor mice (Fig. 2 A–E), colocalizing with lymphatic endothelial markers (arrows in Fig. 2C) and tumor cells (Fig. 2E) within the lymph nodes. No LyP-1 fluorescence was detected in the vessels of normal lymph nodes; the nuclei of a few isolated cells that appeared to be leukocytes were positive (Fig. 2B Inset). Metastatic foci in lungs were also positive for Lyp-1 (Fig. 2F). These results show that metastases can retain the LyP-1 binding of the primary tumor and that the same tumor can induce the LyP-1-binding epitope in the lymphatic vessels of more than one tissue.
Fig. 2.
Lymphatic homing peptide recognizes metastases of VEGF-C-expressing tumors. Fluorescein-conjugated LyP-1 peptide was i.v. injected into mice bearing orthotopic VEGF-C-expressing MDA-MB-435 tumors and allowed to circulate for 15 min. The tumor, lymph nodes, lungs, kidneys, and liver were removed and prepared for immunohistology. Lymphatic vessels in lymph node metastases (A–D) were visualized by staining with anti-LYVE-1 antibodies followed by goat anti-rabbit Alexa 594 (red, A and C). Nuclei were visualized by 4′,6-diamidino-2-phenylindole staining (blue, B and D). A and B and C and D show the same microscopic fields with different staining. LyP-1 peptide (green) is present in the nuclei of cells in and around enlarged lymphatic vessels in lymph node metastases. These cells are tumor cells as judged by their intense staining with anti-VEGF-C antibody (red, E). The peptide is also seen in the nuclei of lymphatic endothelial cells (arrows in C). No LyP-1 accumulated in a tumor-free lymph node (B Inset). A metastatic lung tumor also accumulates LyP-1 (F; LyP-1, green; nuclei, blue). Magnification, ×200; Inset, ×50.
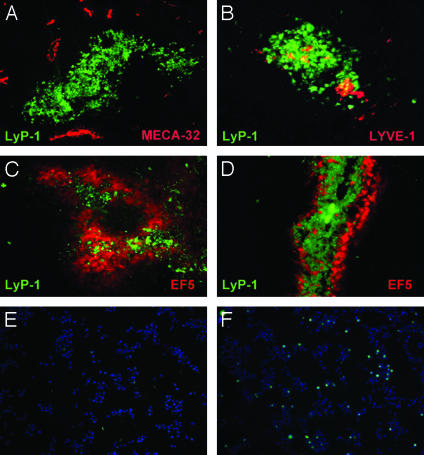
LyP-1 Peptide Recognizes Hypoxic Areas in Tumors. The tumor cells that accumulated LyP-1 formed clusters within the tumors, and these clusters contained few blood vessels (Fig. 3A) but were positive for lymphatic endothelial markers (Fig. 3B). The LyP-1-positive tumor cell clusters were strikingly similar to clusters of tumor cells revealed by uptake of hypoxia markers (23). This similarity, and the lack of blood vessels, led us to examine a possible connection between LyP-1 binding and hypoxia. Intravenously injected hypoxia reagent EF5 and fluorescein-labeled LyP-1 accumulated in the same areas in the tumors, but the staining for the two markers seemed to be mutually exclusive at the level of individual cells (Fig. 3 C and D). If EF5 was injected first, the homing of LyP-1 was reduced (Fig. 3C) and vice versa (Fig. 3D). Moreover, injecting LyP-1 or EF5 alone gave a stronger tumor signal for both compounds than the coinjections. In contrast, the accumulation of EF5 in C8161 melanoma xenografts, which are not recognized by LyP-1 (4), was unaffected by coinjecting LyP-1 (data not shown). These results indicate that LyP-1 preferentially localizes in hypoxic parts of tumors and that LyP-1 and EF5 specifically affect one another's recognition of hypoxic tumor cells.
Fig. 3.
LyP-1 peptide recognizes cell clusters that lack blood vessels but contain lymphatics. Fluorescein-conjugated LyP-1 peptide was i.v. injected into MDA-MB-435 tumor-bearing mice and allowed to circulate for 15 min. LyP-1 peptide was seen in cell clusters throughout the tumor (green, A and B). These areas did not contain blood vessels, as judged by staining with the blood vessel endothelial marker, MECA-32 (red, A) but were often positive for the lymphatic endothelial markers, LYVE-1 (red, B) and podoplanin (not shown). The hypoxia marker EF5 (red), injected 8 h before LyP-1 (green), localized in the LyP-1-positive patches within the tumors (C). Reversing the order of the injections reduced the amount of EF5 in the LyP-1-positive patches (D). The presence of the two compounds at the cellular level seemed to be mutually exclusive. (E and F) LyP-1 binding to cultured cells is increased by serum starvation. Fluorescein-conjugated LyP-1 peptide was added to MDA-MB-435 cells cultured either in 10% (E) or 0.1% (F) serum, and the binding and uptake of the peptide by the cells was determined 3 h later. Serum starvation increased the number of LyP-1-positive cells (LyP-1, green; nuclei, blue). Magnification: A–D, ×200; E and F, ×100.
Serum Starvation Increases Binding of Fluorescein-Conjugated LyP-1 to Cultured MDA-MB-435 Cells. We next sought to reproduce the effect of hypoxia on tumor cell recognition in vitro. We cultured MDA-MB-435 cells under hypoxic conditions but detected no increase in the number of cells that were positive for fluorescein-conjugated LyP-1 (data not shown). However, we did see an increase in the number of cells that had taken up LyP-1 when we maintained the cells in low serum (compare Fig. 3 E and F). Counting of LyP-1-positive cells showed that the difference was 2.5-fold. These results suggest that LyP-1 homing to tumors may not be directly related to hypoxia but may result from the attendant nutrient starvation.
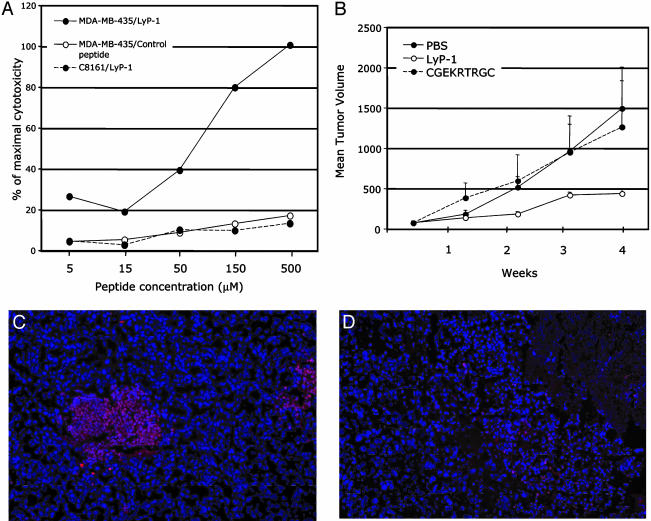
LyP-1 Binding and Internalization Induce Cell Death. Studying the internalization of the fluorescein-conjugated LyP-1 peptide in cultured cells, we noticed that the LyP-1 positive cells tended to round up, and the morphology of their nuclei frequently suggested apoptosis. To investigate whether LyP-1 caused cell death, we incubated MDA-MB-435 cells with unlabeled LyP-1 and monitored cell lysis. Incubation with LyP-1 resulted in a concentration-dependent increase in cell lysis with an IC50 of ≈66 μM (Fig. 4A). C8161 human melanoma cells, which do not bind LyP-1 (4), were not affected by the peptide. Control peptides that resemble LyP-1 in their amino acid composition and/or cyclic structure (CRVRTRSGC, Fig. 4A) and two other peptides [CGEKRTRGC, a variant of LyP-1, which has no cell-binding activity (4); and KECQSRLLSCP, (data not shown)] had no effect on the viability of either cell line. Thus, LyP-1 specifically kills cells that bind this peptide.
Fig. 4.
LyP-1 peptide causes cell death in vitro and inhibits tumor growth in vivo. (A) LyP-1 (•, solid line) causes a dose-dependent release of lactate dehydrogenase from the cultured MDA-MB-435 cells, whereas a control peptide (CRVRTRSGC, ○) has no effect. LyP-1 does not release lactate dehydrogenase from human C8161 melanoma cells (•, dotted line). (B) Mice bearing MDA-MB-435 tumors were injected twice a week with 60 μg of LyP-1 or its inactive variant (CGEKRTRGC), or with PBS. There were five mice/group; the treatment was started 4 weeks after the inoculation of the tumor cells (1–2 weeks after the tumors became palpable) and lasted 4 weeks. One experiment of three is shown. LyP-1 reduced the mean tumor volume by an average of 50% (P < 0.05). (C and D) TUNEL staining (red) reveals clusters of apoptotic cells in the LyP-1-treated (C), but not control-treated (D), tumors. Blue, 4′,6-diamidino-2-phenylindole staining of nuclei. Magnification, ×200. The error bars in B show SEM.
Systemic Treatment with LyP-1 Inhibits Tumor Growth and Reduces the Number of Tumor Lymphatics. Given that LyP-1 had an in vitro cytotoxic effect on the MDA-MB-435 tumor cells, we examined the effect of LyP-1 on tumor growth in vivo. We gave MDA-MB-435 or MDA-MB-435/VEGF-C tumor mice biweekly i.v. injections of the LyP-1 peptide, starting after the mice had established palpable tumors. Fig. 4B shows one of three similar treatment experiments. The LyP-1 peptide inhibited tumor growth formed by both cell lines. The average reduction of tumor volume relative to the control-treated mice was ≈50% and highly significant (P < 0.005). Increasing the dose of the LyP-1 peptide did not improve the efficacy of the compound (data not shown). The tumors of the LyP-1-treated animals contained numerous TUNEL-positive cells, indicating apoptosis, whereas little apoptosis was detected in the tumors of the control-treated mice (Fig. 4 C and D). The increased apoptosis in the LyP-1 group was specific for the tumor tissue; other tissues did not contain significant numbers of TUNEL-positive cells (data not shown).
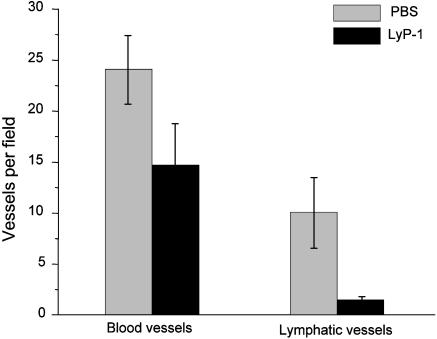
LyP-1-treatment selectively reduced the number of lymphatic vessels in the tumors, while having a less prominent effect on the blood vessel density in the same tumors (Fig. 5). These results are in agreement with the in vivo homing pattern of the fluorescein-conjugated LyP-1 peptide to the lymphatics in MDA-MB-435 tumor-bearing mice (4). It seems that the lymphatic endothelial cells in the tumor are also susceptible to LyP-1.
Fig. 5.
LyP-1 peptide reduces the number of tumor lymphatics. Tumor sections were stained with antibodies against CD-34 and podoplanin to visualize and count tumor-associated blood vessels and lymphatics. LyP-1 reduced the number of lymphatic vessels by an average of 85% (three experiments). Blood vessel density in the same tumors was affected less (average reduction, 39%). The error bars show SD.
Discussion
We report here that LyP-1, a peptide that specifically binds to tumor lymphatics and tumor cells, strongly accumulates in breast cancer xenografts after an i.v. injection. The peptide and a closely related variant of it preferentially localize in hypoxic areas within the tumors. We also show that systemically administered LyP-1 causes tumor cell apoptosis, reduces the number of tumor lymphatics, and inhibits tumor growth in mice bearing breast cancer xenografts. These results suggest that it may be possible to develop LyP-1-based cancer therapies.
The LyP-1 peptide shows strong accumulation in the MDA-MB-435 tumors, including metastases from these tumors. The efficacy and specificity of this peptide was sufficient to allow us to visualize orthotopic tumors in intact mice based on fluorescence. Although fluorescence-based imaging of tumors formed by GFP-producing cells in intact animals is possible (19), achieving it with an i.v. injected material may be unique. The remarkable tumor-homing efficiency of the LyP-1 peptide may be because of the propensity of this peptide to become internalized by cells. Cells that bind the LyP-1 peptide transport it across the cell membrane, into the cytoplasm and the nucleus. In this regard, LyP-1 is similar to the Tat peptide and other cell-penetrating peptides, which are also taken up by cells (24). An important difference is that our LyP-1 peptides are cell type-specific and deliver a payload to specific target cells: the lymphatic endothelial and tumor cells in tumors that display the “receptor” for these peptides. The internalization is likely to contribute to the effectiveness of these peptides in becoming concentrated in the targeted tumors. If this efficacy can be reproduced in clinical settings, LyP-1-directed targeting of contrast agents may become useful in tumor detection.
Our results show that treatment of tumor cells with the LyP-1 peptide causes cell death. This effect is specific because cells that do not bind LyP-1 were not affected. The tumor cell apoptosis we observed in vivo indicates that the LyP-1-binding cells die by apoptosis.
Whereas the mechanism whereby LyP-1 kills cells remains to be elucidated, the proapoptotic effect seems to be directed against tumor cells that are under stress, as LyP-1 colocalized with a tissue hypoxia marker in vivo, and serum starvation enhanced LyP-1 binding and internalization by cultured tumor cells in vitro. It will be important to identify the molecule (receptor) to which LyP-1 binds at the cell surface (and that may mediate the proapoptotic effect of LyP-1). Our efforts to isolate a LyP-1 receptor by affinity chromatography and various cloning methods have not yet been successful.
Treatment of tumor-bearing mice with the LyP-1 peptide suppressed tumor growth. It also drastically reduced the expression of lymphatic endothelial markers in the treated tumors. This latter result suggests that LyP-1 is also cytotoxic/proapoptotic for lymphatic endothelial cells in tumors. As tumor lymphatics have not been shown to be important for tumor growth (25), it is likely that the antitumor activity of LyP-1 is related to its effect on tumor cells rather than tumor lymphatics. However, given the demonstrated role of tumor lymphatics in metastasis (15, 16, 22), destroying tumor lymphatics with LyP-1 may be particularly effective in curtailing lymphatic spread of tumors. As lymphatics appear to be the first target of LyP-1 in tumors (4), the antitumor effect of LyP-1 may be particularly pronounced on tumor cells within and close to the lymphatics, which are likely to be the cells most probable to spread through the lymphatic system.
Hypoxia enhances metastasis (23, 26), and LyP-1 selectively targets tumor cells in the hypoxic areas of tumors. This may be another pathway through which LyP-1 could suppress metastasis. MDA-MB-435 tumors are highly metastatic, and the VEGF-C-expressing cells are even more aggressive in that regard. In this study, we evaluated the effects of LyP-1 on established primary tumors. As metastasis had already occurred at the time the treatment began, we could not evaluate the effect of LyP-1 on the metastatic spread. Studies to determine the effects of LyP-1 on metastasis are underway. Nonetheless, the data already at hand define this peptide as a potentially unique tool for tumor diagnosis and treatment.
Acknowledgments
We thank Dr. Randall Johnson for reagents, Drs. Kari Alitalo and Eva Engvall for comments on the manuscript, and Roslind Varghese for editing. This work was supported by National Cancer Institute Grant CA82713, Department of Defense Grant DAMD 17-02-1-0315 (to E.R.), Cancer Center Support Grant CA30199, and National Cancer Institute Grant CA099258-01 (to AntiCancer, Inc.). P.L. received support from the Academy of Finland and Biocentrum Helsinki. M.E.A. was supported by Department of Defense Fellowship DAMD17-02-1-0308.
Abbreviation: VEGF, vascular endothelial growth factor.
References
- 1.Ruoslahti, E. (2002) Nat. Rev. Cancer 2, 83-90. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman, J. A., Giraudo, E., Singh, M., Zhang, L., Inoue, M., Porkka, K., Hanahan, D. & Ruoslahti, E. (2003) Cancer Cell 4, 383-391. [DOI] [PubMed] [Google Scholar]
- 3.Joyce, J. A., Laakkonen, P., Bernasconi, M., Bergers, G., Ruoslahti, E. & Hanahan, D. (2003) Cancer Cell 4, 393-403. [DOI] [PubMed] [Google Scholar]
- 4.Laakkonen, P., Porkka, K., Hoffman, J. A. & Ruoslahti, E. (2002) Nat. Med. 8, 751-755. [DOI] [PubMed] [Google Scholar]
- 5.Breiteneder-Geleff, S., Soleiman, A., Kowalski, H., Horvat, R., Amann, G., Kriehuber, E., Diem, K., Weninger, W., Tschachler, E., Alitalo, K. & Kerjaschki, D. (1999) Am. J. Pathol. 154, 385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerji, S., Ni, J., Wang, S. X., Clasper, S., Su, J., Tammi, R., Jones, M. & Jackson, D. G. (1999) J. Cell Biol. 144, 789-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wigle, J. T. & Oliver, G. (1999) Cell 98, 769-778. [DOI] [PubMed] [Google Scholar]
- 8.Achen, M. G., Jeltsch, M., Kukk, E., Makinen, T., Vitali, A., Wilks, A. F., Alitalo, K. & Stacker, S. A. (1998) Proc. Natl. Acad. Sci. USA 95, 548-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeltsch, M., Kaipainen, A., Joukov, V., Meng, X., Lakso, M., Rauvala, H., Swartz, M., Fukumura, D., Jain, R. K. & Alitalo, K. (1997) Science 276, 1423-1425. [DOI] [PubMed] [Google Scholar]
- 10.Wigle, J. T., Harvey, N., Detmar, M., Lagutina, I., Grosveld, G., Gunn, M. D., Jackson, D. G. & Oliver, G. (2002) EMBO J. 21, 1505-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson, D. G., Prevo, R., Clasper, S. & Banerji, S. (2001) Trends Immunol. 22, 317-321. [DOI] [PubMed] [Google Scholar]
- 12.Leu, A. J., Berk, D. A., Lymboussaki, A., Alitalo, K. & Jain, R. K. (2000) Cancer Res. 60, 4324-4327. [PubMed] [Google Scholar]
- 13.Karpanen, T., Egeblad, M., Karkkainen, M. J., Kubo, H., Yla-Herttuala, S., Jaattela, M. & Alitalo, K. (2001) Cancer Res. 61, 1786-1790. [PubMed] [Google Scholar]
- 14.Pepper, M. S. (2001) Clin. Cancer Res. 7, 462-468. [PubMed] [Google Scholar]
- 15.Stacker, S. A., Caesar, C., Baldwin, M. E., Thornton, G. E., Williams, R. A., Prevo, R., Jackson, D. G., Nishikawa, S., Kubo, H. & Achen, M. G. (2001) Nat. Med. 7, 186-191. [DOI] [PubMed] [Google Scholar]
- 16.Mandriota, S. J., Jussila, L., Jeltsch, M., Compagni, A., Baetens, D., Prevo, R., Banerji, S., Huarte, J., Montesano, R., Jackson, D. G., et al. (2001) EMBO J. 20, 672-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valtola, R., Salven, P., Heikkila, P., Taipale, J., Joensuu, H., Rehn, M., Pihlajaniemi, T., Weich, H., deWaal, R. & Alitalo, K. (1999) Am. J. Pathol. 154, 1381-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saharinen, P., Tammela, T., Karkkainen, M. J. & Alitalo, K. (2004) Trends Immunol., in press. [DOI] [PubMed]
- 19.Yang, M., Baranov, E., Jiang, P., Sun, F. X., Li, X. M., Li, L., Hasegawa, S., Bouvet, M., Al-Tuwaijri, M., Chishima, T., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 1206-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lord, E. M., Harwell, L. & Koch, C. J. (1993) Cancer Res. 53, 5721-5726. [PubMed] [Google Scholar]
- 21.Schueneman, A. J., Himmelfarb, E., Geng, L., Tan, J., Donnelly, E., Mendel, D., McMahon, G. & Hallahan, D. E. (2003) Cancer Res. 63, 4009-4016. [PubMed] [Google Scholar]
- 22.Skobe, M., Hawighorst, T., Jackson, D. G., Prevo, R., Janes, L., Velasco, P., Riccardi, L., Alitalo, K., Claffey, K. & Detmar, M. (2001) Nat. Med. 7, 192-198. [DOI] [PubMed] [Google Scholar]
- 23.Rofstad, E. K., Rasmussen, H., Galappathi, K., Mathiesen, B., Nilsen, K. & Graff, B. A. (2002) Cancer Res. 62, 1847-1853. [PubMed] [Google Scholar]
- 24.Lundberg, P. & Langel, U. (2003) J. Mol. Recognit. 16, 227-233. [DOI] [PubMed] [Google Scholar]
- 25.He, Y., Kozaki, K., Karpanen, T., Koshikawa, K., Yla-Herttuala, S., Takahashi, T. & Alitalo, K. (2002) J. Natl. Cancer Inst. 94, 819-825. [DOI] [PubMed] [Google Scholar]
- 26.Zhong, H., De Marzo, A. M., Laughner, E., Lim, M., Hilton, D. A., Zagzag, D., Buechler, P., Isaacs, W. B., Semenza, G. L. & Simons, J. W. (1999) Cancer Res. 59, 5830-5835. [PubMed] [Google Scholar]